Abstract
Background
Preoperative synovial fluid culture is pivotal in the early diagnosis of prosthetic joint infection (PJI) but may yield false-positive and false-negative results. We evaluated the predictive value of synovial fluid culture results combined with the measurement of serum anti-staphylococcal antibodies (SASA).
Questions/purposes
(1) For hip and knee PJI, does combining positive SASA results with preoperative synovial culture results improve the positive predictive value (PPV) of preoperative synovial fluid culture alone? (2) Does combining preoperative synovial fluid culture results with a positive cell count and differential result increase the PPV of preoperative synovial fluid culture alone? (3) What proportion of isolated organisms exhibit concordance in antibiotic susceptibility: preoperative aspiration versus intraoperative isolates?
Methods
A prospective study was conducted at two French reference centers that manage bone and joint infections and included 481 adult patients who had a revision or resection arthroplasty between June 25, 2012 and June 23, 2014. Exclusion criteria including no serum sample available for immunoassay, the lack of microbiological documentation, and the absence of preoperative aspiration reduced the patient number to 353. Seven patients with an undetermined SASA result were excluded from the analysis. We also excluded patients with PJI involving more than one Staphylococcus species (polystaphylococcal infection) and those in whom more than one Staphylococcus species was recovered from the preoperative synovial fluid culture (polystaphylococcal synovial fluid culture). In total, 340 patients were included in the analysis (no infection, 67% [226 of 340]; staphylococcal infection, 21% [71 of 340]; other infection, 13% [43 of 340]). The preoperative synovial fluid analysis included a cell count and differential and bacterial culture. SASAs were measured using a multiplex immunoassay. The diagnosis of PJI was determined using the Infectious Diseases Society of America (IDSA) criteria [14] and intraoperative tissue culture at the time of revision surgery was used as the gold standard (at least one positive intraoperative sample for a “virulent” organism (such as S. aureus) or two positive samples for a “non-virulent” (for example S. epidermidis).
Results
SASA increased the PPV compared with synovial fluid culture alone (92% [95% CI 82 to 97] versus 79% [95% CI 68 to 87]; p = 0.04); when stratified by site, an increase in PPV was seen in hip infections (100% [95% CI 89 to 100] versus 77% [95% CI 63 to 88]; p = 0.01) but not in knee infections (84% [95% CI 66 to 95] versus 80% [95% CI 64 to 91]; p = 0.75). A positive cell count and differential result increased the PPV of staphylococcal synovial fluid cultures compared with synovial fluid culture alone (86% [95% CI 70 to 95] versus 79% [95% CI 68 to 87]; p = 0.36); when stratified by site, no difference in hip and knee infections was observed (86% [95% CI 67 to 96] versus 77% [95% CI 63 to 88]; p = 0.42) and 86% [95% CI 70 to 95] versus 80% [95% CI 64 to 91]; p = 0.74).
Conclusion
SASA measurement improves the predictive value of synovial fluid cultures of the hip for all staphylococcal organisms, including coagulase-negative staphylococci, but the PPV of SASA plus synovial fluid culture it is not superior to the PPV of synovial fluid cell count/differential plus synovial culture for the knee.
Level of Evidence
Level III, diagnostic study.
Introduction
The preoperative diagnosis of prosthetic joint infection (PJI) is a key step in the performance of revision arthroplasty and mainly relies on aspiration and a first-line evaluation consisting of an examination, peripheral blood tests, and radiography. A routine analysis of synovial fluid taken by aspiration comprises cell examination and bacterial cultures. The determination of total nucleated cells and differential has high sensitivity and specificity for diagnosing PJI [21, 22]. Synovial fluid culture results support the diagnosis and provide information about the infecting microorganisms, allowing for a more accurate choice of perioperative antimicrobials [1, 16, 20, 21]. Staphylococci, mainly Staphylococcus aureus and Staphylococcus epidermidis, are a frequent cause of PJI at any time after arthroplasty [21, 23]. S. epidermidis and other coagulase-negative staphylococci (CNS) are, however, ubiquitous members of human skin flora and may be cultured from synovial fluid samples as contaminants [8, 11].
Therefore, the recovery of a CNS strain through preoperative aspiration always raises questions about its pathogenicity, particularly because the bacteriologic criteria used for intraoperative liquid and tissue samples (that is, at least two samples whose results are positive for the same organism) lack applicability to preoperative aspiration (only one synovial fluid sample is taken in most patients). Synovial fluid culture results may also be falsely negative in a proportion of patients with staphylococcal PJI, including PJI because of S. aureus [1]. To distinguish between contaminating and pathogenic Staphylococci, we studied the combination of serological assays with synovial fluid culture to enhance the interpretation of CNS culture. A multiplex immunoassay that measures serum anti-staphylococcal antibodies (SASA) has recently been evaluated to diagnose PJI noninvasively [13]. This immunoassay showed good performance in two prospective studies for diagnosing staphylococcal PJI, with sensitivity ranging from 72.3% to 87.5% and specificity from 80.7% to 93.5% [7, 13]. Performances of the assay were studied by site in the first study and no difference of sensitivity and specificity was shown between hip and knee infections. This approach is applicable to staphylococci and, more particularly, the three species for which the test has been validated: S. aureus, S. epidermidis, and S. lugdunensis. However, no prior study investigates the ability of this multiplex assay to improve the performance of preoperative aspiration to diagnose staphylococcal PJI. Here, we aimed to determine whether the measurement of SASA may improve the ability of preoperative aspiration to diagnose staphylococcal PJI of the knee or hip and identify the correct causative organism.
Therefore, we asked: (1) For hip and knee PJI, does combining positive SASA results with preoperative synovial culture results improve the positive predictive value (PPV) of preoperative synovial fluid culture alone? (2) Does combining preoperative synovial fluid culture results with a positive cell count and differential result increase the PPV of preoperative synovial fluid culture alone? (3) What proportion of isolated organisms exhibit concordance in antibiotic susceptibility: preoperative aspiration versus intraoperative isolates?
Patients and Methods
Study Design
A prospective study was conducted at two French reference centers that manage bone and joint infections and included 481 adult patients who had a revision or resection arthroplasty between June 25, 2012 and June 23, 2014. The primary endpoint of this study was to determine the diagnostic accuracy of SASA in PJI patients but previous reports have not addressed the benefit of SASA to enhance the performance of preoperative joint aspiration. Exclusion criteria including no serum sample available for immunoassay, the lack of microbiological documentation, and the absence of preoperative aspiration reduced the patient number to 353. Seven patients with an undetermined SASA result were excluded from the analysis. We also excluded patients with PJI involving more than one Staphylococcus species (polystaphylococcal infection) and those in whom more than one Staphylococcus species was recovered from the preoperative synovial fluid culture (polystaphylococcal synovial fluid culture) (Fig. 1). We included patients who had: (1) a substantial bacteriologic culture of intraoperative samples collected during revision or resection arthroplasty, (2) a preoperative synovial fluid analysis, (3) SASA measurement at the same time as preoperative aspiration between June 25, 2012 and June 23, 2014. Antibiotics were withheld for at least 2 weeks before preoperative and intraoperative samples were collected. We did not exclude nine patients with polymicrobial infection involving a single significant staphylococcal agent (S. epidermidis in six patients and S. aureus in three patients). The protocol and information sheet were approved by our institutional review board, and the database was authorized by the Commission Nationale Informatique Libertés (French privacy watchdog).
Fig. 1.
The flow diagram outlines the figures involved in applying patient inclusion and exclusion criteria. aPJI involving two or more Staphylococcus species. bMore than one Staphylococcus species recovered from the preoperative synovial fluid; SASA = serum anti-staphylococcal antibodies.
Study Sample
Among the 353 patients enrolled in the study, seven were excluded because of undetermined SASA results, three because of polystaphylococcal PJI, and three because of polystaphylococcal synovial fluid culture results (see Table 1; Supplemental Digital Content 1, http://links.lww.com/CORR/A391). A total of 340 patients were thus included in the analysis. Among the 340 analyzed patients, 67% (226 of 340) did not have an infection, 21% (71 of 340) had a staphylococcal infection (S. aureus, 7% [25 of 340], S. epidermidis, 9% [30 of 340]; other staphylococci, 5% [16 of 340]), and 13% (43 of 340) had an infection with another organism. The characteristics of patients with and without infection were different depending on the prosthesis site, the time elapsed since the prosthesis was inserted, the presence of a sinus tract, and the proportion of patients with elevated erythrocyte sedimentation rate and C-reactive protein values (Table 1). There was no difference between patients infected by staphylococci and those infected by other organisms.
Table 1.
Characteristics of the study population
| Characteristics | Total (n = 340) | No infection (n = 226) | Staphylococcal infection (n = 71) | Other infection (n = 43) | p value | |
| Global comparison | Staphylococcal versus othera | |||||
| Age, years (median, interquartile range) | 72 (64 to 79) | 73 (65 to 79) | 69 (62 to 77) | 72 (68 to 79) | 0.2 | 0.1 |
| Female sex, % | 54 (183 of 339) | 60 (134 of 225) | 44% (31 of 71) | 42% (18 of 43) | 0.02 | 1 |
| First implant revision, % | 62 (193 of 313) | 65 (139 of 213) | 56% (37 of 66) | 50% (17 of 34) | 0.1 | 0.7 |
| Site of prosthesis | < 0.001 | 0.01 | ||||
| Hip, % | 67 (227 of 339) | 74 (166 of 225) | 55% (39 of 71) | 51% (22 of 43) | ||
| Knee, % | 31 (104 of 339) | 25 (56 of 225) | 45% (32 of 71) | 37% (16 of 43) | ||
| Shoulder, % | 2 (8 of 339) | 1 (3 of 225) | 0% (0 of 71) | 12% (5 of 43) | ||
| Time since prosthesis insertion | 0.001 | 0.3 | ||||
| ≤ 3 months, % | 5 (15 of 335) | 2 (4 of 222) | 7% (5 of 71) | 14% (6 of 42) | ||
| > 3 months, % | 96 (320 of 335) | 98 (218 of 222) | 93% (66 of 71) | 86% (36 of 42) | ||
| Sinus tract, % | 10 (30 of 298) | 0 (0 of 210) | 32% (18 of 57) | 39% (12 of 31) | < 0.001 | 0.7 |
| CRP ≥ 10 mg/lb, % | 44 (148 of 334) | 25 (55 of 222) | 84% (59 of 70) | 81% (34 of 42) | < 0.001 | 0.9 |
| ESR ≥ 30 mm/hb, % | 37 (113 of 304) | 18 (38 of 210) | 78% (46 of 59) | 83% (29 of 35) | < 0.001 | 0.8 |
Data are presented as the number of total number %, unless otherwise specified.
Staphylococcal infection subgroup versus Other infection subgroup.
Cutoffs are from Tande and Patel [21]; CPR = C-reactive protein; ESR = erythrocyte sedimentation rate.
SASA Measurement
We measured immunoglobulin G (IgG) against eight staphylococcal proteins selected to target three staphylococci (S. aureus, S. epidermidis, S. lugdunensis) was measured using a bead-based multiplex assay (Research Use Only version of BJI Inoplex, Diaxonhit, Paris, France) [13]. Serum samples were diluted to 1:70. IgG binding was detected with R-phycoerythrin-conjugated goat anti-human IgG (MOSS substrates). Median fluorescence intensity values were determined for each antigen using a Magpix instrument (Luminex, Austin, Texas, USA) and Xponent software (Luminex, Austin, Texas, USA). Median fluorescence intensity values were then transformed into a total response index that was graded positive, negative, or undetermined (proprietary software, Diaxonhit, Paris, France).
Preoperative Aspiration and Synovial Fluid Analysis
Aspirations were performed in a clean room using standard aseptic precautions. An average of one synovial fluid sample was obtained (minimum 1; maximum 5). Hip aspirations were performed under echographic or radiologic guidance by an experienced radiologist. Synovial fluid was transmitted to the laboratory and subjected to a cytologic examination, with absolute and differential cell counts and microscopic examination of a gram-stained smear. All samples were cultured on solid media (chocolate agar under 5% CO2 and Columbia media, incubated under aerobic and anaerobic conditions) and on enrichment-liquid Schaedler broth. All isolates were identified with mass spectrometry (Biotyper software on a Microflex LT mass spectrometer, Bruker Daltonik, Bremen, Germany).
Bacteriologic Analysis of Intraoperative Tissue Samples
An average of four intraoperative periprosthetic tissue samples (minimum 1, maximum 11) obtained using a sterile instrument set were placed in sterile, doubly wrapped 30-mL containers and processed within 2 hours, as previously described [17]. Briefly, samples were mechanically disrupted in sterile water with stainless steel beads using a Retsch MM400 bead mill (Verder, Cergy-Pontoise, France). Bead-milled sample suspensions were then cultured on solid media (Columbia sheep’s blood agar incubated under aerobic and anaerobic conditions; chocolate agar under 5% CO2) and on liquid aerobic (Brain heart infusion) and anaerobic (Schaedler’s) broths that were terminally subcultured after 15 days. All experiments were performed using a safety cabinet. Isolates were identified using mass spectrometry, as described above.
Definitions
The study was restricted to patients with a microbiological documentation according to Infectious Diseases Society of America (IDSA) criteria at the time of inclusion [14]. Infection was defined as the presence of a sinus tract and/or the culture of 2 or more of 3 to 5 intraoperative samples with results that were positive for the same microorganism (same species and susceptibility profile). According to IDSA criteria, virulent organisms (S. aureus, Pseudomonas aeruginosa, Enterobacteriaceae, beta-hemolytic streptococci) were considered significant even if cultured from a single sample. Staphylococcal infection was defined as an infection featuring a significant member of the Staphylococcus genus (such as, two or more identical CNS or one or more S. aureus).
A synovial fluid culture result was defined as positive when at least one synovial fluid sample result was positive (we considered only the result of culture and not the cell count criteria).
A synovial fluid cell count/differential result was defined as positive if there were more than 3000/μL leukocytes and more than 80% neutrophils [15].
Staphylococcal Organisms Cultured from Preoperative Synovial Fluid Samples and Intraoperative Tissue Samples
The distribution of Staphylococcus species recovered from preoperative synovial fluid and intraoperative tissue samples was similar (Table 2). S. epidermidis ranked first and S. aureus was ranked second, with the two species accounting for 75% of staphylococcal-positive cultures overall, far ahead of S. lugdunensis, S. capitis, or other CNS.
Table 2.
Staphylococcus species recovered from preoperative synovial fluid and intraoperative tissue samples
| Staphylococcus species | Number (%) of positive culture results | Number (%) of false-positive SF culture resultsc | Number (%) of false-negative SF culture resultsd | |
| Preoperative SF samplesa | Intraoperative tissue samplesb | |||
| S. aureus | 31% (25 of 80)e | 35% (25 of 71)f | 12% (3 of 25) | 12% (3 of 25) |
| S. epidermidis | 44% (35 of 80) | 42% (30 of 71) | 26% (9 of 35) | 13% (4 of 30) |
| S. lugdunensis | 10% (8 of 80) | 11% (8 of 71) | 0% | 0% |
| Other species | 15% (12 of 80) | 11% (8 of 71) | 50% (6 of 12) | 25% (2 of 8) |
| S. capitis | 10% (8 of 80) | 7% (5 of 71) | 38% (3 of 8) | 0% |
| S. caprae | 1% (1 of 80) | 1% (1 of 71) | 0% | 0% |
| S. haemolyticus | 1% (1 of 80) | 0% | 50% (1 of 2) | NA |
| S. hominis | 1% (1 of 80) | 1% (1 of 71) | 100% (1 of 1) | 100% (1 of 1) |
| S. saccharolyticus | 1% (1 of 80) | 0% | 100% (1 of 1) | NA |
| S. xylosus | 0% | 1% | NA | 100% (1 of 1) |
At least one synovial fluid sample has positive culturing results.
At least one tissue sample had culturing results positive for S. aureus and at least two tissue sample results positive for CNS.
Staphylococcus species isolated from synovial fluid and not cultured as a significant organism (as defined in footnote b) from an intraoperative tissue sample.
Staphylococcus species recovered as a significant organism (as defined in footnote b) from an intraoperative tissue sample and not cultured from synovial fluid.
Number/total number of staphylococcal species cultured from synovial fluid.
Number/total number of staphylococcal species cultured from intraoperative samples; SF = synovial fluid.
We found discrepancies between cultures from preoperative synovial fluid and intraoperative tissue samples, with false-positive and false-negative synovial fluid culture results (Table 2). The proportion of false-positive synovial fluid culture results was particularly high for S. epidermidis (26%, 9 of 35) and other CNS that commonly inhabit human skin, such as S. capitis (3 of 8). There was no difference between the hip and the knee (see Table 2; Supplemental Digital Content 2, http://links.lww.com/CORR/A392).
Study Endpoints
The positive predictive value (PPV) was defined as the likelihood of having a PJI involving a given Staphylococcus species if the species was isolated from synovial fluid samples obtained through preoperative aspiration. The negative predictive value (NPV) was defined as the likelihood of not having a PJI involving a given Staphylococcus species if the species was not isolated from synovial fluid samples obtained through preoperative aspiration.
Predictive Value of Staphylococcal Synovial Fluid Culture
The PPV and NPV of synovial fluid cultures were 79% and 97%, respectively, for staphylococci altogether (Table 3). In contrast to the NPV, PPVs varied substantially according to Staphylococcus species; they were the highest for S. aureus (92%) and S. lugdunensis (100%) and the lowest for S. epidermidis (77%) and the “other species” subgroup (46%). The PPV and NPV values were similar between the hip and knee (Table 3).
Table 3.
PPV of staphylococcal synovial fluid culture alone (95% CI)
| All sites | Hip | p value | Knee | p value | |
| All staphylococci | 79% (68 to 87) | 78% (63 to 88) | 1 | 80% (64 to 91) | 1 |
| Staphylococcus aureus | 92% (75 to 99) | 92% (65 to 100) | 1 | 92% (65 to 100) | 1 |
| S. epidermidis | 77% (60 to 88) | 79% (57 to 93) | 1 | 73% (48 to 91) | 1 |
| S. lugdunensis | 100% (69 to 100) | 100% (37 to 100) | 1 | 100% (55 to 100) | 1 |
| Other staphylococci | 46% (21 to 73) | 50% (21 to 79) | 1 | 33% (2 to 87) | 0.62 |
PPV = positive predictive value.
Antibiotic Susceptibility Profiles of Preoperative versus Intraoperative Strains
We compared the antibiotic susceptibility profiles of preoperative and intraoperative staphylococcal strains in infections in which culture results were concordant (same staphylococcal species isolated from preoperative and intraoperative samples). Antibiotic susceptibility data were missing in five samples (S. epidermidis infection, four patients; S. aureus infection, one sample).
Statistical Analysis
In addition to the PPV and NPV, 95% exact binomial confidence intervals (95% CIs) were determined. Categorical and continuous variables were compared using Fisher’s exact test, a t-test, or Wilcoxon’s rank-sum test, as appropriate. P values < 0.01 were considered statistically significant. All calculations were performed using R version 2.13 (R Foundation for Statistical computing, Auckland, New Zealand).
Results
Predictive Value of Staphylococcal Synovial Fluid Culture Combined with SASA
Positive SASA results increased the PPV compared with synovial fluid culture alone (92% [95% CI 82 to 97] versus 79% [95% CI 68 to 87]; p = 0.04) (Fig. 2). There was a substantial increase in PPV for S. epidermidis (88% [95% CI 70 to 97] versus 77% [95% CI 60 to 88]; p = 0.33) and for other CNS (100% [95% CI 22 to 100] versus 46% [95% CI 21 to 100]; p = 0.47) (Table 4). When stratified by site, an increase in PPV was seen in hip infections (100% [95% CI 89 to 100] versus 77% [95% CI 63 to 88]; p = 0.01) but not in knee infections (84% [95% CI 66 to 95] versus 80% [95% CI 64 to 91]; p = 0.75). The PPV decreased to 55% for staphylococci overall when SASA results were negative; the decrease was, however, weak for S. aureus (88% versus 92%). Moreover, a negative SASA result led to a greater decrease in PPV for hips than for knees for all staphylococci (47% [95% CI 26 to 69] versus (70% [95% CI 38 to 92]; p = 0.43), including S. aureus (67% [95% CI 13 to 98] versus (100% [95% CI 55 to 100]; p = 0.38) (Fig. 2).
Fig. 2.
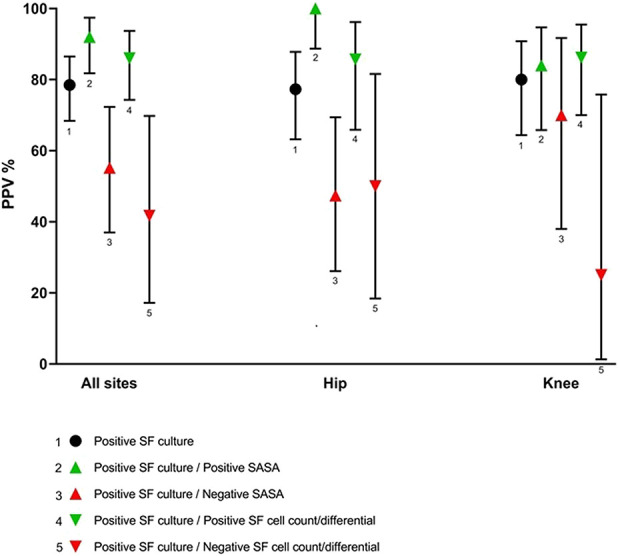
This graph shows the PPV values of synovial fluid cultures alone and combined with SASA or synovial fluid cell count/differential. The PPV values for overall staphylococci are shown. Error bars represent 95% CIs.
Table 4.
PPV of positive synovial fluid culture combined with SASA (95% CI)
| All sites | ||||
| PPV of positive cultures/positive SASA | p valuea | PPV of positive cultures/negative SASA | p valuea | |
| All staphylococci | 92% (82 to 97) | 0.04 | 55% (37 to 72) | 0.02 |
| S. aureus | 94% (73 to 100) | 1 | 88% (52 to 99) | 1 |
| S. epidermidis | 88% (70 to 97) | 0.33 | 50% (21 to 79) | 0.13 |
| S. lugdunensis | 100% (69 to 100) | 1 | ||
| Other staphylococci | 100% (22 to 100) | 0.47 | 36% (13 to 66) | 0.69 |
| Hip | ||||
| All staphylococci | 100% (89 to 100) | 0.01 | 47% (26 to 69) | 0.04 |
| S. aureus | 100% (72 to 100) | 1 | 67% (13 to 98) | 0.37 |
| S. epidermidis | 100% (76 to 100) | 0.27 | 50% (18 to 82) | 0.18 |
| S. lugdunensis | 100% (37 to 100) | 1 | ||
| Other staphylococci | 100% (22 to 100) | 0.47 | 38% (11 to 72) | 0.66 |
| Knee | ||||
| All staphylococci | 84% (66 to 95) | 0.75 | 70% (38 to 92) | 0.67 |
| S. aureus | 86% (41 to 99) | 1 | 100% (55 to 100) | 1 |
| S. epidermidis | 77% (49 to 94) | 1 | 50% (3 to 98) | 0.51 |
| S. lugdunensis | 100% (55 to 100) | 1 | ||
| Other staphylococci | 33.3% (2 to 87) | 1 |
Comparison with PPV of positive cultures alone; SASA = serum anti-staphylococcal antibodies; PPV = positive predictive values.
The SASA results had little impact on the NPV of synovial fluid cultures (see Table 3; Supplemental Digital Content 3, http://links.lww.com/CORR/A393).
Predictive Value of Staphylococcal Synovial Fluid Culture Combined with Synovial Fluid Cell Count and Differential
A positive synovial fluid cell count and differential result increased the PPV compared with synovial fluid culture alone (86% [95% CI 70 to 95] versus 79% [95% CI 68 to 87]; p = 0.36) (Fig. 2). When stratified by site, in contrast to SASAs, this increase in PPV was seen in both hip infections (86% [95% CI 66 to 96] versus 77% [95% CI 63 to 88]; p = 0.42) and knee infections (86% [95% CI 70 to 96] versus 80% [95% CI 64 to 91]; p = 0.74) (Table 5). However, the increase was lower than that observed with SASA measurement for all staphylococcal species subgroups, except for other CNS (PPV of 100% for both). A negative cell count and differential result led to a decrease in PPV for all staphylococci, except for S. aureus (100% [95% CI 22 to 100]) and S. lugdunensis (100% [95% CI 5 to 100]); the number of samples was too small to compare the results for hip and knee arthroplasties. Synovial fluid cell count and differential results had little impact on the NPV of synovial fluid culture results (see Table 3; Supplemental Digital Content 3, http://links.lww.com/CORR/A393), as found for SASA.
Table 5.
PPV of positive synovial fluid (SF) culture combined with synovial fluid (SF) cell count/differential (95% CI)
| All sites | ||||
| PPV of positive cultures/positive SF cell count/differential | p valuea | PPV of positive cultures/ negative SF cell count/differential | p valuea | |
| All staphylococci | 86% (74 to 94) | 0.36 | 42% (17 to 70) | 0.01 |
| Staphylococcus aureus | 90% (71 to 98) | 1 | 100% (22 to 100) | 1 |
| S. epidermidis | 77% (57 to 91) | 1 | 33% (2 to 87) | 0.17 |
| S. lugdunensis | 100% (61 to 100) | 1 | 100% (5 to 100) | 1 |
| Other staphylococci | 100% (22 to 100) | 0.47 | 17% (1 to 59) | 0.33 |
| Hip | ||||
| All staphylococci | 86% (66 to 96) | 0.42 | 50% (18 to 82) | 0.19 |
| S. aureus | 88% (52 to 99) | 1 | 100% (22 to 100) | 1 |
| S. epidermidis | 78% (44 to 96) | 1 | 50% (3 to 98) | 0.43 |
| S. lugdunensis | 100% (37 to 100) | 1 | ||
| Other staphylococci | 100% (5 to 100) | 1 | 25% (1 to 76) | 0.58 |
| Knee | ||||
| All staphylococci | 86% (70 to 96) | 0.74 | 25% (1 to 76) | 0.04 |
| S. aureus | 92% (65 to 100) | 1 | ||
| S. epidermidis | 77% (49 to 94) | 1 | ||
| S. lugdunensis | 100% (37 to 100) | 1 | 100% (5 to 100) | 1 |
| Other staphylococci | 100% (5 to 100) | 1 |
Comparison with PPV of positive cultures alone.
Antibiotic Susceptibility Profiles of Preoperative Versus Intraoperative Strains
We compared the antibiotic susceptibility profiles of preoperative and intraoperative staphylococcal strains in infections in which culture results were concordant (same staphylococcal species isolated from preoperative and intraoperative samples). Antibiotic susceptibility data were missing in five samples (S. epidermidis infection, four patients; S. aureus infection, one sample). The susceptibility profiles of preoperative and intraoperative strains were identical in 90% (56 of 62) of samples and differed by only one marker in the remaining 10% (6 of 62) (aminoglycosides, two samples; penicillin G, two samples; rifampin, one sample; fusidic acid, one sample).
Discussion
In clinical practice, new, reliable, and feasible diagnostic tools to discriminate between septic and aseptic prosthetic failure are needed. Preoperative synovial fluid culture is pivotal in the early diagnosis of PJI but may yield false-positive and false-negative results in particular with staphylococcal PJI. The use of a new assay measuring SASA has been developed to diagnose PJI noninvasively, and its performance in combination with preoperative prosthetic joint aspiration had not been studied. Here, we aimed to determine whether the measurement of SASA may improve the ability of preoperative aspiration to diagnose staphylococcal PJI and identify the correct causative organism. Our study shows that by combining synovial fluid culture with SASA measurement, the PPV was substantially improved. The results are particularly interesting for S. epidermidis and other CNS that may be contaminants because when it is isolated from synovial fluid, it poses a problem in terms of interpretation.
Limitations
Our study had several limitations. First, the prospective study was initiated before the current definition of PJI was established. Therefore, the definition of infection we used is imperfect, and completed datasets could not reliably be obtained retrospectively. However, because we focused on microbiologically documented infections, the lack of sensitivity in PJI diagnosis does not affect our study. Evaluating single cultures of virulent organisms allowed us to examine their significance in the context of SASA testing: we identified 18 patients with a single intraoperative culture with virulent organisms out of 114 patients of infection (16%). Of the 18 patients, 10 had concordant results between intraoperative and preoperative results and were therefore included in the 2018 Musculoskeletal Infection Society (MSIS) definition [15]. The eight other patients had clinical and serological C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) minor criteria of PJI [15]. These observations confirm to a certain extent the relevance of this categorization. Four of eight cases were S. aureus and were included in this study as PJIs with an inconclusive MSIS 2018 score of 5, which could have been positive with the inclusion of any other minor criteria. Sixteen percent (4 of 25) of S. aureus PJIs were equivocal but because our results address a benefit observed for non-aureus staphylococci, this limitation does not impact our findings.
Second, the PPV of any diagnostic test is affected by the prevalence of the disease. Using the same test in a population with a higher prevalence of disease increases the PPV and decreases the NPV. We recruited patients from reference centers that treat bone and joint infections to include a sufficient number of patients with PJI, particularly staphylococcal PJI. The prevalence of PJI in our study was 34%, which was higher than that found in general practice in the United States [4, 5]. Our results are therefore directly applicable only to specialized centers where the management of PJI is a major activity. Nevertheless, our prevalence data are very similar to those reported in most other published studies, most of which were also conducted in specialized centers. For example, the overall prevalence of PJI was 33% in a systematic review and meta-analysis of 30 studies that included 3909 total hip or knee revision arthroplasties to evaluate inflammatory blood markers of PJI [3].
In addition, 62% of the PJIs included in our study were caused by staphylococci, a proportion also widely found in previous studies [21]. The results of diagnostic tests in most previous studies were obtained under conditions similar to ours. Second, despite including a high number of patients with PJI, including more than 70 with staphylococcal infections, the subgroup analyses lacked power, resulting in very wide CIs. This is particularly true for species or subgroup species analyses, and caution should be used when interpreting the results, including the findings of the two most prevalent species, S. aureus and S. epidermidis.
Third, to statistically analyze our data, we needed to exclude patients in whom several species of staphylococci were found, either in preoperative aspirates (polystaphylococcal synovial fluid culture) or as infective organisms from intraoperative specimens (polystaphylococcal infection). These, however, were rare in our series (three synovial fluid polystaphylococcal cultures and three with polystaphylococcal infection) and therefore had no impact on the validity of our results. Anecdotally, the analysis of the three polystaphylococcal synovial fluid cultures highlights that polymicrobial aspirates should be interpreted with caution. These three patients certainly had an infection, but it was because of an organism not found preoperatively in two patients (enteric bacilli) and because of only one of the three staphylococci found preoperatively in the third patient (S. capitis).
Predictive Value of Staphylococcal Synovial Fluid Culture Combined with SASA
A positive SASA result allowed us to affirm that Staphylococcus isolated preoperatively from a hip aspirate was infective in almost all patients, regardless of whether the organism was S. aureus, S. epidermidis, or another CNS.
We found particularly high performance for hip prostheses. The PPV of synovial fluid culture for all staphylococci combined improved from 77% to 100% when the SASA results were positive and was 47% when they were negative, whereas for knee prostheses, it only improved from 80% to 84% when the SASA results were positive and was 70% when they were negative. Despite CI values close to overlap, our study was powered enough to support these results. We have no definitive explanation for such differences in performance between the hip and knee. Some authors have found a particularly high inflammatory response in patients undergoing hip arthroplasty [18], but this may be due to a bias related to a much higher proportion of S. aureus infections than usually encountered in knee arthroplasties [9, 22], which was not the case in our series. It is also possible that the immune response is stronger for hip infections, which is a deep joint involving a greater tissue mass. Essentially, the technical ease of a knee aspiration is such that the risk of contamination or of unproductive aspiration is minimal. Therefore, the validity of the culture results is excellent and less subject to false positives or negatives. In comparison, the complexity of hip aspirations makes it more subject to failures and contaminations and additional immunological data can be informative.
The SASA approach also has several limitations. First, this approach is only applicable to staphylococci and, more particularly, the three species for which the test has been validated: S. aureus, S. epidermidis, and S. lugdunensis [13]. It is thus not applicable to non-staphylococcal contaminants found on the skin, such as corynebacteria and related bacteria. Second, the test only allows for a diagnosis at the genus level (Staphylococcus) and not at the species level (for example, S. aureus and S. epidermidis). We recently showed that the response profile for each of the eight antigens in the test changed depending on the Staphylococcus [2]. There is, therefore, a relationship between the response profile and the species in question. However, the interindividual variability of the responses does not practically and reliably allow for assignment of a particular profile to a given species. Third, the preoperative test may be inconclusive. However, this is rare (2% in this series) and staphylococcal PJI should be suspected when there is a response to an early infection or a PJI involving a CNS other than S. epidermidis or S. lugdunensis [13]. This is what we observed in our series because the seven patients excluded because of an undetermined SASA result had staphylococcal PJI (S. aureus alone, n = 3; S. epidermidis alone, n = 3; S. aureus and S epidermidis, n = 1). For an undetermined SASA result, we recommend performing a new test 4 to 6 weeks later, when it is compatible with the patient’s care [13], which we could not do in this blind prospective study.
Apart from the statistical analysis, S. lugdunensis was the only species for which we found no false positives or false negatives for the preoperative aspirates: the eight patients for whom the synovial fluid culture result was positive for S. lugdunensis alone were infected with this species, and these eight patients accounted for all S. lugdunensis PJIs recorded during the study. This confirms the particular pathogenicity of S. lugdunensis in the group of patients with CNS and the value that must be attributed to a positive culture result, even if it is obtained from a single sample [19]. In comparison, three of the eight PJIs found to be positive for S. capitis by preoperative aspiration were false positives. Twelve percent (3 of 25) of the samples were false-positive for S. aureus and 26% (9 of 35) were false-positive for S. epidermidis.
Staphylococcal Synovial Fluid Culture Combined with Synovial Fluid Cell Count and Differential
The cell count and differential provides a smaller benefit to the hip than that provided by SASA. On the other hand, the measurement of SASA provided no benefit relative to synovial fluid cell count or differential for knee prostheses. Our results thus suggest that the antibody response is a better marker of staphylococcal infection than the leukocyte response for hip prostheses. This deserves confirmation by expanding the analysis to other biomarkers of the inflammatory and leukocyte response. Several synovial fluid biomarkers have shown excellent performance for diagnosing PJI [12]. This is particularly true for alpha-defensin, measured alone or combined with synovial fluid C-reactive protein [6, 12]. It may be informative in future studies to evaluate the contribution of these biomarkers associated with synovial fluid culture to the measurement of SASA, depending on the site of the prosthesis.
Concordance of Antibiotic Susceptibility Profiles of Preoperative versus Intraoperative Strains
There was a high degree of agreement between organisms isolated from both preoperative aspirations and intraoperative samples.
Indeed, in our series, we found a perfect match (identical profiles) in more than 90% of the infections and the few cases of discordance concerned only one marker (aminoglycosides, penicillin G, fusidic acid, or rifampin). Notably, no discordance was found for methicillin resistance in S. aureus, S. epidermidis, or other CNS. These results confirm the relevance of the bacteriological data obtained by preoperative synovial fluid aspiration. However, more than 90% of our PJIs were delayed infections (> 3 months), and there was a lower infection risk based on subtypes of the same species, which may have different susceptibility to antibiotics [10].
Conclusions
In conclusion, SASAs are excellent markers of staphylococcal infection in patients undergoing hip arthroplasties, and their positivity associated with culture-positive synovial fluid with Staphylococcus confirms that the isolated Staphylococcus is responsible for the PJI, even if it is a CNS. In contrast, SASA has no advantage over synovial fluid cell count and differential in patients undergoing knee arthroplasties. These results will need to be confirmed by studies addressing other populations and could include benchmarking the performance of other blood or synovial fluid biomarkers.
Acknowledgments
We thank Isabelle Gentilhomme for her assistance. We thank all the members of the two reference centers of bone and joint infections of Ile de France: Hôpital Ambroise Paré, Boulogne Billancourt, France and Groupe Hospitalier Diaconesses Croix Saint-Simon, Paris, France. We thank all the surgeons, radiologists, microbiologists, and the technical lab team.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed in two reference centers of bone and joint infections of Ile de France : Hôpital Ambroise Paré (APHP), Boulogne Billancourt, France and Groupe Hospitalier Diaconesses Croix Saint-Simon, Paris, France.
The first two authors contributed equally to this manuscript.
References
- 1.Ali F, Wilkinson JM, Cooper JR, Kerry RM, Hamer AJ, Norman P, Stockley I. Accuracy of joint aspiration for the preoperative diagnosis of infection in total hip arthroplasty. J Arthroplasty. 2006;21:221–226. [DOI] [PubMed] [Google Scholar]
- 2.Bauer T, Marmor S, Salomon E, El Sayed F, Ghout I, Heym B, Rottman M, Gaillard J-L, Roux A-L. Multiplex Antibody Measurement for Post-treatment Follow-up of Staphylococcal Prosthetic Joint Infection: A Diagnostic Pilot Study. J Bone Jt Infect . 2019;4:227–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berbari E, Mabry T, Tsaras G, Spangehl M, Erwin PJ, Murad MH, Steckelberg J, Osmon D. Inflammatory blood laboratory levels as markers of prosthetic joint infection: a systematic review and meta-analysis. J Bone Joint Surg Am . 2010;92:2102–2109. [DOI] [PubMed] [Google Scholar]
- 4.Bozic KJ, Kurtz S, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of bearing surface usage in total hip arthroplasty in the United States. J Bone Joint Surg Am . 2009;91:1614–1620. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Kurtz SM, Lau E, Ong K, Chiu V, Vail TP, Rubash HE, Berry DJ. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res . 2010;468:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deirmengian C, Kardos K, Kilmartin P, Cameron A, Schiller K, Parvizi J. Combined measurement of synovial fluid alpha-Defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Joint Surg Am . 2014;96:1439–1445. [DOI] [PubMed] [Google Scholar]
- 7.de Seynes C, de Barbeyrac B, Dutronc H, Ribes C, Cremer P, Dubois V, Fabre T, Dupon M, Dauchy F-A. Contribution of a multiplex serological test for the preoperative diagnosis of prosthetic joint infection: a prospective study. Infect Dis (London, England). 2018;50:609–615. [DOI] [PubMed] [Google Scholar]
- 8.Fehring TK, Cohen B. Aspiration as a guide to sepsis in revision total hip arthroplasty. J Arthroplasty. 1996;11:543–547. [DOI] [PubMed] [Google Scholar]
- 9.Ghanem E, Parvizi J, Burnett RSJ, Sharkey PF, Keshavarzi N, Aggarwal A, Barrack RL. Cell count and differential of aspirated fluid in the diagnosis of infection at the site of total knee arthroplasty. J Bone Joint Surg Am . 2008;90:1637–1643. [DOI] [PubMed] [Google Scholar]
- 10.Hope PG, Kristinsson KG, Norman P, Elson RA. Deep infection of cemented total hip arthroplasties caused by coagulase-negative staphylococci. J Bone Joint Surg Br . 1989;71:851–855. [DOI] [PubMed] [Google Scholar]
- 11.Hughes JG, Vetter EA, Patel R, Schleck CD, Harmsen S, Turgeant LT, Cockerill FR., 3rd Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J Clin Microbiol . 2001;39:4468–4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YS, Koo K-H, Kim HJ, Tian S, Kim T-Y, Maltenfort MG, Chen AF. Synovial Fluid Biomarkers for the Diagnosis of Periprosthetic Joint Infection: A Systematic Review and Meta-Analysis. J Bone Joint Surg Am . 2017;99:2077–2084. [DOI] [PubMed] [Google Scholar]
- 13.Marmor S, Bauer T, Desplaces N, Heym B, Roux A-L, Sol O, Roge J, Mahe F, Desire L, Aegerter P, Ghout I, Ropers J, Gaillard J-L, Rottman M. Multiplex Antibody Detection for Noninvasive Genus-Level Diagnosis of Prosthetic Joint Infection. J Clin Microbiol . 2016;54:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson WR. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis . 2013;56:e1–e25. [DOI] [PubMed] [Google Scholar]
- 15.Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, Shohat N. The 2018 definition of peroprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33:1309-1314. [DOI] [PubMed] [Google Scholar]
- 16.Qu X, Zhai Z, Wu C, Jin F, Li H, Wang L, Liu G, Liu X, Wang W, Li H, Zhang X, Zhu Z, Dai K. Preoperative aspiration culture for preoperative diagnosis of infection in total hip or knee arthroplasty. J Clin Microbiol . 2013;51:3830–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roux A-L, Sivadon-Tardy V, Bauer T, Lortat-Jacob A, Herrmann J-L, Gaillard J-L, Rottman M. Diagnosis of prosthetic joint infection by beadmill processing of a periprosthetic specimen. Clin Microbiol Infect . 2011;17:447–450. [DOI] [PubMed] [Google Scholar]
- 18.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am . 2008;90:1869–1875. [DOI] [PubMed] [Google Scholar]
- 19.Shah NB, Osmon DR, Fadel H, Patel R, Kohner PC, Steckelberg JM, Mabry T, Berbari EF. Laboratory and clinical characteristics of Staphylococcus lugdunensis prosthetic joint infections. J Clin Microbiol . 2010;48:1600–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Somme D, Ziza J-M, Desplaces N, Chicheportiche V, Chazerain P, Leonard P, Lhotellier L, Jacquenod P, Mamoudy P. Contribution of routine joint aspiration to the diagnosis of infection before hip revision surgery. Joint Bone Spine. 2003;70:489–495. [DOI] [PubMed] [Google Scholar]
- 21.Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev . 2014;27:302–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med . 2004;117:556–562. [DOI] [PubMed] [Google Scholar]
- 23.Triffault-Fillit C, Ferry T, Laurent F, Pradat P, Dupieux C, Conrad A, Becker A, Lustig S, Fessy MH, Chidiac C, Valour F. Microbiologic epidemiology depending on time to occurrence of prosthetic joint infection: a prospective cohort study. Clin Microbiol Infect . 2019;25:353–358. [DOI] [PubMed] [Google Scholar]


