Abstract
Context
New approaches are needed to address the evolution of the primary aldosteronism syndrome and to increase its recognition. Herein, we review evidence indicating that primary aldosteronism is a prevalent syndrome that is mostly unrecognized, and present a pragmatic and pathophysiology-based approach to improve diagnosis and treatment.
Methods
Evidence was gathered from published guidelines and studies identified from PubMed by searching for primary aldosteronism, aldosterone, renin, and hypertension. This evidence was supplemented by the authors’ personal knowledge, research experience, and clinical encounters in primary aldosteronism.
Interpretation of Evidence
Renin-independent aldosterone production is a prevalent phenotype that is diagnosed as primary aldosteronism when severe in magnitude, but is largely unrecognized when milder in severity. Renin-independent aldosterone production can be detected in normotensive and hypertensive individuals, and the magnitude of this biochemical phenotype parallels the magnitude of blood pressure elevation, the risk for incident hypertension and cardiovascular disease, and the likelihood and magnitude of blood pressure reduction with mineralocorticoid receptor antagonist therapy. Expansion of the indications to screen for primary aldosteronism, combined with the use of a pathophysiology-based approach that emphasizes inappropriate aldosterone production in the context of renin suppression, will substantially increase the diagnostic and therapeutic yields for primary aldosteronism.
Conclusions
The landscape of primary aldosteronism has evolved to recognize that it is a prevalent syndrome of renin-independent aldosterone production that contributes to the pathogenesis of hypertension and cardiovascular disease. Expanding screening indications and simplifying the diagnostic approach will enable implementation of targeted treatment for primary aldosteronism.
Keywords: aldosterone, renin, primary aldosteronism, adrenal, hypertension
Case Presentation: A 58 year old man was referred to endocrinology for further evaluation of hypertension and hypokalemia. He was diagnosed with hypertension when he was 38 years old but was not treated with medications. At the age of 43 years, his blood pressure was noted to be 200/100 mmHg and he was started on lisinopril. By age 50 years, he had been evaluated in an emergency room and admitted to a hospital more than three times for hypertensive urgency and hypokalemia. Electrocardiograms and echocardiograms had confirmed left ventricular hypertrophy. By age 58 years he was treated with lisinopril 40mg daily, nifedipine 60mg daily, and carvedilol 50mg daily, and his blood pressure ranged from 135-156/80-90 mmHg. However, his serum potassium continued to range from 3.1-3.4 mEq/L and his serum bicarbonate ranged from 25-30 mEq/L. He had never undergone an evaluation for secondary causes of hypertension, and specifically, had never had an assessment of aldosterone or renin.
Author Commentary: This case presentation highlights multiple important questions in the approach to a patient who may have primary aldosteronism. First, what is primary aldosteronism? What is the probability that this patient has primary aldosteronism? When should this patient have been evaluated for primary aldosteronism? How should the evaluation for primary aldosteronism be conducted and interpreted for this patient? In this review, we will provide evidence-based answers to these questions and propose an updated and contemporary diagnostic approach to facilitate increased recognition of the primary aldosteronism syndrome.
When primary aldosteronism was first described more than 60 years ago, it was considered to be a niche form of adrenal hypertension caused by a neoplastic process. In subsequent decades, refinement of screening diagnostics and dynamic confirmatory tests revealed that primary aldosteronism is not rare at all. Rather, it could be detected in 5% to 10% of hypertensive adults. This period of time was characterized by clinical research studies that produced iterative and complementary prevalence estimates for primary aldosteronism. However, in parallel, the clinical diagnostic algorithms that were developed were complex and daunting for most general clinicians and primary care doctors. As a result, and as our understanding of the prevalence of primary aldosteronism grew, awareness among front-line clinicians that primary aldosteronism is a common disorder that warrants diagnostic attention lagged behind. Disappointingly, the pursuit of primary aldosteronism as a diagnosis is so low among general medical providers that, estimates suggest, less than 1% of true cases are ever diagnosed. The public health relevance of this disparity is underscored by the fact that primary aldosteronism is not only a remediable cause of high blood pressure, but also of cardiovascular and metabolic diseases, and death, irrespective of blood pressure (1-13).
The largest gains in our understanding of primary aldosteronism have occurred in the last 2 decades. As will be discussed herein, current prevalence estimates suggest that up to 30% of hypertensive adults have an overt form of primary aldosteronism. Moreover, it has become evident that primary aldosteronism is better characterized as a syndrome of renin-independent aldosterone production that traverses a continuum of severity, ranging from mild to overt, and spanning the blood pressure spectrum from normotension through resistant hypertension. Therefore, it is imperative to raise awareness of these advances by updating the traditional dogma for the general medical community and trainees, and, in addition, to propose a simplified diagnostic strategy capable of diagnosing and/or empirically treating a larger proportion of individuals with unrecognized primary aldosteronism.
In this review, we will highlight the evolving landscape of primary aldosteronism by reviewing the pathophysiological principles that characterize the syndrome. We will review data demonstrating the prevalence of primary aldosteronism across the blood pressure spectrum, from subclinical forms in adults with normal blood pressure to overt forms in resistant hypertension. Finally, we propose a diagnostic algorithm reflecting the current epidemiology and knowledge of primary aldosteronism syndrome that can be employed by general clinicians to maximize the diagnostic and therapeutic yield, thus mitigating cardiometabolic disease and death.
Defining Primary Aldosteronism
There are a number of possible ways to define primary aldosteronism, taking into account pathophysiologic principles, diagnostic methods, and treatment responses. To maximize the detection of the primary aldosteronism, we favor thinking about the syndrome using pathophysiologic principles and avoiding reliance on strict diagnostic thresholds and/or treatment responses. In this regard, 3 cardinal pathophysiologic features of primary aldosteronism, initially described by Conn and colleagues (14), still hold true today (Table 1).
Table 1.
The 3 cardinal features that define primary aldosteronism
| 1. Suppression of baseline renin secretion |
| Primary aldosteronism is a state of extracellular fluid volume expansion (specifically expansion of the effective arterial blood volume) that results in suppression of renin secretion and consequently decreased generation of angiotensin II. |
| 2. Inability to stimulate renin secretion normally |
| The degree of renin suppression by volume expansion due to excess mineralocorticoid activity can be quantified by an inadequate rise in renin in response to common physiologic stimuli, including upright posture, sodium/volume contraction with diuresis or dietary sodium restriction, or the effect of an angiotensin-converting enzyme inhibitor. Patients with primary aldosteronism have a blunted rise in renin to these stimuli. |
| 3. Inappropriate and nonsuppressible aldosterone production |
| Aldosterone production in primary aldosteronism is independent of renin and is relatively nonsuppressible despite volume expansion, inhibition of angiotensin II, and hypokalemia. |
The first obligate manifestation of primary aldosteronism pathophysiology is suppression of baseline renin secretion. As Conn et al described in 1964 (14): “since primary aldosteronism is associated with an increase in intra-vascular volume…the renin-angiotensin system should be suppressed.”
The second cardinal feature is the inability to stimulate renin secretion normally. This feature relates to the first in that baseline renin suppression dampens an appropriate rise in renin. That is, in the face of physiologic stimulants that normally raise renin (such as upright posture, volume contraction, sodium depletion, or angiotensin-converting enzyme inhibition), renin remains relatively low. As initially described by Conn and colleagues in 1964 (14): “…in primary aldosteronism plasma renin activity is not detectable, even under conditions which raise this activity to high levels in normal people.” We have learned over time that this is a relative concept, because subjecting patients with primary aldosteronism to extreme dietary sodium restriction (15, 16), volume contraction with diuretics (2), adequate doses of mineralocorticoid receptor antagonists (4, 17), and high doses of converting enzyme inhibitors (2) can induce an increase in renin, especially in milder instances of primary aldosteronism; however, this increase remains blunted when compared to the normal physiologic response.
The third fundamental feature is inappropriate and nonsuppressible aldosterone production, despite sodium and extracellular volume expansion, in which renin and angiotensin II are suppressed, often in the face of hypokalemia, which normally inhibits aldosterone biosynthesis.
An important note here is that hypertension and hypokalemia are not fundamental characteristics of primary aldosteronism, rather they are dependent features that manifest when intra-arterial volume expansion exceeds the vascular capacity to maintain normal blood pressure, and/or when distal nephron sodium delivery, leading to accelerated sodium-potassium exchange and kaliuresis, exceeds the threshold of potassium intake. Thus, the more severe and prolonged the exposure to renin-independent aldosterone production and a sodium-retention state, the higher the risk of severe hypertension and hypokalemia.
Pursuing these cardinal features of primary aldosteronism in daily practice can be simplified by recognizing that “…the unique combination of overproduction of aldosterone and suppressed plasma renin activity is diagnostic (18).” Numerous diagnostic criteria have been proposed over the last several decades. Herein, we will provide evidence that suggests a simplified and streamlined approach, based on these 3 principal pathophysiologic characteristics, can be employed to maximize the opportunity to diagnose and treat primary aldosteronism.
Evidence from Renin-Physiology Demonstrates a High Prevalence of Primary Aldosteronism
The first 2 cardinal signs of primary aldosteronism have served as biomarkers of unrecognized primary aldosteronism for decades. It has long been established that a substantial proportion of patients with hypertension have a phenotype of low or suppressed renin that was hypothesized to reflect a volume-expanded and/or mineralocorticoid-excess state (19-22). Although a random low renin level may indicate volume expansion due to mineralocorticoid excess, it could also be a physiologic ramification of high dietary sodium intake. A more nuanced method to assess whether renin suppression may be attributable to mineralocorticoid activity is by investigating renin stimulation (23).
Physiologically, dietary sodium restriction induces near-maximal stimulation of renin secretion (24); however, there is a continuum in the ability to stimulate renin secretion in the general population. Normotensive and hypertensive individuals with the greatest inability to stimulate renin have the highest blood pressure, urinary potassium excretion rates, and aldosterone-to-renin ratios (ARRs), and the most nonsuppressible aldosterone production, even within the range of “normal” (16, 25); in other words, greater renin suppression and poorer renin stimulation are biomarkers for unrecognized primary aldosteronism. Indeed, we recognized nearly 50 years ago that the inability to stimulate renin predicted reduction in blood pressure with spironolactone treatment (26). Ultimately, although aldosterone is often the primary focus when assessing for primary aldosteronism, the cardinal signs focusing on renin pathophysiology may be more sensitive biomarkers of inappropriate aldosterone production and responsiveness to mineralocorticoid receptor antagonism. As demonstrated recently in the PATHWAY-2 clinical trials, even after overt primary aldosteronism was supposedly excluded among participants (presumptively because aldosterone levels were not “high” enough to trigger concern), the most robust blood pressure-lowering with spironolactone was observed in individuals with suppressed renin and higher aldosterone levels within the “normal” range (27, 28). These findings underscore that when assessing for primary aldosteronism, attention should be focused on whether aldosterone is appropriate or inappropriate relative to renin, or put another way, that almost any aldosterone level in the setting of renin suppression could represent a phenotype of primary aldosteronism syndrome that may be responsive to mineralocorticoid receptor antagonists (7, 8, 27, 28).
Prevalence of Primary Aldosteronism in Normotension
Although clinical indications for primary aldosteronism screening generally focus on individuals with hypertension, accruing evidence indicates that a primary aldosteronism phenotype can be detected even among those with normal blood pressure (sometimes referred to as subclinical or nonclassical primary aldosteronism [29]). The possibility of primary aldosteronism having origins in normotension was proposed by Conn and colleagues in 1965 (18): “To date, all such patients studied have been hypertensive…It is likely that the future will prove this diagnostic combination to be present before persistent hypertension occurs. It would seem wise to investigate patients in the early phases of fluctuating hypertension with the idea of primary aldosteronism in mind.”
Several studies have demonstrated a primary aldosteronism phenotype in normotensive individuals, and that this phenotype is associated with an increased risk of blood pressure elevation and incident hypertension. Vasan et al reported that 50% of normotensive individuals from the Framingham Offspring Study had plasma aldosterone concentrations greater than 11 ng/dL and that 25% had concentrations greater than 19 ng/dL (30). These authors showed that at least 6.4% of normotensive participants (3.1% of men and 8.9% of women) had a positive or elevated ARR (31, 32). The most important overarching finding, however, was that the phenotype of renin-independent aldosterone production in normotensive individuals was associated with higher risk of increases in blood pressure and development of hypertension (30, 32). In a study from the Multi-Ethnic Study of Atherosclerosis, which enrolled a nationwide sample from the United States, Brown and colleagues showed that normotensive participants with a suppressed renin phenotype had a 68% higher risk of incident hypertension in the next 5 years when compared to normotensive participants with a normal renin phenotype (33). Moreover, 6.7% of the normotensive population had an ARR of at least 35, and among normotensive participants with suppressed renin, at least 15% had an ARR above this threshold. Again, the most notable finding was that the greater the magnitude of the aldosterone level in the context of suppressed renin, the higher the risk for incident hypertension: a clinically relevant spectrum of subclinical primary aldosteronism in adults with normal blood pressure (29, 33).
A recent small study of young men with normal blood pressure (median age, 25.7 years) (34) found that when consuming a diet approximating the high sodium balance needed for an oral sodium suppression test (mean urinary sodium excretion ~ 200 mmol/24 h) (2) and the average American potassium intake (mean urinary potassium excretion. 67.5 mmol/24 h) (35), the 75th percentile for 24-hour urinary aldosterone excretion was 10 mcg/24 h, corresponding to the lower limit for confirming overt primary aldosteronism (2). In other words, even among very young and healthy normotensive people, as many as one-quarter meet the definition of mild primary aldosteronism based on the accepted thresholds of the oral sodium suppression test.
Several well-controlled studies have performed systematic confirmatory testing to robustly demonstrate that primary aldosteronism syndrome can be detected in normotension. Markou et al conducted fludrocortisone-dexamethasone suppression tests in normotensive women, without any prescreening for ARR or renin, discovering that 13% of their cohort had overt primary aldosteronism (36, 37). An important prognostic testament of this phenotype was that 85% of these individuals with normotensive primary aldosteronism developed hypertension within the next 5 years, as compared to only 11% of normotensive individuals without primary aldosteronism. Similarly, Baudrand and colleagues showed that 14% of normotensives with suppressed renin activity in a US cohort had overt primary aldosteronism using oral sodium suppression testing (38). Importantly, the greater the magnitude of aldosterone production in the setting of suppressed renin activity, the greater the kaliuresis and the greater the aldosterone response to angiotensin stimulation (38-40), highlighting a pathologic continuum of primary aldosteronism in normotension. Most recently, in a nationwide sample of American normotensive individuals who underwent oral sodium suppression testing, we observed that the crude prevalence of overt primary aldosteronism was 9% (41); however, beyond this binary prevalence designation, a continuum of renin-independent aldosterone production was evident throughout the normotensive population and well below the conventional definition of overt primary aldosteronism.
Thus, the prevalence of overt primary aldosteronism in normotensive individuals may range from 6% to 14%, and in some instances up to 25%, depending on the specific population demographics and the modality of testing employed (elevated ARR, dynamic confirmatory testing, or both). Importantly, these estimates stem from dynamic confirmatory testing without reliance on antecedent screening with an ARR. However, these prevalence statistics emphasize only the most overt instances, defined using arbitrary diagnostic thresholds, and overlook the continuum of milder manifestations of primary aldosteronism syndrome that have been described (30, 32, 33, 36, 38, 41).
What then is the significance of these biochemical observations in the normotensive population? The magnitude and severity of the primary aldosteronism phenotype in individuals with normotension has been consistently associated with higher risks for incident elevation of blood pressure and hypertension, thereby reinforcing that this prevalent biochemical phenotype is clinically relevant and may play an important role in the pathogenesis of hypertension. Although the explanation underlying why primary aldosteronism is so common in the normotensive population is still under investigation, a leading hypothesis is the accumulation of pathogenic somatic mutations over the lifespan that result in inappropriately high aldosterone synthase expression, visualized as aldosterone-producing cell clusters on immunohistochemistry (42-47), a phenomenon that has been observed in the adrenal cortex of normotensive and hypertensive individuals. Further, this hypothesis represents a paradigm shift in thinking about primary aldosteronism as driven by abnormal aldosterone synthase expression, even in morphologically normal adrenal glands, as opposed to requiring hyperplastic or neoplastic transformation.
Prevalence of Primary Aldosteronism in Hypertension
Population-based studies have used the ARR to approximate the potential prevalence of primary aldosteronism. For example, in the Framingham Offspring Cohort, 12.0% of untreated participants with hypertension had a positive screening ARR (32), and similarly, in a German cohort of hypertensive participants, up to 7.0% had a positive screening ARR (48). In the Multi-Ethnic Study of Atherosclerosis, untreated participants with hypertension had a mean plasma aldosterone concentration of 14 ng/dL, mean plasma renin activity of 0.7 ng/mL/h, and mean ARR of approximately 20 (49). However, hypertensive participants with suppressed renin had a mean ARR of approximately 30, and within this subgroup, higher aldosterone levels were associated with a higher risk for incident coronary artery calcification and all-cause mortality (49).
Numerous studies from across the world have assessed the prevalence of primary aldosteronism using a 2-step approach whereby a screening ARR is first employed to identify those deemed to be at highest risk, followed by dynamic confirmatory testing in only this selected subgroup (50-58). Although we cannot review all of these studies herein, nor dissect the details of each study individually, collectively, the prevalence of overt primary aldosteronism using this 2-step approach has been reported to range from 2% to 19%, with lower prevalence estimates in mild (or stage I) hypertension, and the highest prevalence estimates in those with more severe or resistant hypertension with other comorbidities.
In contrast, studies that conducted dynamic confirmatory tests in hypertensive participants without a prespecified screening ARR threshold have observed much higher prevalence estimates. The insight provided by this approach stems from the fact that many patients with primary aldosteronism can have an unimpressive ARR and/or aldosterone value on a single screening assessment, owing to the variability of aldosterone production (59-62). Therefore, the 2-step approach is likely to underestimate the true prevalence detected by dynamic confirmatory testing by preemptively excluding many potential cases. For example, when Tsiavos et al systematically conducted fludrocortisone-dexamethasone suppression testing, they found that 28% to 30% of participants with hypertension had overt primary aldosteronism (63). Similarly, when Parasiliti-Caprino and colleagues systematically conducted saline suppression tests in a population with confirmed resistant hypertension, they discovered that 29% of their participants had overt primary aldosteronism (64). We evaluated the prevalence of overt primary aldosteronism and milder renin-independent aldosterone production in hypertension using the oral sodium suppression test (41). The crude prevalence of overt primary aldosteronism was 15.7% in stage I hypertension, 20.7% in stage II hypertension, and 24.0% in resistant hypertension. When data were restricted to participants with suppressed renin activity, these prevalence estimates increased to 18.8%, 25.0%, and 52.1%, respectively (41). More informative, was the fact that there was a continuous spectrum of renin-independent aldosterone production in hypertension that was not captured by these prevalence estimates, and the magnitude of aldosterone production was strongly associated with blood pressure severity and potassium wasting, indicating that the primary aldosteronism severity spectrum was a highly prevalent feature in hypertension (41, 65). Another interpretation is that almost any aldosterone production in the context of renin suppression might be inappropriate and represent a manifestation of primary aldosteronism syndrome.
The importance of raising awareness not only of the high prevalence of overt primary aldosteronism in hypertension, but also the prevalent spectrum of renin-independent aldosterone production existing below most diagnostic thresholds (and thus unrecognized), is that this phenotype is associated with cardiometabolic disease (66, 67) and exhibits dramatic blood pressure–lowering when specifically treated with mineralocorticoid receptor antagonists (27, 28, 68). Generally, the higher the aldosterone in the setting of renin suppression, the greater the efficacy of mineralocorticoid receptor antagonists in lowering blood pressure. Although this reproducible observation has resulted in recommendations to use mineralocorticoid receptor antagonists in resistant hypertension (69, 70), we propose as follows that mineralocorticoid receptor antagonists should also be considered in patients with renin suppression and milder phenotypes of hypertension and primary aldosteronism.
The Failure to Implement and Raise Awareness for Screening
Despite the large number of studies indicating that the prevalence of primary aldosteronism syndrome is high and that this disorder can be treated to modify cardiovascular risk, this information has not yet translated to higher screening rates or changes to hypertension-treatment algorithms. Most expert guidelines recommend screening for primary aldosteronism in populations with resistant or severe hypertension and/or hypokalemia (2); however, even in this high-risk group, in which the prevalence of primary aldosteronism syndrome can exceed 25% to 50% (41, 68, 71), less than 3% of eligible patients in the United States (72, 73) and less than 8% in Italy and Germany (74, 75) ever undergo indicated screening. Some official guidelines also recommend screening adults with hypertension and obstructive sleep apnea (2) (although this indication has been questioned (76, 77)), yet less than 2% of eligible patients are screened (73). Because screening for primary aldosteronism is so low, the detection of true cases and the implementation of targeted therapy with mineralocorticoid receptor antagonists or curative unilateral adrenalectomy is abysmal relative to the true prevalence of the syndrome. One cause of this discrepancy is the lack of education provided to primary care providers and front-line general clinicians. Efforts to increase awareness among these clinicians and those managing patients with hypertension are critical to increase the screening rates for primary aldosteronism. A second and concomitant problem is that many clinicians are intimidated by the recommended screening guidelines and worry that the process may involve a tangled web of medication withdrawals and complex diagnostic interpretations, processes that can be confusing, frustrating, and laborious. Efforts to streamline and simplify diagnostic approaches are necessary, and discussed as follows.
Pitfalls in Interpretation of Screening Measurements
Unfortunately, even when screening for primary aldosteronism occurs, there is a relatively high risk that the results will be misinterpreted. This is due to several factors. First, reliance only on the ARR, rather than the individual components (renin and aldosterone), can introduce misinterpretations in which the denominator (renin) may be very low (inflating the ARR) or not low enough (reducing the ARR; due to limitations in the lower limit of assay reporting or certain medication classes that raise renin). Similarly, the numerator (aldosterone) can vary substantially and at times may not be “high” enough to command attention. This leads to the second factor, which is the variability in circulating aldosterone levels. Circulating aldosterone levels vary considerably throughout the day owing to diurnal variation, as well as random peaks and troughs that may be influenced by factors such as corticotropin release/stress, diet, posture, and timing of medication dosing (59-62, 78). Many patients with primary aldosteronism can at times have circulating aldosterone values well below what is conventionally considered to be “high” or consistent with “hyper”aldosteronism, thereby increasing the risk of erroneously excluding the diagnosis (2, 13, 41, 59, 60, 62, 65, 78-80) and a missed opportunity to institute targeted therapy that has been shown to lower blood pressure robustly in this population. For example, patients with confirmed primary aldosteronism can frequently have circulating aldosterone concentrations that are less than 15 ng/dL or even less than 10 ng/dL, and rarely as low as 5 ng/dL (79), levels that are usually interpreted to exclude the possibility of primary aldosteronism. Thus, in parallel with efforts to increase awareness and simplify the diagnostic screening process, efforts to disentangle the interpretation of screening results with actionable steps are needed for general clinicians.
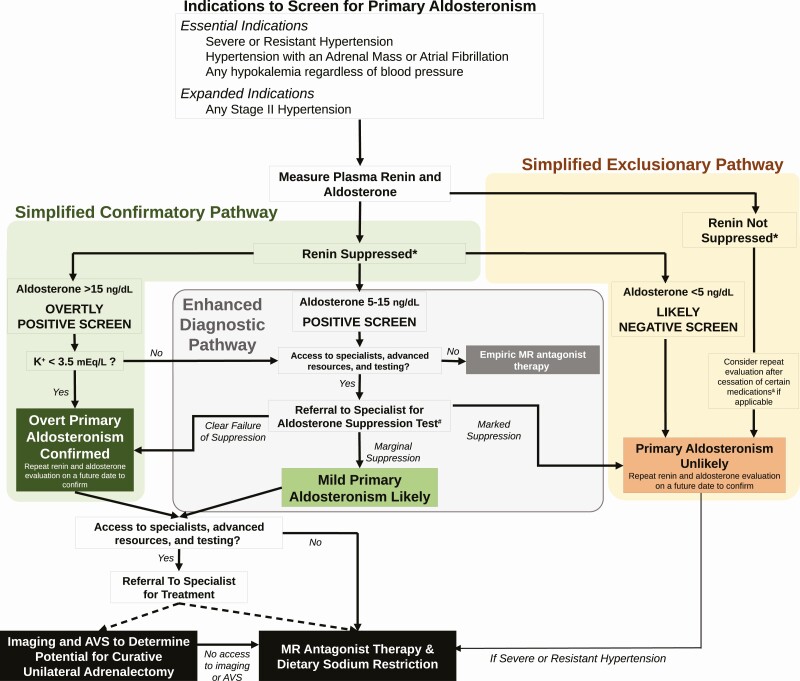
Case Presentation Continued: The patient was screened for primary aldosteronism for the first time at the age of 58 years. While seated in the primary care clinic, and taking lisinopril, nifedipine, and carvedilol, his plasma renin activity was measured to be <0.6 ng/mL/h and his plasma aldosterone concentration was 5.2 ng/dL (ARR greater than 9). The screening results were interpreted as “negative” because the aldosterone and ARR values were considered to be “too low” for primary aldosteronism. Three months later, after another emergency room visit for hypokalemic myopathy, where he presented with muscle weakness and spasms, a serum potassium of 2.9 mEq/L, and a blood pressure of 188/98 mmHg, he was referred to endocrinology. Repeat testing, while seated in the endocrinology clinic revealed a plasma renin activity of <0.6 ng/mL/h and a plasma aldosterone concentration of 9.5 ng/dL. These testing results were considered to represent a “positive screen” (Fig. 1). A 1mg dexamethasone suppression test and plasma metanephrines were normal.
Author Commentary: This case underscores the aforementioned evidence indicating how unappreciated primary aldosteronism is, and how infrequently patients are screened for the condition, even when presenting with hypertension and hypokalemia. Further, this case highlights the emphasis that many clinicians place on arbitrary values indicative of a “high” or “low” aldosterone concentration, rather than focusing on whether there is inappropriate aldosterone production in the context of renin suppression (i.e., renin-independent aldosterone production). Below, we discuss why this patient’s screening results should be considered “positive,” and we outline a simplified and practical approach to interpreting diagnostic studies for primary aldosteronism.
A Simplified Proposal to Maximize Detection of Primary Aldosteronism and Implement Targeted Therapy
Leveraging the evolving knowledge that has informed our current view of primary aldosteronism syndrome, we propose a diagnostic approach to increase the detection of primary aldosteronism syndrome across its severity spectrum and to maximize the opportunity to institute targeted therapy (Fig. 1). The objectives of this algorithm are to simplify the process for primary care and general medical clinicians, regardless of access to advanced resources and specialists, and to increase the likelihood that, at a minimum, mineralocorticoid receptor antagonist therapy is prescribed when it may be beneficial. We recognize that the heterogeneity in global medical practice and access to resources means that no single algorithm will represent the optimum practice for all. However, we consider this approach, or some variation of it, to be straightforward, efficient, and flexible enough to dramatically increase testing and detection of primary aldosteronism, and expand opportunities to initiate targeted treatment.
Figure 1.
Simplified diagnostic algorithm for primary aldosteronism. *There is no uniform definition for suppressed renin. A suppressed, or very low, plasma renin activity or renin concentration is necessary to confirm renin-independent aldosteronism that characterizes primary aldosteronism. A suppressed renin is defined as a plasma renin activity of less than 0.6 ng/mL/h, although a more liberal definition of less than 1.0 ng/mL/h can be used. Alternatively, a renin concentration of less than 5 mU/L is considered suppressed, although a more liberal definition of less than 8.2 mU/L can be used. #The most recommended and widely used dynamic confirmatory tests include the oral sodium suppression test, the seated intravenous saline suppression test, the fludrocortisone suppression test, and the captopril challenge test. &Therapeutic doses of mineralocorticoid receptor antagonists and/or epithelial sodium channel inhibitors can induce an increase in renin in patients with primary aldosteronism. Less commonly, high doses of loop diuretics may induce this increase in renin as well. Rarely, angiotensin-converting enzyme inhibitors and angiotensin receptor blockers can similarly increase renin in patients with primary aldosteronism. When the pretest probability for primary aldosteronism is reasonably high, a medication washout for 4 weeks, followed by repeat renin and aldosterone measurement, can be conducted to assess the true renin phenotype. AVS, adrenal venous sampling; MR, mineralocorticoid receptor.
When to screen
It is essential that populations shown to be most enriched for primary aldosteronism be screened. These include patients with resistant hypertension (requiring 4 or more medications to control blood pressure or uncontrolled blood pressure despite 3 medications), severe hypertension (blood pressure > 150/100 mm Hg), hypertension with an incidental adrenal mass, hypertension with atrial fibrillation (3, 81-84), and those with any hypokalemia irrespective of blood pressure (2, 71). Beyond these critical populations, strong consideration should be given to screening all patients with stage II hypertension (≥140/90 mmHg), among whom the prevalence of primary aldosteronism syndrome is substantial (Fig. 1) (41, 50, 51, 54).
How to screen
The ARR is a reasonable and well-accepted screening metric. If used, we recommend using an ARR threshold of 20 to maximize true case detection. However, our preference is to use the individual components of the ARR; that is, to evaluate the renin and aldosterone values independently to identify phenotypes suggestive of renin-independent aldosterone production. We do not advise medication withdrawal or specific times for testing to minimize burden on patients and clinicians, and to maximize the opportunity to test before it is lost. At the time of the patient encounter, testing should be performed without delay, with the patient in the seated position. If renin is suppressed, the influence of medications is not sufficient to interfere with diagnostic decision making, and the aldosterone level can be interpreted. In other words, if renin is suppressed, a medication washout is not needed to interpret the results of the screening test, subsequent confirmatory tests, and even adrenal venous sampling results (85, 86). If renin is not suppressed, and the pretest probability for primary aldosteronism is high, a medication washout (minimum of 4 weeks) should be considered for mineralocorticoid receptor antagonists and epithelial sodium channel inhibitors with testing repeated following the washout. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers do not significantly raise renin in true primary aldosteronism (in fact, this is the basis of the captopril challenge test [2]), although occasionally they may lower aldosterone concentrations; a washout of these medication classes, and others, is rarely necessary. The use of gliflozins (inhibitors of sodium-glucose transport protein 2) can contract intravascular volume and increase renin in normal physiology (87, 88); however, whether this effect is observable in patients with primary aldosteronism, and could contribute to potential false-negative testing, has not been reported.
Simplified confirmatory pathway
Suppressed renin is a key biomarker for a population that is enriched for primary aldosteronism; that is, at high risk for future cardiovascular events, and most likely to respond to mineralocorticoid receptor antagonists (4, 16, 19, 20, 25-29, 41). Although there is no single definition for what constitutes a suppressed renin, we think the ideal representation is plasma renin activity of less than 0.6 ng/mL/h or plasma renin concentration of less than 5 mU/L; however, more liberal thresholds of less than 1.0 ng/mL/h and less than 8.2 mU/L can be used (2). In this setting, a plasma aldosterone concentration of greater than 15 ng/dL should be considered an “overtly positive screen.” When the pretest probability for primary aldosteronism is high (eg, in a patient with resistant hypertension and/or hypokalemia), these results should be interpreted as explicitly confirmatory for the diagnosis (no further dynamic testing is needed). The presence of hypokalemia with these parameters clinches the diagnosis and no further diagnostic testing is required (Fig. 1). We do recommend repeating the aldosterone and renin measurements on a separate day, when possible, to ensure consistency and to minimize the small risk of false-positive testing.
Simplified exclusionary pathway
The simplest method to exclude the possibility of primary aldosteronism is detection of nonsuppressed renin, which is incompatible with the requisite pathophysiology. Exceptions to this rule include factors that can induce substantial volume contraction in a patient with true primary aldosteronism, such as extreme dietary sodium restriction (15), ongoing and aggressive diuresis (usually with loop diuretics), and the use of therapeutic doses of mineralocorticoid receptor antagonists and/or epithelial sodium channel inhibitors. When the pretest probability for primary aldosteronism is reasonably high, a medication washout can be conducted to assess the true renin phenotype (Fig. 1).
The other pathway to excluding primary aldosteronism is very low aldosterone concentration in the setting of suppressed renin (Fig. 1). We advise using a threshold of less than 5 ng/dL to be conservative and minimize the risk of false negatives given the variability of circulating aldosterone levels and data suggesting that even relatively low circulating aldosterone levels in the context of suppressed renin may be a manifestation of primary aldosteronism syndrome, especially when measured using modern liquid chromatography–tandem mass spectrometry assays (13, 30, 32, 33, 41, 79, 80, 89). We also suggest that the aldosterone and renin be repeated on a separate day to increase confidence that primary aldosteronism can be confidently excluded.
Enhanced diagnostic pathway
When renin is suppressed but aldosterone levels are neither overtly high nor very low (5-15 ng/dL), we suggest considering these values as a “positive screen” for primary aldosteronism (Fig. 1). These patients are likely to either have overt primary aldosteronism or a clinically relevant phenotype of renin-independent aldosterone production (ie, mild primary aldosteronism) that is likely to respond to mineralocorticoid receptor antagonists with a substantial reduction in blood pressure. If access to specialists and advanced health care resources is not available, at a minimum, empiric mineralocorticoid receptor antagonist therapy should be recommended for these patients unless they have an estimated glomerular filtration rate of less than 30 mL/min/1.73 m2 and/or hyperkalemia. When resources permit, we advise referral to specialists who can conduct one of several recommended formal aldosterone suppression tests to confirm or exclude overt primary aldosteronism (Fig. 1).
Confirmed primary aldosteronism
Once primary aldosteronism is confirmed, it is appropriate to refer to an experienced specialist to guide localization tests (cross-sectional imaging and adrenal venous sampling) and candidacy for curative unilateral adrenalectomy or medical therapy with mineralocorticoid receptor antagonists and dietary sodium restriction. When access to specialists or adrenal venous sampling is limited or unavailable, empiric mineralocorticoid receptor antagonist therapy (and advice to restrict dietary sodium intake) should be recommended to control and ideally normalize blood pressure, maintain circulating potassium in the normal range, and normalize renin (4, 90).
Exclusion of primary aldosteronism
Even after excluding primary aldosteronism, we strongly suggest considering the addition of mineralocorticoid receptor antagonist therapy for patients with suppressed renin, particularly if they have uncontrolled blood pressure or resistant hypertension, as for nearly 50 years this treatment approach has been strikingly effective when renin is suppressed (26-28). In this regard, for all patients with suppressed renin, our clinical approach concludes that mineralocorticoid receptor antagonists should be prescribed as the path of least resistance; when resources and clinical context permit, more detailed evaluations could indicate opportunities for curative adrenalectomy.
Case Presentation Conclusion: Based on the clinical history of chronic hypertension and hypokalemia, and the biochemical testing suggesting a “positive screen,” the patient was evaluated using the “Enhanced Diagnostic Pathway” (Fig. 1). An oral sodium suppression test was conducted and revealed a 24-hour aldosterone excretion rate of 15 mcg with a concurrent 24-hour urinary sodium excretion of 276 mEq (an aldosterone excretion rate of > 12 mcg/24h on a high sodium balance is the conventional threshold to confirm primary aldosteronism). Given the failure to suppress aldosterone production in the face of volume and sodium loading, the patient was diagnosed with primary aldosteronism. Abdominal computed tomography revealed bilateral adrenal thickening without a clear adenoma. He was advised to undergo adrenal venous sampling to evaluate his candidacy for a potential curative adrenalectomy. However, the patient declined adrenal venous sampling on the basis of multiple reasons, including: a fear of procedures and surgery, a general distrust of the medical system, and financial concerns given his limited health insurance coverage. He emphasized his preference to try medical therapy first. Therefore, he was counseled to restrict dietary sodium intake and prescribed spironolactone. One year later, while on spironolactone 75mg daily, nifedipine 60mg daily, and carvedilol 25mg daily, his blood pressure was 122-130/66-80 mmHg, his serum potassium was normal without supplemental potassium intake, and his plasma renin activity had increased to 1.3 ng/mL/h. He continues to be monitored for the safety and efficacy of medical treatment, and discussions regarding the potential value of adrenal venous sampling are on-going.
Author Commentary: This patient’s vignette highlights a quintessential example of how case detection for primary aldosteronism is woefully infrequent (in this instance for nearly two decades), and when attempts at screening do occur, how they can be erroneously interpreted to exclude a potential diagnosis due to the reliance on arbitrary diagnostic thresholds. The conclusion of this case highlights how a pathophysiology-based approach and a simplified diagnostic algorithm can increase the likelihood of screening eligible patients, increase the sensitivity of screening results, and prompt more frequent diagnoses and/or empiric treatment with effective targeted medications.
Conclusions
Once considered a rare and neoplastic form of adrenal hypertension, our understanding of primary aldosteronism has evolved substantially. The current state-of-the-science characterizes primary aldosteronism as a syndrome of renin-independent aldosterone production that is highly prevalent and contributes to the pathogenesis of hypertension and cardiovascular disease. The antiquated dogma that deemed primary aldosteronism to be a “secondary” cause of hypertension should be retired; in its stead, primary aldosteronism should be regarded as common cause of primary hypertension, a major contributor to cardiovascular disease, and a condition that can manifest across a severity spectrum ranging from mild to severe phenotypes. In contrast to the scientific advances that have led to better characterization of primary aldosteronism syndrome, the public health efforts needed to increase awareness and education have lagged behind. Herein, we propose a simplified pathophysiology-based diagnostic algorithm that we hope can be used by generalists, as well as specialists, to increase the rate of screening and the ease with which diagnostic testing can be performed and interpreted, and to facilitate the implementation of targeted treatments known to mitigate the downstream adverse consequences of primary aldosteronism.
Acknowledgments
Financial Support: This work was supported by P01-HL-074940 (to R.M.C.), R01-HL-128189 (to R.M.C.), R01DK115392 (to A.V.), R01 HL153004(AV), and R01DK16618 (to A.V.).
Glossary
Abbreviation
- ARR
aldosterone-to-renin ratio
Additional Information
Disclosure Summary: A.V. reports consulting, unrelated to the contents of this work, for Corcept Therapeutics, HRA Pharma, CatalysPacific. R.M.C. has nothing to disclose.
Data Availability
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Vaidya A, Mulatero P, Baudrand R, Adler GK. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr Rev. 2018;39(6):1057-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889-1916. [DOI] [PubMed] [Google Scholar]
- 3. Rossi GP. Primary aldosteronism: JACC state-of-the-art review. J Am Coll Cardiol. 2019;74(22):2799-2811. [DOI] [PubMed] [Google Scholar]
- 4. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 2018;6(1):51-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu VC, Wang SM, Chang CH, et al. Long term outcome of aldosteronism after target treatments. Sci Rep. 2016;6:32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu VC, Chueh SJ, Chen L, et al. ; TAIPAI Study Group . Risk of new-onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35(8):1698-1708. [DOI] [PubMed] [Google Scholar]
- 7. Catena C, Colussi G, Lapenna R, et al. Long-term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50(5):911-918. [DOI] [PubMed] [Google Scholar]
- 8. Catena C, Colussi G, Nadalini E, et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168(1):80-85. [DOI] [PubMed] [Google Scholar]
- 9. Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA. 2006;295(22):2638-2645. [DOI] [PubMed] [Google Scholar]
- 10. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension. 2018;72(3):658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reincke M, Fischer E, Gerum S, et al. ; German Conn’s Registry–Else Kröner-Fresenius-Hyperaldosteronism Registry . Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension. 2012;60(3):618-624. [DOI] [PubMed] [Google Scholar]
- 12. Reincke M, Rump LC, Quinkler M, et al. ; Participants of German Conn’s Registry . Risk factors associated with a low glomerular filtration rate in primary aldosteronism. J Clin Endocrinol Metab. 2009;94(3):869-875. [DOI] [PubMed] [Google Scholar]
- 13. Stowasser M, Gordon RD. Primary aldosteronism: changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev. 2016;96(4):1327-1384. [DOI] [PubMed] [Google Scholar]
- 14. Conn JW, Cohen EL, Rovner DR. Landmark article Oct 19, 1964: suppression of plasma renin activity in primary aldosteronism. Distinguishing primary from secondary aldosteronism in hypertensive disease. By Jerome W. Conn, Edwin L. Cohen and David R. Rovner. JAMA. 1985;253(4):558-566. [DOI] [PubMed] [Google Scholar]
- 15. Baudrand R, Guarda FJ, Torrey J, Williams G, Vaidya A. Dietary sodium restriction increases the risk of misinterpreting mild cases of primary aldosteronism. J Clin Endocrinol Metab. 2016;101(11):3989-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hundemer G, Brown JM, Baudrand R, Curhan G, Williams GH, Vaidya A. Renin phenotypes to characterize vascular disease, autonomous aldosteronism, and mineralocorticoid receptor activity. J Clin Endocrinol Metab. 2017;102(6):1835-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tezuka Y, Turcu AF. Mineralocorticoid receptor antagonists decrease the rates of positive screening for primary aldosteronism. [Published online ahead of print July 13, 2020.] Endocr Pract. doi: 10.4158/EP-2020-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Conn JW, Cohen EL, Rovner DR, Nesbit RM. Normokalemic primary aldosteronism. a detectable cause of curable “essential” hypertension. JAMA. 1965;193:200-206. [DOI] [PubMed] [Google Scholar]
- 19. Adlin EV, Marks AD, Channick BJ. Spironolactone and hydrochlorothiazide in essential hypertension. Blood pressure response and plasma renin activity. Arch Intern Med. 1972;130(6):855-858. [PubMed] [Google Scholar]
- 20. Adlin EV, Marks AD, Channick BJ. The salivary sodium/potassium ratio in hypertension: relation to race and plasma renin activity. Clin Exp Hypertens A. 1982;4(9-10):1869-1880. [DOI] [PubMed] [Google Scholar]
- 21. Jose A, Crout JR, Kaplan NM. Suppressed plasma renin activity in essential hypertension. Roles of plasma volume, blood pressure, and sympathetic nervous system. Ann Intern Med. 1970;72(1):9-16. [DOI] [PubMed] [Google Scholar]
- 22. Laragh JH, Letcher RL, Pickering TG. Renin profiling for diagnosis and treatment of hypertension. JAMA. 1979;241(2):151-156. [PubMed] [Google Scholar]
- 23. Spence JD. Physiologic tailoring of therapy for resistant hypertension: 20 years’ experience with stimulated renin profiling. Am J Hypertens. 1999;12(11 Pt 1):1077-1083. [PubMed] [Google Scholar]
- 24. Oliver WJ, Cohen EL, Neel JV. Blood pressure, sodium intake, and sodium related hormones in the Yanomamo Indians, a “no-salt” culture. Circulation. 1975;52(1):146-151. [DOI] [PubMed] [Google Scholar]
- 25. Adlin EV. Letter: plasma-renin and blood-pressure. Lancet. 1975;1(7908):699. [DOI] [PubMed] [Google Scholar]
- 26. Carey RM, Douglas JG, Schweikert JR, Liddle GW. The syndrome of essential hypertension and suppressed plasma renin activity. Normalization of blood pressure with spironolactone. Arch Intern Med. 1972;130(6):849-854. [PubMed] [Google Scholar]
- 27. Williams B, MacDonald TM, Morant S, et al. ; British Hypertension Society’s PATHWAY Studies Group . Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386(10008):2059-2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams B, MacDonald TM, Morant SV, et al. ; British Hypertension Society programme of Prevention And Treatment of Hypertension With Algorithm based Therapy (PATHWAY) Study Group . Endocrine and haemodynamic changes in resistant hypertension, and blood pressure responses to spironolactone or amiloride: the PATHWAY-2 mechanisms substudies. Lancet Diabetes Endocrinol. 2018;6(6):464-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adlin EV. Subclinical primary aldosteronism. Ann Intern Med. 2017;167(9):673-674. [DOI] [PubMed] [Google Scholar]
- 30. Vasan RS, Evans JC, Larson MG, et al. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351(1):33-41. [DOI] [PubMed] [Google Scholar]
- 31. Perschel FH, Schemer R, Seiler L, et al. Rapid screening test for primary hyperaldosteronism: ratio of plasma aldosterone to renin concentration determined by fully automated chemiluminescence immunoassays. Clin Chem. 2004;50(9):1650-1655. [DOI] [PubMed] [Google Scholar]
- 32. Newton-Cheh C, Guo CY, Gona P, et al. Clinical and genetic correlates of aldosterone-to-renin ratio and relations to blood pressure in a community sample. Hypertension. 2007;49(4):846-856. [DOI] [PubMed] [Google Scholar]
- 33. Brown JM, Robinson-Cohen C, Luque-Fernandez MA, et al. The spectrum of subclinical primary aldosteronism and incident hypertension: a cohort study. Ann Intern Med. 2017;167(9):630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dreier R, Abdolalizadeh B, Asferg CL, et al. Effect of increased potassium intake on the renin-angiotensin-aldosterone system and subcutaneous resistance arteries: a randomized crossover study. [Published online ahead of print June 29, 2020.] Nephrol Dial Transplant. Doi: 10.1093/ndt/gfaa114. [DOI] [PubMed] [Google Scholar]
- 35. Oria M, Harrison M, Stallings VA, eds. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: National Academies Press; 2019. [PubMed] [Google Scholar]
- 36. Markou A, Pappa T, Kaltsas G, et al. Evidence of primary aldosteronism in a predominantly female cohort of normotensive individuals: a very high odds ratio for progression into arterial hypertension. J Clin Endocrinol Metab. 2013;98(4):1409-1416. [DOI] [PubMed] [Google Scholar]
- 37. Piaditis G, Markou A, Papanastasiou L, Androulakis II, Kaltsas G. Progress in aldosteronism: a review of the prevalence of primary aldosteronism in pre-hypertension and hypertension. Eur J Endocrinol. 2015;172(5):R191-R203. [DOI] [PubMed] [Google Scholar]
- 38. Baudrand R, Guarda FJ, Fardella C, et al. Continuum of renin-independent aldosteronism in normotension. Hypertension. 2017;69(5):950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Carey RM, Ayers CR, Vaughan ED Jr, Peach MJ, Herf SM. Activity of [des-aspartyl1]-angiotensin II in primary aldosteronism. J Clin Invest. 1979;63(4):718-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wisgerhof M, Brown RD. Increased adrenal sensitivity to angiotensin II in low-renin essential hypertension. J Clin Invest. 1978;61(6):1456-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brown JM, Siddiqui M, Calhoun DA, et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann Intern Med. 2020;173(1):10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Omata K, Tomlins SA, Rainey WE. Aldosterone-producing cell clusters in normal and pathological states. Horm Metab Res. 2017;49(12):951-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Omata K, Anand SK, Hovelson DH, et al. Aldosterone-producing cell clusters frequently harbor somatic mutations and accumulate with age in normal adrenals. J Endocr Soc. 2017;1(7):787-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nishimoto K, Tomlins SA, Kuick R, et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A. 2015;112(33):E4591-E4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nanba K, Vaidya A, Williams GH, Zheng I, Else T, Rainey WE. Age-related autonomous aldosteronism. Circulation. 2017;136(4):347-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nanba K, Vaidya A, Rainey WE. Aging and adrenal aldosterone production. Hypertension. 2018;71(2):218-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fernandes-Rosa FL, Boulkroun S, Zennaro MC. Genetic and genomic mechanisms of primary aldosteronism. Trends Mol Med. 2020;26(9):819-832. [DOI] [PubMed] [Google Scholar]
- 48. Hannemann A, Bidlingmaier M, Friedrich N, et al. Screening for primary aldosteronism in hypertensive subjects: results from two German epidemiological studies. Eur J Endocrinol. 2012;167(1):7-15. [DOI] [PubMed] [Google Scholar]
- 49. Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: the multi-ethnic study of atherosclerosis. Hypertension. 2020;76(1):113-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811-1820. [DOI] [PubMed] [Google Scholar]
- 51. Mosso L, Carvajal C, González A, et al. Primary aldosteronism and hypertensive disease. Hypertension. 2003;42(2):161-165. [DOI] [PubMed] [Google Scholar]
- 52. Williams JS, Williams GH, Raji A, et al. Prevalence of primary hyperaldosteronism in mild to moderate hypertension without hypokalaemia. J Hum Hypertens. 2006;20(2):129-136. [DOI] [PubMed] [Google Scholar]
- 53. Omura M, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Prospective study on the prevalence of secondary hypertension among hypertensive patients visiting a general outpatient clinic in Japan. Hypertens Res. 2004;27(3):193-202. [DOI] [PubMed] [Google Scholar]
- 54. Rossi GP, Bernini G, Caliumi C, et al. ; PAPY Study Investigators . A prospective study of the prevalence of primary aldosteronism in 1125 hypertensive patients. J Am Coll Cardiol. 2006;48(11):2293-2300. [DOI] [PubMed] [Google Scholar]
- 55. Rossi E, Regolisti G, Negro A, Sani C, Davoli S, Perazzoli F. High prevalence of primary aldosteronism using postcaptopril plasma aldosterone to renin ratio as a screening test among Italian hypertensives. Am J Hypertens. 2002;15(10 Pt 1):896-902. [DOI] [PubMed] [Google Scholar]
- 56. Strauch B, Zelinka T, Hampf M, Bernhardt R, Widimsky J Jr. Prevalence of primary hyperaldosteronism in moderate to severe hypertension in the Central Europe region. J Hum Hypertens. 2003;17(5):349-352. [DOI] [PubMed] [Google Scholar]
- 57. Xu Z, Yang J, Hu J, et al. ; Chongqing Primary Aldosteronism Study (CONPASS) Group . Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. 2020;75(16):1913-1922. [DOI] [PubMed] [Google Scholar]
- 58. Hu Y, Zhang J, Liu W, Su X. Determining the prevalence of primary aldosteronism in patients with new-onset type 2 diabetes and hypertension. J Clin Endocrinol Metab. 2020;105(4):dgz293. [DOI] [PubMed] [Google Scholar]
- 59. Vieweg WV, Veldhuis JD, Carey RM. Temporal pattern of renin and aldosterone secretion in men: effects of sodium balance. Am J Physiol. 1992;262(5 Pt 2):F871-F877. [DOI] [PubMed] [Google Scholar]
- 60. Siragy HM, Vieweg WV, Pincus S, Veldhuis JD. Increased disorderliness and amplified basal and pulsatile aldosterone secretion in patients with primary aldosteronism. J Clin Endocrinol Metab. 1995;80(1):28-33. [DOI] [PubMed] [Google Scholar]
- 61. Kline GA, Darras P, Leung AA, So B, Chin A, Holmes DT. Surprisingly low aldosterone levels in peripheral veins following intravenous sedation during adrenal vein sampling: implications for the concept of nonsuppressibility in primary aldosteronism. J Hypertens. 2019;37(3):596-602. [DOI] [PubMed] [Google Scholar]
- 62. Tanabe A, Naruse M, Takagi S, Tsuchiya K, Imaki T, Takano K. Variability in the renin/aldosterone profile under random and standardized sampling conditions in primary aldosteronism. J Clin Endocrinol Metab. 2003;88(6):2489-2494. [DOI] [PubMed] [Google Scholar]
- 63. Tsiavos V, Markou A, Papanastasiou L, et al. A new highly sensitive and specific overnight combined screening and diagnostic test for primary aldosteronism. Eur J Endocrinol. 2016;175(1):21-28. [DOI] [PubMed] [Google Scholar]
- 64. Parasiliti-Caprino M, Lopez C, Prencipe N, et al. Prevalence of primary aldosteronism and association with cardiovascular complications in patients with resistant and refractory hypertension. J Hypertens. 2020;38(9):1841-1848. [DOI] [PubMed] [Google Scholar]
- 65. Funder JW. Primary aldosteronism: at the tipping point. Ann Intern Med. 2020;173(1):65-66. [DOI] [PubMed] [Google Scholar]
- 66. Vaidya A, Underwood PC, Hopkins PN, et al. Abnormal aldosterone physiology and cardiometabolic risk factors. Hypertension. 2013;61(4):886-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bentley-Lewis R, Adler GK, Perlstein T, et al. Body mass index predicts aldosterone production in normotensive adults on a high-salt diet. J Clin Endocrinol Metab. 2007;92(11):4472-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40(6):892-896. [DOI] [PubMed] [Google Scholar]
- 69. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127-e248. [DOI] [PubMed] [Google Scholar]
- 70. Carey RM, Calhoun DA, Bakris GL, et al. ; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council . Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension. 2018;72(5):e53-e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Burrello J, Monticone S, Losano I, et al. Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension. 2020;75(4):1025-1033. [DOI] [PubMed] [Google Scholar]
- 72. Jaffe G, Gray Z, Krishnan G, et al. Screening rates for primary aldosteronism in resistant hypertension: a cohort study. Hypertension. 2020;75(3):650-659. [DOI] [PubMed] [Google Scholar]
- 73. Ruhle BC, White MG, Alsafran S, Kaplan EL, Angelos P, Grogan RH. Keeping primary aldosteronism in mind: deficiencies in screening at-risk hypertensives. Surgery. 2019;165(1):221-227. [DOI] [PubMed] [Google Scholar]
- 74. Reincke M, Beuschlein F, Williams TA. Progress in primary aldosteronism 2019: new players on the block? Horm Metab Res. 2020;52(6):345-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mulatero P, Monticone S, Burrello J, Veglio F, Williams TA, Funder J. Guidelines for primary aldosteronism: uptake by primary care physicians in Europe. J Hypertens. 2016;34(11):2253-2257. [DOI] [PubMed] [Google Scholar]
- 76. Pecori A, Buffolo F, Pieroni J, et al. Primary aldosteronism and obstructive sleep apnea: casual association or pathophysiological link? Horm Metab Res. 2020;52(6):366-372. [DOI] [PubMed] [Google Scholar]
- 77. Buffolo F, Li Q, Monticone S, et al. Primary aldosteronism and obstructive sleep apnea: a cross-sectional multi-ethnic study. Hypertension. 2019;74(6):1532-1540. [DOI] [PubMed] [Google Scholar]
- 78. Markou A, Sertedaki A, Kaltsas G, et al. Stress-induced aldosterone hyper-secretion in a substantial subset of patients with essential hypertension. J Clin Endocrinol Metab. 2015;100(8):2857-2864. [DOI] [PubMed] [Google Scholar]
- 79. Rossi GP, Gioco F, Fassina A, Gomez-Sanchez CE. Normoaldosteronemic aldosterone-producing adenoma: immunochemical characterization and diagnostic implications. J Hypertens. 2015;33(12):2546-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Stowasser M, Gordon RD. Primary aldosteronism—careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004;217(1-2):33-39. [DOI] [PubMed] [Google Scholar]
- 81. Rossi GP, Maiolino G, Flego A, et al. ; PAPY Study Investigators . Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension. 2018;71(4):585-591. [DOI] [PubMed] [Google Scholar]
- 82. Rossi GP, Seccia TM, Gallina V, et al. Prospective appraisal of the prevalence of primary aldosteronism in hypertensive patients presenting with atrial flutter or fibrillation (PAPPHY Study): rationale and study design. J Hum Hypertens. 2013;27(3):158-163. [DOI] [PubMed] [Google Scholar]
- 83. Seccia TM, Caroccia B, Adler GK, Maiolino G, Cesari M, Rossi GP. Arterial hypertension, atrial fibrillation, and hyperaldosteronism: the triple trouble. Hypertension. 2017;69(4):545-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 2018;3(8): 768-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rossi GP, Ceolotto G, Rossitto G, Maiolino G, Cesari M, Seccia TM. Effects of mineralocorticoid and AT1 receptor antagonism on the aldosterone-renin ratio in primary aldosteronism—the EMIRA study. J Clin Endocrinol Metab. 2020;105(6):2060-2067. [DOI] [PubMed] [Google Scholar]
- 86. Nanba AT, Wannachalee T, Shields JJ, et al. Adrenal vein sampling lateralization despite mineralocorticoid receptor antagonists exposure in primary aldosteronism. J Clin Endocrinol Metab. 2019;104(2):487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schork A, Saynisch J, Vosseler A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Griffin TP, Islam MN, Blake L, Bell M, Griffin MD, O’Shea PM. Effect of sodium glucose co-transporter-2 inhibition on the aldosterone/renin ratio in type 2 diabetes mellitus. Horm Metab Res. 2019;51(2):91-99. [DOI] [PubMed] [Google Scholar]
- 89. Guo Z, Poglitsch M, McWhinney BC, et al. Aldosterone LC-MS/MS assay-specific threshold values in screening and confirmatory testing for primary aldosteronism. J Clin Endocrinol Metab. 2018;103(11):3965-3973. [DOI] [PubMed] [Google Scholar]
- 90. Young WF Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J Intern Med. 2019;285(2):126-148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.