Abstract
Background
Controlling postoperative pain after spinal surgery is important for rehabilitation and patient satisfaction. Wound infiltration with local anesthetics may improve postoperative pain, but true multimodal approaches for achieving analgesia after spinal surgery remain unknown.
Questions/purposes
In this randomized, controlled, double-blind trial after lumbar interbody fusion, we asked: (1) Does multimodal analgesia reduce VAS pain scores by a clinically important amount? (2) Does this analgesic approach reduce the amount of morphine patients consume after surgery? (3) Is this approach associated with fewer opioid-related side effects after surgery?
Methods
This study included 80 adult patients undergoing lumbar interbody fusion who were randomized into two groups: A control group (n = 40) who received infiltration of the surgical incision at the end of the procedure with an injection of 0.5% bupivacaine 100 mg (20 mL) and epinephrine 0.5 mg (0.5 mL), and the multimodal group (n = 40), who received wound infiltration with the same approach but with different medications: 0.5% bupivacaine 92.5 mg (18.5 mL), ketorolac 30 mg (1 mL), morphine 5 mg (0.5 mL), and epinephrine 0.5 mg (0.5 mL). There were no between-group differences in the proportion of patients who were male, nor in the mean age, height, weight, preoperative pain score, or surgical time. All treatments were administered by one surgeon. All patients, the surgeon, and the researchers were blinded to the allocation of patients to each group. Pain at rest was recorded using the VAS. Postoperative morphine consumption (administered using a patient-controlled analgesia pump) and opiod-associated side effects including nausea/vomiting, pruritus, urinary retention, and respiratory depression were assessed; this study was analyzed according to intention-to-treat principles. No loss to follow-up or protocol deviations were noted. We considered a 2-cm change on a 10-cm scale on the VAS as the minimum clinically important difference (MCID). Differences smaller than this were considered unlikely to be important.
Results
At no point were there between-group differences in the VAS scores that exceeded the MCID, indicating no clinically important reductions in pain associated with administering multimodal injections. The highest treatment effect was observed at 3 hours that showed only a -1.3 cm mean difference between the multimodal and the control groups (3.2 ± 1.8 versus 4.5 ± 1.9 [95% CI -1.3 to -0.3]; p < 0.001), which was below the MCID. Morphine consumption was very slightly higher in the control group than in the multimodal group (2.8 ± 2.8 versus 0.3 ± 1.0, mean difference 2.47; p < 0.001). The percentage of patients reporting opioid-related side effects was lower in the multimodal group than in the control group. The proportions of nausea and vomiting were higher in the control group (30% [12 of 40] than in the multimodal group (3% [1 of 40]; p = 0.001). All of these side effects were transient and none was severe.
Conclusions
Multimodal wound infiltration with an NSAID and morphine did not yield any clinically important reduction in pain or opioid consumption. Since no substantial benefit of adding these drugs to a patient’s aftercare regimen was achieved, and considering the potential risks of administering opioids and NSAIDs (such as, polypharmacy in older patients, serious adverse effects of NSAIDs), we recommend against routine use of this approach in clinical practice.
Level of Evidence
Level I, therapeutic study.
Introduction
The management of postoperative pain, particularly after spinal procedures, presents major clinical challenges in orthopaedic surgery as patients often experience acute postoperative pain [1, 3, 6, 17]. Effective pain control leads to earlier mobilization, shorter hospitalization, and improved functional outcomes [3, 4]. Current pain management modalities after spine surgery include oral analgesic, oral NSAIDs, intravenous narcotic (patient-controlled analgesia or on demand), epidural opioids, and local wound infiltration of analgesia. However, these options are often ineffective and may be associated with specific/non-specific complications [5]. The VAS pain scale in the first 24 hours after spinal surgery was reported to be as high as 6 points to 8 points [6, 12].
Infiltration of analgesic and anti-inflammatory medication at the surgical site is commonly used in a variety of procedures, in both orthopaedic and non-orthopaedic settings, such as fracture fixation surgery [9], thoracotomy [16], abdominoplasty [2] and cesarean section [11]. It has been used frequently in arthroplasty and arthroscopy with benefits of improved postoperative pain control. In a randomized controlled trial (RCT) study, Tammachote et al. [15 ] used a periarticular injection cocktail of bupivacaine, morphine, and ketorolac in patients undergoing TKA, and the effectiveness of this combination was the same as intrathecal morphine. In a meta-analysis, Wang et al. [16 ] showed that periarticular multimodal drug injections provided postoperative analgesia compared with a single femoral nerve block. However, the effects of local multimodal drug injections (wound infiltration) after spine surgery have not been well explored. One study by Ozyilmaz et al. [13 ] suggested pain control benefits associated with administering tramadol plus bupivacaine after lumbar discectomy. However, to our knowledge, no randomized trial study has yet investigated the efficacy of morphine and NSAIDs in the spinal surgery setting.
We therefore performed a randomized, controlled, double-blind trial after lumbar interbody fusion, in which we asked: (1) Does multimodal analgesia reduce the VAS pain scores by a clinically important extent? (2) Does this analgesic approach reduce the volume of morphine patients consume after surgery? (3) Is this approach associated with fewer opioid-related side effects after surgery?
Patients and Methods
The study design was an RCT, and the Ethics Committee of the Faculty of Medicine at Chulalongkorn University provided approval. This RCT was registered in the Thai Clinical Trials Registry under registration number TCTR20190829001. Informed consent was obtained from all participants before data collection.
The multimodal group was administered a combination treatment comprising 0.5% bupivacaine 92.5 mg (18.5 mL), ketorolac 30 mg (1 mL), morphine 5 mg (0.5 mL), and epinephrine 0.5 mg (0.5 mL). Similarly, the control group received a combination treatment comprising 0.5% bupivacaine 100 mg (20 mL) and epinephrine 0.5 mg (0.5 mL).
A total of 83 patients were assessed, and three were excluded due to their comorbidities. Finally, 80 patients were included and analyzed (Fig. 1). The patients included were aged between 20 and 80 years; they underwent one-level lumbar interbody fusion, had a good mental status, and provided informed consent before enrollment. Patients with the following criteria were excluded from the study: allergies to any of the drugs used in this study, impaired renal and/or hepatic function, coronary heart disease, and other pain-related comorbidities.
Fig. 1.
This diagram shows the study enrollment process.
The sample size was calculated using the power repeated command in Stata (Stata Corp, College Station, TX, USA). For the control group, we assumed VAS scores of 8 cm, 6.4 cm, 4.5 cm, and 4 cm at each of our four timepoints, respectively. We expected the multimodal arm to have consistent VAS scores of 2 cm (on a 10-cm scale) or lower. We assumed a within-group patient correlation of 0.65 and a variance error of 10. Under these assumptions, 40 patients in each group with give 90% power to detect a minimum clinical difference of 2 cm in VAS scores, at a 2-sided significance level of 5%. A sample size of 80 patients was categorized into the multimodal (n = 40) and the control (n = 40) groups by using computerized block randomization (http://www.randomization.com) and allocation concealment by an independent person (TC). This study was analyzed according to intention-to-treat principles; we noted no patients lost to follow-up and no protocol devations. Hence, all 80 patients were analyzed according to the randomized arm. Envelopes were opened and drugs were prepared in the operating room by nurses. Careful efforts were made to ensure that the patients, surgeon, and the observer were blinded to the allocation of patients to the treatment groups. All operations were performed by one surgeon (WS). All patients received the same premedication, postoperative pain control, and rehabilitation protocols.
The primary outcome was the VAS score at 3, 6, 12, and 24 hours postoperatively, which is the expected period for observing the local infiltration effects. We considered the minimum clinically important difference (MCID) on the VAS pain scale to be 2 cm on a 10-cm scale. Differences smaller were considered unlikely to be important. The secondary outcome variables included: the intravenous morphine consumption in the first 24 hours postoperatively, the number of patients who received intravenous morphine, morphine-related side effects (nausea, vomiting, pruritus, or urinary retention), time to ambulation (from after the operation was finished until the time the patient could walk), and the length of hospitalization (after the surgery until the patient was discharged from the hospital).
After providing consent, the patients were trained on self-recording their VAS scores of postoperative pain at rest. The VAS ranged from 0 (no pain) to 10 (maximum pain).
Infiltration Technique
All combination treatments prepared for administration were divided into two syringes. The first syringe (10 mL) was infiltrated into the cephalad and caudad facet joints at the same level of the procedure under fluoroscopy before an incision was made, using the medial branch block technique. Then, the second syringe (10.5 mL) was injected into the skin and the subcutaneous and thoracolumbar fascia intraoperatively before wound closure.
Anesthetic Protocol and Postoperative Care
The patients were routinely monitored by pulse oximetry, noninvasive blood pressure monitoring, and electrocardiography. Preoxygenation was ensured 3 minutes to 5 minutes before performing anesthesia. Then, general anesthesia was induced using propofol, succinylcholine, or cisatracurium and desflurane. The patient was intubated with a direct laryngoscope and maintained with continuous vaporized desflurane. Intravenous cisatracurium 0.1 to 0.15 mg/kg per bodyweight was infused for muscle relaxation with add on 2 mg each time. Short-acting opioid analgesics (fentanyl 3 mcg/kg IV) were administered at the beginning of the operation in all patients. During wound closure, desflurane and nitrous oxide (NO3) were discontinued; oxygen supply was increased to 6 L/min. Neostigmine 0.05 mg/kgf of bodyweight and atropine 0.02 mg/kg were administered to ensure complete reversal of nondepolarizing muscle relaxants. The patients were extubated after regaining consciousness. All patients were transferred to the postanesthetic care unit and administered patient-controlled analgesic (PCA) infusions consisting of morphine 1 mg/mL, dose 1 mg, lockout 6 min, 1-hour limit 10 mg and no basal rate. Oral medications included 400-mg celecoxib once daily, 75-mg pregabalin before sleeping, and 500-mg paracetamol every 6 hours. Intravenous ondansetron (4 mg/kg per bodyweight) was prescribed to patients if they had nausea/vomiting complaints. The postdischarge medication regimen was the same for groups: 200 mg of celecoxib once daily for 7 days, 75 mg of pregabalin before sleeping and 500 mg of paracetamol every 6 hours when necessary.
Statistical Analysis
All statistical analyses were performed using Stata version 15 (Stata Corp). Consistent with the Consolidated Standards of Reporting Trials (CONSORT) guidelines, we did not compare baseline characteristics of randomized arms. VAS scores were compared at each timepoint using independent t-tests. Then, we used a linear mixed model with a random intercept at the level of subject ID to compare the reduction in VAS scores of the multimodal versus the control group for all timepoints. An unpaired t-test was used to determine the intravenous morphine consumption volume. We used a chi-square test to analyze the number of patients who received intravenous morphine and its related side effects, and we used a Kaplan-Meier curve as a survival analysis to compare time to ambulation and length of hospitalization. Formal comparisons of continuous covariates were made using unpaired t-test, and categorical data were compared using χ2 tests. A logrank test was used to comapre the Kaplan-Meier distributions. The data are presented as means ± SD for the unpaired t-test, and percentage (number) for the χ2 test, and median time to event (95% CI) for Kaplan-Meier data. P values < 0.05 were considered statistically significant.
Results
A total of of patients 40 patients were enrolled into both the multimodal treatment and control groups. Baseline characteristics were comparable between groups (Table 1).
Table 1.
Baseline demographic data
| Data | Multimodal group (n = 40) | Control group (n = 40) |
| Sex (male: female) | 9:31 | 13:27 |
| Age (years) | 66 ± 8 | 66 ± 9 |
| Height (cm) | 158 ± 10 | 158 ± 8 |
| Weight (kg) | 66 ± 12 | 66 ± 14 |
| Preoperative VAS score | 7 ± 3 | 7 ± 2 |
| Operative time (minutes) | 173 ± 33 | 176 ± 32 |
Data are presented as the mean ± SD, except for sex.
Pain Scores
At no timepoint was there a between-group difference in VAS that exceeded the MCID, indicating no clinically important reductions in pain associated with the multimodal injection. The highest treatment effect was observed at 3 hours, but it was only a -1.3 cm mean difference between the multimodal treatment and the control group (3.2 ± 1.8 versus 4.5 ± 1.9 [95% CI -1.3 to -0.3]; p < 0.001), which was below the MCID. There were also no clinically important reduction in pain between the multimodal and control group at 6 hours (2.1 ± 1.3 versus 3.2 ± 1.7 (mean difference -1.1 [95% CI -1.8 to -0.4 cm]; p < 0.002), 12 hours (1.8 ± 1.2 versus 2.6 ± 1.4, mean difference -0.8 [95% CI -2.5 to -0.2 cm]; p < 0.01) and 24 hours (1.5 ± 1.2 versus 2.0 ± 1.1, mean difference -0.5 [95% CI -1.0 to 0.01 cm]; p = 0.08) (Fig. 2). The overall reduction in VAS score over all follow-up in the multimodal versus the control arm from a mixed linear model was -0.8 [95% CI 1.3 to -0.3]; p = 0.001.
Fig. 2.
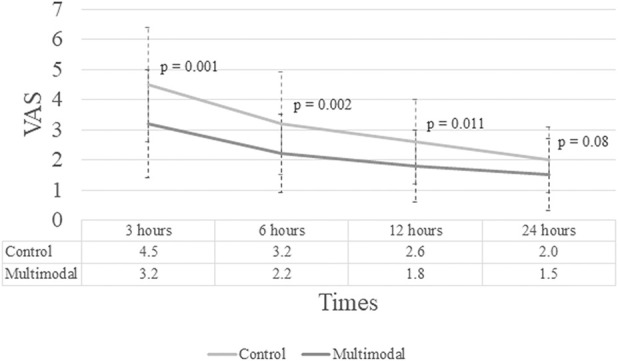
This graph shows VAS scores at 3, 6, 12, and 24 hours.
Opioid Consumption
Morphine consumption was very slightly higher in the control group than in the multimodal group (2.8 ± 2.8 versus 0.3 ± 1.0, mean difference (multimodal versus control) = -2.47 [95% CI -3.6 to -1.4 mg]; p < 0.001) (Table 2). Twenty of 40 patients (50%) in control group compared with 4 of 40 patients (10%) in the multimodal group needed postoperative intravenous morphine.
Table 2.
Comparisons of postoperative outcomes between groups
| Factor | Control group (n = 40) | Multimodal group (n = 40) | p value |
| Postoperative VAS score (mean) | |||
| 3 hours | 4.5 ± 1.9 | 3.2 ± 1.8 | 0.001 |
| 6 hours | 3.2 ± 1.7 | 2.1 ± 1.3 | 0.002 |
| 12 hours | 2.6 ± 1.4 | 1.8 ± 1.2 | 0.011 |
| 24 hours | 2.0 ± 1.1 | 1.5 ± 1.2 | 0.08 |
| Intravenous morphine consumption (n) | 50% (20) | 10% (4) | < 0.001 |
| First 12 hours | 50% (20) | 10% (4) | < 0.001 |
| Second 12 hours | 13% (5) | 0 | 0.02 |
| Intravenous morphine consumption (mg) | 2.8 ± 2.8 | 0.3 ± 1.0 | < 0.001 |
| First 12 hours | 2.4 ± 2.7 | 0.3 ± 1.0 | < 0.001 |
| Second 12 hours | 0.4 ± 1.1 | 0 | 0.02 |
Continuous data are presented as means ± SD; categorical data presented as percentage (number).
Opioid-related Side Effects and Other Secondary Endpoints
A higher proportion of patients in the control group reported nausea that was treated with an anti-emetic drug (30% [12 of 40] versus 2.5% [1 of 40], odds ratio for multimodal versus control group = 0.1 [95% CI 0.01 to 0.5]; p = 0.001), but these symptoms were generally transient. No pruritus or urinary retention occurred in any patient. Using the Kaplan-Meier method, the median time to ambulation and length of hospital stay were not different between the two groups (24 hours [95% CI 18 to 28] in the multimodal group versus 25 hours [95% CI 21 to 29] in the control group, logrank p = 0.65) for time to ambulation and (70 hours [95% CI 68 to 72] in the multimodal group versus 72 hours [95% CI 69 to 84], logrank p = 0.39) for length of hospital stay).
Discussion
Postoperative pain after spinal surgery is a primary challenge for clinicians and an intense concern for patients. Opioids are prescribed most commonly, but their associated side effects can be severe in some patients [10]. Previous studies evidenced the benefits of local wound infiltration after TKA [8], but the application of local wound infiltration approaches after spine surgery has remained unexplored. Moreover, to our knowledge, no randomized controlled trials investigated the efficacy of this approach in patients undergoing lumbar interbody fusion. Therefore, a double-blind, randomized trial was undertaken, which compared a multimodal wound-infiltration treatment with a local anesthetic plus an NSAID plus morphine to another treatment containing only a local anesthetic and epinepherine. However, our results did not show any clinically important reductions in pain scores or opioid consumption at any point. We observed a reduction in nausea, although it was difficult to interpret considering the miniscule differences in opioid consumption between the study groups.
We recognize the limitations of our study methodology. First, we did not assess the efficacy of blinding, which could possibly explain why such small between-group differences in opioid consumption resulted in differences in nausea. However, we made all efforts to ensure that the allocation of the treatment was blinded from the physicians and patients, and that only patients were involved in the VAS score determination. Second, the multimodal injection also included morphine and ketorolac, both of which can be absorbed systemically after local injection. Therefore, we considered that the reduced pain scores could be partly attributable to a systemic effect. Third, some unaccountable between-group differences existed including the preoperative use of opioids, anxiety, depression, workers compensation that could affect postoperative pain outcomes.
The addition of morphine and ketorolac to bupivacaine and epinephrine for surgical wound infiltration did not show a clinically important improvement in pain compared with the control injection (local anesthetic plus epinepherine). Ozyilma et al. [13] reported a prospective randomized study of 80 patients with four different local analgesic treatments injected (100 mg levobupivacaine only, 2 mg/kg tramadol only, 2 mg/kg tramadol plus 100 mg levobupivacaine, and saline) during lumbar disc surgery. They found that adding tramadol to bupivacaine reduced VAS scores compared with the levobupivacaine or saline alone. They concluded that infiltration of the wound site with combined levobupivacaine and tramadol provided better pain control compared with levobupivacaine or tramadol alone. However, a detailed analysis of VAS scores revealed no clinically important difference according to the MCID (> 2 difference in VAS scores at each timepoint) when the data of levobupivacaine plus tramadol were compared with levobupivacaine-only data.
Similarly, we saw a small decrease in the use of postoperative opioids when morphine and ketorolac were added, but the size of this decrease was so small as to likely have been clinically unimportant. In contrast, Ozyilma et al. [13] reported the 24-hour cumulative consumption of PCA with pethidine as 129.0 ± 78.3 mg in the levobupivacaine group compared with 0 in the combined tramadol plus levobupivacaine group. This could be explained by the immediate absorption of intramuscularly injected high-dose tramadol, which converted to the systemic bioequivalent within 30 minutes [14]. Therefore, the patients in tramadol plus levobupivacaine group also received high-dose systemic opioids intraoperatively/preoperatively.
Despite the differences in opioid consumption being very small, there did appear to be a reduction in the proportion of patients who received medication for postoperative nausea and vomiting in the multimodal group compared with the control group. There was no other side effects of morphine such as pruritus, urinary retention, and respiratory depression. This is somewhat difficult to understand; possible explanations might be based on the small differences in opioid consumption, which might have slight effects on fragile older patients, or that blinding was distorted and patients uncovered information regarding their allocation. A chance finding is also possible. The addition of morphine and ketorolac had no effect on the time to ambulation or discharge from the hospital. The similarities in the time to ambulate and length of hospitalization may have occurred due to the slight (clinically unimportant) reduction in pain between groups. A prospective cohort study by Glasser et al. [7] on local wound infiltration in spinal surgery examined the efficacy of methylprednisolone plus bupivacaine combination versus placebo in patients undergoing lumbar microdiscectomy. Patients receiving bupivacaine and corticosteroids had shorter hospital stay (1.4 days) compared with the control group (4.0 days; p = 0.0004). The study group (bupivacaine and corticosteroids) also recieved a macerated fat graft soaked with 80 mg of depomedrol over the affected nerve root after discectomy. This might be the stronger factor than local wound infiltration effect for shorter hospital stay in the study group. In aggregate, there is little reason to use these multimodal injections, and adding NSAIDs does have its own risk.
We found that multimodal wound infiltration with an NSAID and morphine did not yield a clinically important reduction in pain or opioid consumption. In the absence of a substantial benefit to adding these drugs to a patient’s aftercare regimen, and in light of the potential risks (such as polypharmacy in older patients, and the potentially serious side effects of NSAIDs) we recommend against the routine use of this approach in practice.
Acknowledgments
We would like to thank Prof. Steve Kerr for his invaluable suggestion on the statistical analysis of this manuscript.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed at the Department of Orthopedic Surgery, Faculty of Medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand.
References
- 1.Alican MFB, Ver MR, Ramos MRD, Mamaril LJC. Post-operative Single-shot Epidural Fentanyl and Bupivacaine for Post-operative Analgesia after Lumbar Decompression: A Prospective, Double-Blind Randomized Study. Spine. [Published online ahead of print March 19, 2020]. DOI: 10.1097/BRS.0000000000003449. [DOI] [PubMed]
- 2.Bagatin D, Bagatin T, Nemrava J, Ivelj MS, Deutsch J, Sakic K. Influence of Local Infiltration Analgesia on Postoperative Pain in Abdominoplasty Patients. Acta Clinica Croatica. 2019;58:23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajwa SJ, Haldar R. Pain management following spinal surgeries: An appraisal of the available options. J Craniovertebr Junction Spine. 2015;6:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaman DN, Graziano GP, Glover RA, Wojtys EM, Chang V. Substance P innervation of lumbar spine facet joints. Spine. 1993;18:1044-1049. [DOI] [PubMed] [Google Scholar]
- 5.Chung F, Ritchie E, Su J. Postoperative pain in ambulatory surgery. Anesth Analg. 1997;85:808-816. [DOI] [PubMed] [Google Scholar]
- 6.Gerbershagen HJ, Aduckathil S, van Wijck AJ, Peelen LM, Kalkman CJ, Meissner W. Pain intensity on the first day after surgery: a prospective cohort study comparing 179 surgical procedures. Anesthesiology. 2013;118:934-944. [DOI] [PubMed] [Google Scholar]
- 7.Glasser RS, Knego RS, Delashaw JB, Fessler RG. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg. 1993;78:383-387. [DOI] [PubMed] [Google Scholar]
- 8.Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28:1274-1277. [DOI] [PubMed] [Google Scholar]
- 9.Li BL, Liu X, Cui L, Zhang W, Pang H, Wang M, Wang HQ. Local Infiltration Analgesia with Ropivacaine Improves Postoperative Pain Control in Ankle Fracture Patients: A Retrospective Cohort Study. Pain Res Manag. 2020;2020:8542849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy DL, Lebin JA, Severtson SG, Olsen HA, Dasgupta N, Dart RC. Comparative Rates of Mortality and Serious Adverse Effects Among Commonly Prescribed Opioid Analgesics. Drug Saf. 2018;41:787-795. [DOI] [PubMed] [Google Scholar]
- 11.Nasir F, Sohail I, Sadiq H, Habib M. Local Wound Infiltration with Ropivacaine for Postoperative Pain Control in Caesarean Section. Cureus. 2019;11:e5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill P, Knickenberg C, Bogahalanda S, Booth AE. Use of intrathecal morphine for postoperative pain relief following lumbar spine surgery. J Neurosurg. 1985;63:413-416. [DOI] [PubMed] [Google Scholar]
- 13.Ozyilmaz K, Ayoglu H, Okyay RD, Yurtlu S, Koksal B, Hanci V, Erdogan G, Turan IO. Postoperative analgesic effects of wound infiltration with tramadol and levobupivacaine in lumbar disk surgeries. J Neurosurg Anesthesiol. 2012;24:331-335. [DOI] [PubMed] [Google Scholar]
- 14.Shipton EA. Tramadol--present and future. Anaesth Intensive Care. 2000;28:363-374. [DOI] [PubMed] [Google Scholar]
- 15.Tammachote N, Kanitnate S, Manuwong S, Yakumpor T, Panichkul P. Is pain after TKA better with periarticular injection or intrathecal morphine? Clin Orthop Relat Res. 2013;471:1992-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang C, Cai XZ, Yan SG. Comparison of Periarticular Multimodal Drug Injection and Femoral Nerve Block for Postoperative Pain Management in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J Arthroplasty. 2015;30:1281-1286. [DOI] [PubMed] [Google Scholar]
- 17.Weir S, Samnaliev M, Kuo TC, Tierney TS, Manca A, Taylor RS, Bruce J, Eldabe S, Cumming D. Persistent postoperative pain and healthcare costs associated with instrumented and non-instrumented spinal surgery: a case-control study. J Orthop Surg Res. 2020;15:127. [DOI] [PMC free article] [PubMed] [Google Scholar]


