Abstract
The consequences of telomere dysfunction are most apparent in rare inherited syndromes caused by genetic deficiencies in factors that normally maintain telomeres. The principal disease is known as dyskeratosis congenita (DC), but other syndromes with similar underlying genetic defects share some clinical aspects with this disease. Currently, there are no curative therapies for these diseases of telomere dysfunction. Here, we review recent findings demonstrating that dysfunctional (i.e., uncapped) telomeres can downregulate the WNT pathway, and that restoration of WNT signaling helps to recap telomeres by increasing expression of shelterins, proteins that naturally bind and protect telomeres. We discuss how these findings are different from previous observations connecting WNT and telomere biology, and discuss potential links between WNT and clinical manifestations of the DC spectrum of diseases. Finally, we argue for exploring the use of WNT agonists, specifically lithium, as a possible therapeutic approach for patients with DC.
Keywords: WNT, telomeres, telomerase, dyskeratosis congenita, lithium, pulmonary fibrosis
Introduction
Telomeres are the tandemly repeated structures that hide and protect the ends of chromosomes, functions collectively termed capping. Capping is supported by sufficient telomere length and by the action of telomere-associated proteins called shelterins.1,2 Capped telomeres prevent the DNA damage checkpoint machinery from recognizing chromosome ends as double-strand DNA breaks (DSBs). Such checkpoint activation can lead to a host of potentially injurious molecular and cellular consequences including degradation of chromosome ends by exonucleases, recombination of these ends among themselves or to other DNA ends thereby generating unstable chromosomes, and elevated levels of cell cycle arrest and apoptosis, but also rarely the emergence of cancer cells.3,4
Telomeres shorten during chromosomal and cellular replication, and can shorten to the point of uncapping. Shortening typically occurs in a gradual fashion, for example, due to the end replication problem and to exonucleolytic resection of replicated ends to generate the single stranded 3’ overhangs needed for telomere function. But shortening may also be sudden and dramatic, for example, due to oxidative damage of telomere DNA or when a DNA replication fork breaks during replication through a telomere.5–7 Shortening can be countered by the action of the enzyme telomerase, which synthesizes new telomere repeat DNA onto existing ends, and comprises the catalytic subunit TERT, the RNA template component TERC, and several other factors.8 However, in humans, telomerase levels are limited and do not prevent telomere shortening with age.9 Telomere uncapping is thought to contribute to several age-related diseases, including cardiovascular diseases, diabetes mellitus, osteoporosis, cirrhosis, pulmonary fibrosis, and immunosenescence.10 Much of the underlying evidence has been correlational, including epidemiological evidence that people with shorter mean telomere lengths in their peripheral blood cells are at higher risk for these diseases, and that elevated levels of very short and uncapped telomeres are found within pathologic lesions.11–17 However, several other lines of evidence, the most recent of which is Mendelian randomization analysis, move beyond correlation and indicate that short telomeres play a causal role in disease pathogenesis, for example, in the cases of cardiovascular diseases, Alzheimer’s disease, and pulmonary fibrosis.17–20
The consequences of telomere dysfunction are most apparent in rare inherited syndromes caused by genetic deficiencies in factors that normally maintain telomeres. The principal disease is known as dyskeratosis congenita (DC), but other syndromes with similar underlying genetic defects and overlapping signs and symptoms also exist, including aplastic anemia (AA), Hoyeraal–Hreidarsson (HH) syndrome, Coats plus (CP) syndrome, and Revesz syndrome (RS). The clinical presentations and mutations underlying these syndromes have been well reviewed elsewhere (see Refs. 21 and 22), and so we provide only a brief overview here. The classical signs of DC typically present in childhood and include abnormal skin pigmentation, nail dystrophy, and leukoplakia. Bone marrow failure is a major cause of death, which can be treated by transplantation of normal allogeneic bone marrow, but other serious DC pathologies, including pulmonary fibrosis, cancer, vasculopathies, osteoporosis and other bone abnormalities, genitourinary malformations, liver cirrhosis, and gastrointestinal disorders are currently not well treated and contribute to morbidity and mortality. AA can be caused by the same mutations that cause DC, but which nonetheless in AA patients yield primarily bone marrow failure without other DC pathologies. In contrast, HH, CP, and RSs are generally more severe, and display defects characteristic of DC along with additional pathologies. HH typically includes intrauterine growth retardation, microcephaly, and cerebellar hypoplasia, CP includes an exudative retinal vasculopathy (in isolation known as Coats’ disease) plus GI bleeding, bone fractures, poor wound healing, and brain abnormalities including calcified cysts and loss of white matter, whereas RS combines the pathologies in CP with HH-like features including cerebellar hypoplasia and intrauterine growth retardation. Mutations causing these diseases are in genes encoding factors that support the ability of telomerase to extend telomere ends (DKC1, TINF2, TERT, TERC, NOP10, NHP2, PARN, WRAP53, ACD, and POT1), and also encoding factors that may play more complicated roles in telomere replication (CTC1, STN1, and RTEL1). However, it seems likely that even this latter set of factors impact telomere lengthening by telomerase given the similarity of the pathologic consequences of mutations in these genes and those in the first set. We mention this because the DC spectrum of diseases is often called the “telomere syndrome,” but we feel that it might be misleading to imply that these diseases reveal the full range of how telomere dysfunction can contribute to pathology. Telomere defects need not occur only in tissues that express telomerase, which as expected are the tissues that are indeed most affected in the DC spectrum of diseases, and telomere defects can arise for reasons unrelated to telomerase dysfunction. A good example of a disease caused by telomere defects in tissues lacking telomerase may be Werner syndrome (WS). WS is caused by the loss of a DNA helicase, WRN, that is critical for the normal replication of telomeres and which leads to pathologies that are most pronounced in mesenchymal tissues (e.g., dermis, adipose tissue, and bone), which naturally express little to no telomerase.6,23 Although WRN plays roles genome-wide that prevent mutations, which presumably contribute to some WS pathologies such as cancer, there is strong evidence that telomere defects contribute as well. In particular, the facts that WS defects can be suppressed experimentally by telomerase in cells and mice indicate the importance of telomere defects and also can explain the natural restriction of WS pathologies primarily tomesenchymaltissues.24–26 Thus, the DC spectrum and WS provide insight into how telomere dysfunction impacts tissues with higher versus lower levels of telomerase, respectively, and neither is likely to reveal fully how telomere defects contribute to normal age-related pathologies. Importantly, all of these diseases currently suffer from a lack of curative therapies, and thus information from basic studies might provide much needed clues.
A novel connection between WNT signaling and telomere capping
We have recently uncovered links between telomeres and the WNT intercellular signaling pathway. WNTs are a family of 19 similar proteins that are important for normal development and for lifelong tissue homeostasis through the niche-based support of stem cells.27,28 WNTs signal over short-range intercellular distances by secretion from source cells and binding to receptors on the surface of target cells. These receptors include the frizzled (FZD) family of seven-transmembrane spanning proteins and the LRP5/6 coreceptor proteins, and signaling via these receptors is potentiated by binding of the R-spondin proteins to the Lgr4/5/6 family of receptors. Canonical WNT signaling leads to the inhibition of proteins, including GSK-3, that normally cause the degradation of cytoplasmic β-catenin, allowing β-catenin to accumulate in the nucleus and thus regulate the expression of WNT target genes by complexing with the TCF/LEF family of transcription factors. Of note, the signaling pathway contains several feedback loops, for example, the Lgr5 coreceptor is encoded by a WNT-upregulated gene, which allows coordinated expression of pathway components so as to emphasize differences between cellular states having different degrees of WNT signaling. WNT can also signal through β-cateninin-dependent pathways, known as noncanonical signaling, which involves binding to FZD but not LRP receptors and can impact intracellular calcium and the activity of Rho family GTPases. How different combinations of WNT and R-spondin ligands interact with different combinations of receptors to differentially regulate canonical and noncanonical signaling is only poorly understood.27,28
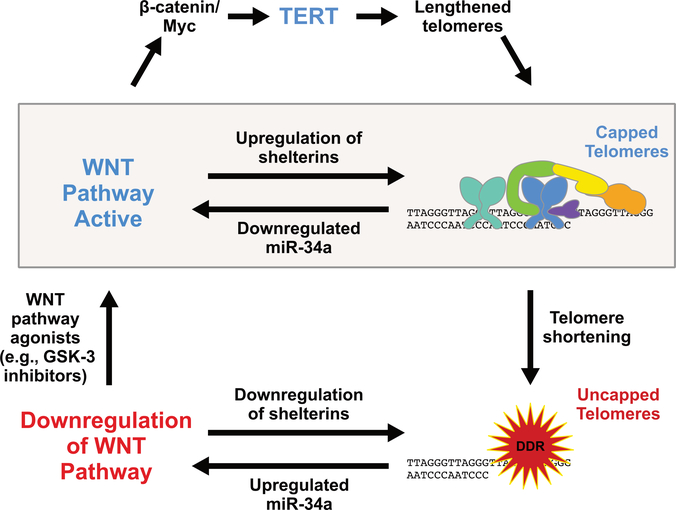
Recently, we published evidence for an unexpected connection between the WNT pathway and the capping of telomeres, which emerged from studies of mice with critical telomere shortening due to homozygous deletion of the gene expressing the RNA template component of telomerase, Terc.29 Because laboratory mice have long telomeres, initial generations of mice lacking telomerase are relatively normal, but the interbreeding of such mice for several generations eventually causes telomeres to shorten to the point of uncapping, causing pathology primarily in tissues with high rates of cell turnover, including the gastrointestinal epithelium. Prior to these published studies,29 we found in pilot studies that transplantation of normal bone marrow into late-generation Terc−/− mutants rescued intestinal pathology (see Ref. 30; and Q. Chen and F.B. Johnson, unpublished). This was surprising because the rescue included improved telomere capping and reduced levels of apoptosis in intestinal epithelial cells, even though the wild-type bone marrow-derived cells were present only in the stroma underlying the epithelium. This raised the possibility that signals coming from the wildtype cells were impacting telomere capping in a non-cell autonomous fashion, and given known roles for WNT in the support of the intestinal stem cell niche, we considered WNT signaling as a candidate. Direct tests of this idea indeed revealed a remarkably broad downregulation in late-generation Terc−/− mutants of genes expressing factors in the canonical WNT signaling pathway and in the targets of WNT signaling that mark and are required for normal function of the so-called crypt base columnar cells (CBCs), including Lgr5, Ascl2, and Sox9.29 CBCs are pluripotent stem cells that cycle frequently and give rise to all cell types in the intestinal epithelium. Consistent with frequent cell turnover, they also express high levels of telomerase.31 The downregulation of WNT signaling in mutant CBCs was not explained by cell losses, and was accompanied by a similar downregulation in the stroma and by the upregulation of WNT pathway inhibitors, indicating a regulatory response to uncapped telomeres. Importantly, pharmacological upregulation of the WNT pathway using exogenous R-spondin1, or the GSK-3 inhibitors CHIR99021 or lithium, all restored expression of WNT pathway factors and targets in the mutant epithelium,29 in the same fashion as the preliminary studies using bone marrow transplantation of normal bone marrow had done (Q. Chen and F.B. Johnson, unpublished). Remarkably, both the pharmacologic treatments and transplants also restored telomere capping in the mutant epithelium. Thus, a positive feedback loop exists in the intestine between telomere capping and WNT pathway activity (Fig. 1, shaded box). Subsequent studies comparing cultured human intestinal organoids derived from iPS cells from normal and DC (DKC1 mutant) donors revealed a similar feedback loop.32 The evolutionary advantage of such a loop may be to remove stem cells that have incurred oncogenic mutations and are thus at risk for forming tumors. Oncogene-induced replication stress would cause premature telomere breakage and uncapping, leading to the withdrawal of WNT support, and thus the death or permanent cell cycle arrest of these cells.33 Mechanistically, how telomere uncapping leads to downregulation of multiple factors in the WNT pathway is explained, at least in part, by the action of the miR-34a, which we found was the most upregulated microRNA in the intestines of late-generation telomerase mutant mice.29 miR-34a expression is p53-dependent, and thus the well-known activation of p53 by uncapped telomeres likely contributes to miR-34a upregulation.1,34 Inhibition or deletion of miR-34a rescued intestinal telomere capping, WNT gene expression, and pathology in the mutants.29 However, even though miR-34a targets WNT pathway transcripts directly, some miR-34a effects on WNT signaling and pathology may be indirect, because it also targets the transcripts of p53 and transcripts of other genes that may be involved including SIRT1.35 Moving to the other arm of the WNT-telomere feedback loop, the mechanism by which WNT signaling supports telomere capping does not involve telomere lengthening (as expected for mice fully lacking telomerase), but rather appears to ensure the proper level of shelterin proteins. Supporting this is the fact that four of six shelterins (TRF1, TRF2, TIN2, and POT1a/b) are encoded by WNT target genes, which were selectively downregulated in mutant intestine and which had their expression restored by WNT pathway agonists.29 Moreover, in cultured human DKC1 mutant intestinal organoids, TRF2 overexpression was sufficient to restore telomere capping and WNT pathway gene expression, consistent with the established capacity of TRF2 overexpression to enable replicatively aged human fibroblasts with telomeres that would be uncapped at normal levels of TRF2 to continue to maintain their capped state and thus shorten even further before activating checkpoint responses.32,36 Additional mechanisms may contribute to the telomere capping-WNT feedback loop, but regardless, the loop exists and may provide a novel approach to the therapy of people suffering from premature telomere shortening or dysfunction.
Figure 1.
A positive feedback loop connects WNT and telomere capping. In normal healthy cells, telomeres have sufficient length and shelterin occupancy to ensure the capped state, which in turn supports normal expression of WNT pathway components. This positive feedback loop is highlighted by the shaded box. As telomeres shorten, they begin to uncap, activating DNA damage responses (DDRs). The DDR upregulates miR-34a, which has many targets including WNT pathway factors, leading to the downregulation of WNT pathway factors and target genes, including those expressing the shelterins TRF1, TRF2, TIN2, and POT1. Thus, the normal mutual support between WNT signaling and telomere capping is lost. Reactivating the WNT pathway using agonists such as GSK-3 inhibitors upregulates shelterins, which promote telomere capping, thus restoring the beneficial feedback. Furthermore, in cells that can express some telomerase (e.g., normal, but also DKC1 mutant, epithelial stem cells), upregulation of TERT by WNT (top pathway) can elevate telomerase activity, thus lengthening telomeres and further promoting capping.
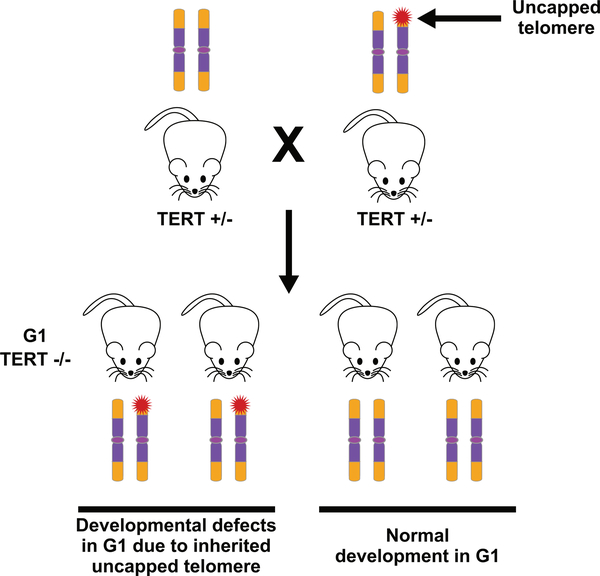
WNT has been connected to telomeres by other studies as well. First, the WNT-to-telomere capping half of the feedback loop, in particular the regulation of TERF2 by β-catenin, was described by Diala et al., who also demonstrated its importance in cancer cells.37 And the telomere capping-to-WNT half of the loop was observed by Tao et al., who observed downregulation of WNT pathway components and target gene expression in late-generation Terc−/− mutants as well as in mice exposed to gamma irradiation.38 Second, TERTis a well-established target of WNT/β-catenin and the transcriptional activator encoded by one of their key upregulated target genes, MYC, and, indeed, WNT signaling can upregulate telomerase activity.39,40 Third, TERT has been reported to complex with the β-catenin/TCF transcription complex and thereby enhance upregulation of WNT target genes, and it was proposed that this activity explains developmental abnormalities reminiscent of WNT3a deficiency that were manifest in first-generation Tert-deficient mice, prior to apparent telomere shortening.41,42 Some of the findings in this third case have been controversial, but we emphasize that this case is distinct from our positive feedback loop.43,44 In particular, the proposed role of TERT in mediating WNT signaling was revealed by TERT deficiency, whereas our mice and human organoid experiments were carried out in the context of normal TERT. Moreover, the proposed role for Tert was apparent in first-generation Terc−/− mice prior to apparent telomere uncapping, whereas our feedback loop does not falter until later generations of Terc−/− mice and at the point where they display uncapped telomeres. However, we would like to suggest a scenario, based on our feedback loop, that could potentially reconcile some of the discrepant findings. In particular, we propose that the WNT-related developmental transformations in the axial skeleton described by Park et al. might be explained by their unknowing use of one unusual Tert+/− animal in the parenting of all the G1 Tert−/− mice in their study, where the unusual characteristic was the presence of a single aberrantly shortened telomere in at least the germline of that parent (Fig. 2). Such a shortened telomere might cause a downregulation in WNT signaling at some particularly sensitive time or place during development of G1 offspring, leading to the skeletal transformations. Its aberrant shortness may have arisen from a low-frequency damaging event, for example, a broken replication fork in a telomere, or perhaps a subtelomeric region, early in the development of that parent, and the known haploinsufficiency of Tert might have prevented its efficient repair.45 Furthermore, such a telomere would be inherited by half of the offspring of the parent, consistent with the 50% penetrance reported for the skeletal transformations in the G1 mice.42 The single dysfunctional telomere would apparently not lead to the adult pathologies observed in later generation telomerase mutants, perhaps because they are unrelated to WNT or because they utilize WNT pathways that are more resistant to perturbation by partial telomere uncapping. This consideration raises the additional idea that the developmental stages and tissues in which uncapped telomeres impact WNT signaling might depend on how they are affected by different degrees of telomere uncapping, which, along with variability in the lengths of inherited telomeres, might contribute to the variable clinical presentations within the DC spectrum even among individuals with the same underlying mutation. Overall, it is clear that there are several links between the biologies of WNT and telomeres, which may reflect their cooperation to optimize the role of each in supporting progrowth states during development and within adult tissues, as well to enhance their mutual failure to block the progression of premalignant cells into cancer.
Figure 2.
Uncapped telomeres in one Tert+/− parent might explain homeotic pathologies in G1 Tert−/− mice. Park et al. (Ref. 42) described developmental transformations in the axial skeletons of half of their G1 Tert−/− mice reminiscent of those seen in Wnt3a-deficient mice, but these changes were not seen in another study using a different cohort of mice (Ref. 43). These transformations might be explained by the presence of a sporadically generated partially uncapped telomere in the germline of one of the Tert+/− parents. Fifty percent of the progeny would inherit this defective telomere, which might be sufficient to suppress WNT expression at a critical period of development, leading to the skeletal transformations.
WNT–telomere interplay may contribute to pathology in tissues beyond intestine
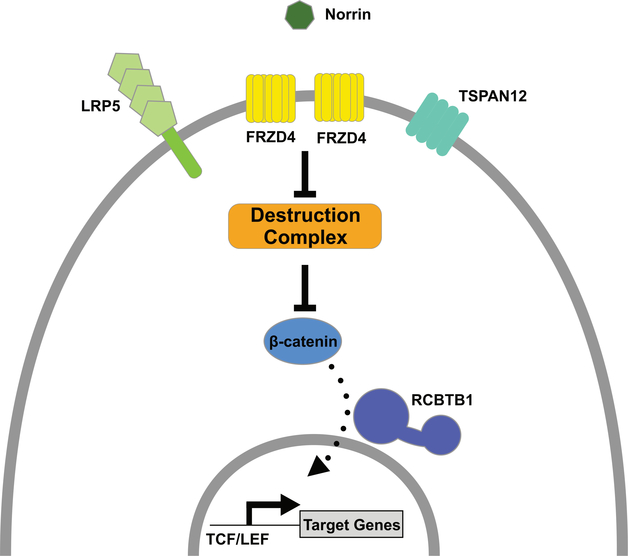
Are there additional ways in which cooperation between WNT and telomeres might impact normal biology and disease pathogenesis in DC spectrum syndromes? Given the numerous roles of WNT in development and tissue homeostasis, it would not be surprising if several potential connections could be argued, at least superficially. For example, the neural tube closure defects in late-generation telomerase mutant mice and the cerebellar defects in the DC spectrum might each be related to established roles for WNT signaling in central nervous system (CNS) development.46–52 How different parts of the CNS would be affected preferentially in these different settings is unknown but might be related to the possibility mentioned above, that how uncapped telomeres and WNT signaling affect one another in different tissues and stages of development could depend on how these settings are affected by different degrees of telomere uncapping. Similar to the CNS idea, WNT signaling plays important roles in hepatocyte regeneration in response to liver injury, and thus defects in these might be related to the development of cirrhosis in DC.53–55 The idea that WNT–telomere connections underlie these examples is speculative, but could be tested. Some additional evidence connecting WNT to DC-related pathologies is more compelling. In particular, perturbed WNT signaling might contribute to vasculopathies in DC, which are increasingly appreciated as a significant component of the syndrome, particularly after bone marrow transplant.56 These include arteriovenous malformations, telangiectasias, exudative retinopathy, and gastrointestinal bleeding. Remarkably, the genetic mutations that appear to underlie certain retinal vasculopathies having substantial phenotypic similarity to those in the DC spectrum are in loci that encode several components of a canonical WNT signaling pathway. For example, familial exudative vitreoretinopathy (FEVR) is caused by mutations in genes encoding WNT receptor proteins (FZD4 and LRP5), in genes encoding proteins that enhance signaling through β-catenin (TSPAN12 and RCBTB1), and in β-catenin itself (CTNNB1) (Fig. 3).57,58 Also, Coats’ disease (from which “Coats plus syndrome” is derived) is associated with mutation in RCBTB1.59 Both FEVR and Coats’ disease are also associated with mutations in Norrin, which signals through the FZD4/LRP5/TSPAN12/RCBTB1/β-catenin pathway, despite the fact that it is not a WNT protein, but rather a TGF-β family member.60 Regardless, its dependence on the downstream machinery of the canonical WNT pathway could make its actions susceptible to telomere dysfunction. The retinal restriction of these diseases, compared with the more widespread vasculopathies observed in DC-spectrum diseases, may reflect telomere-based perturbation of WNT signaling in a broader array of tissues and types of blood vessels than the perturbations caused by FEVR and Coats’ type mutations. Other examples supporting vascular connections between WNT and DC include (1) prominent WNT-related gene expression changes in lesions from hereditary hemorrhagic telangiectasia patients (which have vasculopathies similar to those in DC), (2) the brain and yolk sac vasculopathies observed in mice deficient for WNT7a/b or endothelial β-catenin, and (3) changes in WNT-related gene expression in diabetic retinopathy.61–64 Mechanistically, vascular leakiness in these disorders, including compromised blood–retinal and blood–brain barriers, may be connected with diminished expression of the WNT target genes CLDN1 and CLDN3, encoding tight junction proteins that normally enforce these barriers.65
Figure 3.
WNT pathway mutations underlie vitreoretinopathies that have phenotypic overlap with pathologies seen in the DC spectrum diseases. Exudative retinopathies are a component of two telomere disorders (Coats plus and Revesz syndrome), and their retinal appearance is similar to that observed in familial exudative vitreoretinopathy (FEVR) and Coats’ disease. FEVR is caused by mutations in genes encoding a WNT signaling pathway (LRP5, FRZD4, TSPAN12, RCBTB1, and CTNNB1 (β-catenin)) utilized by the NORRIN protein, and Coats’ diseases is associated with mutations in NORRIN and RCBTB1. It is therefore possible that the retinopathies in telomere disorders are caused by inhibition of WNT pathway activity induced by telomere dysfunction.
The lung is another tissue in which WNT–telomere connections may contribute to disease pathogenesis in DC, particularly pulmonary fibrosis. Mounting evidence suggests that alveolar type II (ATII) cells play a key role in pathogenesis. ATII cells are stem cells whose progeny can differentiate into the ATI cells that occupy most of the lung surface area; they are important in the repair of alveolar injury66 and several lines of evidence link changes in telomeres and WNT signaling in these cells to pulmonary fibrosis. In the normal development of mice, ATII cells emerge after a wave of WNT signaling sweeps through the developing lung; ATII cell development in cultured human alveolar organoids depends on WNT.67,68 Furthermore, there is evidence from organoid culture of ATII cells with LGR5+ fibroblasts that WNT3a and WNT5a are important for expansion and differentiation of ATII cells.69 Experimental telomere dysfunction in mouse ATII cells can induce lung inflammation and fibrosis.70–72 Knockout of the β-catenin gene (Ctnnb1) in ATII cells of adult mice sensitizes them to fibrosis and delays their ability to recover from bleomycin-induced damage.73 ATII cells sorted from lungs of human patients with IPF exhibit upregulation of miR-34a and senescence-associated β-galactosidase activity.74 Recent advances in lung embryology yielded a protocol for generating, from human iPS cells, alveolar epithelial cell organoids containing high levels of ATII cells.68 Such organoids derived from DC patient cells will likely provide a new model in which to test if WNT agonism can provide benefit in the alveolar compartment.
Even though WNT signaling may protect against events that cause telomeres to uncap and thus be recognized as DSBs, it may not be able to prevent the appearance of DNA damage more generally. As mentioned above, a recent investigation of mice possessing uncapped telomeres caused by telomerase deficiency revealed downregulation of WNT pathway and target gene expression in small intestine, consistent with our findings.38 These investigators also reported a similar downregulation in WNT pathway expression in mice exposed to a high dose of gamma irradiation. However, in seeming contrast to the findings in telomerase-deficient mice, activation of WNT signaling enhanced, rather than suppressed, the radiosensitivity of intestinal stem cells. The different results may reflect different consequences of WNT signaling that depend on the intensity or precise molecular nature of the DNA damage in each case, in at least two respects. First, the mice were irradiated with 6–12 Gy of gamma rays, which would be estimated to generate 120–240 DSBs per cell, in addition to numerous other DNA and cellular lesions, whereas many fewer uncapped telomeres (approximately five) are sufficient to activate checkpoint responses.75,76 Therefore, the intensity of DNA damage signals should be much greater in the gamma irradiated cells compared to those with uncapped telomeres, which might affect the requirements to suppress the signals as well as how they are interpreted by cells. Second, and moreover, the WNT-dependent upregulation of shelterin proteins that recaps telomeres would be expected to be of less benefit at nontelomeric DSBs, given the preferential targeting and benefit of shelterin at telomeres versus generic DNA ends.29,32,77 Overall, these findings highlight a special relationship between WNT signaling and telomeres.
The therapeutic potential of WNT pathway agonists in telomere uncapping diseases
What are the prospects for using WNT pathway agonists to improve telomere capping, and thus reduce pathology, in DC spectrum patients? As described above, treatment with agents that enhance β-catenin–dependent signaling, including lithium, improves capping and tissue homeostasis in the intestines of late-generation Terc−/− mice and in human intestinal organoids with DKC1 mutations. Of note, although our studies in mice that completely lack telomerase activity demonstrated that the positive feedback loop between WNT pathway activity and telomere capping did not require telomere lengthening, our studies in DKC1 human intestinal organoids demonstrated a dual benefit of WNT pathway agonist treatment. Telomere capping in these mutant organoids was improved, presumably in part by the feedback loop, but also by the increased telomerase activity leading to telomere lengthening (Fig. 1). The upregulated telomerase activity can be explained by the WNT-dependent expression of the catalytic subunit of telomerase, TERT, given that TERT levels are generally limiting for telomerase activity and given that DC mutations are generally hypormorphic and thus support TERT-dependent effects.32 However, whether DC-causing mutations other than in DKC1 can respond similarly to WNT pathway agonists to upregulate telomerase activity needs further testing. Lithium, which inhibits GSK-3, has been FDA approved since the 1970s for bipolar disorder and, although its therapeutic index is low, its safety and toxicity profile are well known. Furthermore, it is approved for use in pediatric patients, it stimulates granulopoiesis, it shows potential utility in the treatment of AA, and observational studies suggest that lithium leads to telomere lengthening in bipolar patients, indicating that telomeres are impacted by clinically relevant doses of the drug (although we caution that the conclusion related to telomere lengthening might be viewed as tentative, because it relies primarily on case–control studies and on a qPCR-based telomere measurement technique that can suffer from inaccuracy and imprecision).78–85 Therefore, lithium should be considered as a candidate for a clinical trial to test for potential benefits in DC spectrum patients, although it would be helpful to first study the consequences of lithium in additional tissues in mice and human culture models of DC pathology. Also, WNT pathway agonists that are more selective than lithium might provide greater benefit, and we note that the GSK-3 inhibitor tideglusib, which has been studied in a phase II trial for Alzheimer’s disease, has an acceptable safety profile.86
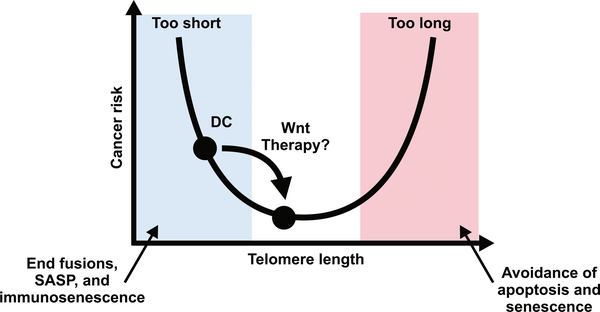
It is important to consider the potential risks associated with lithium (or other WNT pathway agonist) therapy in DC. To our knowledge, these risks are cancer, fibrosis, and unwanted cognitive effects, but there may be others of which we are unaware. DC patients have an elevated risk of cancer, and given the association of mutations that activate the WNT pathway with certain cancers in the general population, this raises concern about GSK-3 inhibitor use in DC patients. However, several considerations temper this concern. First, lithium treatment did not further elevate cancer rates in mice with the Apcmin mutation, which enhances WNT signaling and thus predispose to colorectal tumors.87 Second, long-term studies of people taking lithium for bipolar disorder show no increased cancer risk, and in fact, younger bipolar patients show an overall cancer risk reduction.84,88,89 These and other findings suggest that lithium is unlikely to cause the same level of WNT activation caused by procancer Apc mutations.90–92 Third, cancer can be driven by telomeres that are either too long or too short: overly long telomeres inhibit the apoptosis or cell senescence that would otherwise prevent carcinogenesis, whereas overly shortened and uncapped telomeres drive cancer by causing chromosome instability, immunosenescence, and the release of procancer cytokines from senescent cells (Fig. 4).93 Given premature telomere shortening in DC patients, restoring telomeres to more normal lengths and degrees of capping might diminish cancer risk in DC, and we therefore speculate that lithium might suppress cancer rates in DC even more than it does in bipolar patients.
Figure 4.
Optimization of telomere length and capping. Cancer risk has a U-shaped relationship to telomere length. Telomeres that are too short lead to end-to-end fusions, the engagement of the senescence-associated secretory phenotype (SASP), and immunosenescence, each of which can have procarcinogenic effects. On the other side, when telomeres are too long, cells that acquire oncogenic mutations can bypass apoptosis and senescence programs that shortened telomeres would normally engage, also promoting cancer. We propose that the elevated cancer risk in DC is caused by telomeres that are too short. By restoring optimal telomere lengths and capping, cancer risk in DC might be reduced.
Another potential risk of therapeutic use of WNT agonists in the DC spectrum is the exacerbation of fibrosis, particularly in the lung. There is evidence for enhanced WNT/β-catenin signaling in both alveolar epithelium and in fibroblastic foci in the lungs of individuals with idiopathic pulmonary fibrosis (but without apparent DC).94,95 Furthermore, some findings from mouse studies argue that excess WNT signaling promotes pulmonary fibrosis.96–98 However, as noted above, canonical WNT signaling clearly plays important roles in normal alveolar homeostasis, and it is possible that a primary defect in WNT signaling in alveolar epithelial cells, leading to failure to maintain alveolar integrity, could signal a secondary fibrotic response that is also WNT-dependent.70–72 Re-establishing epithelial homeostasis might prevent activation of fibrosis entirely, thus also blocking any potential direct effects of WNT on fibrosis.73 Furthermore, the mouse studies used bleomycin, a potent DSB inducer, to induce damage that is more acute and severe than the chronic and gradual accumulation of uncapped telomeres underlying pulmonary fibrosis in DC, and it is thus unclear how accurately the bleomycin model reflects pathogenic mechanisms in DC. Of note, there is evidence that lithium can be helpful in promoting lung repair in a model of emphysema induced by elastase, including a restoration of levels of nuclear β-catenin in alveolar epithelial cells, expression of WNT target genes, and reduced collagen levels.99 Differential activation of canonical over noncanonical WNT signaling might also be beneficial, given that enhanced noncanonical signaling contributes to TGF-β-induced pulmonary fibrosis in mice.100 Overall, more must be understood before it can be predicted what is the balance of potential positive and negative effects of lithium or other WNT agonists on the pathogenesis of pulmonary fibrosis in DC.
As for the cognitive effects of lithium, its effects in nonpsychiatric patients are not well studied, but one study reported that normal subject given lithium generally reported a decrease in subjective feelings of wellbeing and experienced other problems including memory impairment.101 However, the doses and administration schedule used in this study were higher and different from those typically used in the treatment of bipolar disorder. Furthermore, it is possible that the dose of lithium required to improve telomere capping is lower than that used for the treatment of bipolar disorder. Consistent with this possibility, the ratio of lithium in CSF to plasma in bipolar patients is generally less than 0.3, indicating that sufficiently high lithium levels might be achieved in the periphery without major CNS side effects.102
More work needs to be done to assess the potential risks and benefits of WNT-related therapies in DC spectrum diseases and to study how WNT agonism might affect diseases that affect other aspects of telomere biology such as WS. Furthermore, it will be important to determine if such therapies might also be of benefit for diseases in which normal age-related telomere shortening is a contributing factor.
Acknowledgments
We would like to thank Chris Lengner, Peter Klein, Robert Pignolo, Ting Yang, John Lynch, Anil Rustgi, John Sedivy, Peter Adams, Alison Bertuch, Suneet Agarwal, Mary Armanios, Lea Harrington, Calvin Kuo, Vincent Geli, Foteini Mourkiati, Ergun Sahin, Ed Morrisey, and Klaus Kaestner for helpful discussions, and also thank Qijun Chen for her efforts in conducting many of the experiments described herein. This work was supported by NIH grants R21 AG054209 (F.B.J.) and 5T32AG000255 (R.J.F.), and a grant from DC Outreach and the Penn Orphan Disease Center. F.B.J. and R.J.F. cowrote the review.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Herbig U, Jobling WA, Chen BPC, et al. 2004. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21CIP1, but not p16INK4a. Mol. Cell 14: 501–513. [DOI] [PubMed] [Google Scholar]
- 2.Doksani Y & de Lange T. 2014. The role of double-strand break repair pathways at functional and dysfunctional telomeres. Cold Spring Harb. Perspect. Biol 6: a016576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossiello F, Herbig U, Longhese MP, et al. 2014. Irreparable telomeric DNA damage and persistent DDR signalling as a shared causative mechanism of cellular senescence and ageing. Curr. Opin. Genet. Dev. 26: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suram A & Herbig U. 2014. The replicometer is broken: telomeres activate cellular senescence in response to genotoxic stresses. Aging Cell 13: 780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harley CB, Futcher AB & Greider CW. 1990. Telomeres shorten during ageing of human fibroblasts. Nature 345: 458–460. [DOI] [PubMed] [Google Scholar]
- 6.Crabbe L, Verdun RE, Haggblom CI, et al. 2004. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science 306: 1951–1953. [DOI] [PubMed] [Google Scholar]
- 7.Sfeir A, Kosiyatrakul ST, Hockemeyer D, et al. 2009. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell 138: 90–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt JC & Cech TR. 2015. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev. 29: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright WE, Piatyszek MA, Rainey WE, et al. 1996. Telomerase activity in human germline and embryonic tissues and cells. Dev. Genet. 18: 173–179. [DOI] [PubMed] [Google Scholar]
- 10.Yang T-LB, Song S & Johnson FB. 2016. Contributions of telomere biology to human age-related disease In Handbook of the Biology of Aging. 8th ed. Kaeberlein MR & Martin GM, Eds.: 205–239. Academic Press. [Google Scholar]
- 11.Jeyapalan JC, Ferreira M, Sedivy JM, et al. 2007. Accumulation of senescent cells in mitotic tissue of aging primates. Mech. Ageing Dev. 128: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan RG, Ives SJ, Lesniewski LA, et al. 2013. Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. Am. J. Physiol. Heart Circ. Physiol. 305: H251–H258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbo M, Delaisse JM, Kjaersgaard-Andersen P, et al. 2013. The relationship between ultra-short telomeres, aging of articular cartilage and the development of human hip osteoarthritis. Mech. Ageing Dev. 134: 367–372. [DOI] [PubMed] [Google Scholar]
- 14.Wiemann SU, Satyanarayana A, Tsahuridu M, et al. 2002. Hepatocyte telomere shortening and senescence are general markers of human livercirrhosis. FASEB J. 16: 935–942. [DOI] [PubMed] [Google Scholar]
- 15.Friis-Ottessen M, Bendix L, Kølvraa S, et al. 2014. Telomere shortening correlates to dysplasia but not to DNA aneuploidy in longstanding ulcerative colitis. BMC Gastroenterol. 14: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cawthon RM, Smith KR, O’Brien E, et al. 2003. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet 361: 393–395. [DOI] [PubMed] [Google Scholar]
- 17.Haycock PC, Burgess S, Nounu A, et al. 2017. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 3: 636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagg S, Zhan Y, Karlsson R, et al. 2017. Short telomere length is associated with impaired cognitive performance in European ancestry cohorts. Transl. Psychiatry 7: e1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atzmon G, Cho M, Cawthon RM, et al. 2010. Genetic variation in human telomerase is associated with telomere length in Ashkenazi centenarians. Proc. Natl. Acad. Sci. USA 107: 1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Codd V, Nelson CP, Albrecht E, et al. 2013. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet. 45: 422–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savage SA 2009. Dyskeratosis congenita In Gene Reviews. Adam MP, Ardinger HH, Pagon RA, et al. , Eds. Seattle, WA: GeneReviews®; [Internet] Accessed February 26, 2018 https://www.ncbi.nlm.nih.gov/books/NBK22301/. [Google Scholar]
- 22.Bertuch AA 2016. The molecular genetics of the telomere biology disorders. RNA Biol. 13: 696–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shamanna RA, Croteau DL, Lee J-H, et al. 2017. Recent advances in understanding Werner syndrome. F1000Res. 6: 1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson FB, Marciniak RA, McVey M, et al. 2001. The Saccharomyces cerevisiae WRN homolog Sgs1p participates in telomere maintenance in cells lacking telomerase. EMBO J. 20: 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du XB, Shen J, Kugan N, et al. 2004. Telomere shortening exposes functions for the mouse Werner and bloom syndrome genes. Mol. Cell. Biol. 24: 8437–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabbe L, Jauch A, Naeger CM, et al. 2007. Telomere dysfunction as a cause of genomic instability in Werner syndrome. Proc. Natl. Acad. Sci. USA 104: 2205–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nusse R & Clevers H. 2017. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169: 985–999. [DOI] [PubMed] [Google Scholar]
- 28.Mah AT, Yan KS & Kuo CJ. 2016. Wnt pathway regulation of intestinal stem cells. J. Physiol. 594: 4837–4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang T-LB, Chen Q, Deng JT, et al. 2017. Mutual reinforcement between telomere capping and canonical Wnt signalling in the intestinal stem cell niche. Nat. Commun. 8: 14766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh L, Brennan TA, Kim J-H, et al. 2013. Brief report: long-term functional engraftment of mesenchymal progenitor cells in a mouse model of accelerated aging. Stem Cells 31: 607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schepers AG, Vries R, van den Born M, et al. 2011. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 30: 1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo D-H, Chen Q, Yang T-LB, et al. 2016. Enhancing a Wnt-telomere feedback loop restores intestinal stem cell function in a human organotypic model of dyskeratosis congenita. Cell Stem Cell 19: 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suram A, Kaplunov J, Patel PL, et al. 2012. Oncogene-induced telomere dysfunction enforces cellular senescence in human cancer precursor lesions. EMBO J. 31: 2839–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li XJ, Ren ZJ & Tang JH. 2014. MicroRNA-34a: a potential therapeutic target in human cancer. Cell Death Dis. 5: e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Navarro F & Lieberman J. 2015. miR-34 and p53: new insights into a complex functional relationship. PLoS One 10: e0132767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karlseder J, Smogorzewska A & de Lange T. 2002. Senescence induced by altered telomere state, not telomere loss. Science 295: 2446–2449. [DOI] [PubMed] [Google Scholar]
- 37.Diala I, Wagner N, Magdinier F, et al. 2013. Telomere protection and TRF2 expression are enhanced by the canonical Wnt signalling pathway. EMBO Rep. 14: 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao S,Tang D,Morita Y,et al. 2015Wntactivity and basal niche position sensitize intestinal stem and progenitor cells to DNA damage. EMBO J. 34: 624–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmeyer K, Raggioli A, Rudloff S, et al. 2012. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science 336: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 40.Khattar E & Tergaonkar V. 2017. Transcriptional regulation of telomerase reverse transcriptase (TERT) by MYC. Front. Cell Dev. Biol. 5: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi J, Southworth LK, Sarin KY, et al. 2008. TERT promotes epithelial proliferation through transcriptional control of a Myc-and Wnt-related developmental program. PLoS Genet. 4: e10–0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J-I, Venteicher AS, Hong JY, et al. 2009. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature 460: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strong MA, Vidal-Cardenas SL, Karim B, et al. 2011. Phenotypes in mTERT(+/−) and mTERT(−/−) mice are due to short telomeres, not telomere-independent functions of telomerase reverse transcriptase. Mol. Cell. Biol. 31: 2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Listerman I, Gazzaniga FS & Blackburn EH. 2014. An investigation of the effects of the core protein telomerase reverse transcriptase on Wnt signaling in breast cancer cells. Mol. Cell. Biol. 34: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erdmann N, Liu Y & Harrington L.2004. Distinct dosage requirements for the maintenance of long and short telomeres in mTert heterozygous mice. Proc. Natl. Acad. Sci. USA 101: 6080–6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carter M, Chen X, Slowinska B, et al. 2005. Crooked tail (Cd) model of human folate-responsive neural tube defects is mutated in Wnt coreceptor lipoprotein receptor-related protein 6. Proc. Natl. Acad. Sci. USA 102: 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamblet NS, Lijam N, Ruiz-Lozano P, et al. 2002. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129: 5827–5838. [DOI] [PubMed] [Google Scholar]
- 48.Zhao T, Gan Q, Stokes A, et al. 2014. β-Catenin regulates Pax3 and Cdx2 for caudal neural tube closure and elongation. Development 141: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pei Y, Brun SN, Markant SL, et al. 2012. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development 139:1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Subashini C, Dhanesh SB, Chen C-M, et al. 2017. Wnt5a is a crucial regulator of neurogenesis during cerebellum development. Sci. Rep. 7: 42523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang Y, Zhang Q, Song N-N, et al. 2016. Lrp5/6 are required for cerebellar development and for suppressing TH expression in Purkinje cells via β-catenin. Mol. Brain 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herrera E, Samper E & Blasco MA. 1999. Telomere shortening in mTR(−/−) embryos is associated with failure to close the neural tube. EMBO J. 18: 1172–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Apte U, Singh S, Zeng G, et al. 2009. Beta-catenin activation promotes liver regeneration after acetaminophen-induced injury. Am. J. Pathol. 175: 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang B, Zhao L, Fish M, et al. 2015. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature 524: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell JO & Monga SP. 2018. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu. Rev. Pathol. 13: 351–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khincha P, Savage S, Alter B, et al. 2016. Pulmonary arteriovenous malformations: an uncharacterized phenotype of dyskeratosis congenita. Pediatr. Blood Cancer 63: S60. [Google Scholar]
- 57.Lai MB, Zhang C, Shi J, et al. 2017. TSPAN12 is a norrin co-receptor that amplifies frizzled4 ligand selectivity and signaling. Cell Rep. 19: 2809–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Panagiotou ES, Soriano CS, Poulter JA, et al. 2017. Defects in the cell signaling mediator β-catenin cause the retinal vascular condition FEVR. Am. J. Hum. Genet. 100: 960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J-H, Liu J-H, Ko Y-C, et al. 2016. Haploinsufficiency of RCBTB1 is associated with Coats disease and familial exudative vitreoretinopathy. Hum. Mol. Genet. 25: 1637–1647. [DOI] [PubMed] [Google Scholar]
- 60.Chen ZY, Battinelli EM, Fielder A, et al. 1993. A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat. Genet. 5: 180–183. [DOI] [PubMed] [Google Scholar]
- 61.Stenman JM, Rajagopal J, Carroll TJ, et al. 2008. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science 322: 1247–1250. [DOI] [PubMed] [Google Scholar]
- 62.Birdsey GM, Shah AV, Dufton N, et al. 2015. The endothelial transcription factor ERG promotes vascular stability and growth through Wnt/β-catenin signaling. Dev. Cell 32: 82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Torring PM, Larsen MJ, Kjeldsen AD, et al. 2015. Global gene expression profiling of telangiectasial tissue from patients with hereditary hemorrhagic telangiectasia. Microvasc. Res. 99: 118–126. [DOI] [PubMed] [Google Scholar]
- 64.Drenser KA 2016. Wnt signaling pathway in retinal vascularization. Eye Brain 8: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tran KA, Zhang X, Predescu D, et al. 2016. Endothelial β-catenin signaling is required for maintaining adult blood–brain barrier integrity and central nervous system homeostasis. Circulation 133: 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barkauskas CE, Cronce MJ, Rackley CR, et al. 2013. Type 2 alveolar cells are stem cells in adult lung. J. Clin. Invest. 123: 3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frank DB, Peng T, Zepp JA, et al. 2016. Emergence of a wave of Wnt signaling that regulates lung alveologenesis by controlling epithelial self-renewal and differentiation. Cell Rep. 17: 2312–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacob A, Morley M, Hawkins F, et al. 2017. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21: 472–488. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee J-H, Tammela T, Hofree M, et al. 2017. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell 170: 1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Povedano JM, Martinez P, Flores JM, et al. 2015. Mice with pulmonary fibrosis driven by telomere dysfunction. Cell Rep. 12: 286–299. [DOI] [PubMed] [Google Scholar]
- 71.Naikawadi RP, Disayabutr S, Mallavia B, et al. 2016. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight 1: e86704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alder JK, Barkauskas CE, Limjunyawong N, et al. 2015. Telomere dysfunction causes alveolar stem cell failure. Proc. Natl. Acad. Sci. USA 112: 5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tanjore H, Degryse AL, Crossno PF, et al. 2013. Β-Catenin in the alveolar epithelium protects from lung fibrosis after intratracheal bleomycin. Am. J. Respir. Crit. Care Med. 187: 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Disayabutr S, Kim EK, Cha S-I, et al. 2016. miR-34 miRNA sregulate cellular senescence in type II alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One 11: e0158367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asaithamby A & Chen DJ. 2009. Cellular responses to DNA double-strand breaks after low-dose gamma-irradiation. Nucleic Acids Res. 37: 3912–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaul Z, Cesare AJ, Huschtscha LI, et al. 2012. Five dysfunctional telomeres predict onset of senescence in human cells. EMBO Rep. 13: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams ES, Stap J, Essers J, et al. 2007. DNA double-strand breaks are not sufficient to initiate recruitment of TRF2. Nat. Genet. 39: 696–698. [DOI] [PubMed] [Google Scholar]
- 78.Köse C¸ inar R. 2017. Telomere length and hTERT in mania and subsequent remission. Rev. Bras. Psiquiatr 351 10.1590/1516-4446-2017-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barrett AJ 1980. Haematological effects of lithium and its use in treatment of neutropenia. Blut 40: 1–6. [DOI] [PubMed] [Google Scholar]
- 80.Dempsey GM 1980. Lithium carbonate in aplastic anemia. Arch. Gen. Psychiatry 37: 720. [DOI] [PubMed] [Google Scholar]
- 81.Amano I, Morii T, Yamanaka T, et al. 1999. Successful lithium carbonate therapy for a patient with intractable and severe aplastic anemia. Rinsho Ketsueki 40: 46–50. [PubMed] [Google Scholar]
- 82.Powell TR, Dima D, Frangou S, et al. 2018. Telomere length and bipolar disorder. Neuropsychopharmacology 43: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Squassina A, Pisanu C, Congiu D, et al. 2016. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur. Neuropsychopharmacol. 26: 1241–1247. [DOI] [PubMed] [Google Scholar]
- 84.Martinsson L, Wei Y, Xu D, et al. 2013. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl. Psychiatry 3: e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aviv A, Hunt SC, Lin J, et al. 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 39: e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lovestone S, Boada M, Dubois B, et al. 2015. A phase II trial of tideglusib in Alzheimer’s disease. J. Alzheimers Dis. 45: 75–88. [DOI] [PubMed] [Google Scholar]
- 87.Gould TD, Gray NA & Manji HK. 2003. Effects of aglycogen synthase kinase-3 inhibitor, lithium, in adenomatous polyposis coli mutant mice. Pharmacol. Res. 48: 49–53. [PubMed] [Google Scholar]
- 88.Martinsson L, Westman J, Hallgren J, et al. 2016. Lithium treatment and cancer incidence in bipolar disorder. Bipolar Disord. 18: 33–40. [DOI] [PubMed] [Google Scholar]
- 89.Huang R-Y, Hsieh K-P, Huang W-W, et al. 2016. Use of lithium and cancer risk in patients with bipolar disorder: population-based cohort study. Br. J. Psychiatry 209: 395–401. [DOI] [PubMed] [Google Scholar]
- 90.Luis TC, Naber BA, Roozen PP, et al. 2011. Canonical Wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell 9: 345–356. [DOI] [PubMed] [Google Scholar]
- 91.Kielman MF, Rindapaa M, Gaspar C, et al. 2002. Apc modulates embryonic stem-cell differentiation by controlling the dosage of beta-catenin signaling. Nat. Genet. 32: 594–605. [DOI] [PubMed] [Google Scholar]
- 92.Sievers S, Fritzsch C, Grzegorczyk M, et al. 2006. Absolute beta-catenin concentrations in Wnt pathway-stimulated and non-stimulated cells. Biomarkers 11: 270–278. [DOI] [PubMed] [Google Scholar]
- 93.Campisi J 2013. Aging, cellular senescence, and cancer. Annu. Rev. Physiol 75: 685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chilosi M, Poletti V, Zamo A, et al. 2003. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am. J. Pathol. 162: 1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Königshoff M, Balsara N, Pfaff E-M, et al. 2008. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One 3: e2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Henderson WRJ, Chi EY, Ye X, et al. 2010. Inhibition of Wnt/beta-catenin/CREB binding protein (CBP) signaling reverses pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 107: 14309–14314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ulsamer A, Wei Y, Kim KK, et al. 2012. Axin pathway activity regulates in vivo pY654-β-catenin accumulation and pulmonary fibrosis. J. Biol. Chem. 287: 5164–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang C, Zhu H, Sun Z, et al. 2014. Inhibition of Wnt/β-catenin signaling promotes epithelial differentiation of mesenchymal stem cells and repairs bleomycin-induced lung injury. Am. J. Physiol. Cell Physiol. 307: C234–C244. [DOI] [PubMed] [Google Scholar]
- 99.Kneidinger N, Yildirim AÖ, Callegari J, et al. 2011. Activation of the WNT/β-catenin pathway attenuates experimental emphysema. Am. J. Respir. Crit. Care Med. 183: 723–733. [DOI] [PubMed] [Google Scholar]
- 100.Baarsma HA, Skronska-Wasek W, Mutze K, et al. 2017. Noncanonical WNT-5A signaling impairs endogenous lung repair in COPD. J. Exp. Med. 214: 143–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Karniol IG, Dalton J & Lader MH. 1978. Acute and chronic effects of lithium-chloride on physiological and psychological measures in normals. Psychopharmacology 57: 289–294. [DOI] [PubMed] [Google Scholar]
- 102.Platman SR & Fieve RR. 1968. Biochemical aspects of lithium in affective disorders. Arch. Gen. Psychiatry 19: 659–663. [DOI] [PubMed] [Google Scholar]