Abstract
Background
Radiation-induced fibrosis is a long-term adverse effect of external beam radiation therapy for cancer treatment that can cause pain, loss of function, and decreased quality of life. Transforming growth factor beta (TGF-β) is believed to be critical to the development of radiation-induced fibrosis, and TGF-β inhibition decreases the development of fibrosis. However, no treatment exists to prevent radiation-induced fibrosis. Therefore, we aimed to mitigate the development of radiation-induced fibrosis in a mouse model by inhibiting TGF-β.
Question/purposes
Does TGF-β inhibition decrease the development of muscle fibrosis induced by external beam radiation in a mouse model?
Methods
Twenty-eight 12-week-old male C57BL/6 mice were assigned randomly to three groups: irradiated mice treated with TGF-βi, irradiated mice treated with placebo, and control mice that received neither irradiation nor treatment. The irradiated mice received one 50-Gy fraction of radiation to the right hindlimb before treatment initiation. Mice treated with TGF-c (n = 10) received daily intraperitoneal injections of a small-molecule inhibitor of TGF-β (1 mg/kg) in a dimethyl sulfoxide vehicle for 8 weeks (seven survived to histologic analysis). Mice treated with placebo (n = 10) received daily intraperitoneal injections of only a dimethyl sulfoxide vehicle for 8 weeks (10 survived to histologic analysis). Control mice (n = 8) received neither radiation nor TGF-β treatment. Control mice were euthanized at 3 months because they were not expected to exhibit any changes related to treatment. Mice in the two treatment groups were euthanized 9 months after radiation, and the quadriceps of each thigh was sampled. Masson’s trichome stain was used to assess muscle fibrosis. Slides were viewed at 10 × magnification using bright-field microscopy, and in a blinded fashion, five representative images per mouse were used to quantify fibrosis. The mean ± SD fibrosis pixel densities in the TGF-βi and radiation-only groups were compared using Mann-Whitney U tests. The ratio of fibrosis to muscle was calculated using the mean fibrosis per slide in the TGF-βi group to standardize measurements. Alpha was set at 0.05.
Results
The mean (± SD) percentage of fibrosis per slide was greater in the radiation-only group (1.2% ± 0.42%) than in the TGF-βi group (0.14% ± 0.09%) (odds ratio 0.12 [95% CI 0.07 to 0.20]; p < 0.001). Among control mice, mean fibrosis was 0.05% ± 0.02% per slide. Mice in the radiation-only group had 9.1 times the density of fibrosis as did mice in the TGF-βi group.
Conclusion
Our study provides preliminary evidence that the fibrosis associated with radiation therapy to a quadriceps muscle can be reduced by treatment with a TGF-β inhibitor in a mouse model.
Clinical Relevance
If these observations are substantiated by further investigation into the role of TGF-β inhibition on the development of radiation-induced fibrosis in larger animal models and humans, our results may aid in the development of novel therapies to mitigate this complication of radiation treatment.
Introduction
As part of their treatment plans, patients with cancer diagnoses often receive external beam radiation in addition to chemotherapy. As many as half of the approximately 14 million individuals who have been treated for cancer in the United States likely received radiation as part of their treatment [33]. Radiation therapy damages tumor cells, and it may have deleterious effects on healthy tissue in the radiation field. Adverse effects of radiation therapy include dermatitis, edema, inflammation, necrosis [19, 30], and radiation-induced fibrosis. Radiation-induced fibrosis typically occurs 4 to 12 months after radiation therapy and worsens with time. The manifestations of radiation-induced fibrosis include skin thickening, lymphedema, pain, muscle thickening, muscle atrophy, and limited joint mobility, which affect patients’ quality of life [15, 16, 27].
Radiation-induced fibrosis is characterized by fibroblast proliferation, myofibroblast differentiation, and excess synthesis of collagen, proteoglycans, and the extracellular matrix [42, 43]. Radiation induces direct DNA damage because of the formulation of reactive oxygen species and oxidative stress [9, 34]. These injured cells release chemoattractant molecules that stimulate inflammation through the release of inflammatory cytokines and chemokines [13, 34, 40]. The first inflammatory cells that arrive to the injury site are neutrophils, which release cytokines such as tumor necrosis factor alpha and IL-1 and IL-6 [1, 2, 42]. Monocytes and lymphocytes then interact with damaged cells and stimulate differentiation of monocytes into two subsets of macrophages: M1 and M2 [18, 32, 36]. M2 macrophages secrete platelet-derived growth factor, which stimulates the migration of fibroblasts to the site of injured tissue, neoangiogenesis, and the secretion of transforming growth factor beta (TGF-ß) from many cell types, including macrophages [22, 23]. TGF-ß is responsible for the production and differentiation of fibroblasts into myofibroblasts [47] and is believed to be the main effector in radiation-induced fibrosis [23]. In the TGF-ß family, TGF-ß1 is predominantly upregulated in radiation-induced fibrosis [10, 11, 20].
Over-expression of TGF-ß results in over-activation of myofibroblasts, causing excess deposition of collagen, fibronectin, and proteoglycans [8]. This upregulation is believed to cause stiffness and thickening of irradiated tissue [21, 26]. Deposition of collagen downregulates progressive vascularization of tissue, causing loss of cell function, tissue atrophy, and tissue necrosis [12, 13]. Several studies have shown that radiation-induced activation of TGF-ß1 is rapid and more strongly activated on Day 14 after irradiation than on Day 1, indicating an early tissue response [3-5, 14, 35]. Furthermore, repeated irradiation (as is typical in radiation fractionation in cancer treatment) causes continued autoinduction of local TGF-ß1 production, creating a chronic cycle of TGF-ß1 over-production [6].
Although TGF-ß inhibitors (TGF-ßi) have been used successfully as antifibrotic agents capable of neutralizing the activity of TGF-ß in the joints, arterial wall, brain, lung, kidney, and skin [7, 17, 25, 31, 37, 41], no studies to our knowledge have shown the role of TGF-ß inhibition in the mitigation of radiation-induced myofibrosis. Given the successful use of TGF-ßi in mitigating fibrotic responses previously in many animal models, as well as in humans [7, 17, 25, 31, 37, 41], and because derivations of TGF-ßi are currently produced for human use for reasons other than radiation-induced fibrosis, we chose to investigate whether TGF-ßi would successfully mitigate the fibrotic response seen in radiation-induced fibrosis and potentially be a therapeutic target in the treatment of radiation-induced fibrosis. Studies have used mouse models to address radiation-induced fibrosis because of the similar, yet expedited, fibrotic response in mice compared with that in humans [39, 45, 46]. We therefore investigated whether TGF-β inhibition decreased the development of muscle fibrosis induced by external beam radiation in a mouse model.
Specifically, we asked: Does TGF-β inhibition decrease the development of muscle fibrosis induced by external beam radiation in a mouse model?
Materials and Methods
All animals were maintained in our institution’s animal facility under a 12-hour light/dark cycle with ad libitum access to food and water. The mice were housed according to experimental group, with four or five mice per cage. The experimental protocols were reviewed and approved by our institutional animal care and use committee.
We purchased 12-week-old male C57BL/6 (wild-type) mice from the Jackson Laboratory (Bar Harbor, ME, USA). We chose C57BL/6 mice because of familiarity with dosing of TGF-βi from the work of Zhen et al. [48]. An ascending radiation dose trial was first conducted to determine at what dosage a consistent level of myofibrosis could be achieved. Mice received irradiation to the right hindlimb at an ascending dosage on a small-animal radiation research platform, developed by the Johns Hopkins Department of Radiation Oncology and Molecular Radiation Sciences. The small-animal radiation research platform was equipped with on-board cone-beam CT for accurate positioning of the mice and localization of target volumes. We used doses of 15 Gy, 20 Gy, 30 Gy, 40 Gy, 50 Gy, and 60 Gy. Two mice per radiation dose were tested and subsequently euthanized at 12 weeks. Histologic assessment showed that 50 Gy dosing was adequate to induce fibrosis and allow for quantification.
Next, 20 12-week-old mice received 50 Gy of external beam radiation to the right hindlimb in one dose using the small-animal radiation research platform. They were then randomized into two groups. The TGF-βi group (n = 10) received daily intraperitoneal injections of 1 mg/kg of a TGF-β inhibitor (SB-505124 hydrochloride hydrate, Millipore Sigma, St. Louis, MO, USA) in a dimethyl sulfoxide and phosphate-buffered saline solution vehicle (per the dosing protocol proposed by Zhen et al. [48]) beginning on the day of irradiation. Daily injections were delivered for 8 weeks under our laboratory’s fume hood. The radiation-only group (n = 10) received a placebo consisting of a daily injection of a dimethyl sulfoxide and phosphate-buffered saline (vehicle only without TGF-ßi) for 8 weeks. Injections were given daily between 10:00 am and 12:00 pm using light isoflurane sedation administered in a gas chamber before injection. A separate group of eight mice that did not receive radiation or TGF-ßi was used as a control group (Fig. 1).
Fig. 1.
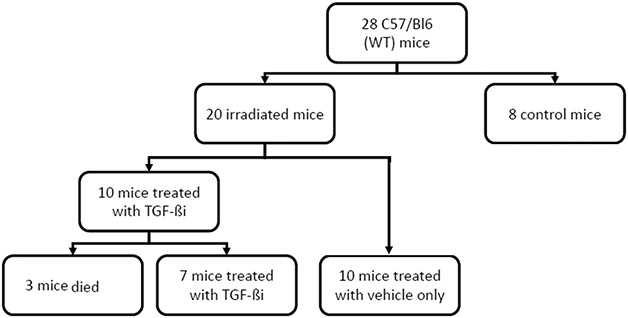
This flow chart shows mouse allocation to each of the groups. Twenty-eight mice were assigned to three groups: a control group, a TGF-ßi, group (irradiated and treated with intraperitoneal injections of a small-molecule inhibitor of TGF-β in a dimethyl sulfoxide vehicle), and a radiation-only group (irradiated and injected with vehicle only); WT = wild type.
Of the 10 mice in the TGF-ßi group, three died during anesthesia before the injection and thus were excluded from further analysis. The remaining seven mice completed the 8-week course of TGF-ßi injections and were subsequently euthanized 9 months after radiation. All 10 mice in the radiation-only group survived until euthanasia was administered at 9 months after radiation. The control group of eight non-irradiated, non-injected mice all survived until they were euthanized at 3 months. We chose to euthanize the control mice at an earlier point because of budgetary constraints. The control mice were not expected to exhibit any changes related to treatment. All mice were euthanized by CO2 inhalation.
Histologic Assessment
The quadriceps of each thigh was sampled. Muscle samples were washed in phosphate-buffered saline and preserved using the fresh-frozen technique. For each mouse, three representative samples in the center of the irradiated field were sectioned to obtain samples at a 6-µm thickness at 60-µm intervals. All slides were stained using Masson’s trichrome stain to assess for muscle fibrosis. Masson’s trichrome staining showed muscle in red and collagen (indicating fibrosis) in blue. Two reviewers (AWJ, JMEA) blinded to the treatment group viewed the slides at 10 × magnification using bright-field microscopy on a Leica DM6 microscope (Leica, Wetzlar, Germany), and five representative images were captured per mouse using Application Suite X, version 3.4.2, software (Leica). We did not do intra- and/or interobserver reliability testing. One reviewer (JMEA) quantified the slides. Fibrosis was quantified using Photoshop CC 2019 software (Adobe, San Jose, CA, USA) using the Magic Wand tool to quantify pixel density in the red spectrum (muscle) and blue spectrum (fibrosis).
Statistical Analysis
Data are presented as the mean ± SD. We compared the mean percentages of fibrosis pixel density among the TGF-βi, radiation-only, and control groups using Mann-Whitney U tests. We calculated the ratio of fibrosis between the TGF-ßi and radiation-only mice using the mean fibrosis in the TGF-ßi group as a referent. Similarly, the ratio of fibrosis between the TGF-ßi and control mice was calculated using the mean fibrosis in the control group as a referent. Significance was set at p < 0.05. All analyses were performed using SPSS, version 15.0, software (IBM Corp, Armonk, NY, USA).
Results
The mean (± SD) percentage of fibrosis per slide was greater in the radiation-only group (1.2% ± 0.42%) (Fig. 2) than in the TGF- βi group (0.14% ± 0.09%) (Fig. 3) (odds ratio 0.12 [95% CI 0.07 to 0.20]; p < 0.001). Mean fibrosis in the control group was 0.05% ± 0.02% (range 0.02 to 0.06) (Fig. 4). Most of the fibrosis was located in the perivascular space, although there was interstitial myofibrosis, as well. Additionally, the TGF-ßi group had a greater rate of fibrosis than the control group did (OR 24 [95% CI 9.9 to 59]; p = 0.009) (Table 1).
Fig. 2.
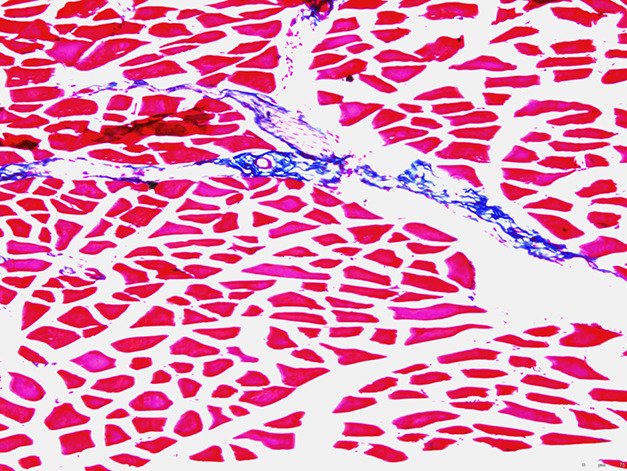
This histologic image (at 10 × magnification) is of the quadriceps muscle of a mouse 9 months after irradiation, treated with the dimethyl sulfoxide vehicle only (no TGF-ß inhibitor). Fresh-frozen tissue was stained with Masson’s trichrome (red indicates muscle; blue indicates fibrosis).
Fig. 3.
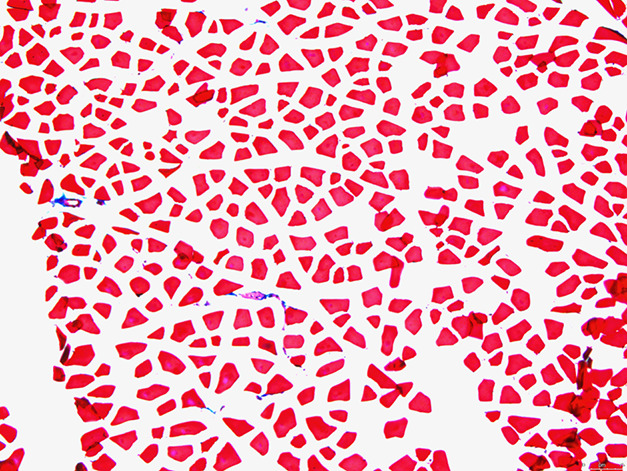
This histologic image (at 10 × magnification) is of the quadriceps muscle of a mouse 9 months after irradiation, treated with the TGF-ß inhibitor (1 mg/kg daily for 8 weeks) after radiation. Fresh-frozen tissue was stained with Masson’s trichrome (red indicates muscle; blue indicates fibrosis).
Fig. 4.
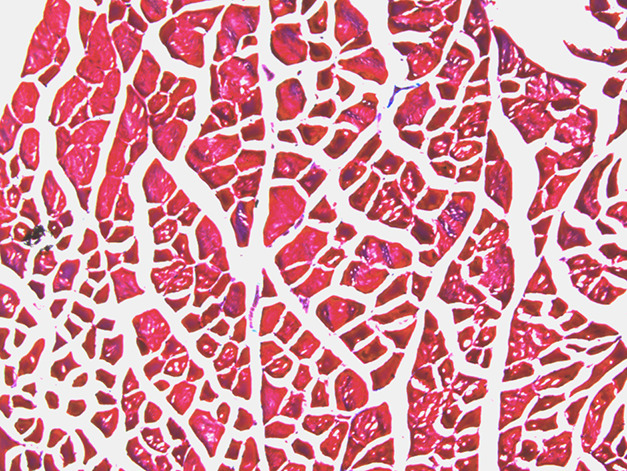
This histologic image (at 10 × magnification) is of the quadriceps muscle of a control mouse, neither irradiated nor treated with a TGF-ß inhibitor. Fresh-frozen tissue was stained with Masson’s trichrome (red indicates muscle; blue indicates fibrosis).
Table 1.
Mean radiation-induced fibrosis and SD per representative high-power field in a C57Bl/6 mouse model
| Group | Mean fibrosis | SD |
| Control | 0.05% | 0.02% |
| Radiation + placebo | 0.14% | 0.09% |
| Radiation + TGF-ßi | 1.20% | 0.42% |
Discussion
Radiation-induced fibrosis may cause thickening of the skin and muscle, muscle atrophy, and limitation of joint mobility, decreasing quality of life in patients receiving life-saving radiation treatment [15, 16, 27]. This fibrosis may, in part, be regulated by TGF-ß overexpression, resulting in stiffness and thickening of the irradiated tissue caused by overexpression of chemokines inducing a fibrotic response [8]. Because of the involvement of TGF-ß in the fibrotic pathway, we aimed to determine whether inhibition of TGF-ß would limit the fibrotic response induced by radiation exposure in a mouse model. In our study, mice treated with radiation demonstrated more fibrosis in the quadriceps muscle than did control mice. In irradiated mice, the administration of TGF-ßi was associated with less fibrosis than use of placebo.
We acknowledge several limitations of our study that may be addressed in future studies. In our model, no tumor was present in the irradiated field. It is unclear how myofibrosis would react to treatment with TGF-ßi in the setting of a tumor or whether the tumor was excised surgically before or after radiation. We also cannot predict how administration of TGF-ßi would affect wound healing if a tumor bed were resected before irradiation and administration of TGF-ßi therapy. Future studies should investigate the role of inhibition TGF-ß on the fibrotic response to surgically altered tissue or tumor bed resection in irradiated tissue. In our study, we used a male mouse model. It is possible that these findings would differ in a female model because of hormonal differences. However, given the pivotal role of expression of TGF-ß in the fibrotic pathway, we believe these findings would likely occur in a female model, as well.
TGF-ß causes systemic immune suppression and inhibits host immunosurveillance, which can promote tumor formation. Inhibition of TGF-ß enhances the CD8+ T-cell and natural killer cell–mediated antitumor immune response [44]; therefore, future studies should investigate whether TGF-ß inhibition has a dual role in the treatment of tumors treated with radiation by promoting the innate and adaptive immune response, as well as mitigating radiation-induced fibrosis. Further investigation should focus on the interactions of TGF-ßi, chemotherapy, and radiation in treatment response of a malignant tumor. Although our sample size was small, a post-hoc power analysis revealed adequate statistical power. Additionally, multiple sections from each quadriceps sample were analyzed to increase the amount of objective data acquired.
There are concerns regarding systemic use of TGF-ßi. Given the ubiquitous nature of the TGF-ß cytokine, systemic inhibition in humans may have deleterious effects if continued for a prolonged period. Previous studies using systemic TGF-ß antisense tumor vaccination resulted in a 10% rate of adverse events, including pain, fatigue, nausea, headache, cough, and weakness [28]. However, several treatment regimens using inhibition of TGF-ß are currently used in humans. The mice in our study received one 50-Gy fraction of radiation at a single point. In human treatment, this radiation is typically fractionated into smaller doses to avoid adverse events; however, we were able to limit the adverse effects of high-dose radiation while using a single-dose radiation fractionation as determined by an ascending dose trial before initiation of our study. It is possible, however, that the high dose of radiation caused functional limitations in our mice that reduced their activity level and altered fibrosis development. Such alteration in activity would be analogous to changes in activity and limb use seen in human patients who develop radiation-induced fibrosis after high-dose radiation treatment.
In our study, we analyzed myofibrosis 9 months after radiation, whereas previous studies have analyzed shorter-term effects on tissue after irradiation, from days to weeks, rather than the long-term consequences that we aimed to study [38, 39, 45]. Our model of myofibrosis may provide an opportunity to examine the effects of early TGF-ßi therapy on later stages of myofibrosis. Finally, we recognize that the amount of radiation fibrosis produced in our model was modest. Nonetheless, fibrosis was seen in the perivascular and interstitial spaces, which is coincident with a previous study of radiation-induced fibrosis [39]. The percentage of radiation-induced fibrosis needed to create a clinically relevant manifestation is unclear.
We found that, at 9 months after irradiation, the early use of TGF-ßi resulted in decreased fibrosis. Previous work using a cytokine analysis showed that radiation causes a substantial increase in TGF-ß expression compared with nonirradiated muscle [46]. A study by Park et al. [29] investigated the role of SKI2162, a small-molecule inhibitor of TGF-ß1 phosphorylation and nuclear transcription of Smad2 and Smad3, in the mitigation of radiation-induced fibrosis in mice. In vitro, SKI2162 decreased the transcription of radiation-induced transcriptional expression of fibrosis genes, including MMP2, MMP8, SERPINE1, LOX, and PLAU. In vivo, using a leg contraction assay, the authors found that the mean length of the irradiated limb was much greater in mice treated with SKI2162 than in mice that did not receive the treatment between 6 and 16 weeks after irradiation. Real-time polymerase chain reaction analysis using mRNA from the legs of irradiated mice with or without SKI2162 treatment at 16 weeks after irradiation showed a reduction in fibrosis genes SERPINE1 and SMN1. Although they showed mitigation of early fibrosis at up to 4 months after radiation as a result of inhibition of TGF-ß1 via SK12162, the later effects of TGF-ß1 inhibition on mitigation of fibrosis were not considered. Because of the rapid maturation of mice, we believe that observing the effects of radiation-induced fibrosis 9 months after radiation may better reflect the clinical manifestations of radiation-induced fibrosis in humans.
The role of TGF-ß inhibition in the mitigation of fibrosis in other systems has been well-established. In a rat model, antibodies against TGF-ß reduced the size of intimal hyperplasia after carotid balloon injury and down-regulator expression of extracellular matrix components integral in the fibrosis pathway, including extradomain-A, viscerin, and fibronectin [41]. Additionally, in the central nervous system, TGF-ß inhibition has been shown to attenuate the deposition of fibrous scar tissue and formation of a limiting glial membrane in the brains of injured rats [24, 25]. Others have investigated the role of TGF-ß in radiation-induced skin fibrosis. Inhibition of TGF-ß1 phosphorylation, via SKI2162, ameliorated radiation-induced skin damage, including skin necrosis and aberrant collagen accumulation in the dermis and epidermis [29]. However, we believe a reduction in myofibrosis with the administration of TGF-ßi therapy has not been previously reported.
In conclusion, TGF-β inhibition was associated with a lower percentage of myofibrosis in radiated mouse muscle than in an irradiated placebo group. The hindlimb muscles of mice in the placebo group had nine times the radiation-induced fibrosis as those treated with TGF-β inhibition. The clinical importance of these findings remains to be shown because we have not examined the functional aspects of these radiated mice with and without TGFßi and have not studied the relationship to fibrosis in mice with tumor and/or major resections. Future studies should investigate the potential role of TGF-ß inhibition in models with a tumor in the radiation field. Of interest would be whether perioperative administration of TGF-ßi, either before or after surgical tumor resection, mitigates radiation effects. Before considering clinical trials, work would need to be done on the administration of TGF-ßi in humans to determine the implications they may have on a tumor or other clinical effects, but we think this preliminary observation provides avenues for further investigation in animal models and potentially humans.
Acknowledgments
We thank Xu Cao PhD, Thomas L. Clemens PhD, and John Wong PhD, for their contributions to the development and planning of this study. We also thank Rachel Box, Jenni Weems, and Kerry Kennedy for their editorial assistance in preparing this manuscript.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the animal protocol of this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453-4460. [DOI] [PubMed] [Google Scholar]
- 2.Amadeu TP, Seabra AB, de Oliveira MG, Monte-Alto-Costa A. Nitric oxide donor improves healing if applied on inflammatory and proliferative phase. J Surg Res. 2008;149:84-93. [DOI] [PubMed] [Google Scholar]
- 3.Barcellos-Hoff MH, Derynck R, Tsang ML, Weatherbee JA. Transforming growth factor-beta activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2017;127:1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286-1292. [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor beta 1. Nature. 1990;346:371-374. [DOI] [PubMed] [Google Scholar]
- 8.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the glycosaminoglycans in the matrix of healing dermal wounds in rats. J Ethnopharmacol. 1998;59:179-186. [DOI] [PubMed] [Google Scholar]
- 9.Cohen SR, Cohen EP. Chronic oxidative stress after irradiation: An unproven hypothesis. Med Hypotheses. 2013;80:172-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dadrich M, Nicolay NH, Flechsig P, Bickelhaupt S, Hoeltgen L, Roeder F, Hauser K, Tietz A, Jenne J, Lopez R, Roehrich M, Wirkner U, Lahn M, Huber PE. Combined inhibition of TGFbeta and PDGF signaling attenuates radiation-induced pulmonary fibrosis. Oncoimmunology. 2016;5:e1123366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Andrade CBV, Ramos IPR, de Moraes ACN, do Nascimento ALR, Salata C, Goldenberg R, de Carvalho JJ, de Almeida CEV. Radiotherapy-Induced Skin Reactions Induce Fibrosis Mediated by TGF-beta1 Cytokine. Dose Response. 2017;15:1559325817705019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delanian S, Martin M, Bravard A, Luccioni C, Lefaix JL. Abnormal phenotype of cultured fibroblasts in human skin with chronic radiotherapy damage. Radiother Oncol. 1998;47:255-261. [DOI] [PubMed] [Google Scholar]
- 13.Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiother Oncol. 2002;63:129-145. [DOI] [PubMed] [Google Scholar]
- 14.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dorr W, Hendry JH. Consequential late effects in normal tissues. Radiother Oncol. 2001;61:223-231. [DOI] [PubMed] [Google Scholar]
- 16.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giri SN, Hyde DM, Hollinger MA. Effect of antibody to transforming growth factor beta on bleomycin induced accumulation of lung collagen in mice. Thorax. 1993;48:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [DOI] [PubMed] [Google Scholar]
- 19.Harper JL, Franklin LE, Jenrette JM, Aguero EG. Skin toxicity during breast irradiation: pathophysiology and management. South Med J. 2004;97:989-993. [DOI] [PubMed] [Google Scholar]
- 20.Koerdt S, Rohleder NH, Rommel N, Nobis C, Stoeckelhuber M, Pigorsch S, Duma MN, Wolff KD, Kesting MR. An expression analysis of markers of radiation-induced skin fibrosis and angiogenesis in wound healing disorders of the head and neck. Radiat Oncol. 2015;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefaix JL, Daburon F. Diagnosis of acute localized irradiation lesions: review of the French experimental experience. Health Phys. 1998;75:375-384. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Jendrossek V, Belka C. The role of PDGF in radiation oncology. Radiat Oncol. 2007;2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99-146. [DOI] [PubMed] [Google Scholar]
- 24.Lichtman MK, Otero-Vinas M, Falanga V. Transforming growth factor beta (TGF-beta) isoforms in wound healing and fibrosis. Wound Repair Regen. 2016;24:215-222. [DOI] [PubMed] [Google Scholar]
- 25.Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A. Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci. 1994;6:355-363. [DOI] [PubMed] [Google Scholar]
- 26.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47:277-290. [DOI] [PubMed] [Google Scholar]
- 27.Muller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 2007;35:96-104. [DOI] [PubMed] [Google Scholar]
- 28.Nemunaitis J, Dillman RO, Schwarzenberger PO, Senzer N, Cunningham C, Cutler J, Tong A, Kumar P, Pappen B, Hamilton C, DeVol E, Maples PB, Liu L, Chamberlin T, Shawler DL, Fakhrai H. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24:4721-4730. [DOI] [PubMed] [Google Scholar]
- 29.Park JH, Ryu SH, Choi EK, Ahn SD, Park E, Choi KC, Lee SW. SKI2162, an inhibitor of the TGF-beta type I receptor (ALK5), inhibits radiation-induced fibrosis in mice. Oncotarget. 2015;6:4171-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pires AM, Segreto RA, Segreto HR. RTOG criteria to evaluate acute skin reaction and its risk factors in patients with breast cancer submitted to radiotherapy. Rev Lat Am Enfermagem. 2008;16:844-849. [DOI] [PubMed] [Google Scholar]
- 31.Shah M, Foreman DM, Ferguson MW. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339:213-214. [DOI] [PubMed] [Google Scholar]
- 32.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stubblefield MD. Radiation fibrosis syndrome: neuromuscular and musculoskeletal complications in cancer survivors. PM R. 2011;3:1041-1054. [DOI] [PubMed] [Google Scholar]
- 34.Travis EL. Organizational response of normal tissues to irradiation. Semin Radiat Oncol. 2001;11:184-196. [DOI] [PubMed] [Google Scholar]
- 35.Travis EL, Rachakonda G, Zhou X, Korhonen K, Sekhar KR, Biswas S, Freeman ML. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic Biol Med. 2011;51:1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology. 2009;214:630-641. [DOI] [PubMed] [Google Scholar]
- 37.Wahl SM, Allen JB, Costa GL, Wong HL, Dasch JR. Reversal of acute and chronic synovial inflammation by anti-transforming growth factor beta. J Exp Med. 1993;177:225-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Westbury CB, Haviland J, Davies S, Gothard L, Abdi BA, Sydenham M, Bowen J, Stratton R, Short SC, Yarnold JR. Cytokine levels as biomarkers of radiation fibrosis in patients treated with breast radiotherapy. Radiat Oncol. 2014;9:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willey JS, Bracey DN, Gallagher PE, Tallant EA, Wiggins WF, Callahan MF, Smith TL, Emory CL. Angiotensin-(1-7) Attenuates Skeletal Muscle Fibrosis and Stiffening in a Mouse Model of Extremity Sarcoma Radiation Therapy. J Bone Joint Surg Am. 2016;98:48-55. [DOI] [PubMed] [Google Scholar]
- 40.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11:1386-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf YG, Rasmussen LM, Ruoslahti E. Antibodies against transforming growth factor-beta 1 suppress intimal hyperplasia in a rat model. J Clin Invest. 1994;93:1172-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Pang Y, Moses HL. TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 2010;31:220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yano H, Hamanaka R, Nakamura M, Sumiyoshi H, Matsuo N, Yoshioka H. Smad, but not MAPK, pathway mediates the expression of type I collagen in radiation induced fibrosis. Biochem Biophys Res Commun. 2012;418:457-463. [DOI] [PubMed] [Google Scholar]
- 46.Yano H, Hamanaka R, Nakamura-Ota M, Zhang JJ, Matsuo N, Yoshioka H. Regulation of type I collagen expression by microRNA-29 following ionizing radiation. Radiat Environ Biophys. 2018;57:41-54. [DOI] [PubMed] [Google Scholar]
- 47.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol 2010;97:149-161. [DOI] [PubMed] [Google Scholar]
- 48.Zhen G, Wen C, Jia X, Li Y, Crane JL, Mears SC, Askin FB, Frassica FJ, Chang W, Yao J, Carrino JA, Cosgarea A, Artemov D, Chen Q, Zhao Z, Zhou X, Riley L, Sponseller P, Wan M, Lu WW, Cao X. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19:704-712. [DOI] [PMC free article] [PubMed] [Google Scholar]


