Abstract
Background
Early detection of soft-tissue sarcoma recurrences may decrease the morbidity of reoperation and improve oncologic outcomes. The benefit of imaging compared with clinical surveillance for detecting local recurrences remains controversial, as prior studies have varied in terms of inclusion criteria, factors analyzed, and outcomes reported.
Questions/purposes
(1) What proportion of local recurrences were detected by surveillance imaging compared with clinical signs and symptoms? (2) Were local recurrences detected by imaging smaller than those detected by clinical surveillance? (3) Were relevant tumor, patient, or operative characteristics associated with clinically occult local recurrence?
Methods
Over a 20-year period ending in 2018, we treated 545 patients for soft-tissue sarcoma. During that period, we recommended that patients receive a surgical excision as well as radiation therapy based on current clinical guidelines. Of those we treated, 9% (51 of 545) were excluded for having a low-grade liposarcoma, and 4% (21 of 545) were excluded for being metastatic at the time of presentation. Of the remaining patients, 22% (107 of 473) were lost to follow-up before 2 years but were not known to have died. There were a remaining 366 patients for analysis in this retrospective study of electronic medical records from a single center. Patients routinely underwent advanced imaging and clinical follow-up at intervals based on currently available guidelines for sarcoma surveillance. We recommended that patients with high-grade sarcomas be followed every 3 months until 2 years, then every 6 months until 3 years, then annually thereafter. In contrast, we recommended that patients with low-grade sarcomas be followed every 6 months until 2 years, then annually thereafter. In addition, patients were encouraged to return for evaluation if they noted a new mass or other symptoms. In general, patients with high-grade sarcomas received postoperative radiation therapy unless they underwent amputation, while intermediate- and low-grade sarcomas were radiated according to clinical concern for local recurrence, as determined by the multidisciplinary sarcoma team. Seventeen percent (61 of 366) of patients developed or presented with a local recurrence. Of the local recurrences detected by surveillance imaging, 17 were detected by MRI, three were detected by position emission tomography, and one was detected by CT scan. The proportion of local recurrences first identified by advanced imaging versus clinical detection (physical examination, self-detection, or symptomatic presentation) were compared. Logistic regression with a Wald chi-square test was performed to evaluate if tumor, patient, or operative characteristics are associated with clinical versus imaging detection of local recurrences.
Results
A higher proportion of local recurrences were detected by clinical signs and symptoms than by routine imaging (66% (40 of 61) versus 34% (21 of 61), binomial proportion 0.66 [95% CI 0.55 to 0.77]; p = 0.007). With the numbers available, there was no difference in the tumor size detected by clinical signs and symptoms compared with surveillance imaging. The median (interquartile range) largest tumor dimension was 3.9 cm (2.5 to 7.8) for clinical surveillance versus 4.5 cm (2.7 to 6.2) for imaging surveillance (p = 0.98). We were unable to identify any associated factors, alone or in combination, with detection by physical exam, including patient age, tumor size, tumor depth, tumor location, operative closure type, or radiation status. Characteristics such as larger tumors, more superficial tumors, low BMI, the absence of a flap reconstruction or radiation treatment, were not associated with a greater likelihood of detection by physical examination.
Conclusions
We found that although a high proportion of local recurrences were detected by clinical signs and symptoms, approximately one-third were detected by imaging. Although not all patients may benefit equally from routine imaging, we were unable to identify any patient, tumor, or operative characteristics to define a subgroup of patients that are more or less likely benefit from this surveillance technique. These findings support current surveillance guidelines that recommend the use of advanced imaging; however, other factors may also warrant consideration. Futher insight could be gained by studying surveillance imaging in terms of optimal frequency, cost-effectiveness, and psychosocial implications for patients.
Level of Evidence
Level III, diagnostic study.
Introduction
Soft-tissue sarcomas are a group of uncommon cancers accounting for less than 1% of all malignancies. Initial management of soft-tissue sarcoma includes surgical resection combined with radiation therapy and, in some patients, systemic treatments. Even with improving adjuvant treatments, local recurrence affects 5% to 9% of patients undergoing limb-sparing surgical resection at 5 years [5, 14]. Monitoring for local recurrence is critical, as early detection may decrease the morbidity of re-excision and improve survival and physical function. Advanced imaging studies, including MRI, CT, and position emission tomography (PET) scans, are highly sensitive for identifying local recurrence; however, they are costly in terms of time and resources for both patients and the health care systems.
Surveillance guidelines, such as those issued by the National Comprehensive Cancer Network and the MD Anderson Cancer Center, recommend advanced imaging every 3 to 6 months for the first 2 to 3 years, then every 6 months for the following 2 years, and then annually until 10 years after initial treatment [4, 8, 11]. However, evidence supporting these practices is limited. Clinical guidelines, such as those issued by the National Comprehensive Cancer Network in 2018, report that there is limited data on effective soft-tissue sarcoma surveillance strategies [11]. Similarly, the American Academy of Orthopaedic Surgeons/Musculoskeletal Tumor Society Appropriate Use Criteria on Sarcoma Surveillance provides vague guidance on the use of MRI based on a general lack of evidence to support definitive recommendations.
Existing research on this topic has been limited by the rare and diverse nature of soft-tissue sarcoma, and it has produced conflicting findings. Most prior studies included only one to 13 recurrent tumors [1, 2, 3, 6, 10]; local recurrence has been reported in 5.3% to 20% of patients, and among these 8.8% to 60% were clinically occult. Although each of these studies included at least one patient with a clinically occult local recurrence (that is, a local recurrence first detected on imaging rather than clinical signs or symptoms), most observed minimal advantage of imaging over physical examination on the population level [3, 13]. Moreover, those finding a benefit of imaging surveillance reported variable proportions of clincally occult local recurrences [13, 18]. Inclusion criteria also vary, with most studies focusing on extremity tumors, and few including the neck, torso, pelvis, or sacrum. Finally, although a few studies have accounted for tumor size and location as potential confounding factors, none have comprehensively investigated tumor, patient, and operative charactertistics that may be associated with clinically occult local recurrence. Thus, the use of routine advanced imaging to detect local recurrence remains controversial [1, 10, 18].
We sought to address this evidence gap by asking (1) What proportion of local recurrences were detected by surveillance imaging compared with clinical signs and symptoms? (2) Were local recurrences detected by imaging smaller than those detected by clinical surveillance? (3) Were relevant tumor, patient, or operative characteristics associated with clinically occult local recurrence?
Patients and Methods
We retrospectively evaluated the electronic medical records of all patients who underwent excision of a nonmetastatic soft-tissue sarcoma by the orthopaedic oncology service at our sarcoma referral center between August 1999 and December 2018.
Over the 20-year period ending in 2018, we treated 545 patients for soft-tissue sarcoma. During that period, patients underwent surgical excision and radiation therapy based on current clinical guidelines. Of those we treated, 9% (51 of 545) were excluded for having a low-grade liposarcoma and 4% (21 of 545) were excluded for metastases at time of presentation (Fig. 1). Of the remaining 473 patients meeting the inclusion criteria, 22% (107 of 473) were lost to follow-up before 2 years but were not known to have died, leaving 366 patients available for analysis. The patients lost to follow-up were compared with the included patient population in terms of the relevant patient, tumor, and surgical characteristics analyzed. The only differences between these populations were age, with younger patients being more likely to follow-up, and surgical margin, with patients having grossly positive margins being more likley to follow-up than those with widely negative (≥ 5 mm) margins (Table 1).
Fig. 1.
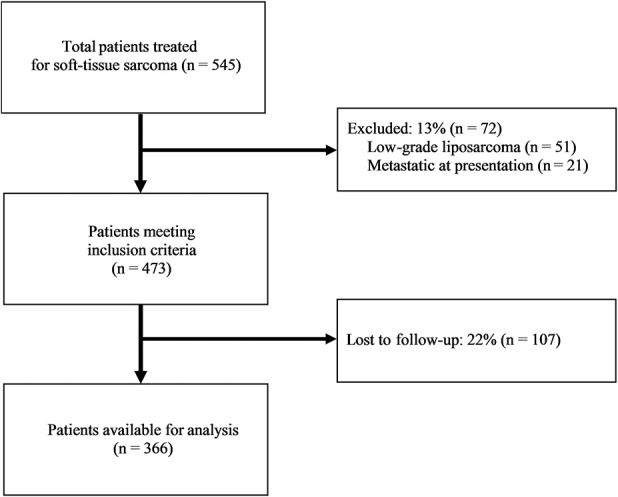
The STROBE flow chart of inclusion criteria is shown here.
Table 1.
Distribution of patients included in the study versus excluded for loss of follow, with patient, tumor, and operative characteristics
| Parameter | Included patients (n = 366) | Patients lost to follow-up (n = 107) | Odds ratio (95% CI) or mean difference ± SD | p value |
| Patient gendera | 0.51b | |||
| Female | 45 (164) | 41(44) | ||
| Male | 55 (202) | 59 (63) | 1.16 (0.74 to 1.8) | |
| Patient age (years)c | 59.0 (43.0 to 70.0) | 63.0 (50.0 to 76.0) | 0.003d | |
| Patient BMI (kg/m2)e | 28.9 ± 7.0 | 28.9 ± 6.9 | -0.02 ± 6.95 | 0.98f |
| Largest tumor dimension (cm)c | 7.0 (4.0 to 11.2) | 7.5 (4.5 to 15.0) | 0.32d | |
| Categorical tumor sizea | 0.71d | |||
| ≤ 5 cm | 37 (137) | 41 (44) | ||
| > 5, ≤ 10 cm | 32 (118) | 30 (32) | 0.91 (0.54 to 1.54) | |
| > 10 cm | 30 (111) | 29 (31) | 0.94 (0.55 to 1.61) | |
| Tumor locationa | 0.15b | |||
| Lower extremity | 56 (206) | 67 (72) | ||
| Upper extremity | 18 (67) | 18 (19) | 0.79 (0.44 to 1.42) | |
| Back/torso/neck | 10 (37) | 3 (4) | 0.33 (0.11 to 0.95) | |
| Pelvis/sacrum | 9 (32) | 6 (6) | 0.55 (0.22 to 1.38) | |
| Acral (hand/foot) | 7 (24) | 6 (6) | 0.74 (0.29 to 1.87) | |
| Tumor deptha | 0.63b | |||
| Superficial | 14 (50) | 11 (12) | ||
| Deep | 86 (316) | 89 (94) | 1.21 (0.62 to 2.4) | |
| Tumor gradea | 0.13d | |||
| Low | 12 (43) | 17 (18) | ||
| Intermediate | 2 (7) | 3 (3) | 0.98 (0.23 to 4.21) | |
| High | 86 (315) | 80 (86) | 0.61 (0.33 to 1.11) | |
| Tumor marginsa | 0.020d | |||
| Positive margins | 29 (104) | 22 (23) | ||
| < 0.1 cm | 21 (76) | 18 (19) | 1.15 (0.58 to 2.25) | |
| < 0.5 cm | 20 (72) | 18 (19) | 1.07 (0.53 to 2.14) | |
| ≥ 0.5 cm | 30 (111) | 42 (45) | 1.87 (1.06 to 3.31) | |
| Operative closurea | 0.30b | |||
| Primary | 95 (346) | 93 (99) | ||
| Flap | 5 (20) | 7 (8) | 1.41 (0.60 to 3.3) | |
Data are presented as: aN (%), cmedian (IQR), or emean 6 SD; p values were calculated using the following: +Fisher's Exact test, dKruskal-Wallis test, fANOVA. p < 0.05 indicates significance.
All patients underwent surgical excision of their primary tumor. Most patients with high-grade sarcomas received adjuvant radiation therapy, with the exception of some who had a small, superficial sarcoma or who underwent an amputation. Intermediate- and low-grade sarcomas were radiated according to clinical concern for local recurrence, as determined by the multidisciplinary sarcoma team. Overall, 83% (303 of 366) of patients received radiation; 89% (281 of 316) of high-grade sarcomas, 29% (2 of 7) of intermediate-grade sarcomas, and 47% (20 of 43) of low-grade sarcomas received radiation therapy.
Patients were routinely followed by our clinical sarcoma team, including surgical, medical, and radiation oncologists, according to standard guidelines. This included ongoing patient self-monitoring, as well as physician examination and imaging at routine intervals. Imaging consisted of local MRI, and CT of the chest, with occasional use of abdomen/pelvis CT or PET/CT if indicated. We recommended that patients with high-grade sarcomas be followed every 3 months until 2 years, then every 6 months until 3 years, then annually thereafter. In contrast, we recommended that patients with low-grade sarcomas be followed every 6 months until 2 years, then annually thereafter. Patients who presented with symptoms between routine follow-up intervals were evaluated with local advanced imaging. All imaging findings sugestive of local recurrence underwent biopsy.
Variables and Outcome Measures
Local recurrence was defined as a malignant tumor, histologically similar to the original soft-tissue sarcoma confirmed by surgical pathology, present in the bed of the previous surgical resection. Local recurrence was considered to be detected clinically if the patient presented with mass, pain or other symptoms; or if it was detected on physical examination by a surgical or medical oncologist.
Revelant tumor characteristics included size, location, depth, histological subtype and grade, and resection margins. Tumor size was recorded as the largest tumor dimension and categorized as ≤ 5 cm, > 5 cm and ≤ 10 cm, and > 10 cm. Tumor depth was classified as superficial or deep relative to fascia. Histological grade was determined by the French Federation of Cancer Centers Sarcoma Group grading guidelines, and tumors were grouped according to histologic subtype (Table 2). Pathologic margins were classified into one of four categories: positive margins, < 1 mm from tumor, < 5 mm from tumor, and ≥ 5 mm from tumor.
Table 2.
Frequency of recurrent tumor histological subtypes
| Histopathology | % (n) (n = 61) |
| Malignant fibrous histiocytoma | 43 (26) |
| Fibrosarcoma | 21 (13) |
| Leiomyosarcoma | 18 (11) |
| Sarcoma, unspecified | 7 (4) |
| Ewing’s/round cell sarcoma | 3 (2) |
| Rhabdomyosarcoma | 3 (2) |
| Liposarcoma | 3 (2) |
| Synovial sarcoma | 2 (1) |
Demographics and Description of Local Recurrences
In this study, 17% (61 of 366) of patients developed a local recurrence at a median (interquartile range) of 12 months (3 to 33). The median (IQR) age for a patient with local recurrence was 68 years (54 to 76). Thirty patients with a local recurrence died of their disease, 14 within 24 months of initial treatment. The median (IQR) length of follow-up from the initial operation until death for local recurrence patients was 28 months (9 to 64). Eleven percent of patients with local recurrence had metastatic disease at the time of detection, and an additional 36% (22 of 61) of patients with local recurrence went on to develop metastasic disease.
The most common local recurrence subtypes were as follows: malignant fibrous histiocytoma, fibrosarcoma, and leiomyosarcoma (Table 2). A total of 89% (54 of 61) of tumors were high grade and 90% (55 of 61) were considered deep to fascia. The lower extremity had the highest number and proportion of local recurrence, followed by the upper extremity (Table 3). Ten percent (36 of 366) of patients had an unplanned excision with positive margins before referral to our institution and underwent re-excision of the primary tumor for definitive surgical treatment. The median (IQR) time from initial surgery until identification of local recurrence was 12 months (5 to 34) for clinical detection and 9.5 months (1.3 to 29.5) for imaging detection (p = 0.23). In addition, there was no difference between the proportion of local recurrences detected by surveillance imaging in the last 5 years of the study compared with the entire study population (OR 0.21 [95% CI 0.02 to 1.78]; p = 0.15).
Table 3.
Distribution of the study population’s patient, tumor, and operative characteristics
| Parameter | No recurrence (n = 305) | Recurrence (n = 61) | Odds ratio (95% CI) or mean difference ± SD | p value |
| Patient age (years)a | 57 (41 to 68) | 68 (54 to 76) | 0.001b | |
| Patient BMI (kg/m2)c | 28.3 ± 7.3 | 27.4 ± 4.9 | 1.57 ± 7.0 | 0.21b |
| Largest tumor dimension (cm)a | 7 (4 to 11.5) | 7 (4.5 to 10.5) | 0.93b | |
| Categorical Tumor Sized | 0.58b | |||
| ≤ 5 cm | 38 (116) | 34 (21) | ||
| > 5, ≤ 10 cm | 32 (95) | 38 (23) | 1.09 (0.59 to 1.99) | |
| > 10 cm | 30 (94) | 28 (17) | 0.89 (0.49 to 1.7) | |
| Tumor locationd | 0.96e | |||
| Lower extremity | 56 (172) | 56 (34) | ||
| Upper extremity | 19 (57) | 16 (10) | 0.82 (0.4 to 1.68) | |
| Back/torso/neck | 10 (30) | 11 (7) | 1.19 (0.49 to 2.89) | |
| Pelvis/sacrum | 8 (25) | 11 (7) | 1.27 (0.52 to 3.09) | |
| Acral (hand/foot) | 7 (21) | 5 (3) | 0.87 (0.29 to 2.64) | |
| Tumor depthd | 0.34e | |||
| Superficial | 14 (44) | 10 (6) | ||
| Deep | 86 (261) | 90 (55) | 1.44 (0.63 to 3.3) | |
| Tumor graded | 0.64b | |||
| Low | 12 (36) | 11 (7) | ||
| High | 86 (262) | 89 (54) | 0.88 (0.42 to 1.83) | |
| Intermediate | 2 (7) | 0 | ||
| Tumor marginsd | 0.001b | |||
| Positive | 23 (71) | 54 (33) | ||
| < 0.1 cm | 20 (62) | 23 (14) | 0.55 (0.28 to 1.07) | |
| < 0.5cm | 22 (68) | 7 (4) | 0.16 (0.06 to 0.43) | |
| ≥ 0.5 cm | 33 (101) | 16 (10) | 0.29 (0.15 to 0.57) | |
| Operative closured | 0.10e | |||
| Primary | 96 (292) | 89 (54) | ||
| Flap | 4 (13) | 11 (7) | 2.39 (0.96 to 6.02) | |
| Months to recurrencea | N/A | 12 (3 to 33) | N/A | |
| Months of follow-upa | 44 (27 to 72.5) | 42 (22 to 81) | 0.91b | |
| Months from first operation until deatha | 21 (11 to 37) | 28 (9 to 64) | 0.20b | |
Data are presented as: amedian (IQR), cmean 6 SD, or dN (%); p values were calculated using the following: bKruskal-Wallis test, or eFisher's Exact test. p < 0.05 indicates significance.
Of the 40 clinically-detected recurrences, 34 were detected by patient monitoring (26 noticed a new mass and eight reported symptoms but no mass), and six were detected by physician examination.
Statistical Analysis
To answer our first study question, whether the proportions of local recurrences detected by imaging versus clinical surveillance differ, we compared the proportion of local recurrences detected by each modality using a binomial proportion test.
To answer our second study question, whether local recurrences detected by imaging were smaller than those detected by clinical surveillance, we compared the median largest tumor dimension detected by each surveillance technique (imaging and clinical) using a Kruskal-Wallis Test, because the distributions of the tumor dimensions were right skewed and not compatible with the use of a t-test.
To answer our third study question, whether modality of local recurrence detection was associated with clinically relevant factors (tumor dimension, location, or depth, patient age or BMI, flap closure of index excision, radiation status) or combinations of these variables, we performed a t-test or Kruskal-Wallis test as appropriate for continuous variables and a chi-square test (or for comparisons with inadequately populated cases, Fisher’s exact test) for categorical variables.
To assess the potential for transfer bias, patients who were lost to follow-up were compared with the included patient population in terms of all tumor, patient, and operative charactertistics evaluated in this study (tumor dimension, location, or depth; patient age or BMI; and primary excision flap closure). This analysis was performed using t-tests or Shapiro-Wilkes tests for continuous variables, and chi-square or Fisher’s exact tests for categorical variables, as appropriate. The results of these analyses were that median age was greater among who were lost to follow-up and those who were included in the study (patient lost to follow-up median (IQR) age: 63 [50 to 76], included patient median age: 59 [43 to 70]; p = 0.003). In addition, operative tumor margins were wider among patients who were lost to follow-up when compared with those included in the study (Table 1).
To investigate the potential for confounding related to imaging utilization and quality over time, we compared the proportion of local recurrences detected by imaging surveillance from the last 5 years to the overall study population. The results of this analysis indicate that the detection method was not different over time (OR 0.21 [95% CI 0.02 to 1.78]; p = 0.15).
All statistical analyses were conducted using SPSS statistical software (IBM Corp, Armonk, NY, USA). A significance threshold of 0.05 was considered to be statistically significant.
Results
Detection of Local Recurrences by Imaging Compared to Clinical Surveillance
A higher proportion of local recurrences were detected by clinical signs and symptoms than routine imaging. Sixty-six percent (40 of 61) of local recurrences were detected clinically, whereas 34% (21 of 61) were first noted on imaging (binomial proportion 0.66 [95% CI 0.55 to 0.77]; p = 0.007).
Size of Local Recurrences Detected by Imaging Compared to Clinical Surveillance
With the numbers available, there was no difference in the size of local recurrences detected by clinical signs and symptoms compared with routine imaging. The median (IQR) size of local recurrence was 3.9 cm (2.5 to 7.8) for those detected clinically versus 4.5 cm (2.7 to 6.2; p = 0.98) for those detected on imaging.
Association of Relevant Tumor, Patient, or Operative Characteristics with Occult Local Recurrence
We did not detect any association between clinically relevant factors (tumor dimension, location, or depth, patient age or BMI, flap closure at the time of initial excision, or radiation status), or combinations of these characteristics, and modality of local recurrence detection (Table 4). For example, larger tumors, more superficial tumors, low patient BMI, and absence of flap coverage, alone or in combination, were not associated with a greater likelihood of detection by physical examination.
Table 4.
Association of relevant patient, tumor, or operative characteristics with local recurrence detection method
| Variable | Odds ratio | 95% CI | p value |
| Age | 0.99 | 0.95 to 1.03 | 0.66 |
| BMI | 1.03 | 0.9 to 1.12 | 0.70 |
| Tumor size (largest dimension) | 0.99 | 0.9 to 1.09 | 0.83 |
| Tumor depth (superficial vs deep) | 1.15 | 0.16 to 8.18 | 0.89 |
| Acral vs upper extremity | 3.06 | 0.09 to 108.47 | 0.54 |
| Back/torso/neck vs upper extremity | 0.39 | 0.03 to 4.9 | 0.46 |
| Lower extremity vs upper extremity | 1.58 | 0.3 to 8.34 | 0.59 |
| Pelvis/sacrum vs upper extremity | 1.27 | 0.12 to 13.35 | 0.84 |
| Closure type (primary vs flap) | 2.08 | 0.26 to 16.41 | 0.49 |
| Radiation status | 1.97 | 0.47 to 8.19 | 0.35 |
95% confidence intervals are Wald’s confidence intervals.
Discussion
The appropriateness of using routine advanced imaging to monitor local recurrence of soft-tissue sarcomas remains controversial [4]. In the absence of strong data to support practice guidelines, it may seem that sarcoma patients receive excessive surveillance imaging after treatment of their tumors. Many poorly understood variables factor into this debate, including the questionable benefit of early detection on morbidity and mortality, appropriate utilization of patient and health care system resources, and psychosocial implications for patients. We sought to provide evidence for one aspect of this controversy by comparing the proportion, size, and characteristics potentially associated with local recurrences detected clinically versus on routine imaging. Although it may seem that most local recurrences would be clinically detectable, that local recurrences would become clinically detectable once they reach a certain size, or that certain characteristics can predict which local recurrences will be clinically detectable, we did not find this to be the case. Instead, we found that although a high proportion of local recurrences were detected by signs and symptoms, more than one-third were detected on routine imaging. Local recurrances detected clinically and on imaging were comparable in size. Lastly, we were unable to identify any patient, tumor, or operative characteristics to define a subgroup of patients that would be more likely to benefit from routine imaging surveillance. Although other elements of the surveillance controversy should be explored before definitive recommendations are issued, our findings suggest that it is difficult to detect local recurrences clinically, or to predict which patients will present with signs or symptoms of local recurrence rather than having their recurrence detected on imaging tests used for surveillance.
Limitations
Foremost, our study is not designed to answer definitively the question of whether soft-tissue sarcoma surveillance should routinely include advanced imaging, as it does not account for other considerations such as the morbidity/mortality benefit of early detection, nor have we performed a cost-benefit analysis of patient and health care system resource use. Second, this study is limited by sample size, related to the rarity of soft-tissue sarcoma and local recurrence, which restricts the power of our analyses. In particular, our study may have been underpowered to detect an association between the modality of local recurrence detection and the tumor, patient, and treatment characteristics that we evaluated. It is also likely that other factors we did not consider, such as unrecognized or poorly-understood variables related to specific tumor biology, may be useful in identifying patients at risk for clinically occult local recurrence. Thirdly, the study period spans 20 years, during which imaging use and technology has advanced; our results might therefore tend to underestimate the current sensitivity of imaging for detecting local recurrence. Nonetheless, we found no difference in local recurrence detection by surveillance imaging in the last 5 years compared with the entire study population.
Finally, this study is limited by certain biases related to its retrospective design. With 22% of patients having less than 2 years of follow-up, it is possible that some had local recurrences that were not included in this analysis. Older patients were less likely to follow-up but more likely to have local recurrences, while patients with positive margins were more likely to follow-up and more likely to have local recurrences. However, neither age nor surgical margin was associated with local recurrence detection modality, so this potential confounding was not likely to affect the main findings of our study. In general, if patients missed routine surveillance imaging but later developed a symptomatic local recurrence prompting follow-up, this could artificially elevate the proportion of clinically detectable versus occult local recurrences in our population. In such patients, if imaging could have detected the tumor earlier, our data would underestimate the its value in early diagnosis of local recurrence. Finally, co-treatment bias was also likely, as theraputic interventions (such as radiation) varied and sarcoma management evolved over the 20-year course of this study. Given the number and intersectionality of these factors, their effects on our findings are unclear.
Detection of Local Recurrences by Imaging Compared to Clinical Surveillance
Most local recurrences in our study were first detected by signs and symptoms, confirming that clinical follow-up is a valuable surveillance modality. However, more than one-third of the local recurrences in our population were clinically occult and only identified on routine imaging. In the context of recurrent sarcoma, wih imaging may translate into a clinically meaningful treatment advantage for a substantial proportion of local recurrences. Most prior studies have not demonstrated a convincing diagnostic benefit of routine MRIs compared with physical examination for surveillance of extremity soft-tissue sarcoma [9, 15, 18]. One study reported that MRIs detected only one asymptomatic extremity local recurrence in 114 MRI scans [1]. In contrast, the largest, most recent, and most inclusive study demonstrated that surveillance with MRI and thorax-abdomen-pelvis CT scans detected 60% of all extremity and trunk local recurrence in their patient cohort of 113 patients (10 recurrent) [3]. Similarly, we found that clinically occult local recurrences may be more frequent than previously recognized. If more than one-third of local recurrences can be detected on imaging before they present with signs or symptoms, our findings would suggest that advanced imaging is an important component of routine surveillance.
Size of Local Recurrences Detected by Imaging Compared to Clinical Surveillance
Detecting local recurrences at smaller sizes theoretically reduces the morbidity associated with re-excision and the mortality associated with delayed diagnosis. Our analysis revealed no difference in the largest diameter of local recurrences detected by clinical versus imaging surveillance. The majority of prior studies on this topic have not investigated whether advanced imaging impacts the size at which recurrent tumors are detected. The notable exception is a study by Park et al. [13] including 94 local recurrences, of which 60% were clinically occult . They found that local recurrences first detected with MRI were smaller than those first detected clinically (2.3 ± 1.3 cm and 4.0 ± 3.4 cm, respectively; p = 0.001); however, this study compared several factors of the clinically occult versus clinically apparent tumors without a regression analysis, making the design suceptible to confounding. Although our study did not demonstrate that routine imaging detects recurrences at smaller sizes, we are also unable to exclude this possibillity, since it is unclear how large the tumors detected on imaging would have grown before becoming clinically apparent. Our findings suggest that most local recurrences are clinically apparent by the time they grow to approximately 4 cm; however, even at that size a substantial minority require advanced imaging to detect.
Association of Relevant Tumor, Patient, or Operative Characteristics with Occult Local Recurrence
We found no association between patient age, BMI, tumor size, depth, location, secondary flap closure, radiation status, and the modality by which local recurrence was identified. Tumor characteristics, including size, depth, and grade, have been identified as risk factors for the development of local recurrence in general [3, 6, 7, 9, 15]; however, whether these could be risk factors for clinically occult versus apparent recurrence has not been thoroughly explored. Previous authors have suggested that surveillance MRI should be reserved for patients who have tumors that are not easily evaluated by physical examination, but strong evidence to support this conclusion is lacking [1, 4]. Prior studies have detected an age-related effect on self detection in melanoma, with older patients being less likely to notice a lesion, but this has not been studied in sarcoma [16, 17]. Elevated BMI could also have a negative impact on rates of clinical detection and has been shown to be associated with larger sarcoma size at time of diagnosis, presumably for this reason [12]. Park et al. [13] observed that recurrent tumors located in the thigh/buttock or in patients over the age of 40 were more commonly detected by MRI than tumors in other locations and younger patients; however, per above, confounding could have been present. In our study none of these factors, including patient age, tumor size, depth, location, or flap coverage, were associated with clinicallly occult local recurrence. Between our findings and the existing evidence, there is insufficient data to predict which, if any, sarcoma patients are more likely to benefit from routine imaging. Thus, although future research may identify a subgroup of patients that are at low risk for clinically occult recurrences, currently there are no data to support narrowing the indications of advanced imaging in this population.
Clinical follow-up, including patient-reported symptoms and physician-conducted examination, detected most recurrent tumors in this series; however, approximately one-third were detected on advanced imaging before manifesting through signs or symptoms. These clinically occult local recurrences were not smaller, nor were they associated with relevent patient, tumor, or operative characteristics; as such, our findings did not define a patient subgroup that could be followed clinically without risk for occult local recurrence. Our data, therefore, support current sarcoma guidelines, but important evidence gaps remain regarding the benefits and repercussions of imaging surveillance. Although randomized trials comparing morbidity and mortality in this context may not be feasible, future studies could investigate other aspects of surveillance strategies such as frequency, cost-effectiveness, and psychosocial implications for patients.
Footnotes
The author certifies that neither he, nor any members of his immediate family, have funding or commercial associations (such as consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Investigation performed at Washington University School of Medicine, St. Louis, MO, USA.
References
- 1.Cheney MD, Giraud C, Goldberg SI, Rosenthal DI, Hornicek FJ, Choy E, Mullen JT, Chen YL, Delaney TF. MRI surveillance following treatment of extremity soft tissue sarcoma. J Surg Oncol. 2014;109:593–596. [DOI] [PubMed] [Google Scholar]
- 2.Chou YS, Liu CY, Chen WM, Chen TH, Chen PC, Wu HT, Chiou HJ, Shiau CY, Wu YC, Liu CL, Chao TC, Tzeng CH, Yen CC. Follow-up after primary treatment of soft tissue sarcoma of extremities: impact of frequency of follow-up imaging on disease-specific survival. J Surg Oncol. 2012;106:155-161. [DOI] [PubMed] [Google Scholar]
- 3.De Angelis F, Guy F, Bertaut A, Méjean N, Varbedian O, Hervieu A, Truc G, Thibouw D, Barra CC, Fraisse J, Burnier P, Isambert N, Causeret S. Limbs and trunk soft tissue sarcoma systematic local and remote monitoring by MRI and thoraco-abdomino-pelvic scanner: A single-centre retrospective study. Eur J Surg Oncol. 2019;45:1274-1280. [DOI] [PubMed] [Google Scholar]
- 4.Ezuddin NS, Pretell-Mazzini J, Yechieli RL, Kerr DA, Wilky BA, Subhawong TK. Local recurrence of soft-tissue sarcoma: issues in imaging surveillance strategy. Skeletal Radiol. 2018;47:1595-1606. [DOI] [PubMed] [Google Scholar]
- 5.Felderhof JM, Creutzberg CL, Putter H, Nout RA, Bovée JVMG, Dijkstra PDS, Hartgrink HH, Marijnen CAM. Long-term clinical outcome of patients with soft tissue sarcomas treated with limb-sparing surgery and postoperative radiotherapy. Acta Oncol. 2013;52:745-752. [DOI] [PubMed] [Google Scholar]
- 6.Fujiki M, Miyamoto S, Kobayashi E, Sakuraba M, Chuman H. Early detection of local recurrence after soft tissue sarcoma resection and flap reconstruction. Int Orthop . 2016;40:1975–1980. [DOI] [PubMed] [Google Scholar]
- 7.Gerrand CH, Wunder JS, Kandel RA, O’Sullivan B, Catton CN, Bell RS, Griffin AM, Davis AM. Classification of positive margins after resection of soft-tissue sarcoma of the limb predicts the risk of local recurrence. J Bone Jt Surg. 2001;83:1149-1155. [DOI] [PubMed] [Google Scholar]
- 8.Grimer R, Judson I, Peake D, Seddon B. Guidelines for the management of soft tissue sarcomas. Sarcoma.2010;2010:506182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Italiano A, Le Cesne A, Mendiboure J, Blay JY, Piperno-Neumann S, Chevreau C, Delcambre C, Penel N, Terrier P, Ranchere-Vince D, Lae M, Le Guellec S, Michels JJ, Robin YM, Bellera C, Bonvalot S. Prognostic factors and impact of adjuvant treatments on local and metastatic relapse of soft-tissue sarcoma patients in the competing risks setting. Cancer. 2014;120:3361-3369. [DOI] [PubMed] [Google Scholar]
- 10.Labarre D, Aziza R, Filleron T, Delannes M, Delaunay F, Marques B, Ferron G, Chevreau C. Detection of local recurrences of limb soft tissue sarcomas: Is magnetic resonance imaging (MRI) relevant? Eur J Radiol. 2009;72:50-53. [DOI] [PubMed] [Google Scholar]
- 11.Mehren M Von, Randall RL, Benjamin RS, Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane JM, Keedy V, Kim E, Koon H, Mayerson J, McCarter M, McGarry S V., Meyer C, Morris ZS, O’Donnell RJ, Pappo AS, Paz IB, Petersen IA, Pfeifer JD, Riedel RF, Ruo B, Schuetze S, Tap WD, Wayne JD, Bergman MA, Scavone JL. Soft tissue sarcoma, version 2.2018: Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16:536-563. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery C, Harris J, Siegel E, Suva L, Wilson M, Morell S, Nicholas R. Obesity is associated with larger soft-tissue sarcomas, more surgical complications, and more complex wound closures (obesity leads to larger soft-tissue sarcomas). J Surg Oncol. 2018;118:184-191. [DOI] [PubMed] [Google Scholar]
- 13.Park JW, Yoo HJ, Kim HS, Choi JY, Cho HS, Hong SH, Han I. MRI surveillance for local recurrence in extremity soft tissue sarcoma. Eur J Surg Oncol. 2019;45:268-274. [DOI] [PubMed] [Google Scholar]
- 14.Patel SA, Royce TJ, Barysauskas CM, Thornton KA, Raut CP, Baldini EH. Surveillance Imaging Patterns and Outcomes Following Radiation Therapy and Radical Resection for Localized Extremity and Trunk Soft Tissue Sarcoma. Ann Surg Oncol. 2017;24:1588-1595. [DOI] [PubMed] [Google Scholar]
- 15.Sabolch A, Feng M, Griffith K, Rzasa C, Gadzala L, Feng F, Biermann JS, Chugh R, Ray M, Ben-Josef E. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity. Am J Clin Oncol . 2012;35:151-157. [DOI] [PubMed] [Google Scholar]
- 16.Swetter SM, Pollitt RA, Johnson TM, Brooks DR, Geller AC. Behavioral determinants of successful early melanoma detection: Role of self and physician skin examination. Cancer. 2012;118:3725-3734. [DOI] [PubMed] [Google Scholar]
- 17.Trolle L, Henrik-Nielsen R, Gniadecki R. Ability to self-detect malignant melanoma decreases with age. Clin Exp Dermatol . 2011;36:499-501. [DOI] [PubMed] [Google Scholar]
- 18.Watts AC, Teoh K, Evans T, Beggs I, Robb J, Porter D. MRI surveillance after resection for primary musculoskeletal sarcoma. J Bone Joint Surg. 2008;90:484-487. [DOI] [PubMed] [Google Scholar]


