Abstract
Background
Adverse discharge disposition, which is discharge to a long-term nursing home or skilled nursing facility is frequent and devastating in older patients after lower-extremity orthopaedic surgery. Predicting individual patient risk allows for preventive interventions to address modifiable risk factors and helps managing expectations. Despite a variety of risk prediction tools for perioperative morbidity in older patients, there is no tool available to predict successful recovery of a patient’s ability to live independently in this highly vulnerable population.
Questions/purposes
In this study, we asked: (1) What factors predict adverse discharge disposition in patients older than 60 years after lower-extremity surgery? (2) Can a prediction instrument incorporating these factors be applied to another patient population with reasonable accuracy? (3) How does the instrument compare with other predictions scores that account for frailty, comorbidities, or procedural risk alone?
Methods
In this retrospective study at two competing New England university hospitals and Level 1 trauma centers with 673 and 1017 beds, respectively; 83% (19,961 of 24,095) of patients 60 years or older undergoing lower-extremity orthopaedic surgery were included. In all, 5% (1316 of 24,095) patients not living at home and 12% (2797 of 24,095) patients with missing data were excluded. All patients were living at home before surgery. The mean age was 72 ± 9 years, 60% (11,981 of 19,961) patients were female, 21% (4155 of 19,961) underwent fracture care, and 34% (6882 of 19,961) underwent elective joint replacements. Candidate predictors were tested in a multivariable logistic regression model for adverse discharge disposition in a development cohort of all 14,123 patients from the first hospital, and then included in a prediction instrument that was validated in all 5838 patients from the second hospital by calculating the area under the receiver operating characteristics curve (ROC-AUC).Thirty-eight percent (5360 of 14,262) of patients in the development cohort and 37% (2184 of 5910) of patients in the validation cohort had adverse discharge disposition. Score performance in predicting adverse discharge disposition was then compared with prediction scores considering frailty (modified Frailty Index-5 or mFI-5), comorbidities (Charlson Comorbidity Index or CCI), and procedural risks (Procedural Severity Scores for Morbidity and Mortality or PSS).
Results
After controlling for potential confounders like BMI, cardiac, renal and pulmonary disease, we found that the most prominent factors were age older than 90 years (10 points), hip or knee surgery (7 or 8 points), fracture management (6 points), dementia (5 points), unmarried status (3 points), federally provided insurance (2 points), and low estimated household income based on ZIP code (1 point). Higher score values indicate a higher risk of adverse discharge disposition. The score comprised 19 variables, including socioeconomic characteristics, surgical management, and comorbidities with a cutoff value of ≥ 23 points. Score performance yielded an ROC-AUC of 0.85 (95% confidence interval 0.84 to 0.85) in the development and 0.72 (95% CI 0.71 to 0.73) in the independent validation cohort, indicating excellent and good discriminative ability. Performance of the instrument in predicting adverse discharge in the validation cohort was superior to the mFI-5, CCI, and PSS (ROC-AUC 0.72 versus 0.58, 0.57, and 0.57, respectively).
Conclusion
The Adverse Discharge in Older Patients after Lower Extremity Surgery (ADELES) score predicts adverse discharge disposition after lower-extremity surgery, reflecting loss of the ability to live independently. Its discriminative ability is better than instruments that consider frailty, comorbidities, or procedural risk alone. The ADELES score identifies modifiable risk factors, including general anesthesia and prolonged preoperative hospitalization, and should be used to streamline patient and family expectation management and improve shared decision making. Future studies need to evaluate the score in community hospitals and in institutions with different rates of adverse discharge disposition and lower income. A non-commercial calculator can be accessed at www.adeles-score.org.
Level of Evidence
Level III, diagnostic study.
Introduction
Demographic changes in the Western world have yielded a steadily increasing proportion of older patients [10, 19]. Two of the three most frequently performed surgical procedures [12] in this population (THA and TKA) are lower-extremity musculoskeletal procedures that are associated with a high risk of postoperative mortality and prolonged hospitalization [37, 56, 59]. Adverse discharge disposition [23], which typically results in long-term loss of independent living after surgery through discharge to a long-term nursing facility or long-term professional care, is a devastating event that carries an inherent risk of loss of sense of life [25, 42]. Adverse discharge disposition after hospitalization is further associated with increased healthcare use, costs, and hospital readmission [61].
Prospective identification of high-risk patients allows for channeling of healthcare resources and risk mitigation through modification of modifiable factors, [57] with the goal of avoiding an adverse postoperative outcome and reducing hospitalizations [2, 27, 45, 46]. Furthermore, estimating an individual patient’s risk of adverse discharge helps streamline preoperative discussions with patients and families and shared-decision making on whether to perform elective procedures. Although previous frailty-based instruments in older patients have been used to predict less-frequent outcomes, such as mortality and cardiopulmonary complications [3, 5, 13, 18, 24, 32, 33, 38, 41, 49, 53], many of these instruments were designed to facilitate epidemiologic comparisons rather than clinical decision-making. Previous instruments designed to predict adverse discharge disposition specifically excluded most of these high-risk procedures, such as THA and TKA [22] or trauma surgery [34]. Additionally, they were not tailored to older patients. Thus, there is a current gap of knowledge regarding which factors predict adverse discharge disposition in older patients undergoing lower-extremity orthopaedic surgery. These factors could then be used to design a tool to predict an individual patients’ risk of adverse discharge disposition. The instrument then needs to be validated and, by integrating all relevant factors, should prove superior to other tools that account for frailty [49], comorbidities [7] or procedural risk [9] alone.
We therefore asked: (1) What factors predict adverse discharge disposition in patients older than 60 years after lower-extremity surgery? Based on the answer to this, we developed and validated a prediction score for adverse discharge disposition in a high-risk cohort of older patients (> 60 years) after lower extremity surgery (ADELES). We then asked: (2) Can the prediction instrument be applied to another patient population with reasonable accuracy? (3) How does the instrument compare with other predictions scores considering frailty [49], comorbidities [7], or procedural risk [9] alone?
Patients and Methods
After approval by our institutional review board (2019P000316, Committee on Clinical Investigations, Beth Israel Deaconess Medical Center, Boston, MA, USA) and establishment of a reliance agreement with the institutional review board at Massachusetts General Hospital, Boston, MA, USA (SMART IRB 2163), we included patients older than 60 years undergoing lower-extremity orthopaedic surgery (pelvis, femur, tibia, fibula, and foot) in this study. Surgeries only involving soft tissue were not included. Patients with an American Society of Anesthesiologists physical status classification of more than IV and patients not living at home before surgery were excluded (Fig. 1).
Fig. 1.
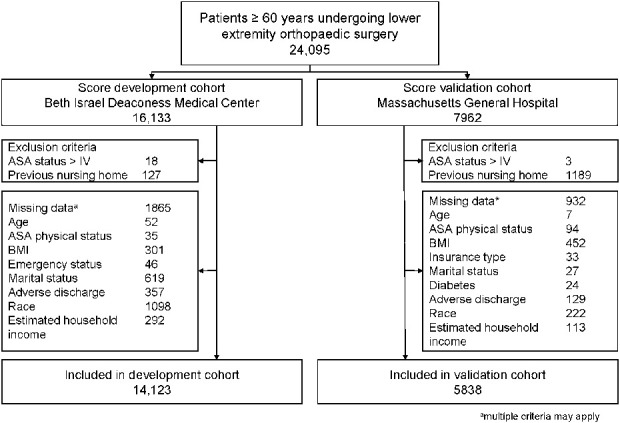
This study flowchart details patient inclusion and the reasons for excluding patients. ASA = American Society of Anesthesiologists. aMultiple criteria may apply.
Investigation of Factors Predicting Adverse Discharge Disposition in Patients Older Than 60 Years after Lower-extremity Surgery
Data were collected from hospital databases comprising patient-, surgery-, anesthesia-, and outcome-related data (Supplemental Digital Content 1; http://links.lww.com/CORR/A456). We investigated a priori-defined candidate predictors for adverse discharge disposition using multivariable logistic regression. Candidate predictors were identified based on a review of relevant studies [22, 34] as well as physiologic and clinical plausibility. Comorbidities were derived from the ICD-9 and the ICD-10 (see Table 1, Supplemental Digital Content 2; http://links.lww.com/CORR/A457). Adverse discharge disposition after surgery was defined as discharge to a skilled nursing facility, chronic or long-term care facility, or living assisted by a skilled healthcare worker, as previously published [17, 23, 42]. As was done previously, patients who died in hospital were included in the adverse discharge disposition group [23]. Discharges to rehabilitation, short-term care facilities, and nonprofessional assisted living (such as intermediate or custodial care) were not counted as adverse discharge disposition.
Patients undergoing surgery between October 2005 and September 2017 at Beth Israel Deaconess Medical Center in Boston, MA, USA, (development cohort), a 673-bed university teaching hospital and Level 1 trauma center were considered. In this cohort, 88% (14,123 of 16,133 patients) undergoing lower extremity orthopaedic surgery were included (Fig.1). Patients were on average 72 ± 9 years old, 60% (8513 of 14,123) were female, 81% (11,451 of 14,123) were white, and the median estimated household income was USD 78,414 (interquartile range [IQR] USD 59,306 to USD 102,342). In all, 4% (585 of 14,123 patients) underwent emergency surgery and 20% (2770 of 14,123) underwent fracture surgery (Table 1). Overall, 38% (5309 of 14,123 patients) in this cohort had adverse discharge disposition (Table 2).
Table 1.
Patient and perioperative characteristics in the development and validation cohort
| Variable | Development cohort | Validation cohort | ||
| No adverse discharge (n = 8814) | Adverse discharge (n = 5309) | No adverse discharge (n = 3674) | Adverse discharge (n = 2164) | |
| Age in years, mean ± SD | 69 ± 7 | 76 ± 10 | 70 ± 8 | 74 ± 9 |
| Female, % (n) | 56 (4935) | 67 (3578) | 55 (2019) | 67 (1449) |
| BMI in kg/m2, mean ± SD | 28.64 ± 6.03 | 28.41 ± 7.01 | 28.99 ± 6.21 | 29.29 ± 6.93 |
| ASA physical status, % (n) | ||||
| I | 3 (299) | 0 (23) | 2 (60) | 1 (14) |
| II | 53 (4645) | 23 (1226) | 63 (2284) | 48 (1039) |
| III | 40 (3522) | 66 (3522) | 35 (1285) | 49 (1061) |
| IV | 4 (348) | 11 (561) | 1 (45) | 2 (50) |
| Emergency surgery, % (n) | 2 (215) | 7 (370) | 2 (77) | 4 (76) |
| Not married, % (n) | 41 (3615) | 63 (3331) | 38 (1394) | 55 (1193) |
| Federal insurance, % (n) | 54 (4764) | 81 (4298) | 63 (2323) | 74 (1594) |
| Medicare | 91 (4344) | 97 (4157) | 97 (2251) | 96 (1535) |
| MassHealth | 8 (394) | 3 (127) | 2 (52) | 2 (33) |
| Medicaid and other | 1 (26) | 0 (14) | % (20) | 2 (26) |
| Lowest tertile of estimated household income, % (n) | 32 (2790) | 36 (1921) | 31 (1121) | 38 (827) |
| Federal insurance | 57 (1581) | 78 (1493) | 68 (761) | 75 (623) |
| Race, % (n) | ||||
| White | 7175 (81%) | 81 (4276) | 92 (3395) | 90 (1950) |
| Hispanic | 3 (305) | 4 (185) | 2 (83) | 2 (52) |
| Black | 10 (837) | 11 (588) | 2 (89) | 4 (83) |
| Other | 6 (497) | 5 (260) | 3 (106) | 4 (77) |
| Comorbidities, % (n) | ||||
| Diabetes | 30 (2631) | 38 (2007) | 20 (721) | 27 (584) |
| Anemia | 20 (1727) | 42 (2242) | 28 (1033) | 45 (973) |
| COPD | 7 (611) | 14 (737) | 8 (305) | 12 (256) |
| Smoking | 11 (1004) | 13 (692) | 19 (681) | 21 (460) |
| Chronic kidney disease | 11 (1009) | 24 (1268) | 11 (386) | 17 (357) |
| Dementia | 1 (111) | 10 (526) | 1 (39) | 4 (84) |
| Depression | 12 (1047) | 21 (1129) | 16 (586) | 23 (490) |
| Atrial fibrillation | 13 (1165) | 26 (1385) | 14 (503) | 20 (438) |
| Chronic heart failure | 10 (880) | 24 (1297) | 11 (402) | 18 (381) |
| Coronary artery disease | 28 (2478) | 40 (2100) | 25 (899) | 31 (668) |
| Hypertension | 63 (5577) | 76 (4042) | 73 (2676) | 83 (1786) |
| History of myocardial infarction | 8 (716) | 12 (662) | 6 (235) | 9 (196) |
| CAVD | 3 (269) | 5 (270) | 5 (190) | 8 (168) |
| Hemiparesis or hemiplegia | 1 (42) | 1 (46) | 1(52) | 2 (38) |
| Solid cancer | 10 (867) | 11 (585) | 19 (707) | 19 (406) |
| Metastatic cancer | 1 (119) | 3 (154) | 5 (193) | 6 (137) |
| Liver disease | 5 (423) | 6 (318) | 6 (203) | 7 (145) |
| Osteoporosis | 13 (1143) | 23 (1222) | 18 (670) | 25 (543) |
| Surgery-related factors, % (n) | ||||
| Concomitant infection | 18 (1587) | 18 (945) | 5 (181) | 4 (94) |
| Hospitalization longer than 48 hours | 13 (1171) | 27 (1424) | 12 (435) | 19 (405) |
| Preoperative opioid use | 21 (1886) | 19 (1012) | 12 (429) | 11 (242) |
| Surgical location, % (n) | ||||
| Pelvis or hip surgery | 20 (1736) | 44 (2358) | 40 (1464) | 50 (1070) |
| Femur or knee surgery | 23 (2059) | 36 (1887) | 35 (1275) | 39 (849) |
| Leg or ankle surgery | 10 (854) | 10 (515) | 11 (397) | 8 (173) |
| Foot or toe surgery | 24 (2147) | 10 (542) | 6 (201) | 3 (62) |
| Type of surgery, % (n) | ||||
| Periprosthetic fracture | 1 (43) | 2 (81) | 4 (152) | 8 (163) |
| Fracture management | 12 (1049) | 32 (1721) | 14 (521) | 20 (425) |
| Joint replacement | 27 (2357) | 39 (2060) | 39 (1441) | 47 (1024) |
| Arthroscopy | 23 (2018) | 0 (7) | 9 (337) | 1 (10) |
| Revision surgery | 2 (203) | 5 (264) | 10 (369) | 11 (230) |
| Other | 36 (3144) | 22 (1176) | 23 (854) | 14 (312) |
| Type of anesthesia | ||||
| General anesthesia | 73 (6429) | 87 (4618) | 100 (3674) | 100 (2164) |
| Peripheral nerve block | 22 (1928) | 31 (1626) | 13 (484) | 13 (274) |
| Neuraxial anesthesia | 6 (539) | 5 (242) | 2 (90) | 2 (41) |
| Intraoperative factors, % (n) | ||||
| Duration of surgery in minutes, mean ± SD | 99.70 ± 63.75 | 127.61 ± 61.31 | 144.87 ± 66.19 | 160.42 ± 63.49 |
| Fluid volume in liters, median (IQR) | 2 (1.25 to 3.25) | 2.5 (1.75 to 3.75) | 1.25 (1 to 2) | 1.5 (1 to 2.05) |
| Work relative value units, median (IQR) | 9.63 (7.03 to 20.72) | 17.61 (13.65 to 20.72) | 19.65 (11.21 to 23.25) | 21.79 (17.61, 23.25) |
| MAP below 55 mmHg, min, median (IQR) | 0 (0 to 2) | 1 (0 to 4) | 0 (0 to 1) | 0 (0 to 2) |
| Vasopressor equivalenta dose in mg, median (IQR) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) |
| Opioid equivalentb dose in mg, median (IQR) | 25 (7 to 48) | 38 (19 to 63) | 63 (42 to 84) | 65 (44 to 84) |
In norepinephrine equivalents.
In oral morphine equivalent.
ASA = American Society of Anesthesiologists; COPD = chronic obstructive pulmonary disease; CAVD = cerebral arterial vascular disease; IQR = interquartile range; MAP = mean arterial pressure.
Table 2.
Characteristics of discharge disposition for patients in the development and validation cohort
| Discharge disposition | Development cohort (n = 14,123) | Validation cohort (n = 5838 ) |
| Nonadverse discharge, % (n) | ||
| Home (including custodial care) | 52% (7319) | 46 % (2653) |
| Rehabilitation facility | 10% (1420) | 17% (1003) |
| Other hospital/medical facility | 0% (47) | 0% (11) |
| Other | 0% (28) | 0% (3) |
| Adverse discharge, % (n) | ||
| Skilled nursing facility | 34% (4820) | 33% (1952) |
| Chronic/long-term care facility | 3% (349) | 3% (195) |
| Death in hospital | 1% (140) | 0% (21) |
Multivariable logistic regression to predict adverse discharge disposition was performed including all a priori-defined candidate predictors. Stepwise elimination was conducted using a cutoff p value ≤ 0.01 for retaining predictors in the logistic regression model [47, 50], followed by bootstrap resampling with 100 samples to eliminate predictors that did not contribute to the model’s fit. Potential overfitting was addressed by applying penalized maximum likelihood estimation. Then, we used findings from the logistic regression analysis to create a prediction score for adverse discharge disposition. We weighted variables in the model by dividing the beta coefficient by the overall smallest coefficient of all predictors. The corresponding score point value was derived by rounding the calculated value to the nearest integer. To assess the performance of the ADELES score, we calculated the area under the receiver operating characteristic (ROC) curve and the Brier Score. The ROC curve represents a plot of sensitivity (y-axis) versus 1-specificity (x-axis) for all possible thresholds of the score. The area under the ROC curve may range from 0 to 1, with a greater area indicating a higher probability that an individual who experiences adverse discharge has a higher score point value than an individual who does not [21]. An area under the ROC curve of 0.5 indicates no ability to distinguish, while above 0.7 is considered as good, above 0.8 as excellent and above 0.9 as outstanding discriminative ability [21]. The Brier score is another method to verify the accuracy of a prediction model. It incorporates discrimination as well as calibration and may take values ranging from 0 to 1, while smaller scores (below 0.44) indicate better prediction, with the best possible Brier score being 0 [20].
We then determined the cutoff point value of the ADELES score to differentiate between high- and low-risk patients. Any cutoff is always a tradeoff between sensitivity and specificity, and we applied the Youden index [39], which determines the threshold that maximizes the sum of both. [44] We then calculated the corresponding positive and negative predictive value at the determined cutoff, which is the probability that individuals classified as high-risk will experience adverse discharge and that individuals classified as low-risk will not experience adverse discharge.
We performed a decision curve analysis to determine the score’s value toward clinical decision making. This analysis indicates in what range of probability for adverse discharge the score provides a net benefit, and the net benefit is assessed for all possible score thresholds to differentiate between high- and low-risk individuals. Results are presented in a graph and compared with the extreme strategies of considering everyone or no one to be at high risk of adverse discharge [52].
To confirm the robustness of the score, we conducted a series of sensitivity analyses (Section 3 of Supplemental Digital Content 1; http://links.lww.com/CORR/A456). Data management and statistical analysis were performed in Stata SE 15 (StataCorp, College Station, TX, USA), Prism 8 (GraphPad Software, San Diego, CA, USA), and SigmaPlot 14.0 (Systat Software Inc, San Jose, CA, USA).
Validation of the Prediction Instrument
We validated the performance of the ADELES score in an independent sample (validation cohort) of patients undergoing surgery at Massachusetts General Hospital, Boston, MA, USA, between January 2007 and December 2015. Massachusetts General Hospital is a competing 1017-bed university teaching hospital and Level 1 Trauma Center. In this cohort, 73% (5838 out of 7962) of patients undergoing lower extremity orthopaedic surgery were included (Fig. 1). This cohort differed from the validation cohort (Table 1) as patients were more often white (92%, [5345 of 5838]), had a slightly lower estimated household income (USD 74,768 [IQR 56,530 to 93,337]), underwent fewer emergency procedures (3%, [153 of 5838]) and had a lower proportion of fracture surgery (16%, [946 of 5838]). The rate of adverse discharge disposition in this cohort was comparable to the development cohort (37%, 2164 of 5838, Table 2).
Comparison with Other Instruments
As a secondary analysis, we compared the ADELES score performance in predicting adverse discharge disposition against prediction scores accounting for frailty (modified Frailty Index-5, mFI-5 [49]), comorbidities (Charlson Comorbidity Index, CCI [7]), and procedural risks (Procedural Severity Scores for Morbidity and Mortality, PSS [9]), as has been proposed previously [22]. This analysis was done within the validation cohort. C-statistics of the different prediction instruments were compared in a subset of 5208 patients when complete data for all scores were available, using the “roccomp/rocgold” command of Stata (StataCorp).
Results
What Factors Predict Adverse Discharge Disposition in Patients Older Than 60 Years after Lower-extremity Orthopaedic Surgery?
In our multivariable analysis, we found that the most prominent predictive factors were age older than 90 years (10 points), dementia (5 points), unmarried status (3 points), an estimated household in the lowest tertile based on ZIP code (1 point), federally provided insurance (2 points), hip or knee surgery (7 or 8 points), and fracture management (6 points, Fig. 2). Penalized maximum likelihood estimation confirmed the results from the logistic regression analysis (Table 2, Supplemental Digital Content 3; http://links.lww.com/CORR/A458). Higher score point values indicate higher risk of adverse discharge disposition.
Fig. 2.

This figure shows how the ADELES score was calculated. Variables included in the ADELES score are shown with corresponding score point values; PPF = periprosthetic fracture; ASA = American Society of Anesthesiologists.
The predictive performance of the score was characterized by an area under the ROC curve of 0.85 (95% confidence interval 0.84 to 0.85) (Fig. 3) and a Brier score of 0.155 (reliability < 0.001), indicating excellent discriminative ability, which is typically assumed with an area under the ROC curve > 0.80 [21]. Calibration plot confirmed excellent calibration (Fig. 4A).
Fig. 3.

This graph shows the score performances assessed with receiver operating characteristic curve for predicting adverse discharge by the ADELES score (blue) in the development cohort. Dark green: reference line. A color image accompanies the online version of this article.
Fig. 4.

A-B These graphs show the calibration plots for the ADELES score. (A) The calibration plots for predicting adverse discharge by the ADELES in the development cohort is shown here. (B) The calibration plots for predicting adverse discharge by the ADELES in the validation cohort is shown here. The calibration plots indicate the observed over expected fraction of adverse discharge disposition. The reference line (dashed dark blue) indicates optimum calibration with the equal expected and observed fraction of adverse discharge disposition. A color image accompanies the online version of this article.
The cutoff point for score dichotomization determined by Youden J-statistics [39] was ≥ 23 points out of a maximum possible value of 61 points; thus 48% (6713 of 14,123) of patients in the development cohort were classified as being at a high risk of having adverse discharge. This cutoff revealed good discrimination, with a sensitivity of 0.80 and specificity of 0.73.
The decision curve analysis revealed a positive net benefit for threshold probabilities between 0% and 83% (Fig. 1, Supplemental Digital Content 4; http://links.lww.com/CORR/A459), indicating that in patients with a risk of adverse discharge disposition between 0 and 83%, using the score provides additional benefit compared with the extreme assumptions that either no or every patient is at maximum risk of adverse discharge. All sensitivity analyses confirmed the robustness of the score (Section 3 of Supplemental Digital Content 1; http://links.lww.com/CORR/A456). A noncommercial calculator that is free online can be accessed at www.adeles-score.org.
Validation of the Prediction Instrument
Score characteristics in the validation cohort revealed an area under the ROC curve of 0.72 (95% CI 0.71 to 0.73) (Fig. 2, Supplemental Digital Content 5; http://links.lww.com/CORR/A460) and a Brier score of 0.20 (reliability 0.004), indicating good discriminative ability in this cohort, which is usually assumed with an area under the ROC curve > 0.70. In the decision curve analysis, there was a positive net benefit for predicted probability thresholds between 0% and 57% (Fig. 3, Supplemental Digital Content 6; http://links.lww.com/CORR/A461) and good calibration between 0% and 70% risk of adverse discharge disposition (Fig. 4B).
Comparison to Other Instruments
Secondary comparison of the ADELES score to instruments taking only into account frailty (mFI-5) [49], comorbidities (CCI) [7] and procedural severity (PSS) [9] indicated superior discriminative ability of the ADELES score (area under the ROC curve 0.72 versus 0.58, 0.57, and 0.57 respectively; Fig. 4, Supplemental Digital Content 7; http://links.lww.com/CORR/A462).
Discussion
Adverse discharge disposition, defined as discharge to a nursing home or long-term care facility implicates the loss of the ability to live independently, is a devastating event for patients and their families that is also associated with increased health care costs and hospital use. The ADELES prediction instrument identifies modifiable risk factors [57], including general anesthesia and prolonged preoperative hospitalization. Knowledge about which patients are at high risk for losing the ability to live independently after surgery is important for managing patient and family expectations.
We investigated clinically available potential predictors, including socioeconomic variables, surgical factors, and patient characteristics. Predictors were then combined into a score to predict adverse discharge disposition in older patients undergoing lower extremity surgery (ADELES) and the instrument was validated in an independent cohort of patients from a separate hospital.
Limitations
Our findings have limitations. First, our study is limited by its retrospective design. Using routinely collected hospital registry data such as billing codes and administrative clinical data carries the inherent risk of unaccounted confounding by unmeasured variables and bias through missing data that were unavailable due to lack of prospective follow-up. To address these issues, we conducted a series of sensitivity analyses including imputation of missing data, which yielded very robust results. We further used independent cohorts from two competing hospitals for score development and validation, as opposed to splitting a single cohort into a development and validation population. This approach yields a more reliable estimation of how the ADELES score performs outside the development cohort, and we found a good predictive value in this validation cohort. We now advocate for prospective evaluation of the score in different settings, which might include prospective investigation and follow-up in patient cohorts from community hospitals across southern or western parts of the United States.
We acknowledge that our data stem from Massachusetts, where the median annual income is USD 79,835, as opposed to USD 61,937 across the United States [51], which might impair comparability of socioeconomic factors to poorer parts of the country. To account for these substantial differences [51], we normalized household income by calculating equally sized tertiles rather than using absolute thresholds. When investigating score performance in the validation cohort, these tertiles were recalculated to adjust the variable to the slightly lower income, and we were able to demonstrate good score performance in this cohort. In addition, we found comparable score performance in subgroups of patients from the lowest, middle, and highest tertiles of household income. We now advocate for prospective evaluation of the score in areas with very low, as well as high income to confirm score validity in these populations.
A high between-hospital variability for loss of independent living after total joint arthroplasty has previously been reported [4], and these procedures were therefore excluded from previous scores. [22] In a previous study, variances between centers might have been explained by differences in patient characteristics among institutions, as the authors found that patients older than 80 years were 20 times more likely to be discharged to a nonhome environment than patients younger than 40 years [4]. When validating the ADELES score in an independent cohort from a competing hospital, we observed comparable rates of adverse discharge (37% and 38%). We believe that this observation illustrates the high vulnerability of this patient collective across hospitals and does not support a hospital-specific effect.
Although we found good score performance and score calibration in both patient cohorts, future studies should evaluate the ADELES score in locations with lower incidence of adverse discharge. This can then be used for optimization, as has been done for other scores such as the American College of Surgeons National Surgical Quality Improvement Program calculator [29] using simple methods of recalibration such as described by Wussler et al. [60].
Variables not readily available to clinicians such as social vulnerability [6] and the patients’ preoperative activity level might be associated with adverse discharge. However, there is a strong association between preoperative activity level and patient frailty [8], and our score contains several variables such as older age [14], a history of dementia [31], or depression [16], which predict frailty. Future prospective investigations might evaluate the predictive value of these factors. Finally, adverse discharge often implies a loss of independent living; although we excluded patients not living at home before surgery, we did not assess whether all patients lived independently in their homes before surgery.
Factors Associated with Adverse Discharge
We found that socioeconomic factors including low household income, federally provided insurance, unmarried status, and being a woman; surgical factors such as surgical management, location, concomitant infection and emergency status; and comorbidities including dementia, ASA physical status, heart failure and depression were associated with adverse discharge. Our study shows that socioeconomic risk factors, which are not included in other instruments [34], explain similar variance in adverse discharge disposition as do comorbidities and surgical approach [22, 34, 48]. For example, a patient with a high risk of adverse discharge based on socioeconomic factors has 4.7 times higher odds of adverse discharge than a patient who does not have these risk factors. Although there is evidence that low socioeconomic status hinders access to healthcare [40, 54] and is associated with worsened patient outcome [15, 28], the impact of social disparities might be even stronger than traditional surgical predictor variables [1, 11, 15].
The importance of marriage in maintaining a self-dependent status was emphasized by a study in 1576 older patients undergoing cardiac surgery, which reported a 40% higher odds of dying or developing a new functional disability within 24 months after surgery in divorced, separated or widowed patients than in patients who did not have these factors [35]. In patients 50 years and older, living alone more than doubles the odds of discharge to a nursing facility after hospitalization for medical reasons [26]. Cross-sectional [58] and longitudinal studies [36] confirmed marriage as an important protective predictor from admission to a nursing home.
We identified general anesthesia as a potentially modifiable risk factor that could be targeted to mitigate a patients’ risk of adverse discharge. This finding is corroborated by a recent study that reported higher odds of severe complications, including mortality, in patients undergoing revision THA under general anesthesia, as opposed to spinal anesthesia [57]. Based on these findings, physicians might be encouraged to use regional or neuraxial anesthesia and avoid general anesthesia in high-risk patients, if possible, although this still warrants confirmation by randomized, controlled trials. Many other risk factors included in the score are nonmodifiable, which is common among risk prediction instruments [34]. Physicians may use the score in these patients to streamline preoperative discussions and manage expectations from patients and families.
Validation of a Predictive Tool
We validated the ADELES score with data from an independent patient cohort from a competing hospital. Validation revealed a good predictive value. We further found good calibration between 0% and 70%, risk of adverse discharge in the external cohort. This means that we were able to demonstrate good score performance in patients with a risk of adverse discharge between very low (0%) and very high (70%). We believe the information in this range is clinically meaningful as it comprises the most relevant range of patients at medium risk of adverse discharge, where the greatest uncertainty exists. We emphasize the application of the proposed cutoff value of ≥ 23 out of 61 points for identification of high-risk patients and to facilitate decision making.
Comparison of ADELES to Other Tools
We found superiority of the ADELES score in our secondary analysis comparing it to previous tools that have been validated to depict frailty [49], comorbidities [7] or procedural severity [9] alone. These findings illustrate the specific ability of the score to predict adverse discharge disposition, rather than reflecting the well-documented finding that patients with a high degree of frailty, comorbidity load or undergoing extensive procedures suffer from increased postoperative hospitalization. We would like to emphasize the use of the ADELES score to specifically predict this important patient-centered outcome, adverse discharge disposition [25], and we feel that this validated risk calculator may add substantial value to the care of older patients undergoing lower-extremity orthopaedic surgery.
Conclusion
The ADELES score predicts adverse discharge disposition after lower-extremity surgery, reflecting loss of the ability to live independently in patients older than 60 years. The discriminative ability is better than other predictions scores that for account frailty, comorbidities, or procedural risk alone. ADELES identifies modifiable risk factors, including general anesthesia and prolonged preoperative hospitalization, and should be used to streamline patient and family expectation management and improve shared decision making. Future studies might evaluate the score in community hospitals and in institutions with different rates of adverse discharge disposition and lower income. A noncommercial calculator can be accessed at www.adeles-score.org.
Supplementary Material
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The institution of one or more of the authors (ME) has received, during the study period, institutional funding from a philanthropic donation from Jeff and Judy Buzen.
One of the authors certifies that he (EKR), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in an amount of less than USD 10,000 from Synthes (West Chester, PA, USA); in an amount of less than USD 10,000 from Zimmer (Warsaw, IN, USA); in an amount of less than USD 10,000 from Riverside Partners (Boston, MA, USA), all outside the submitted work.
Each remaining author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Beth Israel Deaconess Medical Center, Boston, MA, USA.
The first two authors contributed equally to this work.
References
- 1.Allen Butler R, Rosenzweig S, Myers L, Barrack RL. The Frank Stinchfield Award: the impact of socioeconomic factors on outcome after THA: a prospective, randomized study. Clin Orthop Relat Res . 2011;469:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein DN Liu TC Winegar AL, et al. Evaluation of a preoperative optimization protocol for primary hip and knee arthroplasty patients. J Arthroplasty. 2018;33:3642–3648. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY Liu Y Paruch JL, et al. Development and evaluation of the Universal ACS NSQIP Surgical Risk Calculator: A decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833-842.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozic KJ, Wagie A, Naessens JM, Berry DJ, Rubash HE. Predictors of discharge to an inpatient extended care facility after total hip or knee arthroplasty. J Arthroplasty. 2006;21:151–156. [DOI] [PubMed] [Google Scholar]
- 5.Cancio JM, Vela E, Santaeugènia S, Clèries M, Inzitari M, Ruiz D. Long-term impact of hip fracture on the use of healthcare resources: a population-based study. J Am Med Dir Assoc. 2019;20:456–461. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael H, Moore A, Steward L, Velopulos CG. Disparities in emergent versus elective surgery: comparing measures of neighborhood social vulnerability. J. Surg Res. 2020;256:397–403. [DOI] [PubMed] [Google Scholar]
- 7.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 8.Da Silva VD, Tribess S, Meneguci J, Sasaki JE, Garcia-Meneguci CA, Carneiro JAO, Virtuoso JS. Association between frailty and the combination of physical activity level and sedentary behavior in older adults. BMC Public Health. 2019;19:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton JE, Kurz A, Turan A, Mascha EJ, Sessler DI, Saager L. Development and validation of a risk quantification index for 30-day postoperative mortality and morbidity in noncardiac surgical patients. Anesthesiology. 2011;114:1336–1344. [DOI] [PubMed] [Google Scholar]
- 10.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National hospital discharge survey. Natl Health Stat Report. 2008:1–20. [PubMed] [Google Scholar]
- 11.Dyer SM, Crotty M, Fairhall N, Magaziner J, Beaupre LA, Cameron ID, Sherrington C, Fragility Fracture Network (FFN) Rehabilitation Research Special Interest Group. A critical review of the long-term disability outcomes following hip fracture. BMC Geriatr. 2016;16:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fingar KR, Stocks C, Weiss AJ, Steiner CA. Most frequent operating room procedures performed in U.S. Hospitals, 2003–2012: Statistical Brief #186. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. Available at: http://www.ncbi.nlm.nih.gov/books/NBK274246/. Accessed November 11, 2019. [Google Scholar]
- 13.Fried LP Tangen CM Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. [DOI] [PubMed] [Google Scholar]
- 14.Fu MC Ondeck NT Nwachukwu BU, et al. What associations exist between comorbidity indices and postoperative adverse events after total shoulder arthroplasty? Clin Orthop Relat Res . 2019;477:881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George J, Navale SM, Schiltz NK, Siccha M, Klika AK, Higuera CA. Racial disparities in above-knee amputations after TKA: A national database study. Clin Orthop Relat Res . 2017;475:1809–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givens JL, Sanft TB, Marcantonio ER. Functional recovery after hip fracture: the combined effects of depressive symptoms, cognitive impairment, and delirium. J Am Geriatr Soc. 2008;56:1075–1079. [DOI] [PubMed] [Google Scholar]
- 17.Gosling AF, Hammer M, Grabitz S, Wachtendorf LJ, Katsiampoura A, Murugappan KR, Sehgal S, Khabbaz KR, Mahmood F, Eikermann M. Development of an Instrument for Preoperative Prediction of Adverse Discharge in Patients Scheduled for Cardiac Surgery. J Cardiothorac Vasc Anesth. 2020;16:S1053-0770(20)30822-3. [DOI] [PubMed] [Google Scholar]
- 18.Hall DE Arya S Schmid KK, et al. Development and initial validation of the Risk Analysis Index for measuring frailty in surgical populations. JAMA Surg. 2017;152:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall MJ, DeFrances CJ, Williams SN, Golosinskiy A, Schwartzman A. National hospital discharge survey: 2007 summary. Natl Health Stat Report. 2010:1–20, 24. [PubMed] [Google Scholar]
- 20.Harrell FE. Regression Modeling Strategies, 2nd Ed. Berlin, Germany: Springer; 2015. [Google Scholar]
- 21.Hosmer D, Lemeshow S. Applied logistic regression. Wiley Series in Probability and Statistics; New York, NY, USA: John Wiley & Sons, Inc; 2000. Available at: http://resource.heartonline.cn/20150528/1_3kOQSTg.pdf. [Google Scholar]
- 22.Hyder JA, Wakeam E, Habermann EB, Hess EP, Cima RR, Nguyen LL. Derivation and validation of a simple calculator to predict home discharge after surgery. J Am Coll Surg. 2014;218:226–236. [DOI] [PubMed] [Google Scholar]
- 23.Joseph B Pandit V Zangbar B, et al. Superiority of frailty over age in predicting outcomes among geriatric trauma patients: a prospective analysis. JAMA Surg. 2014;149:766–772. [DOI] [PubMed] [Google Scholar]
- 24.Kim S Han H-S Jung H, et al. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149:633–640. [DOI] [PubMed] [Google Scholar]
- 25.Koppitz AL, Dreizler J, Altherr J, Bosshard G, Naef R, Imhof L. Relocation experiences with unplanned admission to a nursing home: a qualitative study. Int Psychogeriatr. 2017;29:517–527. [DOI] [PubMed] [Google Scholar]
- 26.Lage DE Jernigan MC Chang Y, et al. Living alone and discharge to skilled nursing facility care after hospitalization in older adults. J Am Geriatr Soc. 2018;66:100–105. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Jung SH, Lee S-U, Ha Y-C, Lim J-Y. Effect of balance training after hip fracture surgery: a systematic review and meta-analysis of randomized controlled studies. J Gerontol A Biol Sci Med. Sci. 2019;74:1679–1685. [DOI] [PubMed] [Google Scholar]
- 28.Lieber AM, Kirchner GJ, Kerbel YE, Moretti VM, Vakil JJ, Brahmabhatt S. Socioeconomic status is associated with risk of above-knee amputation after periprosthetic joint infection of the knee. Clin Orthop Relat Res . 2019;477:1531–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Cohen ME, Hall BL, Ko CY, Bilimoria KY. Evaluation and enhancement of calibration in the American College of Surgeons NSQIP Surgical Risk Calculator. J Am Coll Surg . 2016;223:231–239. [DOI] [PubMed] [Google Scholar]
- 30.Manson S, Schroeder J, Van Riper D, Ruggles S. IPUMS National Historical Geographic Information System: Version 14.0 [Database]. Minneapolis, MN: 2019. Available at: 10.18128/D050.V14.0. Accessed December 21, 2019. [DOI] [Google Scholar]
- 31.Mariconda M Costa GG Cerbasi S, et al. Factors predicting mobility and the change in activities of daily living after hip fracture: a 1-year prospective cohort study. J Orthop Trauma. 2016;30:71–77. [DOI] [PubMed] [Google Scholar]
- 32.Maxwell MJ, Moran CG, Moppett IK. Development and validation of a preoperative scoring system to predict 30 day mortality in patients undergoing hip fracture surgery. Br J Anaesth. 2008;101:511–517. [DOI] [PubMed] [Google Scholar]
- 33.McIsaac DI, Wong CA, Huang A, Moloo H, van Walraven C. Derivation and validation of a generalizable preoperative frailty index using population-based health administrative data. Ann. Surg. 2019;270:102–108. [DOI] [PubMed] [Google Scholar]
- 34.Mohanty S Liu Y Paruch JL, et al. Risk of discharge to postacute care: a patient-centered outcome for the american college of surgeons national surgical quality improvement program surgical risk calculator. JAMA Surg. 2015;150:480–484. [DOI] [PubMed] [Google Scholar]
- 35.Neuman MD, Werner RM. Marital status and postoperative functional recovery. JAMA Surg. 2016;151:194–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noël-Miller C. Spousal loss, children, and the risk of nursing home admission. J Gerontol B Psychol Sci Soc Sci. 2010;65B:370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panula J Pihlajamäki H Mattila VM, et al. Mortality and cause of death in hip fracture patients aged 65 or older: a population-based study. BMC Musculoskelet Disord. 2011;12:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel KV, Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin Orthop Relat Res . 2014;472:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol . 2006;163:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabah NM, Knusel KD, Khan HA, Marcus RE. Are there nationwide socioeconomic and demographic disparities in the use of outpatient orthopaedic services? Clin Orthop Relat Res . 2020;478:979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rolfson DB, Majumdar SR, Tsuyuki RT, Tahir A, Rockwood K. Validity and reliability of the Edmonton Frail Scale. Age Ageing. 2006;35:526–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rostin P Teja BJ Friedrich S, et al. The association of early postoperative desaturation in the operating theatre with hospital discharge to a skilled nursing or long-term care facility. Anaesthesia. 2019;74:457–467. [DOI] [PubMed] [Google Scholar]
- 43.Royston P, Moons KGM, Altman DG, Vergouwe Y. Prognosis and prognostic research: developing a prognostic model. BMJ. 2009;338:b604. [DOI] [PubMed] [Google Scholar]
- 44.Ruopp MD, Perkins NJ, Whitcomb BW, Schisterman EF. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom J. 2008;50:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaller SJ Anstey M Blobner M, et al. International early SOMS-guided mobilization research initiative. Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388:1377–1388. [DOI] [PubMed] [Google Scholar]
- 46.Schaller SJ Scheffenbichler FT Bose S, et al. Influence of the initial level of consciousness on early, goal-directed mobilization: a post hoc analysis. Intensive Care Med. 2019;45:201–210. [DOI] [PubMed] [Google Scholar]
- 47.Shin CH Grabitz SD Timm FP, et al. Development and validation of a score for preoperative prediction of obstructive sleep apnea (SPOSA) and its perioperative outcomes. BMC Anesthesiol. 2017;17:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AB, Bronsert MR, Henderson WG, Lambert-Kerzner A, Hammermeister KE, Meguid RA. Accurate preoperative prediction of discharge destination using 8 predictor variables: a NSQIP analysis. J Am Coll Surg . 2020;230:64-75.e2. [DOI] [PubMed] [Google Scholar]
- 49.Subramaniam S, Aalberg JJ, Soriano RP, Divino CM. New 5-Factor Modified Frailty Index using American College of Surgeons NSQIP Data. J Am Coll Surg . 2018;226:173-181.e8. [DOI] [PubMed] [Google Scholar]
- 50.Teja B Raub D Friedrich S, et al. Incidence, prediction, and causes of unplanned 30-Day hospital admission after ambulatory procedures. Anesth Analg . 2020;131:497–507. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Census Bureau, U.S. Department of Commerce. 2018. median household income in the United States. 2018 Median Household Income in the United States. 2019. Available at: https://www.census.gov/library/visualizations/interactive/2018-median-household-income.html. Accessed August 12, 2020.
- 52.Van Calster B Wynants L Verbeek JFM, et al. Reporting and interpreting decision curve analysis: a guide for investigators. Eur Urol . 2018;74:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velanovich V, Antoine H, Swartz A, Peters D, Rubinfeld I. Accumulating deficits model of frailty and postoperative mortality and morbidity: its application to a national database. J Surg Res . 2013;183:104–110. [DOI] [PubMed] [Google Scholar]
- 54.Wang AY, Wong MS, Humbyrd CJ. Eligibility criteria for lower extremity joint replacement may worsen racial and socioeconomic disparities. Clin Orthop Relat Res . 2018;476:2301–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.WHO Expert Committee. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 56.Williams SN, Wolford ML, Bercovitz A. Hospitalization for total knee replacement among inpatients aged 45 and over: United States, 2000-2010. NCHS Data Brief; 2015:1–8. [PubMed] [Google Scholar]
- 57.Wilson JM, Farley KX, Bradbury TL, Guild GN. Is spinal anesthesia safer than general anesthesia for patients undergoing revision THA? Analysis of the ACS-NSQIP Database. Clin Orthop Relat Res . 2020;478:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winder JY, Achterberg WP, Roos RAC. Marriage as protector for nursing home admission in Huntington’s Disease. J Huntingtons Dis. 2018;7:251–257. [DOI] [PubMed] [Google Scholar]
- 59.Wolford ML, Palso K, Bercovitz A. Hospitalization for total hip replacement among inpatients aged 45 and over: United States, 2000-2010. NCHS Data Brief; 2015:1–8. [PubMed] [Google Scholar]
- 60.Wussler D Kozhuharov N Sabti Z, et al. External validation of the MEESSI Acute Heart Failure Risk Score: A Cohort Study. Ann Intern Med . 2019;170:248–256. [DOI] [PubMed] [Google Scholar]
- 61.Xiao R, Miller JA, Zafirau WJ, Gorodeski EZ, Young JB. Impact of home health care on health care resource utilization following hospital discharge: A cohort study. Am J Med . 2018;131:395-407.e35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


