Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic, and its associated lockdowns in many parts of the world, have changed our daily lives and may have a psychological impact on around the globe. However, it is unknown how this influences the patient-reported outcome measures (PROMs) of patients involved in ongoing clinical research and medical care. For both the current and potential future lockdowns, it is important to determine if PROMs collected during such a period can be interpreted with confidence.
Questions/purposes
(1) Is there a difference in quality of life between patients in the COVID-19 period group (March 23, 2020 to May 4, 2020) and patients in a reference period group (from the same period in 2018 or 2019)? (2) Is there a difference in pain, hand function, anxiety, depression, and illness perception between patients in the COVID-19 period group and patients in the reference period group?
Methods
This study was part of a large cohort study with routine outcome measures of patients with hand and wrist conditions. To answer our research questions, we analyzed two samples because not all PROMs were sent to participants at the same time points after treatment. The first sample consisted of all participants who completed PROMs on quality of life (QoL), pain, and hand function at their final follow-up time point, which was either 3, 6, or 12 months post-treatment. The second sample consisted of participants who completed PROMs 3 months post-treatment on anxiety, depression, and illness perception. Each sample consisted of two groups: a COVID-19 period group and a reference period group. We included 1613 participants in the first sample (COVID-19 period group: n = 616; reference period group: n = 997) and 535 participants in the second sample (COVID-19 period group: n = 313; reference period group: n = 222). The primary outcome was QoL, expressed in the EuroQol 5-Dimensions questionnaire (EQ-5D) index score. Secondary outcomes were the other domains on the EQ-5D, as well as pain, hand function, anxiety, depression, and illness perception.
Results
We found no between-group differences in the EQ-5D index score (standardized mean difference 0.035; p = 0.98). Furthermore, there were no between-group differences in PROM scores for hand function, anxiety, or depression. There were, however, a few small differences in subdomain items regarding pain and illness perception, but we believe in aggregate that these are unlikely to make a clinically important difference in our main finding.
Conclusion
The COVID-19 pandemic and its associated lockdown had no influence on QoL and had little influence on secondary outcomes in participants who were part of the Hand-Wrist Study Cohort. This finding implies that PROMs data collected during this period can be used with confidence in clinical research. Our findings indicate that when a pandemic like this occurs again, we can continue to use PROMs for analysis in clinical research or routine outcome measures.
Level of Evidence
Level III, therapeutic study.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic affects people all around the world [20, 21]. To control the virus, multiple countries have instituted their own version of a government order to stay-at-home and avoid social contact, often referred to as a “lockdown” [8, 9, 10, 12, 14]. In the Netherlands, an “intelligent lockdown” has been in effect since March 15, 2020 [13, 14], which instructed social distancing, and more specifically, to stay at home as much as possible, avoid busy places, and maintain a physical distance from each other.
Because a pandemic resulting in lockdowns in many affected countries is unprecedented in modern history, the effects of such measures on quality of life (QoL) and other health status domains are unknown. Brooks et al. [2] recently reviewed pre-COVID-19 studies on people in quarantine for an infection and reported an increased prevalence of depression, anxiety, insomnia, and emotional disturbance. More specifically, for the COVID-19 pandemic, one study reported a mild stress response in 263 Chinese citizens because of the COVID-19 pandemic [23], whereas another study reported no changes in emotional state in most participants during a 2-week study period during the COVID-19 pandemic [22]. As such, the influence of the COVID-19 pandemic and associated lockdowns on psychological distress is still unclear, as is the influence of COVID-19 on important outcomes such as QoL. More knowledge of psychological distress during the COVID-19 pandemic is important for both medical care and clinical research, because distress is known to influence QoL, pain, and function [3, 11, 17, 18].
In this study, we asked: (1) Is there a difference in QoL between patients in the COVID-19 period group (March 23, 2020 to May 4, 2020) and patients in a reference period group (from the same period in 2018 or 2019)? (2) Is there a difference in pain, hand function, anxiety, depression, and illness perception between patients in the COVID-19 period group and patients in the reference period group?
Patients and Methods
Study Design and Setting
This was a cohort study using a sample of patients with hand and wrist conditions and the study was reported per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [19]. In this study, we compared the outcomes of a COVID-19 period group and a reference period group at 3, 6, or 12 months after the start of treatment.
Data collection was part of usual care and occurred between January 2012 and May 2020 at Xpert Clinic and Handtherapie Nederland, comprising 28 clinics for hand surgery and therapy in the Netherlands. Our treatment centers employ 23 surgeons certified by the Federation of European Societies for Surgery of the Hand and more than 150 hand therapists.
In this study, we compared PROMs gathered at 3, 6, or 12 months after the start of a treatment for their hand or wrist conditions between two study groups: One was from a COVID-19 period group (collected from March 23, 2020 to May 4, 2020), and the other was a reference period group (collected during the same period in 2018 and 2019). We used two samples in this study, since not all PROMs of interest were sent to participants at each time point.
The first sample comprised participants who completed PROMs on QoL, pain, and hand function at final follow-up, at either 3, 6, or 12 months post-treatment. These predefined time points for final follow-up were dependent on the measurement track [16], specified per diagnosis-treatment combination and dependent on disease severity and invasiveness of the treatment. For example, a trigger finger release had a final follow-up of 3 months, Dupuytren’s surgery had a final follow-up of 6 months, and thumb base resection arthroplasty had a final follow-up of 12 months. The second sample consisted of participants who completed PROMS on anxiety, depression, and illness perception. These PROMs were distributed at 3 months post-treatment for all patients. More details on our routine outcome measurements are described elsewhere [16]. The study was performed in accordance with the Declaration of Helsinki and approved by the local Medical Research Ethical Committee at Erasmus Medical Center (reference number: MEC-2018-1088). Written informed consent was obtained from all patients.
Patients in the COVID-19 period group completed PROMs during the so-called intelligent lockdown in the Netherlands. This lockdown was designed to control the spread of the virus, and citizens were instructed to follow hygiene rules, stay home and work from home as much as possible, and avoid visiting families and friends. Forming groups was forbidden and people were instructed to keep a 1.5-meter distance from others. As a result, during the lockdown, schools and public venues such as cafes and restaurants were closed, but shops were open [13, 14]. In addition, during the lockdown, all other usual healthcare was minimalized or postponed; for example, outpatient department appointments were held via telephone, and physical therapy appointments were cancelled or converted to video consultations.
Participants
Participants were eligible for inclusion if there were complete baseline sociodemographic data and complete PROMs data completed during either the COVID-19 period (March 23, 2020 to May 4, 2020) or the reference period (same period but in 2018 or 2019).
As mentioned before, we used two data samples in this study. The first sample included patients who completed PROMs for QoL, pain, and hand function at their final pre-defined follow-up time point. The second sample included patients who completed PROMs for anxiety, depression, and illness perception at 3 months follow-up.
Study Population
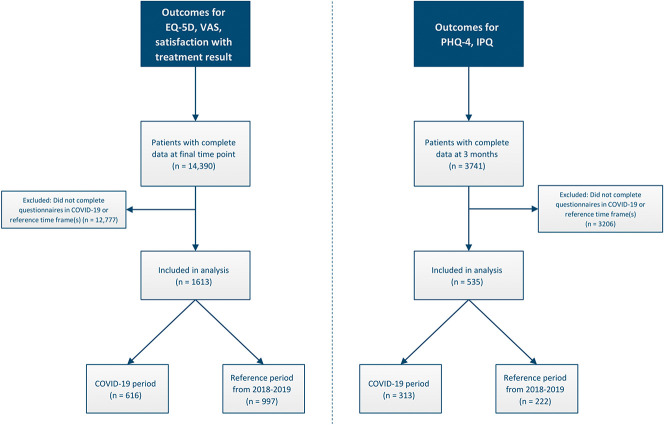
After applying the inclusion criteria, we included 1613 patients in the first sample (616 patients in the COVID-19 period group and 997 patients in the reference period group) and 535 patients in the second sample (313 patients in the COVID-19 period group and 222 patients in the reference period group) (Fig. 1). The baseline characteristics of patients in the COVID-19 period group and those in the reference period group were highly similar, except for the distribution between measurement tracks in the second sample (Table 1).
Fig. 1.
This flow chart shows the patients who were included in this study. The left side describes the sample for the first analysis and the right side describes the sample for the second analysis.
Table 1.
Pretreatment sociodemographic characteristics
| First sample | Second sample | |||||
| (EQ-5D, VAS Pain, and VAS Hand function) | (IPQ, PHQ-4) | |||||
| Variable | COVID-19 period (n = 616) | Reference period (n = 997) | p value | COVID-19 period (n = 313) | Reference period (n = 222) | p value |
| Age, mean ± SD | 52 ± 16 | 53 ± 14 | 0.17 | 57 ± 13 | 59 ± 12 | 0.15 |
| Sex % men (n) | 37% (228) | 35% (348) | 0.42 | 33% (104) | 38% (85) | 0.27 |
| Hand dominance, % right hand (n) | 86% (527) | 87% (867) | 0.30 | 88% (275) | 90% (199) | 0.84 |
| Treatment side, % right hand (n) | 45% (279) | 46% (455) | 0.51 | 48% (151) | 53% (117) | 0.13 |
| Measurement track, % (n) | 0.19 | < 0.001 | ||||
| Thumb regular | 17% (103) | 18% (184) | 32% (101) | 45% (99) | ||
| Thumb extended | 6% (34) | 8% (76) | 12% (39) | 17% (38) | ||
| Dupuytren’s | 5% (30) | 5% (47) | 15% (48) | 24% (54) | ||
| Nerve (de)compression | 15% (95) | 14% (141) | 40% (125) | 14% (31) | ||
| Wrist regular | 22% (135) | 24% (238) | ||||
| Wrist extended | 12% (75) | 8% (83) | ||||
| Finger regular | 20% (125) | 20% (196) | ||||
| Finger extended | 3% (19) | 3% (32) | ||||
| Nonsurgical treatment, % (n) | 30% (187) | 33% (332) | 0.24 | 28% (87) | 35% (78) | 0.09 |
| Second opinion = no, % (n) | 95% (585) | 94% (939) | 0.34 | 98% (306) | 98% (218) | 1.00 |
| Type of Work, % (n) | 0.21 | 0.50 | ||||
| Unemployed | 32% (196) | 32% (321) | 38% (119) | 44% (97) | ||
| Light physical labor | 32% (196) | 28% (279) | 31% (97) | 27% (59) | ||
| Moderate physical labor | 25% (157) | 26% (261) | 22% (70) | 20% (45) | ||
| Heavy physical labor | 11% (67) | 14% (136) | 8% (26) | 9% (21) | ||
| Duration of symptoms in months, median (IQR) | 6 (4 to 18) | 8 (4 to 18) | 0.16 | 9 (5 to 24) | 12 (6 to 24) | 0.26 |
Variables, Data Sources, and Measurement
Primary Outcome
The primary outcome was the index score of the 5-level EuroQol-5 Dimensions (EQ-5D), which is a validated and widely used tool for measuring QoL [6]. The index score ranges from 0 (death) to 1 (perfect health). The questionnaire consists of five dimensions (mobility, self-care, usual activities, pain and discomfort, and anxiety and depression) with 5 levels (no problems, slight problems, moderate problems, severe problems, and unable/extreme problems). The individual domain scores and the self-reported health on a VAS scale (range 0 to 100; a higher scores indicate a better health state) were secondary outcomes [6].
Secondary Outcomes
We used the VAS to measure pain (range 0 to 100; higher scores indicate more pain), hand function (range 0 to 100; higher scores indicate better function), [4].
Anxiety and depression were measured using the Patient Health Questionnaire for anxiety and depression ([PHQ-4]; score range: 0 to 6 for the subscales of anxiety and depression; higher scores indicate more anxiety and depression), which is a screening tool for detecting depressive disorders [7].
Illness perceptions were measured using the Brief Illness Perception Questionnaire ([IPQ]; item scores range from 0 to 10; higher scores indicate worse illness perception except for the items of personal control, treatment control, and coherence) [1]. As an indication of the patient’s overall illness perception, a sum score can be calculated after converting the items of personal control, treatment control, and coherence (range 0 to 80; higher scores indicate worse illness perception) [18].
Study Size
A power analysis for a multiple linear model with 10 covariates, a conventional effect size of f = 0.15, α = 0.05, and power of 0.80 suggested that 118 participants were required, which was well below the included sample of 1613 patients in the first sample and 535 patients in the second sample.
Statistical Methods
We investigated between-group differences in pretreatment sociodemographic characteristics using independent sample t-tests for normally distributed continuous data, Kruskal-Wallis tests for non-normally distributed continuous data and chi-square tests for dichotomous or categorical data. Each sample was analyzed separately.
To inspect possible trends over time, we created scatter plots of the primary outcome as a function to the number of days since the lockdown as well as for the reference period in 2018 and 2019. A best fitting, second-order polynomial was fitted for each of the three time intervals.
We investigated differences in PROM scores between the COVID-19 period group and reference period group using multivariable linear mixed models. We used the multivariable linear mixed model to allow adjusting for potential differences in baseline characteristics between both groups. More specifically, we adjusted for the following sociodemographic characteristics to determine whether there was an adjusted group effect: age, sex, hand dominance, treatment side, measurement track, whether a surgical treatment or a nonsurgical treatment was performed, whether it was a second opinion or not, type of work, and duration of symptoms. Assumptions were checked using residual plots and normal probability plots. If data were not normally distributed, we reported medians and interquartile ranges. A p value < 0.05 was considered statistically significant.
Results
Quality of Life Between Patients in the COVID-19 Period Group and the Reference Period Group
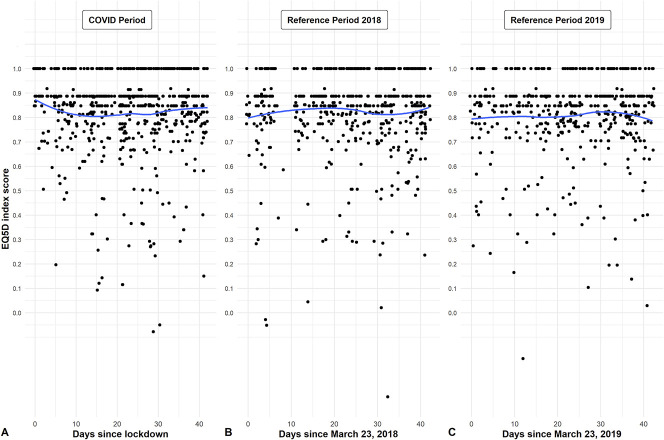
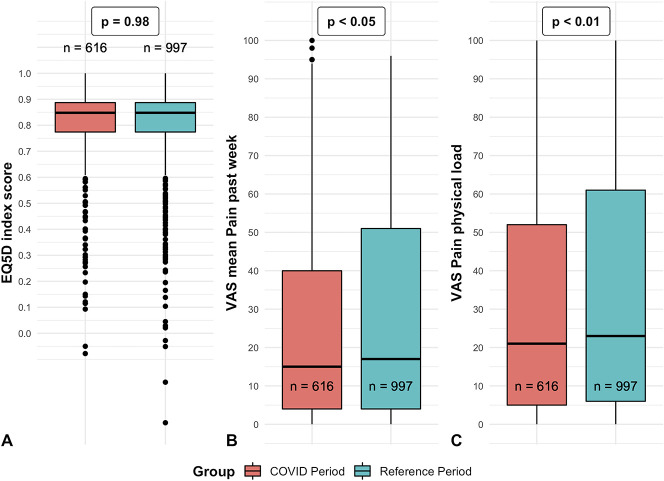
The primary outcome, EQ-5D index score, did not show a clear change over time (Fig. 2). When comparing groups, there was no difference in the index EQ-5D score between the COVID-19 period group and reference period group (standardized mean difference: 0.035; adjusted p = 0.98) (Fig. 3A). Also, there were no differences in the other EQ-5D items (Table 2).
Fig. 2.
A-C These scatterplots and second-order polynomial regression line show the individual index EQ-5D scores as a function of (A) the numbers of days since the lockdown on March 23, 2020 and for the reference periods in (B) 2018 (C) and 2019.
Fig. 3.
A-C These boxplots demonstrate the distribution of the (A) index EQ-5D score, (B) mean VAS score for pain in the past week, and (C) VAS score for pain during physical load. The p values refer to the significance of the adjusted group factor from the linear mixed model analyses. The X represents the mean, whereas the horizontal line represents the median. The dots represent outliers.
Table 2.
Between-group differences for the 5-Level EuroQol Group 5-Dimensions (EQ-5D) and pain and hand function measured with a VAS from the first sample
| Variable | COVID period group (n = 616) | Reference period group (n = 997) | Standardized mean difference | p value |
| Primary outcome | ||||
| EQ-5D index score | 0.85 (0.77 to 0.89) | 0.85 (0.77 to 0.89) | 0.035 | 0.98 |
| Secondary outcomes | ||||
| EQ-5D VAS general health | 86 (71 to 94) | 85 (73 to 93) | 0.005 | 0.90 |
| EQ-5D subscale mobility | 1 (1 to 1) | 1 (1 to 1) | 0.006 | 0.92 |
| EQ-5D subscale self-care | 1 (1 to 1) | 1 (1 to 1) | 0.014 | 0.76 |
| EQ-5D subscale usual activities | 1 (1 to 2) | 2 (1 to 2) | 0.039 | 0.37 |
| EQ-5D subscale pain/ discomfort | 2 (1 to 3) | 2 (2 to 3) | 0.059 | 0.19 |
| EQ-5D subscale anxiety/ depression | 1 (1 to 1) | 1 (1 to 1) | 0.003 | 0.73 |
| VAS hand function | 84 (59 to 96) | 81 (53 to 94) | 0.108 | 0.37 |
| VAS mean pain past week | 15 (4 to 40) | 17 (4 to 51) | 0.096 | 0.046 |
| VAS pain during rest | 7 (0 to 27) | 7 (0 to 30) | 0.077 | 0.07 |
| VAS pain during physical load | 21 (5 to 52) | 23 (6 to 61) | 0.093 | 0.008 |
Reported values per group are medians (interquartile range); in addition, the unadjusted standardized mean difference and the p values for the adjusted group factor from the linear mixed model analyses are shown.
Comparing Pain, Hand Function, Anxiety, Depression, and Illness Perception in the COVID-19 Period Group and the Reference Period Group
Similarly, for the secondary outcomes, we found no differences in VAS pain during rest, VAS score for hand function (Table 2), and PHQ-4 scores (Table 3) between the COVID-19 period group and reference period group. However, we did find less pain during the past week in the COVID-19 period (median = 15) group than in the reference period group (median = 17, p = 0.046) (Fig. 3B) and less pain during physical load in the COVID-19 period (median = 21) group compared to the reference period group (median = 23, p = 0.008) (Fig. 3C). In addition, the IPQ items of treatment control (p = 0.03) (Fig. 4A) and emotional representation (p = 0.02) (Fig. 4B) were different, although all these differences were small for both and so we believe in aggregate that these are unlikely to make a clinically important difference to our main finding.
Table 3.
Between-group differences for the secondary outcomes Patient Health Questionnaire (PHQ-4) and the Brief Illness Perception Questionnaire (IPQ) from the second sample
| Variable | COVID period group (n = 313) | Reference period group (n = 222) | Standardized mean difference | p value |
| PHQ-4 total score, median (IQR) | 0 (0 to 1) | 0 (0 to 1) | 0.11 | 0.14 |
| PHQ-4 subscale anxiety, median (IQR) | 0 (0 to 0) | 0 (0 to 1) | 0.09 | 0.28 |
| PHQ-4 subscale depression, median (IQR) | 0 (0 to 0) | 0 (0 to 0) | 0.12 | 0.11 |
| IPQ consequences: How much does your illness affect your life? (10 = severely affects my life), median (IQR) | 4 (2 to 7) | 4 (1 to 7) | 0.048 | 0.79 |
| IPQ timeline: How long do you think your illness will continue? (10 = forever), median (IQR) | 5 (3 to 8) | 6 (3 to 8) | 0.03 | 0.24 |
| IPQ personal control: How much control do you feel you have over your illness? (0 = absolutely no control), median (IQR) | 6 (4 to 8) | 6 (5 to 8) | 0.01 | 0.91 |
| IPQ treatment control: How much do you think your treatment can help your illness? (10 = extremely helpful), median (IQR) | 8 (5 to 9) | 8 (6 to 9) | 0.15 | 0.03 |
| IPQ identity: How much do you experience symptoms from your illness? (10 = many severe symptoms), median (IQR) | 3 (1 to 6) | 4 (1 to 7) | 0.16 | 0.14 |
| IPQ concern: How concerned are you about your illness? (10 = extremely concerned), median (IQR) | 3 (1 to 6) | 3 (1 to 7) | 0.14 | 0.37 |
| IPQ coherence: How well do you feel you understand your illness? (10 = understand very clearly), median (IQR) | 9 (7 to 10) | 9 (7 to 10) | 0.008 | 0.35 |
| IPQ emotional representation: How much does your illness affect you emotionally? e.g. does it make you angry. scared. upset or depressed? (10 = extremely affected emotionally), median (IQR) | 1 (0 to 5) | 2 (0 to 5) | 0.22 | 0.02 |
| IPQ total score, mean (SD) | 28.5 (15.3) | 29.9 (15.9) | 0.09 | 0.62 |
Reported values per group are medians plus interquartile range (IQR) or means plus SD; in addition, the unadjusted standardized mean difference and the p values for the adjusted group factor from the linear mixed model analyses are shown.
Fig. 4.
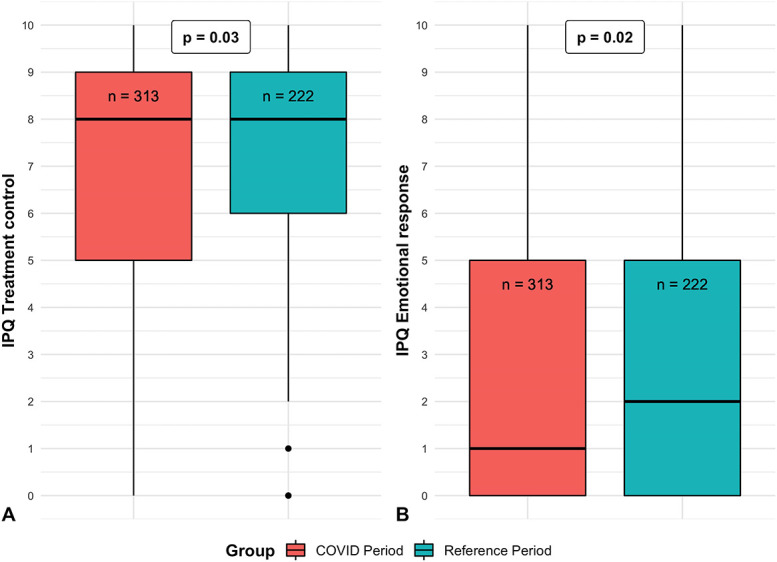
A-B These boxplots demonstrate the distribution of the IPQ items (A) “treatment control” and (B) “emotional response” for the COVID-19 period group and reference period group. The p values refer to the significance of the adjusted group factor from the linear mixed model analyses. The horizontal line represents the median. The dots represent outliers; IPQ = Illness Perception Questionnaire.
Discussion
Several countries have declared their own versions of a lockdown due to the COVID-19 pandemic. During this lockdown, patients may continue to report PROMs. Because these PROMs are used in ongoing clinical research, it is important to know whether these outcomes are influenced by the COVID-19 pandemic and if they can be analyzed with confidence. We found that the COVID-19 pandemic and its associated intelligent lockdown in the Netherlands had no influence on QoL, hand function, anxiety, and depression in patients treated for hand or wrist conditions. For some of the secondary outcomes of pain and illness perception, we found adjusted group differences, but these differences were small. These findings suggest that PROMs collected during the COVID-19 pandemic are comparable to those collected before the COVID-19 pandemic and can be used with confidence in future analyses of ongoing clinical studies or routinely collected outcomes of medical care.
Limitations
This study has several limitations. First, we analyzed the early stage of the intelligent lockdown in the Netherlands. As such, the long-term effects of this lockdown on PROMs are still unclear, and these may be present, such as the financial consequences that were not present on the short-term. For example, the review by Brooks et al. [2] reported that the longer-lasting quarantine during the Severe Acute Respiratory Syndrome (SARS) outbreak was associated with worse mental health. One of the included studies reported worse mental health already after 10 days of quarantine [5]. A larger study evaluated the emotional state of 845 Chinese individuals over a 2-week span during the COVID-19 pandemic, and found that most participants reported no change [22]. This is in line with our analysis over time, which indicated no change in QoL during our study period from March 23, 2020 to May 4, 2020 (Fig. 2). With that in mind, future studies should further investigate the influence of the lockdown duration on PROMs. Another limitation is that our analysis was performed in one country (the Netherlands), and as such, it may have a limited generalizability elsewhere, since other countries imposed different approaches to stay-at-home orders and lockdowns. However, across countries, there may be similar stressors during quarantine and post-quarantine that apply to all countries, including possible fears of infection, fears about inadequate supplies, (fear of) loss of finance, lack of adequate information, frustration, and boredom due to social isolation [2]. Therefore, we believe that our results are generalizable to other countries with a similar form of a lockdown based on these overlapping stressors. Furthermore, a limitation of this study is that we used the PHQ-4 for evaluating anxiety and depression, which is a screening tool that may not be able to detect more subtle changes in anxiety and depression that may have occurred during the COVID-19 pandemic. However, it is a commonly used instrument that should be able to detect clinically important changes.
In addition, this study was a between-group comparison without randomization. In theory, baseline differences in unidentified covariates may have been present, possibly resulting in bias. As randomizing patients to either group was impossible, and since we adjusted for pretreatment sociodemographic characteristics, we believe that this has not influenced our results. An additional limitation is that our patients completed all questionnaires in light of their hand or wrist condition. This context may have influenced the way patients interpreted questions when they completed PROMs for the more generic outcome domains such as QoL, anxiety, or depression.
Quality of Life Between Patients in the COVID-19 Period Group and the Reference Period Group
Our study found no difference in QoL, measured with the EQ-5D, during the COVID-19 pandemic. This is important because QoL is a valuable health outcomes endpoint that is commonly measured in clinical studies and economic evaluations of health care. We are unable to state if the EQ-5D is able to detect changes in QoL due to the COVID-19 pandemic. However, the EQ-5D is often used to measure QoL in research. Our findings confirm that EQ-5D data collected during the COVID-19 pandemic in ongoing clinical research can be used with confidence.
Comparing Pain, Hand Function, Anxiety, Depression, and Illness Perception in the COVID-19 Period Group and the Reference Period Group
Our findings are in contrast with two studies that found a higher prevalence of depression or anxiety during quarantine [5, 23]; we did not find any differences in anxiety or depression between the COVID-19 period group and reference period group. These disparate findings may be explained by differences in lockdown approach compared with the Netherlands, the extent to which the country is affected by COVID-19, or because patients in other studies may have been isolated because of their illness, whereas our study included predominantly non-COVID-19 patients. During our study period, only 3% of the blood donors in the Netherlands had antibodies against COVID-19 [15].
Although there were no differences in almost all PROMs, we did find that patients in the COVID-19 period group reported better outcomes on the IPQ item emotional response than those in the reference period group. This implies that during the COVID-19 pandemic, patients may have a higher capability to put their non-COVID-19 condition into perspective. This theory may be supported by our finding that patients in the COVID-19 period group reported less pain than did those in the reference period group. However, the unadjusted mean difference for the IPQ items were less than 1 on a 10-point scale, and the unadjusted mean difference for the VAS pain items were less than 3 on a 100-point scale. These differences were very small (and likely not clinically important), and there may have been a risk of multiple testing for our secondary outcomes. Thus, these findings should be considered exploratory and need a more in-depth analysis in future research.
Conclusion
In conclusion, we found no meaningful differences in QoL and secondary outcomes between patients during the COVID-19 pandemic and a reference period from 2018 to 2019 in a large cohort of patients with hand and wrist conditions. Our study implies that PROMs collected during this period are not scored differently by patients during the COVID-19 pandemic, and can be used with confidence in future analyses of ongoing research. Our findings suggest that if another similar pandemic occurs, we can continue to collect these PROMs. Future research should determine whether these findings are generalizable to populations with different cultural traits, socioeconomic support systems, severity of the outbreak of COVID-19, or differences in the type or duration of the lockdown.
Acknowledgments
We thank all the patients who completed questionnaires as part of their clinical care and who agreed that their data could be used anonymously for the present study. We thank the members of the Hand-Wrist Study Group, the caregivers, and the personnel of Xpert Clinic, Handtherapie Nederland, and Equipe Zorgbedrijven for assisting in the routine outcome measurements that are the basis for this study.
Footnotes
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Hand–Wrist Study Group Collaborators: R. A. M. Blomme MD, B. J. R. Sluijter MD PhD, D. J. J. C. van der Avoort MD, A. Kroeze MD, J. Smit MD PhD, J. Debeij MD PhD, E. T. Walbeehm MD PhD, G. M. van Couwelaar MD, G. M. Vermeulen MD PhD, J. P. de Schipper MD, J. F. M. Temming MD, J. H. van Uchelen MD PhD, H. L. de Boer MD, K. P. de Haas MD, K. Harmsen MD, O. T. Zöphel MD PhD, R. Feitz MD, R. Koch MD, S. E. R. Hovius MD PhD, T. M. Moojen MD PhD, X. Smit MD PhD, R. van Huis PT, P. Y. Pennehouat PT, K. Schoneveld PT MSc, Y. E. van Kooij PT MSc, P. Zagt PT, F. J. van Ewijk PT, J. J. Veltkamp PT, A. Fink PT MSc, H. P. Slijper PhD, J. T. Porsius PhD, R. Poelstra MD, M. J. W. van der Oest BSc, L. Hoogendam BSc, J. M. Zuidam MD PhD, L. Duraku MD PhD, E. P. A. van der Heijden MD PhD, J. W. Colaris MD PhD, J. S. Teunissen, J. Dekker MSc, M. Jansen-Landheer MD PhD.
The institution of one or more of the authors (AC, RW, RS) has received, during the study period funding from ZonMW (The Hague, The Netherlands) and CZ (Tilburg, The Netherlands).
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This study was performed at the Xpert Clinic, Eindhoven, the Netherlands.
The first two authors contributed equally to this manuscript.
References
- 1.Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60:631-637. [DOI] [PubMed] [Google Scholar]
- 2.Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, Rubin GJ. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunillera O Tresserras R Rajmil L,Vilagut G Brugulat P Herdman M Mompart A Medina A Pardio Y Alonso J Brazier J Ferrer M. Discriminative capacity of the EQ-5D, SF-6D, and SF-12 as measures of health status in population health survey. Qual Life Res. 2010;19:853-864. [DOI] [PubMed] [Google Scholar]
- 4.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):S240-252. [DOI] [PubMed] [Google Scholar]
- 5.Hawryluck L, Gold WL, Robinson S, Pogorski S, Galea S, Styra R. SARS control and psychological effects of quarantine, Toronto, Canada. Emerg Infect Dis. 2004;10:1206-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen MF, Bonsel GJ, Luo N. Is EQ-5D-5L better than EQ-5D-3L? A head-to-head comparison of descriptive systems and value sets from seven countries. Pharmacoeconomics. 2018;36:675-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroenke K, Spitzer RL, Williams JB, Lowe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50:613-621. [DOI] [PubMed] [Google Scholar]
- 8.Lau H Khosrawipour V Kocbach P,Mikolajczyk A Schubert J Bania J Khosrawipour T. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. J Travel Med. 2020;27:taaa037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lancet. India under COVID-19 lockdown. Lancet. 2020;395:1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitjà O Arenas À Rodó X,Tobias A Brew J Benlloch JM. Experts' request to the Spanish Government: move Spain towards complete lockdown. Lancet. 2020;395:1193-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Payakachat N, Ali MM, Tilford JM. Can the EQ-5D detect meaningful change? A systematic review. Pharmacoeconomics. 2015;33:1137-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peto J Alwan NA Godfrey KM,Burgess RA Hunter DJ Riboli E Romer P. Universal weekly testing as the UK COVID-19 lockdown exit strategy. Lancet. 2020;395:1420-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rijksoverheid. Additional measures education, catering industry, sport. Available at: https://www.rijksoverheid.nl/actueel/nieuws/2020/03/15/aanvullende-maatregelen-onderwijs-horeca-sport. Accessed May 10, 2020.
- 14.Goverment of the NetherlandsDutch measures against coronavirus. Available at: https://www.government.nl/topics/coronavirus-covid-19/tackling-new-coronavirus-in-the-netherlands Accessed April 29, 2020.
- 15.Sanquin Sanquin.: Approximately 3% of donors have antibodies against corona. Available at: https://www.sanquin.nl/over-sanquin/nieuws/2020/04/sanquin-ongeveer-3-van-donors-heeft-corona-antistoffen. Accessed May 15, 2020.
- 16.Selles RW Wouters RM Poelstra R,van der Oest MJW Porsius JT Hovius SER Moojen TM van Kooij Y Pennehouat PY van Huis R Vermeulen GM Feitz R Slijper HP, Hand-Wrist Study Group. Routine health outcome measurement: Development, design, and implementation of the Hand and Wrist Cohort. Plast Reconstr Surg. 2020. [Published online ahead of print April 27, 2020]. DOI: 10.1097/ PRS.0000000000007008. [DOI] [PubMed] [Google Scholar]
- 17.Sun PO Walbeehm ET Selles RW,Jansen MC Slijper HP Ulrich DJO Porsius JT, Hand-Wrist Study Group. Influence of illness perceptions, psychological distress and pain catastrophizing on self-reported symptom severity and functional status in patients with carpal tunnel syndrome. J Psychosom Res. 2019;126:109820. [DOI] [PubMed] [Google Scholar]
- 18.van der Oest MJW, Poelstra R, Feitz R, Vranceanu AM, Slijper HP, Selles RW, Porsius JT, Hand-Wrist Study Group. Illness perceptions of patients with first carpometacarpal osteoarthritis, carpal tunnel syndrome, Dupuytren contracture, or trigger finger. J Hand Surg Am. 2020;45:455 e451-455 e458. [DOI] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP l. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453-1457. [DOI] [PubMed] [Google Scholar]
- 20.WHO. Novel Coronavirus Situation Report - 22. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200211-sitrep-22-ncov.pdf?sfvrsn=fb6d49b1_2. Accessed February 11, 2020, 2020.
- 21.WHO. Novel Coronavirus Situation Report - 52. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200312-sitrep-52-covid-19.pdf?sfvrsn=e2bfc9c0_4. Accessed March 12, 2020.
- 22.Yuan S, Liao Z, Huang H, Jiang B, Zhang X, Wang Y, Zhao M. Comparison of the indicators of psychological stress in the population of Hubei province and non-endemic provinces in China during two weeks during the coronavirus disease 2019 (COVID-19) Outbreak in February 2020. Med Sci Monit. 2020;26:e923767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Ma ZF. Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning province, China: a cross-sectional study. Int J Environ Res Public Health. 2020;17:2381. [DOI] [PMC free article] [PubMed] [Google Scholar]