Abstract
Aim
This case series demonstrated that phthalocyanine derivate mouthwash is a promising alternative for reducing the viral load of SARS-CoV-2 and for clinical improvement of infected patients who presented mild and moderate symptoms.
Purpose
The aim of this study was to report a case series of patients diagnosed with COVID-19 that used the phthalocyanine derivate mouthwash to reduce clinical symptoms.
Patients and Methods
Eight patients used 5mL of phthalocyanine derivate mouthwash gargling/rinsing for one minute, five times daily, over a fourteen day period. Two measurement scales were applied for each patient in different periods to verify sore throat – VAS – Visual Analogue Scale for Pain and the clinical conditions – PS – Performance Status.
Results
All patients presented a significant reduction in clinical symptoms with the use of the mouthwash for gargling/rinsing after few days of use, without hospitalization.
Conclusion
The phthalocyanine derivate mouthwash protocol appears as a potential alternative for clinical improvement of COVID-19 infected patients. Daily use of this mouthwash rapidly reduced clinical symptoms such as sore throats, cough and mouth ulcers. Large, high-quality randomized controlled trials with larger sample size are necessary to confirm the effectiveness of this mouthwash protocol against COVID-19.
Keywords: mouthwash, sore throats, mouth ulcers, coronavirus infections
Introduction
The SARS-CoV-2 is responsible for COVID-19, disease that may manifest asymptomatically, but can lead to severe complications and death in a small proportion of the population.1 The oral cavity is an important site for the understanding of COVID-19 pathways, and positivity rate of COVID-19 in patients’ saliva can reach 91.7%.2,3 In addition, saliva collection for diagnosis is more comfortable for the patient, easier, inexpensive and non-invasive method. Few equipment are necessary for the diagnosis and saliva composition, peptides, proteins, can be isolated allowing the comparison of the state of physiology or pathology of human body.4
Therefore, gargling/rinsing with active substances represent a significant tool for the reduction of viral load in saliva and oral cavity.3,5 Consequently, the use of mouthwash could reduce the severity of the local and overall disease and prevent its transmission to other individuals.2,3,5–8 Thus, therapeutic oral rinses have been indicated for antimicrobial control in oral cavity with active ingredients such as phthalocyanine derivate.6,8,9 Also, the importance of pre-dental procedures solutions such as Povidone-Iodine mouthwash can be an important tool to reduce contamination of professionals.7
The phthalocyanine derivative is a compound that presents inferior cytotoxicity compared to fluorine and is classified as inert due to the negative toxicological and carcinogenicity effects.9
In this clinical research design protocol, patients diagnosed with COVID-19 have used the mouthwash to evaluate the role of this support therapy in the reduction of damage caused by SARS-CoV-2 pandemic.
Patients and Methods
Case Series
A convenience sample of eight SARS-CoV-2 positive patients diagnosed by RT-PCR analysis was monitored for fourteen days (approved by the Ethics Committee for Human Research of the Bauru School of Dentistry - University of São Paulo - CAAE: 36493520.1.0000.5417) and presented the registration code in the Brazilian Registry of Clinical Trials (REBEC): RBR-58ftdj. All patients signed an informed consent form before the initiation of the study and agreed to participate in the research and that their case data would be published. The data would be used for scientific purposes, maintaining their confidentiality.
All patients used the protocol of phthalocyanine derivate mouthwash - (PPM) for gargling/rinsing (5mL) during 1 minute/five times a day during 14 days. The Visual Analogue Scale – VAS - was used to assess sore throat and the Performance Status Scale – PS - for clinical conditions. Both measurement scales were applied to each patient at different times to check predetermined conditions.10,11 Also, a tolerability questionnaire was applied to verify the conditions of use and side effects.12
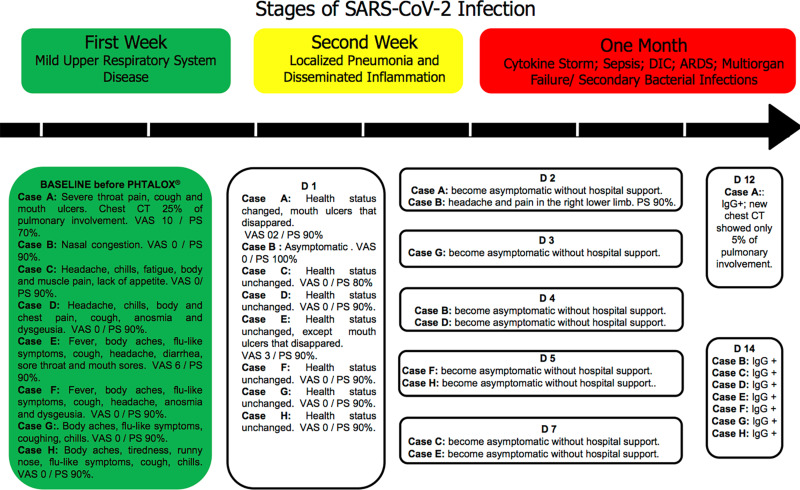
Figure 1 describes the stages of the infection caused by SARS-CoV-2, the initial symptoms of the patients and their respective clinical evolution until the positive test for Immunoglobulin G (IgG).
Figure 1.
Stages of SARS-CoV-2 infection and the evolution in days (D) of the patients, with baseline being the initial moment of patient assessment.
Abbreviations: DIC, disseminated intravascular coagulation; ARDS, acute respiratory distress syndrome; CT, computed tomography; VAS, Visual Analogue Scale for pain; PS, performance status.
Characteristics of All Patients
Patient A, a 52-year-old man, former smoker for over 20 years, presented severe sore throat, cough, mouth ulcers and pulmonary impairment of 25%. Patient B, a 28-year-old woman, presented nasal congestion and headache. Patient C, a 63-year-old woman, hypertensive, type II Diabetic, presented headache, chills, fatigue, body and muscle pain and lost appetite. Patient D, a 35-year-old man, presented headache, chills, fatigue, severe body pain, coughing, anosmia and dysgeusia. Patient E, a 30-year-old woman presented flu-like symptoms, cough, diarrhea, severe sore throat and mouth sores. Patient F, a 73-year-old man, with 1 stent and cardiac arrhythmia reported flu-like symptoms, cough, headache, dysgeusia and anosmia. Patient G, a 32-year-old man, reported flu-like symptoms, coughing, chills and the presence of mouth sores. Patient H, a 32-year-old woman, reported flu-like symptoms, coughing and chills.
COVID-19 treatment for the majority of these patients included antibiotics, anti-inflammatories, analgesics and vitamins. Some patients used Nitazoxanide and Azithromycin. Patients started the use of PPM after 2 days (patient D), 3 days (patients A, B, E and H), 4 days (patients C and F) and 5 days (patient G) of the onset of symptoms. All patients presented a significant reduction in clinical symptoms with the PPM after few days of use, without hospitalization.
According to the tolerability questionnaire, almost all patients stated that the PPM was possible and tolerable, except patient B. All participants reported a pleasant and sweet taste after the use of the mouthwash.
Discussion
In the evaluated cases, the phthalocyanine derivate mouthwash proved to be an alternative to reduce the clinical symptoms of coronavirus manifestation in the upper airway region, resulting in a faster recovery, without adverse effects.
The oral cavity environment is directly related to COVID-19, mainly because SARS-CoV-2 virus alive was found in the mouth and the virus spread may occur through saliva.2,3 Therefore, actions to reduce the amount of virus in the oral cavity and oropharynx are necessary, since the viral load is directly related to the evolution to a critical condition of COVID-19.3,5 One important step to reduce the viral load is gargles with an ingredient that demonstrates antiviral effect such as the active oxygen.5,8 In this way, the phthalocyanine derivative showed antimicrobial activity.9 This mouthwash promotes a self-activation and continuous production of reactive oxygen in the presence of molecular oxygen.9 Therefore, the phthalocyanine derivate mouthwash appears to be a promising alternative for reducing the viral load of SARS-CoV-2.
Considering this case series, no patient evolves to more severe COVID-19 conditions as shown in Figure 1. Conversely, patients showed a significant reduction of clinical symptoms. Then, the use of this present mouthwash seems to positively contribute to the local and systemic clinical improvement of patients infected with SARS-CoV-2.
Considering the limitations of the present clinical research design protocol, the lack of a comparative placebo control could influence the interpretation of the results. The disadvantages of using mouthwashes may be related to the lack of professional control regarding the number of mouthwashes recommended per day and the correct steps of mouthwash and gargle. Also, patients that eat or drink soon after the use of the product, can reduce the residual effect in the oral cavity. More broadly, the use of the product may result in a feeling of protection, reducing the use of the widely publicized recommendations, such as wearing masks, hand washing, social distance and medical care. Conversely, the rapid pain/cough relief for patient A, D, E, F, G, H and absence of mouth ulcers after twelve hours of the use of the protocol for patients A and E suggested the positive action of the phthalocyanine derivate.
The PPM was well tolerated by the patients and demonstrated a good performance against COVID-19 symptoms. Hence, no contraindications could be found in the use of the mouthwash used in this research as a support therapy to reduce the damage in the SARS-CoV-2 pandemic.
Conclusion
The findings of this case series suggested that the use of phthalocyanine derivate mouthwash in individuals infected with SARS-CoV-2 provided clinical improvement, regardless of other medical treatments needed for COVID-19. Further clinical trials using this mouthwash are necessary.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil (Finance Code 001). In editing the image by colleague Marco Aurélio Rosi de Podestá.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Vilhena reports personal fees from TRIALS Inc, during the conduct of the study; in addition, Dr. Vilhena has a patent classified pending. Dr. Araki reports grants from CNPq 401581/2016-0, grants from FAPESP 18/21489-1, during the conduct of the study; in addition, Dr. Araki has a patent null pending. Dr. Da Silva Santos reports personal fees and scholarships in CNPq process nº. 309525/2018-7 during the conduct of the study; another null, outside the submitted work. The other authors claim there are no conflicts of interest.
References
- 1.Hussain A, Kaler J, Tabrez E, Tabrez S, Tabrez SSM. Novel COVID-19: a comprehensive review of transmission, manifestation, and pathogenesis. Cureus. 2020;12(5):e8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.To KK-W, Tsang OT-Y, Yip CC-Y, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon JG, Yoon J, Song JY, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35(20):e195. doi: 10.3346/jkms.2020.35.e195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khurshid Z, Asiri FYI, Al Wadaani H. Human saliva: non-invasive fluid for detecting novel coronavirus (2019-nCoV). Int J Environ Res Public Health. 2020;17(7):2225. doi: 10.3390/ijerph17072225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton MJ, Clarkson JE, Goulao B, et al. Use of antimicrobial mouthwashes (gargling) and nasal sprays by healthcare workers to protect them when treating patients with suspected or confirmed COVID-19 infection. Cochrane Database Syst Rev. 2020;9:CD013626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imran E, Khurshid Z, Qadhi AAMA, Al-Quraini AAA, Tariq K. Preprocedural use of povidone-iodine mouthwash during dental procedures in the COVID-19 pandemic. Eur J Dent. 2020;14(S 01):S182–S184. doi: 10.1055/s-0040-1717001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meister TL, Brüggemann Y, Todt D, et al. Virucidal efficacy of different oral rinses against acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222(8):1289–1292. doi: 10.1093/infdis/jiaa471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teodoro GR, Santos CA, Carvalho MA, Koga-Ito CY, Khouri S, Vilhena FV. PHTALOX® antimicrobial action and cytotoxicity: in vitro study. J Dent Res. 2020;99(SpecIss A). abstract number, 0839, 2020 IADR/AADR/CADR General Session (Washington, D.C., USA). [Google Scholar]
- 10.Kelly A. The minimum clinically significant difference in visual analogue scale pain score does not differ with severity of pain. Emerg Med J. 2001;18(3):205–207. doi: 10.1136/emj.18.3.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer In: MacLeod CM, editor. Evaluation of Chemotherapeutic Agents. Columbia University Press; 1949:191–205. [Google Scholar]
- 12.Guerra F, Pasqualotto D, Rinaldo F, et al. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: performance-related evaluation of mouthwashes added with anti-discoloration system and cetylpyridinium chloride. Int J Dent Hyg. 2019;17(3):229–236. doi: 10.1111/idh.12371 [DOI] [PubMed] [Google Scholar]