Abstract
Background
Psychological distress can negatively influence disability, quality of life, and treatment outcomes for individuals with hip and knee osteoarthritis (OA). Clinical practice guidelines recommend a comprehensive disease management approach to OA that includes the identification, evaluation, and management of psychological distress. However, uncertainty around the best psychological screening and assessment methods, a poor understanding of the heterogeneity of psychological distress in those with OA, and lack of guidance on how to scale treatment have limited the growth of OA care models that effectively address individual psychological needs.
Questions/purposes
(1) Across which general and pain-related psychological distress constructs do individuals seeking conservative care for hip or knee OA report higher scores than the general population of individuals seeking conservative care for musculoskeletal pain conditions? (2) What common psychological phenotypes exist among nonsurgical care-seeking individuals with hip or knee OA?
Methods
The sample included participants from the Duke Joint Health Program (n = 1239), a comprehensive hip and knee OA care program, and the Optimal Screening for Prediction of Referral and Outcome (OSPRO) cohort studies (n = 871) comprising individuals seeking conservative care for knee, shoulder, low back, or neck pain. At the initial evaluation, patients completed the OSPRO Yellow Flag (OSPRO-YF) Assessment Tool, which assesses 11 general and pain-related psychological distress constructs (depression, anxiety, fear of movement, self-efficacy for managing one’s own pain). We used OSPRO-YF scores to compare levels of psychological distress between the cohorts. Cohen’s d effect sizes were calculated to determine the magnitude of differences between the groups, with d = 0.20, d = 0.50, and d = 0.80 indicating small, medium, and large effect sizes, respectively. We used a latent class analysis to derive psychological distress phenotypes in people with OA based on the 11 OSPRO-YF psychological distress indicators. Psychological distress phenotypes are characterized by specific mood, belief, and behavioral factors that differentiate subgroups within a population. Phenotyping can help providers develop scalable treatment pathways that are better tailored to the common needs of patients.
Results
Patients with OA demonstrated higher levels of general and pain-related psychological distress across all psychological constructs except for trait anxiety (that is, anxiety level as a personal characteristic rather than as a response to a stressful situation, like surgery) with small-to-moderate effect sizes. Characteristics with the largest effect sizes in the OA and overall OSPRO cohort were (Cohen’s d) general anxiety (-0.66, lower in the OA cohort), pain catastrophizing (the tendency to ruminate over, maginfiy, or feel helpless about a pain experience, 0.47), kinesiophobia (pain-related fear of movement, 0.46), pain self-efficacy (confidence in one’s own ability to manage his or her pain, -0.46, lower in the OA cohort), and self-efficacy for rehabilitation (confidence in one’s own ability to perform their rehabilitation treatments, -0.44, lower in the OA cohort). The latent class analysis yielded four phenotypes (% sample): high distress (52%, 647 of 1239), low distress (26%, 322 of 1239), low self-efficacy and acceptance (low confidence in managing and willingness to accept pain) (15%, 186 of 1239), and negative pain coping (exhibiting poor pain coping skills) (7%, 84 of 1239). The classification error rate was near zero (2%), and the median of posterior probabilities used to assign subgroup membership was 0.99 (interquartile range 0.98 to 1.00), both indicating excellent model performance. The high-distress group had the lowest mean age (61 ± 11 years) and highest levels of pain intensity (6 ± 2) and disability (HOOS JR: 50 ± 15; KOOS JR: 47 ± 15), whereas the low-distress group had the highest mean age (63 ± 10 years) and lowest levels of pain (4 ± 2) and disability (HOOS JR: 63 ± 15; KOOS JR: 60 ± 12). However, none of these differences met or exceeded anchor-based minimal clinically important difference thresholds.
Conclusions
General and pain-related psychological distress are common among individuals seeking comprehensive care for hip or knee OA. Predominant existing OA care models that focus on biomedical interventions, such as corticosteroid injection or joint replacement that are designed to directly address underlying joint pathology and inflammation, may be inadequate to fully meet the care-related needs of many patients with OA due to their underlying psychological distress. We believe this because biomedical interventions do not often address psychological characteristics, which are known to influence OA-related pain and disability independent of joint pathology. Healthcare providers can develop new comprehensive hip and knee OA treatment pathways tailored to these phenotypes where services such as pain coping skills training, relaxation training, and psychological therapies are delivered to patients who exhibit phenotypes characterized by high distress or negative pain coping. Future studies should evaluate whether tailoring treatment to specific psychological phenotypes yields better clinical outcomes than nontailored treatments, or treatments that have a more biomedical focus.
Level of Evidence
Level III, diagnostic study.
Introduction
Hip and knee osteoarthritis (OA) is one of the leading causes of pain and disability in adults, and a direct contributor to the increasing economic burden of health care in the United States and worldwide [47, 79]. Psychological distress can be general (such as depression and anxiety) or pain-related (such as catastrophic thinking about pain and low self efficacy or confidence for managing pain). Increased psychological distress manifests behaviorally by having an individual do less in reponse to his/her pain experience [73]. As a result, recent clinical practice guidelines recommended a comprehensive approach to OA treatment that includes the identification, evaluation, and management of general and pain-related psychological distress [4]. However, a poor understanding of psychological distress, heterogeneity among those with hip and knee OA, uncertainty around the best psychological screening and assessment methods, and lack of guidance on how to scale treatment have limited the growth of OA care models that effectively address individual psychological needs [38].
Psychological processes are highly interconnected and collectively function in a variety of ways to impact pain and disability related to OA [59]. Healthcare providers must understand variability in the clinical presentation of psychological distress among care-seeking people with OA to develop comprehensive, personalized care plans. To date, the full breadth and scope of psychological distress among individuals with hip and knee OA is unclear. Prior OA studies have predominantly focused on a limited number of individual characteristics such as depression or pain catastrophizing [74]. These characteristics can substantially affect treatment outcomes for patients with hip and knee OA, but they represent only a few of the characteristics that comprise an individual’s broader psychological profile. This knowledge gap is critical because recent research of other musculoskeletal conditions suggests that multiple psychological characteristics provide greater prognostic information on clinical outcomes than individual characteristics alone do [48, 73]. These studies highlight the cumulative risk conferred by psychological distress and reinforce the need to evaluate and address multiple psychological characteristics as part of a comprehensive approach to hip and knee OA treatment [2].
Therefore, we asked: (1) Across which general and pain-related psychological distress constructs do individuals seeking care for hip or knee OA report higher scores than the general population of individuals seeking care for musculoskeletal pain conditions? (2) What common psychological distress phenotypes exist among care-seeking individuals with hip or knee OA?
Patients and Methods
Study Design and Setting
This was a retrospective, cross-sectional evaluation of patients with hip or knee OA treated in the Duke Joint Health Program. Guided by the biopsychosocial model and behavior change theories [8, 62], the Joint Health Program is an OA management program focused on comprehensive evaluation and integrated, multidisciplinary management of hip or knee OA [51]. A primary OA provider provides direct treatment and functions as an “OA home” by coordinating services with other healthcare partners such as orthopaedic surgeons, registered dieticians, weight loss specialists, and behavioral health providers, as well as other medical specialists when appropriate. Primary OA providers are physical therapists with advanced training specific to OA care that includes disease education, sleep health, OA-specific nutrition and weight management, and cognitive-behavioral theory-based strategies for mitigating pain-associated psychological distress [51]. Primary OA providers work with patients to address their specific needs (such as exercise and nutritional and psychological needs) to develop a holistic OA management regimen. The program operates within the Duke University Health System, which consists of three hospitals, 14 ambulatory orthopaedic clinics, and 17 ambulatory physical therapy sites. This study was approved by the Duke University Health System’s institutional review board.
Participants
This study included baseline (that is, initial evaluation) data from individuals with hip or knee OA referred from a physician to the Joint Health Program between October 2017 and November 2019. A total of 1364 patients entered the Joint Health Program during the study timeframe. Of those, 125 patients did not have complete data on the questionnaire used for the phenotyping analysis and were excluded. This left a final sample of 1239 patients in the final analysis (Fig. 1). Patients were generally referred to the Joint Health Program if they were not immediate candidates for total joint arthroplasty or elected to undergo nonsurgical management. To provide context for psychological assessment scores (that is, are scores high, low, or average among those with OA?) and better understand the potentially unique distress characteristics and health care delivery needs of patients with OA undergoing conservative care, we derived a comparison cohort from the Optimal Screening for Prediction of Referral and Outcome (OSPRO) development and validation cohort studies [24, 42]. Descriptions of these cohorts were previously published and include intake data from individuals (n = 871) seeking conservative care for a variety of knee, shoulder, low back, or neck pain conditions within the Orthopaedic Physical Therapy Investigator Network, a national network of outpatient physical therapy clinics. Briefly, the OSPRO development cohort (n = 431) was a cross-sectional cohort used to develop the OSPRO Yellow Flag (OSPRO-YF) Assessment tool, described in further detail below. The OSPRO validation cohort (n = 440) was subsequently and separately assembled as a longitudinal cohort to validate the newly-developed OSPRO-YF tool and establish its ability to predict clinical outcomes [24, 42]. Eligibility criteria for both the development and validation cohorts were identical and designed to make the cohort generalizable to outpatient orthopaedic rehabilitation populations. Because these two cohorts had identical eligibility criteria and both had baseline OSPRO-YF scores, we developed a combined sample that included baseline data from both cohorts as a comparison group for this study.
Fig 1.
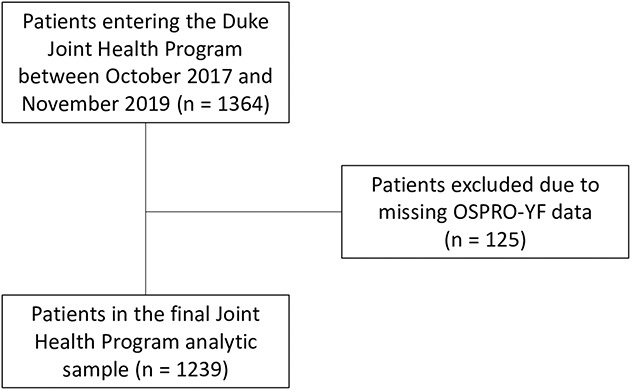
This diagram shows the number of patients who entered the Joint Health Program and those that were excluded due to missing OSPRO data; OSPRO-YF = Optimal Screening for Prediction of Referral and Outcome Yellow Flag Assessment Tool.
Description of Experiment, Treatment, or Surgery Variables, Outcome Measures, Data Sources, and Bias
Demographic information (age, gender, self-reported race, and employment status) and self-reported measures of pain intensity, joint-specific disability, and general and pain-related psychological distress were collected at baseline Joint Health Program assessments [49, 50]. In the OSPRO cohort, baseline demographic variables, pain intensity, and psychological distress measures were available.
Pain Intensity
Pain intensity was assessed with a numerical rating scale ranging from 0 (“no pain”) to 10 (“worst pain imaginable”) [7, 12, 33]. Participants rated their current, best, and worst pain intensity over the preceding 24 hours. The minimum clinically important difference (MCID) for the numeric rating scale is 2.0 points [67].
Joint-specific Disability
Joint-specific disability was assessed with the Hip Osteoarthritis Outcome Scale Joint Reconstruction (HOOS JR) or the Knee Osteoarthritis Outcome Scale Joint Reconstruction (KOOS JR), depending on the involved anatomic region. The HOOS JR was developed from the original version of the HOOS survey [50]. The HOOS JR contains six items evaluating hip pain and functioning over the past week. The HOOS JR exhibits high internal consistency (Pearson’s separation index = 0.86) and responsiveness in individuals with OA [50]. The KOOS JR was developed from the original long version of the KOOS survey [49]. The KOOS JR contains seven items from the original KOOS survey evaluating knee stiffness, pain, and functioning. In individuals with OA, the KOOS JR has excellent internal consistency (Pearson’s separation index = 0.84) and responsiveness for individuals undergoing TKA [49]. HOOS JR and KOOS JR scales range from 0 to 100 where 0 represents total hip or knee disability and 100 represents perfect hip or knee health [49, 50]. MCID values are 18.0 for the HOOS JR, and 15.1 for the KOOS JR [29].
General and Pain-related Psychological Distress
The OSPRO-YF Assessment Tool is a multidimensional screening tool for general and pain-related psychological distress. Using patient responses to each item, it accurately calculates estimates for what a patient would score on 11 full-length questionnaires measuring psychological constructs across three different domains. The three domains along with their associated psychological constructs (in parentheses) are: negative mood (depression [56], trait anxiety [25], trait anger [11]), negative coping (fear-avoidance for work [41], fear-avoidance for physical activity [41], pain catastrophizing [70], kinesiophobia [40], and pain anxiety [53]), and positive affect and coping (pain self-efficacy [58], self-efficacy for rehabilitation [77], and pain acceptance [52]) (Table 1) [24, 42]. The OSPRO-YF then uses those score estimates to identify the presence of a yellow flag for each of the 11 constructs. A yellow flag is a psychosocial prognostic factor for the development of disability after the onset of musculoskeletal pain [59]. The presence of a yellow flag is based on meeting a score threshold for each of the full-length questionnaire score estimates [42]. Score thresholds were established based on the sample distribution of full-length questionnaire scores in the previously described OSPRO development cohort [42]. Score estimates for negative mood and negative coping questionnaires within the top quartile of OSPRO development cohort scores indicate a yellow flag, whereas score estimates for positive affect and coping questionnaires that fall into the bottom quartile (suggesting higher psychological distress) indicate a yellow flag. For instance, if the quartile threshold for the Tampa Scale of Kinesiophobia (a negative coping questionnaire) is 30, then patients with a score estimate greater than 30 on the Tampa Scale of Kinesiophobia would have a yellow flag for kinesiophobia. This process results in 0 to 11 possible yellow flags. There are different versions of the tool, with the 10-item version representing the ideal combination of high accuracy and low response burden. The 10-item OSPRO-YF version was used for all analyses and has good internal validity, reliability, and predictive validity for persistent pain, disability, quality of life, and healthcare use [5, 24, 42, 43].
Table 1.
Psychological domains and constructs evaluated by the OSPRO yellow flag assessment tool
| Domain | Construct | Description |
| Negative mood | Depression | A mood disorder that causes severe symptoms that affect how you feel, think, and handle daily activities, such as sleeping, eating, or working depression causes feelings of sadness and/or a loss of interest in activities once enjoyed [3]. |
| Trait anxiety | The stable tendency to attend to, experience, and report negative emotions such as fears, worries, and anxiety across many situations [27]. | |
| Trait anger | A dispositional characteristic where one experiences frequent anger, with varying intensity (mild irritability, intense rage), and is often accompanied by related negative emotions such as envy, resentment, hate, and disgust [13]. | |
| Negative coping | Fear-avoidance beliefs for physical activity and work | Fear that emerges when stimuli that are related to pain are perceived as a main threat, resulting in psychophysiological (heightened muscle reactivity), behavioral (escape and avoidance behavior), and/or cognitive (catastrophizing thoughts) responses. This includes fear avoidance related to general physical activity and fear avoidance related to work-related activities [43]. |
| Pain catastrophizing | An exaggerated negative orienta-tion toward actual or anticipated pain experiences and catastrophic cognitions due to musculoskeletal pain [71]. | |
| Kinesiophobia | An excessive, irrational, and debilitating fear of physical movement and activity resulting from a feeling of vulnerability due to painful injury or re-injury [42]. | |
| Pain anxiety | The degree of pain-related anxi-ety and fear for individuals with pain conditions [55]. | |
| Positive affect and coping | Pain self-efficacy | One’s confidence regarding the ability to function effectively while in pain [59]. |
| Self-efficacy for rehabilitation | The degree of confidence associated with performing various tasks during rehabilitation [77]. | |
| Chronic pain acceptance | A willingness to experience continuing pain without needing to reduce, avoid, or otherwise change it [54]. |
OSPRO = Optimal Screening for Prediction of Referral and Outcomes.
Using OSPRO-YF responses at the initial evaluation, we calculated 11 full-length psychological questionnaire score estimates and the presence or absence of a yellow flag based on each of the 11 score estimates. We developed a composite pain rating by creating a mean of three pain-intensity ratings—current, best, and worst—for the preceding 24-hour period. This approach was selected because a mean pain rating provides better reliability and validity than single pain ratings do [31–34], and we have used this approach in several clinical pain research studies [5, 6, 23, 24].
Statistical Analysis, Study Size
To be eligible for inclusion in the OA cohort analytic sample, patients must have fully completed the OSPRO-YF at initial evaluation. This criterion was necessary since OSPRO scores were required for the phenotype analysis. We did not exclude patients for missing pain intensity or disability scores. To address our first question, we compared OSPRO-YF full-length questionnaire score estimate means and 95% confidence intervals between the OA cohort and the OSPRO cohort. As a sensitivity analysis to account for age-related differences between the cohorts, we developed a subset of the OSPRO cohort older than 50 years (n = 360) to provide a comparison group with a similar age distribution as the Joint Health Program cohort. Although MCIDs are a preferred metric for interpreting the magnitude of differences between groups [46], MCIDs for OA populations do not exist for many of the full-length psychological questionnaires estimated by the OSPRO-YF. In the absence of MCIDs, we used Cohen’s d, which standardizes mean differences so they can be easily compared across different questionnaires. In the social sciences, Cohen’s d values have been described as large (0.8), medium (0.5), and small (0.2) [13]. The larger the value the potentially more clinically meaningful the difference may be; however, interpretation is still highly context-dependent. Context can come from understanding the size of treatment effects, including a meta-analysis of randomized controlled trials of psychological interventions for arthritis which reported pooled effect sizes for psychological status (0.15), coping (0.46), and self efficacy (0.35) [3]. A recent randomized trial testing a positive psychological intervention for OA reported an effect size of d = 0.50 for negative affect outcomes [27]. These effects provide context around what size differences might be considered meaningful.
To address our second question, we used a latent class analysis to derive psychological distress phenotypes in the OA cohort based on the presence or absence of 11 yellow flag indicators. Psychological distress phenotypes are characterized by specific mood, belief, and behavioral factors that differentiate subgroups within a population. Phenotyping can help providers develop scalable treatment pathways that are better tailored to the common needs of patients. Latent class analysis is a probability clustering technique that identifies unobserved latent classes defined by the distribution of binary indicators; in this case, the presence or absence of 11 different yellow flags [76]. To allow for proper model identification, sparsely distributed indicators were excluded if present in less than 5% of the analytic sample to ensure that a sufficient number of respondents with each psychological characteristic were included [13, 75].
To select the best class size, model fit was first assessed for different numbers of classes using the model-fit likelihood ratio chi-square statistic (L2), which is a measure of how similar model-based frequencies are to observed frequencies [75]. The associated p value formally assesses the model’s fit, with p < 0.05 indicating poor fit. Additional measures of model parsimony include the Bayesian information criterion and Akaike information criterion, based on the L2, with lower values indicating better models [75]. Additional criteria used to determine the optimal class solution were the proportion of classification errors, class size (more than 5% of the sample), and interpretability of classes (clinical relevance of psychological characteristic combinations), particularly when other criteria did not produce a clearly superior model [15, 21, 30, 54]. We accounted for pairwise associations among variables (local dependence) by modeling the direct effect pf parameters associated with large bivariate residuals (that is, more than 1) in the model [76]. After selecting a best-fit latent class model, we assigned participants to the groups based on the highest posterior probability estimates. Age, gender, BMI, involved joint, baseline pain intensity, and baseline KOOS JR or HOOS JR scores were compared across the different psychological characteristic phenotypes.
Missing data within the OA cohort included BMI (n = 10), pain intensity (n = 12), HOOS JR (n = 11) and KOOS JR (n = 62). The OA cohort was older than the OSPRO cohort (61.4 ± 11.0 versus 44.9 ± 15.7, respectively, p < 0.001) but not different in age than the OSPRO cohort subset older than 50 years (61.4 ± 11.0 versus 60.5 ± 6.0, p = 0.14) (Table 2). All analyses were conducted using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA), except for the latent class analysis, which was conducted with Latent Gold software version 5.1 (Statistical Innovations, Belmont, MA, USA).
Table 2.
Descriptive information for the JHP, OSPRO, and OSPRO older subset cohorts
| Variable | JHP cohort (n = 1239) | OSPRO cohort (n = 871) | OSPRO subset (older than 50 years) (n = 360) |
|
| Age in yearsa | 61 ± 11 | 45 ± 16 | 61 ± 6 | |
| Gender, % (n)b | Women | 73 (904) | 62 (536) | 65 (233) |
| Race, % (n)b | White | 66 (818) | 76 (659) | 80 (288) |
| Black | 28 (352) | 18 (154) | 16 (58) | |
| Other | 6 (69) | 7 (58) | 4 (14) | |
| Employment status, % (n)b | Employed | 47 (584) | 64 (561) | 51 (187) |
| Unemployed | 15 (181) | 19 (166) | 15 (54) | |
| Retired | 37 (455) | 14 (121) | 31 (112) | |
| Joint, % (n)b | Knee OA | 80 (993) | ||
| Hip OA | 20 (246) | |||
| Knee pain | 26 (228) | 25 (89) | ||
| Low back pain | 27 (237) | 27 (98) | ||
| Shoulder pain | 25 (215) | 28 (99) | ||
| Neck pain | 22 (191) | 21 (74) | ||
Data are presented as mean ± SD
Data are percentage of sample (n); JHP = Joint Health Program; OSPRO = Optimal Screening for Prediction of Referral and Outcome.
Results
Across Which Psychological Distress Constructs do Individuals Seeking Care for Hip or Knee OA Report Higher Scores than the General Population of Individuals Seeking Care for Musculoskeletal Pain Conditions?
The OA cohort demonstrated higher levels of psychological distress than did the OSPRO group of patients with general musculoskeletal pain conditions and across all questionnaire score estimates except for the State-Trait Anxiety Inventory (STAI) (trait anxiety), which was lower among the OA cohort (Table 3). Effect sizes were generally small or medium. Characteristics with the largest effect sizes in the OA and overall OSPRO cohorts were (Cohen’s d) general anxiety (-0.66; lower in the OA cohort than in the OSPRO cohort), pain catastrophizing (0.47), kinesiophobia (0.46), pain self-efficacy (-0.46; lower in the OA cohort than in the OSPRO cohort), and self-efficacy for rehabilitation (-0.44; lower in the OA cohort than in the OSPRO cohort). Although most group differences were small, some of the larger effect sizes corresponded with group differences that are similar to MCIDs reported for these questionnaires in other populations [26, 60, 61]. Mean questionnaire score estimates were not different between the overall OSPRO cohort and the older OSPRO cohort subset for any estimate; therefore, we do not report separate results for the sensitivity analysis using the older OSPRO subset. Across all psychological questionnaires, the OA cohort had a higher proportion of patients with a yellow flag compared to the OSPRO cohort (Fig. 2). We also observed a higher prevalence of yellow flags among the JHP cohort (median six flags) compared to the OSPRO cohort (median three flags) (Fig. 3).
Table 3.
Mean psychological questionnaire score estimates from the OSPRO-YF for each cohort
| Questionnaire | JHP (n = 1239) | OSPRO (n = 831) | Mean difference (95% CI) | p value | Effect size (Cohen’s d) |
| Mean ± SD | Mean ± SD | ||||
| PHQ-9 (0-27)a | 7 ± 5 | 6 ± 4 | 0.9 (0.5 to 1.2) | < 0.001 | 0.20 |
| STAI (20-80) | 38 ± 8 | 43 ± 6 | -4.6 (-5.2 to -4.0) | < 0.001 | -0.66 |
| STAXI (10-40) | 16 ± 3 | 15 ± 4 | 0.5 (0.1 to 0.8) | 0.006 | 0.13 |
| FABQ-PA (0-24) | 16 ± 4 | 14 ± 6 | 1.8 (1.3 to 2.2) | < 0.001 | 0.35 |
| FABQ-W (0-42) | 14 ± 10 | 11 ± 10 | 3.3 (2.4 to 4.1) | < 0.001 | 0.34 |
| PCS (0-52) | 19 ± 10 | 14 ± 10 | 4.5 (3.7 to 5.3) | < 0.001 | 0.47 |
| TSK-11 (11-44) | 25 ± 5 | 23 ± 5 | 2.5 (2.0 to 2.9) | < 0.001 | 0.46 |
| PASS (0-100) | 34 ± 14 | 29 ± 16 | 4.3 (3.1 to 5.6) | < 0.001 | 0.30 |
| PSEQ (0-60) | 35 ± 10 | 40 ± 11 | -4.7 (-5.6 to -3.8) | < 0.001 | -0.46 |
| SER (0-120) | 93 ± 17 | 101 ± 16 | -7.3 (-8.8 to -5.9) | < 0.001 | -0.44 |
| CPAQ (0-120) | 62 ± 14 | 66 ± 12 | -4.1 (-5.2 to -2.9) | < 0.001 | -0.30 |
Information in the column is questionnaire name (range of potential scores). Cohen’s d effect sizes were calculated to determine the magnitude of differences between the groups, with d = 0.20, 0.50, and 0.80 indicating small, medium, and large effect sizes, respectively. Effect size is the difference between the two sample means divided by the pooled SD, with positive values indicating higher average scores in the JHP cohort; OSPRO = Optimal Screening for Prediction of Referral and Outcome; JHP = Joint Health Program; PHQ-9 = Patient Health Questionnaire-9; STAI = State-Trait Anxiety Inventory; STAXI = State-Trait Anger Expression Inventory; FABQ-PA = Fear-Avoidance Beliefs Questionnaire physical activity subscale; FABQ-W = Fear-Avoidance Beliefs Questionnaire work subscale; PCS = Pain Catastrophizing Scale; TSK-11 = Tampa Scale of Kinesiophobia; PASS-20 = Pain Anxiety Symptoms Scale; PSEQ = Pain Self-Efficacy Questionnaire; SER = Self-Efficacy for Rehabilitation; CPAQ = Chronic Pain Acceptance Questionnaire.
Fig. 2.
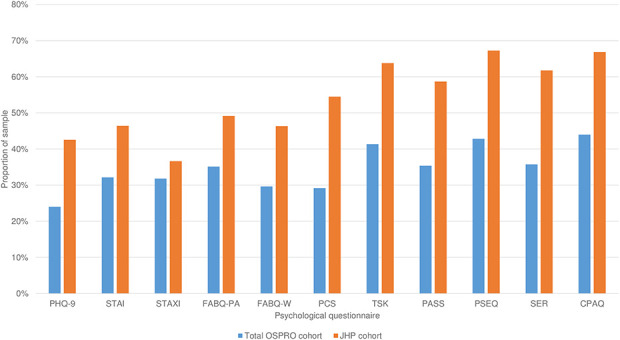
This graph shows the proportion of OSPRO and Joint Health Program cohort samples with a yellow flag for each psychological questionnaire score estimate. A yellow flag is a psychological questionnaire score estimate in the top quartile for negative mood and negative coping domain questionnaires, and in the bottom quartile for positive affect and coping questionnaires. Quartile thresholds were set based on questionnaire scores originally collected in the OSPRO development cohort. PHQ-9 = Patient Health Questionnaire-9; STAI = State-Trait Anxiety Inventory; STAXI = State-Trait Anger Expression Inventory; FABQ-PA = Fear-Avoidance Beliefs Questionnaire physical activity subscale; FABQ-W = Fear-Avoidance Beliefs Questionnaire work subscale; PCS = Pain Catastrophizing Scale; TSK-11 = Tampa Scale of Kinesiophobia; PASS-20 = Pain Anxiety Symptoms Scale; PSEQ = Pain Self-Efficacy Questionnaire; SER = Self-Efficacy for Rehabilitation; CPAQ = Chronic Pain Acceptance Questionnaire; OSPRO-YF = Optimal Screening for Prediction of Referral and Outcome Yellow Flag Assessment Tool; JHP = Joint Health Program.
Fig. 3.
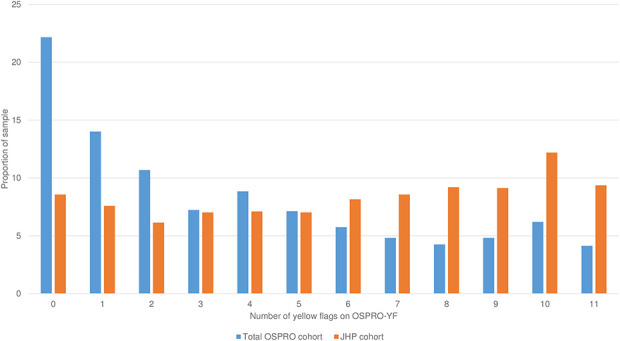
This graph shows the distribution of total yellow flags in the OSPRO and Joint Health Program cohorts. A yellow flag is a psychological questionnaire score estimate in the top quartile for negative mood and negative coping domain questionnaires, and in the bottom quartile for positive affect and coping questionnaires. Quartile thresholds were set based on questionnaire scores originally collected in the OSPRO development cohort. OSPRO-YF = Optimal Screening for Prediction of Referral and Outcome Yellow Flag Assessment Tool; JHP = Joint Health Program.
Common Psychological Phenotypes Among Care-seeking Individuals with Hip or Knee OA
After considering multiple indices for fit and performance, we found that the latent class analysis resulted in four phenotypes (Table 4). The classification error rate was near zero (0.015), and the median of posterior probabilities used to assign subgroup membership was 0.99 (interquartile range 0.98 to 1.00), both indicating excellent model performance (see Supplemental Table 1; http://links.lww.com/CORR/A440). We characterized phenotypes based on their predominant psychological profile (percentage of the sample): high distress (52%), low distress (26%), low self-efficacy and acceptance (15%), and negative pain coping (7%). The high-distress group had the lowest mean age (60.7 ± 11.3 years) and highest levels of pain intensity (6.0 ± 2.3) and disability (HOOS JR: 49.8 ± 15.5; KOOS JR: 46.8 ± 14.8), whereas the low-distress group had the highest mean age (63.0 ± 10.3 years) and lowest levels of pain (4.2 ± 2.3) and disability (HOOS JR: 63.2 ± 14.6; KOOS JR: 60.0 ± 12.3) (Table 5). All differences were statistically significant (p < 0.01) but pain intensity and disability differences did not meet established anchor-based MCID [29, 67].
Table 4.
Probability of reporting a yellow flag on OSPRO-YF full-length questionnaires for each phenotype identified by latent class analysisa
| Questionnaire | High distress (n = 646; 52%)b | Low distress (n = 322; 26%) | Low self-efficacy and acceptance (n = 187; 15%) | Negative pain coping (n = 84; 7%) |
| PHQ-9c | 59% | 17% | 37% | 30% |
| STAI | 69% | 15% | 28% | 36% |
| STAXI | 50% | 20% | 22% | 31% |
| FABQ-PA | 65% | 24% | 46% | 33% |
| FABQ-W | 67% | 15% | 33% | 36% |
| PCS | 89% | 9% | 4% | 81% |
| TSK-11 | 97% | 13% | 26% | 82% |
| PASS | 98% | 0% | 8% | 96% |
| PSEQ | 100% | 0% | 100% | 2% |
| SER | 82% | 22% | 86% | 9% |
| CPAQ | 97% | 6% | 92% | 18% |
A yellow flag is a psychological questionnaire score estimate in the top quartile for negative mood and negative coping domain questionnaires, and in the bottom quartile for positive affect and coping questionnaires. Quartile thresholds were set based on questionnaire scores originally collected in the OSPRO development cohort.
Values are sample size; percentage of overall population.
For each questionnaire in this column, percentages in the table represent the probability of reporting a yellow flag on that questionnaire for each phenotype identified by latent class analysis; OSPRO = Optimal Screening for Prediction of Referral and Outcome; PHQ-9 = Patient Health Questionnaire-9; STAI = State-Trait Anxiety Inventory; STAXI = State-Trait Anger Expression Inventory; FABQ-PA = Fear-Avoidance Beliefs Questionnaire physical activity subscale; FABQ-W, Fear-Avoidance Beliefs Questionnaire work subscale; PCS = Pain Catastrophizing Scale; TSK-11 = Tampa Scale of Kinesiophobia; PASS = Pain Anxiety Symptoms Scale; PSEQ = Pain Self-Efficacy Questionnaire; SER = Self-Efficacy for Rehabilitation; CPAQ = Chronic Pain Acceptance Questionnaire.
Table 5.
Demographic and health-related information for each phenotype
| Factor | High distress (n = 646) | Low distress (n = 322) | Low self-efficacy and acceptance (n = 187) | Negative pain coping (n = 84) |
| BMI in kg/m2a | 36 ± 9 | 32 ± 7 | 35 ± 8 | 34 ± 8 |
| Age in yearsa | 61 ± 11 | 63 ± 10 | 61 ± 11 | 62 ± 11 |
| HOOS JRa | 50 ± 15 | 63 ± 15 | 60 ± 10 | 59 ± 11 |
| KOOS JRa | 47 ± 15 | 60 ± 12 | 54 ± 14 | 52 ± 13 |
| Pain intensitya | 6.0 ± 2.3 | 4.2 ± 2.3 | 4.7 ± 2.4 | 5.5 ± 2.4 |
| Categorical variables | ||||
| Gender, % (n) | ||||
| Women | 72 (468) | 72 (232) | 75 (141) | 75 (63) |
| Joint, % (n) | ||||
| Knee | 78 (504) | 83 (266) | 84 (157) | 79 (66) |
| Hip | 22 (142) | 17 (56) | 16 (30) | 21 (18) |
Data are presented as mean ± SD; HOOS JR = Hip Osteoarthritis Outcome Scale Joint Reconstruction; KOOS JR = Knee Osteoarthritis Outcome Scale Joint Reconstruction.
Discussion
General and pain-related psychological distress can negatively influence disability, quality of life, and treatment outcomes for individuals with OA. However, uncertainty around the best psychological screening and assessment methods, a poor understanding of the heterogeneity of psychological distress in those with OA, and lack of guidance on how to scale treatment have limited the growth of OA care models that effectively address individual psychological needs. This study aimed to better understand the unique presentation of psychological distress among individuals with hip or knee OA in an effort to inform treatment in comprehensive OA management programs. We found that distress levels were higher across almost all psychological constructs in those with OA than in the general cohort of healthcare-seeking individuals in this study. Moreover, approximately 75% of the OA cohort demonstrated a profile characterized by evidence of yellow flags in one or more of the negative mood, negative coping, and positive affect domains, with over half characterized by multiple yellow flags across all domains. These findings highlight a potential discrepancy between the high prevalence of pain-related psychological distress among individuals with OA and the generally low prevalence of OA care models directly structured to address these characteristics. Predominant OA care models focus on biomedical interventions, which could be inadequate to fully meet the needs of many adults seeking nonoperative OA care. Although speculative, this potential discrepancy between patient characteristics and care delivery could partially explain high rates of opioid use, persistent disability, and failed surgical interventions in this population [57, 64, 65].
Limitations
This study had a number of limitations. First, these data are cross-sectional and therefore we are unable to comment on the response to treatment or prognosis for individuals with these phenotypes. Future work is needed to better understand the extent to which phenotype membership accounts for variation in pain intensity and joint-specific disability scores, especially in longitudinal studies that explore whether phenotype membership can predict clinical outcomes of nonoperative care. Future work should also determine the potential for these phenotypes to aid in risk adjustment. Second, there was a potential selection bias from the referring provider and the participating patient. Patients in the Joint Health Program cohort are commonly referred to the program by an arthritis care provider (such as a physician) and likely do not represent the broader population of individuals with symptomatic OA. The structure of the Joint Health Program may not appeal to some patients, and they may choose to not enter the program. The Joint Health Program cohort comprised individuals with a wide range of goals including preoperative rehabilitation and risk mitigation, avoiding or prolonging time to surgery, and postoperative management. Over the last 12 months, we have begun to track the proportion of patients referred into the program that do not initiate care and that proportion is approximately 49%. We do not have specific attendance rates from the study timeframe and are unable to assess psychological differences between those referred who attended and those who did not. Readers should understand this could bias the sample. The potential impact of these biases is that our results may not apply to the broader population of individuals with hip or knee OA, including those who opt for surgery instead of nonsurgical care. There is some evidence that those who opt for surgery may have a worse psychological profile [78]; however, that is not something we were able to evaluate in this cohort. Although this cohort may not represent all individuals with OA, it likely does represent most healthcare-seeking individuals who are appropriate for and will engage in a comprehensive care program. Informing the treatment pathways of these programs is a primary goal of this study.
A third limitation is that we combined patients with hip and knee OA in the analytic cohort. We did this because the hip OA cohort alone would not meet minimum sample size requirements for latent class analysis [17]. The drawback of this approach is that we cannot determine whether there were psychological profiles specific to hip OA or knee OA. Although this is a limitation, it also should be noted that based on prior evidence and examples from other patient populations (such as those with low-back pain) we did not hypothesize there to be separate phenotypes for hip and knee OA [38, 45]. Future work in larger samples should confirm whether phenotypes need to be tailored for specific anatomical regions. A fourth limitation is that we did not have access to measures of pathophysiology (like Kellgren-Lawrence classifications or ROM). These measures could help readers better understand the extent to which pathophysiological features are associated with individual phenotypes. Although our intention was to derive phenotypes solely based on psychological measures, a more comprehensive approach to phenotyping that includes pathophysiological features could enhance the tailoring of treatment pathways for OA. Finally, we used a generalizable comparison sample from a nationwide cohort with musculoskeletal pain to contextualize the psychological distress questionnaire score estimates seen in the OA cohort. Both cohorts are comprised of patients that have been evaluated by a physician and referred to a physical therapist for a musculoskeletal condition. Those referred to the Joint Health Program at our institution would generally be referred to a physical therapist in most other health care systems. Therefore, both cohorts represent patients at the point of initiating conservative care for their musculoskeletal condition. However, we acknowledge that this cohort may not represent all patients seeking care for musculoskeletal pain. Nevertheless, because there are no known Joint Health Program equivalents for a general population of patients with musculoskeletal pain, we believe the OSPRO cohort provides a suitable population comparison.
Across Which Psychological Distress Constructs do Individuals Seeking Care for Hip or Knee OA Report Higher Scores than the General Population of Individuals Seeking Care for Musculoskeletal Pain Conditions?
A key finding was that high levels of distress across multiple psychological constructs (such as yellow flags) were more prevalent in patients with hip or knee OA compared with a more general care-seeking sample of individuals with musculoskeletal pain conditions. This is an interesting finding because the patients with hip or knee OA intentionally opted for conservative treatment and may be expected to have lower psychological distress than observed in this cohort. Common, biomedically-focused pain treatments may be appropriate for most patients in the general musculoskeletal care-seeking population where the prevalence of psychological distress is relatively low, such as in theOSPRO cohort. But our findings suggest these treatments may be inadequate for a larger proportion of patients seeking conservative care for hip or knee OA due to the higher prevalence of psychological distress in this population and its known influence on pain and disability. Despite strong evidence supporting the impact of psychological distress on clinical outcomes for patients with hip or knee OA [22, 35, 38, 68], few prior studies have examined the prevalence of psychological distress in these patients compared with other populations. One study examined the relationship between OA and mental health and found that patients with OA reported higher rates of perceived stress and depression than did their non-OA counterparts [35]. A study by Gandhi et al. [22] of patients with hip or knee OA undergoing total joint arthroplasty found that with each additional symptomatic joint, the odds of self-reporting depression increased by 19%, and patients with six or more painful joints had a 150% (or greater) rate of depression than patients with symptoms in only the surgical joint. Ackerman et al. [1] studied young people with hip and knee OA and found they have elevated levels of psychological distress compared with the general population. These findings are consistent with the results of our study, which identified higher levels of psychological distress across all measured psychological characteristics (except anxiety) in the OA cohort than in the OSPRO cohort. A systematic review found high levels of anxiety among individuals with OA, but studies included in the review made this determination based on the use of established screening measures and did not directly compare these patients’ anxiety levels to those of the general population [68].
Prior studies have established a link between OA and pain-related psychological distress [22, 35, 38, 68], but our study better characterizes the extent of psychological distress among a health care seeking population. The high prevalence of psychological distress, across 11 different measures, may not be surprising if one considers that OA can also be a chronic pain condition [37, 72], especially for individuals who seek care. Pain is a primary reason individuals with OA seek care, and OA is often comorbid with other chronic pain conditions (such as low-back pain) [66, 71]. Furthermore, OA-related pain is likely multifactorial, associated with central neurological changes in pain processing as well as psychological and cognitive factors [37]. Although not directly assessed in this analysis, we suspect that psychological distress scores and yellow flag distributions in the OA cohort would be similar to other chronic pain cohorts, instead of the comparision cohort we used in this analysis.
Given these findings, we strongly advocate for routine assessment of psychological distress in patients with OA. Mood disorders such as depression and anxiety are familiar to many clinicians as distinct medical diagnoses and, therefore, may be screened for more commonly in clinical practice. However, our data suggest that less-familiar or often overlooked pain-specific constructs such as catastrophizing, self-efficacy, and acceptance should also be assessed. Comprehensive, multidimensional screening tools such as the OSPRO-YF may be used to identify the myriad psychological characteristics that could influence treatment outcomes but are not routinely assessed nor easy to identify through patient interactions alone in a typical clinical visit (such as patients with poor scores for self-efficacy). Results of these brief psychological assessments would help clinicians classify patients into psychological phenotypes like those derived in this study, which is currently not standard practice. As further described below, clinicians would use a patient’s phenotype to match them to a treatment pathway that offers services addressing their specific psychological and behavioral needs.
Common Psychological Phenotypes Among Care-seeking Individuals with Hip or Knee OA
Our study identified four psychological phenotypes among individuals with hip or knee OA: high overall distress, low overall distress, low self-efficacy and acceptance, and negative pain coping. These phenotypes are helpful to clinicians because they provide opportunities to classify an otherwise heterogenous group of patients into more defined groups with similar psychological traits. The benefit of this classification is clinicians could develop “personalized” treatment pathways that address the specific psychological and behavioral needs of each phenotype, and deploy those pathways at scale, since each phenotype is represented by a sizeable propotion of patients. Numerous studies have analyzed the characteristics of patients with knee and/or hip OA to develop common phenotypes using a variety of methods including latent class analysis, cluster analysis, and a priori hypotheses [10, 14, 16, 18, 38, 39, 55]. The number of phenotypes developed in other studies ranged from two to six, with most studies identifying four or five distinct phenotypes, a result consistent with our study (Table 6). In a study perhaps most similar to ours, Cruz-Almeida et al. [14] used psychological and pain processing characteristics to derive phenotypes by a cluster analysis. Although our study used a different methodology, its findings align those of Cruz-Almeida et al. [14] on the number and general composition of phenotypes. Our study builds on the work of Cruz-Almeida et al. [14] and others [10, 18, 38, 39, 55] by focusing on a clinical sample and considering a broader range of psychological distress characteristics, many of which are modifiable and treatable. The phenotype with high yellow flag probabilities across all psychological constructs comprised 52% of the sample, in contrast with prior studies suggesting that a phenotype predominantly defined by psychological distress would comprise between 9% and 35% of the study sample [14, 38, 39]. This discrepancy could be explained by multiple factors, including different methods for evaluating psychological distress, different phenotyping variables including a smaller number of distress characteristics, and different populations (such as laboratory versus healthcare-seeking samples). Identifying effective management strategies is a priority for this high-distress group because they have high levels of pain and disability and are likely to have high and persistent healthcare costs [44]. Perhaps most noteworthy is the relatively young average age of individuals with this phenotype. Experiencing symptoms of OA, a condition often associated with aging, could be particularly distressing for younger individuals. Those who are diagnosed with OA at a younger age may generally have a longer timeline before surgical intervention, making comprehensive OA management for this phenotype especially important. Individuals with this high-distress phenotype may benefit the most from multidisciplinary OA management models focused on early and comprehensive management to improve negative mood, negative coping, and positive affect. Given the strong potential for psychological characteristics to mediate pain and disability related to OA [69], interventions focused on the biomedical aspects of the condition, such as medication, injection, or surgery, could be less effective among those with this phenotype.
Table 6.
Comparison of methodology and results to other OA phenotyping studies
| Study | Target population | Criteria used to develop phenotypes | Psychological characteristics assessed | Analytical method used to determine phenotypes | Phenotypes developed |
| Cardoso et al. [10] | Individuals with mild-to-moderate knee OA pain | QST, psychological characteristics | Coping, pain catastrophizing, pain vigilance, self-reported pain and function | Hierarchical cluster analysis | 1. Low pressure pain sensitivity 2. Average pain sensitivity 3. High temporal summation of punctate pain 4. High cold pain sensitivity 5. High heat pain sensitivity |
| Cruz-Almeida et al. [14] | Adults with knee OA recruited for a study of racial differences in knee OA pain | QST, psychological characteristics | Depression, coping, pain vigilance, optimism, positive/negative affect, anger, self-reported pain and function | Hierarchical cluster analysis | 1. High optimism, low negative affect 2. Low positive affect 3. Low optimism 4. Somatic sensitivity/pain hypervigilance |
| Egsgaard et al. [18] | Adult patients with knee pain | QST, psychological characteristics | Self-reported pain and function, pain catastrophizing | Hierarchical cluster analysis | 1. Low pain sensitivity 2. Early phase sensitization 3. Presence of pain sensitization 4. Presence of pain sensitization and catastrophizing |
| Kittleson et al. [38] | Adults with knee OA from the OAI | Knee OA pathology, psychological distress, joint sensitivity, CCI, muscle strength | Coping, pain catastrophizing, self-reported pain and function | LCA | 1. High CCI scores 2. High knee joint sensitivity 3. High psychological distress 4. Less radiographic OA, lower psychological distress, greater strength, less pain sensitivity |
| Knoop et al. [39] | Adults with knee OA from the OAI | Severity of radiographic OA, lower limb muscle strength, body mass index, psychological characteristics | Depression | K-means cluster analysis | 1. Minimal joint disease 2. Strong muscle 3. Nonobese and weak muscle 4. Obese and weak muscle 5. Depressive |
| Murphy et al. [55] | Adults with symptomatic knee and/or hip OA | Physical function, psychological characteristics, illness burden | Self-reported pain and physical function, depression, fatigue, sleep quality | Hierarchical cluster analysis | 1. High depression, pain, fatigue, illness burden, sleep problems 2. Intermediate depression, moderate fatigue and illness burden, low pain and sleep problems 3. High sleep problems, low pain, fatigue, illness burden, depression |
OA = osteoarthritis; OAI = Osteoarthritis Initiative; LCA = latent class analysis; QST = quantitative sensory testing; CCI = Charlson comorbidity index.
A final note of caution is that categorization by psychological and mental health characteristics can reinforce stigma and shame among patients [20]. Categorization can also send clinicians the false message that psychological, emotional, or cognitive aspects of health can be ignored if patients do not meet categorization thresholds. Use of non-stigmatizing language and inquiring about a patient’s overall psychological and emotional well-being during patient assessment are two methods of ensuring the success of a psychologically-based approach to evaluation and treatment [20, 36].
Conclusions
Individuals with knee and hip OA scored higher in multiple general and pain-associated psychological distress domains than did a general cohort of individuals with musculoskeletal pain conditions, suggesting healthcare delivery and resource distribution designed for general populations with musculoskeletal pain may need reconfiguring to adequately address the psychological and behavioral needs of many people with OA. Many of the common psychological characteristics identified in this study are familiar treatment targets in other chronic pain conditions. Stratified care models, such as those used to guide tailored treatment of low back pain, are expanding to other musculoskeletal conditions and may be well-suited for delivering care tailored to the four specific OA phenotypes identified in this analysis [2, 28, 63]. For instance, psychological interventions may be unnecessary and potentially wasteful for the approximately 25% of individuals with a low psychological distress profile. Conversely, integration of psychologically informed treatment into existing OA pathways may benefit the nearly 75% of healthcare-seeking individuals with OA who fit a phenotype characterized by some level of psychological distress. Importantly, the development of these pathways would be tailored to phenotype. For instance, coordinated multidisciplinary psychological and behavioral healthcare may be necessary for those with high-distress phenotypes. For those with poor coping or low self-efficacy phenotypes, treatment may consist of strategies to enhance mindfulness, positive thinking, and the ability to better cope with and independently manage pain [9, 19]. Future work should focus on designing, implementing, and testing scalable, stratified hip and knee OA care models that deliver high-quality healthcare at low costs for individuals with OA.
Footnotes
Each author certifies that he or she has no commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ackerman I, Bucknill A, Page R, Broughton N, Roberts C, Cavka B, Schoch P, Brand C. High levels of psychological distress among young people with hip and knee osteoarthritis. Osteoarthritis and Cartilage. 2015;23:A179–A180. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Choong PF, Davis AM, Dowsey MM, Dziedzic KS, Emery C, Hunter DJ, Losina E, Page AE, Roos EM, Skou ST, Thorstensson CA, van der Esch M, Whittaker JL. Osteoarthritis: Models for appropriate care across the disease continuum. Best Pract Res Clin Rheumatol. 2016;30:503–535. [DOI] [PubMed] [Google Scholar]
- 3.Astin JA, Beckner W, Soeken K, Hochberg MC, Berman B. Psychological interventions for rheumatoid arthritis: A meta-analysis of randomized controlled trials. Arthritis Care & Research. 2002;47:291–302. [DOI] [PubMed] [Google Scholar]
- 4.Bannuru RR, Osani MC, Vaysbrot EE, Arden NK, Bennell K, Bierma-Zeinstra SMA, Kraus VB, Lohmander LS, Abbott JH, Bhandari M, Blanco FJ, Espinosa R, Haugen IK, Lin J, Mandl LA, Moilanen E, Nakamura N, Snyder-Mackler L, Trojian T, Underwood M, McAlindon TE. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019;27:1578–1589. [DOI] [PubMed] [Google Scholar]
- 5.Beneciuk JM, Lentz TA, He Y, Wu SS, George SZ. Prediction of persistent musculoskeletal pain at 12 months: a secondary analysis of the optimal screening for prediction of referral and outcome (OSPRO) Validation Cohort Study. Phys Ther. 2018;98:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beneciuk JM, Robinson ME, George SZ. Subgrouping for patients with low back pain: a multidimensional approach incorporating cluster analysis and the STarT Back Screening Tool. J Pain. 2015;16:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolton JE. Accuracy of recall of usual pain intensity in back pain patients. Pain. 1999;83:533–539. [DOI] [PubMed] [Google Scholar]
- 8.Borrell-Carrió F, Suchman AL, Epstein RM. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Fam Med. 2004;2:576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Büssing A, Ostermann T, Neugebauer EA, Heusser P. Adaptive coping strategies in patients with chronic pain conditions and their interpretation of disease. BMC Public Health. 2010;10:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso JS, Riley JL, Glover T, Sibille KT, Bartley EJ, Goodin BR, Bulls HW, Herbert M, Addison AS, Staud R, Redden DT, Bradley LA, Fillingim RB, Cruz-Almeida Y. Experimental pain phenotyping in community-dwelling individuals with knee osteoarthritis. Pain. 2016;157:2104–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carroll J. Trait Anger. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. New York, NY: Springer; 2013:1987–1989. Available at: 10.1007/978-1-4419-1005-9_854. Accessed August 12, 2020. [DOI] [Google Scholar]
- 12.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine. 2005;30:1331–1334. [DOI] [PubMed] [Google Scholar]
- 13.Cohen J. Statistical power analysis for the behavioral sciences . New York: Academic Press; 1969. [Google Scholar]
- 14.Cruz-Almeida Y, King CD, Goodin BR, Sibille KT, Glover TL, Riley JL, Sotolongo A, Herbert MS, Schmidt J, Fessler BJ, Redden DT, Staud R, Bradley LA, Fillingim RB. Psychological profiles and pain characteristics of older adults with knee osteoarthritis. Arthritis Care Res (Hoboken). 2013;65:1786–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dean N, Raftery AE. Latent class analysis variable selection. Ann Inst Stat Math. 2010;62:11–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deveza LA, Melo L, Yamato TP, Mills K, Ravi V, Hunter DJ. Knee osteoarthritis phenotypes and their relevance for outcomes: a systematic review. Osteoarthr. Cartil. 2017;25:1926–1941. [DOI] [PubMed] [Google Scholar]
- 17.Dziak JJ, Lanza ST, Tan X. Effect size, statistical power and sample size requirements for the bootstrap likelihood ratio test in latent class analysis. Struct Equ Modeling. 2014;21:534–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egsgaard LL, Eskehave TN, Bay-Jensen AC, Hoeck HC, Arendt-Nielsen L. Identifying specific profiles in patients with different degrees of painful knee osteoarthritis based on serological biochemical and mechanistic pain biomarkers: a diagnostic approach based on cluster analysis. Pain. 2015;156:96–107. [DOI] [PubMed] [Google Scholar]
- 19.Firth AM, Cavallini I, Sütterlin S, Lugo RG. Mindfulness and self-efficacy in pain perception, stress and academic performance. The influence of mindfulness on cognitive processes. Psychol Res Behav Manag. 2019;12:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flink IK, Reme S, Jacobsen HB, Glombiewski J, Vlaeyen JWS, Nicholas MK, Main CJ, Peters M, Williams AC de C, Schrooten MGS, Shaw W, Boersma K. Pain psychology in the 21st century: lessons learned and moving forward. Scand J Pain. 2020;20:229–238. [DOI] [PubMed] [Google Scholar]
- 21.Fop M, Smart K, Murphy TB. Variable selection for latent class analysis with application to low back pain diagnosis. Ann. Appl. Stat. 2017;11:2080–2110. [Google Scholar]
- 22.Gandhi R, Zywiel MG, Mahomed NN, Perruccio AV. Depression and the overall burden of painful joints: an examination among individuals undergoing hip and knee replacement for osteoarthritis. Arthritis. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George SZ, Beneciuk JM. Psychological predictors of recovery from low back pain: a prospective study. BMC Musculoskelet Disord. 2015;16:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.George SZ, Beneciuk JM, Lentz TA, Wu SS, Dai Y, Bialosky JE, Zeppieri G. Optimal screening for prediction of referral and outcome (OSPRO) for musculoskeletal pain conditions: results from the validation cohort. J Orthop Sports Phys Ther. 2018;48:460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gidron Y. Trait Anxiety. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. New York, NY: Springer; 2013:1989–1989. Available at: 10.1007/978-1-4419-1005-9_1539. Accessed August 12, 2020. [DOI] [Google Scholar]
- 26.Hapidou EG, O’Brien MA, Pierrynowski MR, de Las Heras E, Patel M, Patla T. Fear and avoidance of movement in people with chronic pain: Psychometric properties of the 11-Item Tampa Scale for Kinesiophobia (TSK-11). Physiother Can. 2012;64:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausmann LRM, Youk A, Kwoh CK, Ibrahim SA, Hannon MJ, Weiner DK, Gallagher RM, Parks A. Testing a positive psychological intervention for osteoarthritis. Pain Med. 2017;18:1908–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill JC, Garvin S, Chen Y, Cooper V, Wathall S, Saunders B, Lewis M, Protheroe J, Chudyk A, Dunn KM, Hay E, van der Windt D, Mallen C, Foster NE. Stratified primary care versus non-stratified care for musculoskeletal pain: findings from the STarT MSK feasibility and pilot cluster randomized controlled trial. BMC Family Practice. 2020;21:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung M, Bounsanga J, Voss MW, Saltzman CL. Establishing minimum clinically important difference values for the Patient-Reported Outcomes Measurement Information System Physical Function, hip disability and osteoarthritis outcome score for joint reconstruction, and knee injury and osteoarthritis outcome score for joint reconstruction in orthopaedics. World J Orthop. 2018;9:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Islam MM, Valderas JM, Yen L, Dawda P, Jowsey T, McRae IS. Multimorbidity and comorbidity of chronic diseases among the senior Australians: prevalence and patterns. PLoS ONE. 2014;9:e83783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen MP, Castarlenas E, Tomé-Pires C, de la Vega R, Sánchez-Rodríguez E, Miró J. The number of ratings needed for valid pain assessment in clinical trials: Replication and extension. Pain Med. 2015;16:1764–1772. [DOI] [PubMed] [Google Scholar]
- 32.Jensen MP, McFarland CA. Increasing the reliability and validity of pain intensity measurement in chronic pain patients. Pain. 1993;55:195–203. [DOI] [PubMed] [Google Scholar]
- 33.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–162. [DOI] [PubMed] [Google Scholar]
- 34.Jensen MP, Turner LR, Turner JA, Romano JM. The use of multiple-item scales for pain intensity measurement in chronic pain patients. Pain. 1996;67:35–40. [DOI] [PubMed] [Google Scholar]
- 35.Jung JH, Seok H, Kim J-H, Song GG, Choi SJ. Association between osteoarthritis and mental health in a Korean population: a nationwide study. Int J Rheum Dis. 2018;21:611–619. [DOI] [PubMed] [Google Scholar]
- 36.Keefe FJ, Main CJ, George SZ. Advancing psychologically informed practice for patients with persistent musculoskeletal pain: promise, pitfalls, and solutions. Phys Ther. 2018;98:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kittelson AJ, George SZ, Maluf KS, Stevens-Lapsley JE. Future directions in painful knee osteoarthritis: harnessing complexity in a heterogeneous population. Phys Ther. 2014;94:422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kittelson AJ, Stevens-Lapsley JE, Schmiege SJ. Determination of pain phenotypes in knee osteoarthritis: a latent class analysis using data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2016;68:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knoop J, van der Leeden M, Thorstensson CA, Roorda LD, Lems WF, Knol DL, Steultjens MPM, Dekker J. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2011;63:1535–1542. [DOI] [PubMed] [Google Scholar]
- 40.Kori S. Kinisophobia : A new view of chronic pain behavior. Pain Manage. 1990:35–43. [Google Scholar]
- 41.Leeuw M, Goossens MEJB, Linton SJ, Crombez G, Boersma K, Vlaeyen JWS. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. [DOI] [PubMed] [Google Scholar]
- 42.Lentz TA, Beneciuk JM, Bialosky JE, Zeppieri G, Dai Y, Wu SS, George SZ. Development of a yellow flag assessment tool for orthopaedic physical therapists: results from the optimal screening for prediction of referral and outcome (OSPRO) cohort. J Orthop Sports Phys Ther. 2016;46:327–343. [DOI] [PubMed] [Google Scholar]
- 43.Lentz TA, Beneciuk JM, George SZ. Prediction of healthcare utilization following an episode of physical therapy for musculoskeletal pain. BMC Health Serv Res. 2018;18:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lentz TA, Harman JS, Marlow NM, Beneciuk JM, Fillingim RB, George SZ. Factors associated with persistently high-cost health care utilization for musculoskeletal pain. PLoS ONE. 2019;14:e0225125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lentz TA, Marlow NM, Beneciuk JM, Fillingim RB, George SZ. Comorbidity subgroups among Medicare beneficiaries seeking health care for musculoskeletal pain. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leopold SS, Porcher R. Editorial: The minimum clinically important difference—the least we can do. Clin Orthop Relat Res. 2017;475:929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lowry V, Ouellet P, Vendittoli P-A, Carlesso LC, Wideman TH, Desmeules F. Determinants of pain, disability, health-related quality of life and physical performance in patients with knee osteoarthritis awaiting total joint arthroplasty. Disability and Rehabilitation. 2018;40:2734–2744. [DOI] [PubMed] [Google Scholar]
- 49.Lyman S, Lee Y-Y, Franklin PD, Li W, Cross MB, Padgett DE. Validation of the KOOS, JR: A Short-form Knee Arthroplasty Outcomes Survey. Clin. Orthop. Relat. Res. 2016;474:1461–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyman S, Lee Y-Y, Franklin PD, Li W, Mayman DJ, Padgett DE. Validation of the KOOS, JR: a short-form knee arthroplasty outcomes survey. Clin. Orthop. Relat. Res. 2016;474:1472–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malay MR, Lentz TA, O’Donnell J, Coles T, Mather RC, III, Jiranek WA. Development of a comprehensive, nonsurgical joint health program for people with osteoarthritis: a case report. Phys Ther. 2020;100:127–135. [DOI] [PubMed] [Google Scholar]
- 52.McCracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain. 2004;107:159–166. [DOI] [PubMed] [Google Scholar]
- 53.McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50:67–73. [DOI] [PubMed] [Google Scholar]
- 54.Miaskowski C, Dunn L, Ritchie C, Paul SM, Cooper B, Aouizerat BE, Alexander K, Skerman H, Yates P. Latent class analysis reveals distinct subgroups of patients based on symptom occurrence and demographic and clinical characteristics. J Pain Symptom Manage. 2015;50:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Subgroups of older adults with osteoarthritis based upon differing comorbid symptom presentations and potential underlying pain mechanisms. Arthritis Research & Therapy. 2011;13:R135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Institute of Mental Health. Depression. Available at: https://www.nimh.nih.gov/health/topics/depression/index.shtml. Accessed August 12, 2020.
- 57.National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Sciences Policy, Committee on Pain Management and Regulatory Strategies to Address Prescription Opioid Abuse. Pain Management and the Opioid Epidemic: Balancing Societal and Individual Benefits and Risks of Prescription Opioid Use. Phillips JK, Ford MA, Bonnie RJ, eds. Washington (DC): National Academies Press (US); 2017. Available at: http://www.ncbi.nlm.nih.gov/books/NBK458660/. Accessed August 12, 2020. [PubMed] [Google Scholar]
- 58.Nicholas MK. The pain self-efficacy questionnaire: Taking pain into account. Eur J Pain. 2007;11:153–163. [DOI] [PubMed] [Google Scholar]
- 59.Nicholas MK, Linton SJ, Watson PJ, Main CJ. Early identification and management of psychological risk factors (“yellow flags”) in patients with low back pain: a reappraisal. Phys Ther. 2011;91:737–753. [DOI] [PubMed] [Google Scholar]
- 60.Ogunlana MO, Odole AC, Adejumo A, Odunaiya N. Catastrophising, pain, and disability in patients with nonspecific low back pain. Hong Kong Physiotherapy Journal. 2015;33:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palomo-López P, Becerro-de-Bengoa-Vallejo R, Losa-Iglesias ME, López-López D, Rodríguez-Sanz D, Romero-Morales C, Calvo-Lobo C, Mazoteras-Pardo V. Kinesiophobia and pain intensity are increased by a greater hallux valgus deformity degree- kinesiophobia and pain intensity in hallux valgus. Int J Environ Res Public Health. 2020;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997:12:38-48. [DOI] [PubMed] [Google Scholar]
- 63.Protheroe J, Saunders B, Bartlam B, Dunn KM, Cooper V, Campbell P, Hill JC, Tooth S, Mallen CD, Hay EM, Foster NE. Matching treatment options for risk sub-groups in musculoskeletal pain: a consensus groups study. BMC Musculoskeletal Disorders. 2019;20:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66:2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riddle DL, Perera RA, Jiranek WA, Dumenci L. Using surgical appropriateness criteria to examine outcomes of total knee arthroplasty in a United States sample. Arthritis Care Res (Hoboken). 2015;67:349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rundell SD, Goode AP, Suri P, Heagerty PJ, Comstock BA, Friedly JL, Gold LS, Bauer Z, Avins AL, Nedeljkovic SS, Nerenz DR, Kessler L, Jarvik JG. Effect of comorbid knee and hip osteoarthritis on longitudinal clinical and health care use outcomes in older adults with new visits for back pain. Arch Phys Med Rehabil. 2017;98:43–50. [DOI] [PubMed] [Google Scholar]
- 67.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain. 2004;8:283–291. [DOI] [PubMed] [Google Scholar]
- 68.Sharma A, Kudesia P, Shi Q, Gandhi R. Anxiety and depression in patients with osteoarthritis: impact and management challenges. Open Access Rheumatol. 2016;8:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shelby RA, Somers TJ, Keefe FJ, Pells JJ, Dixon KE, Blumenthal JA. Domain specific self-efficacy mediates the impact of pain catastrophizing on pain and disability in overweight and obese osteoarthritis patients. J Pain. 2008;9:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychological Assessment. 1995;7:524–532. [Google Scholar]
- 71.Suri P, Morgenroth DC, Kwoh CK, Bean JF, Kalichman L, Hunter DJ. Low back pain and other musculoskeletal pain comorbidities in individuals with symptomatic osteoarthritis of the knee: data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 2010;62:1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB, Giamberardino MA, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Perrot S, Scholz J, Schug S, Smith BH, Svensson P, Vlaeyen JWS, Wang S-J. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. 2019;160:19–27. [DOI] [PubMed] [Google Scholar]
- 73.Urquhart DM, Phyomaung PP, Dubowitz J, Fernando S, Wluka AE, Raajmaakers P, Wang Y, Cicuttini FM. Are cognitive and behavioural factors associated with knee pain? A systematic review. Semin. Arthritis Rheum. 2015;44:445–455. [DOI] [PubMed] [Google Scholar]
- 74.van Baar ME, Dekker J, Lemmens JA, Oostendorp RA, Bijlsma JW. Pain and disability in patients with osteoarthritis of hip or knee: the relationship with articular, kinesiological, and psychological characteristics. J Rheumatol . 1998;25:125–133. [PubMed] [Google Scholar]
- 75.Vermunt JK, Magidson J. Latent class models for classification. Comput Stat Data Anal. 2003;41:531–537. [Google Scholar]
- 76.Vermunt JK, Magidson J. Latent Gold 4.0 User’s Guide. Belmont, Massachusetts: Statistical Innovations Inc; 2004. [Google Scholar]
- 77.Waldrop D, Lightsey OR, Jr., Ethington CA, Woemmel CA, Coke AL. Self-efficacy, optimism, health competence, and recovery from orthopedic surgery. J Counsel Psychol. 2001;48:233–238. [Google Scholar]
- 78.Wouters RM, Vranceanu A-M, Slijper HP, Vermeulen GM, van der Oest MJW, Selles RW, Porsius JT, Hand-Wrist Study Group. Patients with thumb-base osteoarthritis scheduled for surgery have more symptoms, worse psychological profile, and higher expectations than nonsurgical counterparts: a large cohort analysis. Clin. Orthop. Relat. Res. 2019;477:2735–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhao X, Shah D, Gandhi K, Wei W, Dwibedi N, Webster L, Sambamoorthi U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthritis and Cartilage. 2019;27:1618–1626. [DOI] [PubMed] [Google Scholar]


