ABSTRACT
Aim: The aims of the study were to evaluate the non-inferiority of the safety and immunogenicity of a new trial purified vero cell-cultured rabies vaccine (trial vaccine) in healthy subjects comparing with the control purified vero cell-cultured rabies vaccine (control vaccine) following Essen regimen and to evaluate the non-inferiority of the safety and immunogenicity of the trial vaccine following two intramuscular regimens, between Zagreb and Essen regimen. Methods: Serum samples were collected before vaccination and on d 7, 14, 35/42 post vaccination. Adverse events (AEs) were recorded for 30 d following each vaccination. This study was registered in the Chinese Clinical Trial Registry (ChiCTR-PPR-15007057). Results: There was no significant difference in the incidence of AEs, local and systemic reactions, among Zagreb group, Essen group, and control group. But the incidence of solicited AEs was a significant difference among the three groups (p = 0.0498). The incidence of solicited AEs was higher in Essen group than that in control group and Zagreb group (p = 0.0278, p = 0.0248). In the subjects whose antibodies were seronegative before vaccination, the seroconversion rates of antibodies among three groups were all 100.0% on d 14 and d 35/42. The Essen group was not inferior to the control group, and the Zagreb group was not inferior to the Essen group on d 14. On d 14 and d 35/42, the geometric mean concentration of the three groups was much higher than the immune protection level of 0.5 IU/ml. Conclusions: The trial vaccine had good safety and immunogenicity, and the trial vaccine is not inferior to the control vaccine.
Abbreviations: PVRV: purified vero cell-cultured rabies vaccine; AE: adverse event; CI: confidence interval; GMC: geometric mean concentration; IM: intramuscular; NIFDC: National Institutes for Food and Drug Control; PPS: per-protocol set; SS: safety set; REFIT: Rapid Fluorescent Focus Inhibition Test; RVNA: rabies virus neutralizing antibody; WHO: World Health Organization.
KEYWORDS: Rabies vaccine, Zagreb regimen, Essen regimen, immunogenicity, safety
Introduction
Rabies is a zoonosis caused by the infection of the rabies virus, with a mortality rate of nearly 100%. Although rabies is basically extinct among industrialized countries, it is estimated that nearly 59,000 people died of rabies each year worldwide, especially in Asia and Africa.1 China is one of the countries threaten severely by rabies, around 40 million people were bitten by animals each year.2 Rabies is one of the notifiable infectious diseases in China, with the top one death report of all infectious diseases, which severely threaten the health of the Chinese people.3 China has also been recognized by the World Health Organization (WHO) as one of the countries with a high risk of rabies epidemic. Due to the high number of animal bites, there is an increasing demand for safe and cost-effective rabies vaccines in China.
Contrary to other human infectious diseases, timely and effectively rabies vaccination could prevent rabies even after exposure to the virus. Therefore, vaccination is considered an effective way to prevent rabies after post-exposure. Purified vero cell rabies vaccine (PVRV) was first approved to be used in humans in 1992.4 Because PVRV has both excellent immunogenicity and safety, it is recommended to be used for human rabies prevention by the WHO.5,6 Since the year 2001, PVRV has been successfully manufactured in China. Liaoning ChengDa company has received approval from the Health Ministry of China and the State Food and Drug Administration of China (SFDA) in 2004 and began to sell across China and widely used.
Now, the WHO recommended two intramuscular post-exposure prophylaxis regimens: the Essen regimen (1-1-1-1-1), a 5-dose regimen that one dose of the vaccine was given on each of D0, D3, D7, D14, and D28; the Zagreb regimen (2-1-1), a 4-dose regimen that two doses of the vaccine were given on D0 (one dose at each of the arms), followed by one dose of vaccine given on D7 and D21.6 According to the previous research, both regimens performed well in safety and immunogenicity.7–9 Zagreb regimen was promoted in clinical application because of the fewer injections, earlier protection titers, cost-effective and convenient operation.10,11 The PVRV manufactured by Chengda Biotechnology has been most extensively used in China. But this vaccine under the Zagreb regimen was not approved to be used until 2010.12 Although the Zagreb regimen has been approved by the SFDA later, it still lacked direct evidence to prove the safety, immunogenicity, and antibody persistence of this regimen.
This study evaluated the safety and immunogenicity of a new freeze-dried vero cell rabies vaccine produced in China by using a randomized, double-blind, phase 3 clinical trial and compared it with the rabies vaccine manufactured by Liaoning Chengda Biotechnology Co., Ltd. (Speeda).
Results
Study population
The trial profile is shown in Figure 1. Overall, 1800 subjects were enrolled and evenly distributed into three groups (Zagreb group, Essen group, and control group), of whom 1798 (99.9%) had vaccinated at least one dose against rabies and entered the safety set (SS) and 1600 (88.9%) of these subjects had finished the study and entered the per-protocol set (PPS). Reasons why some subjects were not included in the PPS were voluntary withdrawal due to missing visits, serious adverse events (AEs), incomplete full-course vaccination or blood collection after vaccination; protocol violation due to not meeting inclusion/exclusion criteria, incorrect vaccination, and failure to vaccinate or collect blood at the prescribed time.
Figure 1.
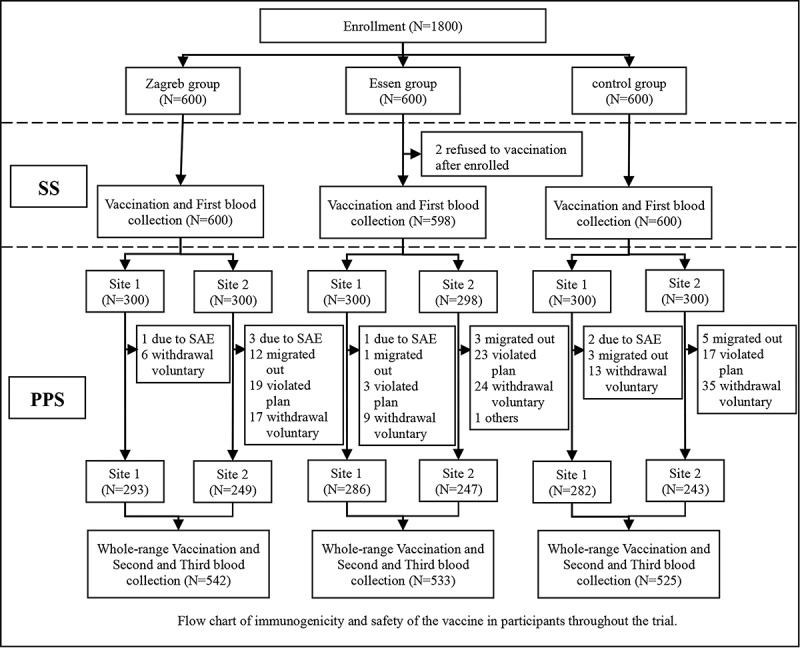
Flowchart of immunogenicity and safety of the vaccine in the subjects throughout the trial
The baseline demographic characteristics are presented in Table 1. There was no statistical difference for gender and age distribution among the three groups and between the subgroups of site 1 (7-d blood collection group) and site 2 (14-d blood collection group). The distributions of gender and age for SS and PPS were comparable.
Table 1.
Summary of demographic characteristics of subjects enrolled in the study
| Cohort | Characteristics | SS |
p | PPS |
p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Zagreb group |
Essen group |
Control group |
Zagreb group |
Essen group |
Control group |
||||
| Total | Gender, n (%) | ||||||||
| Male | 298 (49.7) | 290 (48.5) | 264 (44.0) | 0.1161 | 264 (48.7) | 256 (48.0) | 227 (43.2) | 0.1507 | |
| Female | 302 (50.3) | 308 (51.5) | 336 (56.0) | 278 (51.3) | 277 (52.0) | 298 (56.8) | |||
| Age, y | |||||||||
| Mean ± SD | 31.9 ± 12.5 | 32.1 ± 12.7 | 33.1 ± 12.4 | 0.2136 | 32.0 ± 12.4 | 31.6 ± 12.7 | 33.2 ± 12.4 | 0.1468 | |
| Median | 34.3 | 34.7 | 36.2 | 34.3 | 34.0 | 36.9 | |||
| Site 1 | Gender, n (%) | ||||||||
| Male | 154 (51.3) | 144 (48.0) | 136 (45.3) | 0.3376 | 149 (50.9) | 140 (49.0) | 126 (44.7) | 0.3183 | |
| Female | 146 (48.7) | 156 (52.0) | 164 (54.7) | 144 (49.2) | 146 (51.1) | 156 (55.3) | |||
| Age, y | |||||||||
| Mean ± SD | 32.6 ± 11.8 | 32.2 ± 12.4 | 33.3 ± 11.8 | 0.4891 | 32.7 ± 11.8 | 32.3 ± 12.3 | 33.3 ± 11.8 | 0.6085 | |
| Median | 34.6 | 34.7 | 36.2 | 34.7 | 34.9 | 36.2 | |||
| Site 2 | Gender, n (%) | ||||||||
| Male | 144 (48.0) | 146 (49.0) | 128 (42.7) | 0.2482 | 115 (46.2) | 116 (47.0) | 101 (41.6) | 0.4308 | |
| Female | 156 (52.0) | 152 (51.0) | 172 (57.3) | 134 (53.8) | 131 (53.0) | 142 (58.4) | |||
| Age, y | |||||||||
| Mean ± SD | 31.3 ± 13.1 | 31.9 ± 13.1 | 32.9 ± 13.0 | 0.3264 | 31.1 ± 13.1 | 31.3 ± 13.1 | 33.1 ± 13.2 | 0.1882 | |
| Median | 33.4 | 33.9 | 36.6 | 32.6 | 32.8 | 38.2 | |||
Safety
The total incidence of AEs was 30.7%, 36.8%, and 32.3%, respectively, in the Zagreb group, Essen group, and control group, and there was no significant difference among the three groups (p = 0.0677). The incidence of solicited AEs was 28.2%, 34.3%, and 29.2%, respectively, in the three groups, and there was a significant difference among the three groups (p = 0.0498). The incidence of solicited AEs was higher in the Essen group than that in the control group and Zagreb group (p = 0.0278, p = 0.0248).
A total of 256 subjects had 402 local reactions, including 84 (14.0%) in Zagreb group, 100 (16.7%) in Essen group, and 72 (12.0%) in control group. The incidence of local reactions was not significantly different among the three groups (p = 0.0658). According to the statistics of inoculation times, the incidence of local reaction was 4.9%, 6.0%, and 4.1% in the Zagreb group, Essen group, and control group, respectively. The incidence of the main symptoms of local reactions in the trial was pain, erythema, itch, swell, and induration from high to low. The incidence of itch was significantly different among the three groups (p = 0.0140), of which was no significant difference between the Essen group and the control group (p = 0.1440), while Essen group was higher than Zagreb group (p = 0.0037). The incidence of other symptoms was not different among groups. Almost all local reactions occurred within 3 d after vaccination (99.6%).
A total of 410 subjects had 676 systemic reactions, including 123 (20.5%) in the Zagreb group, 151 (25.3%) in the Essen group, and 136 (22.7%) in the control group. The incidence of systemic reactions was not significantly different among the three groups (p = 0.1478). According to the statistics of inoculation times, the incidence of systemic reaction was 8.4%, 9.0%, and 7.9% in the Zagreb group, Essen group, and control group, respectively. The incidence of the main symptoms of systemic reaction in the trial was fever, headache, and fatigue from high to low. The incidence of all symptoms was not significantly different among groups. 93.9% of systemic reactions occurred within 3 d after vaccination.
AEs in the Zagreb group, Essen group, and control group are shown in Table 2.
Table 2.
Adverse event list after any vaccination
| Zagreb group (N = 600) |
Essen group (N = 598) |
Control group (N = 600) |
Total | p | |
|---|---|---|---|---|---|
| Total AEs, n (%) | 184 (30.7) | 220 (36.8) | 194 (32.3) | 598 (33.3) | 0.0677 |
| Solicited AEs, n (%) | 169 (28.2) | 205 (34.3) | 175 (29.2) | 549 (30.5) | 0.0498 |
| Local AEs, n (%) | 84 (14.0) | 100 (16.7) | 72 (12.0) | 256 (14.2) | 0.0658 |
| Pain | 78 (13.0) | 86 (14.4) | 60 (10.0) | 224 (12.5) | 0.0605 |
| Erythema | 16 (2.7) | 19 (3.2) | 14 (2.3) | 49 (2.7) | 0.6507 |
| Itch | 5 (0.8) | 19 (3.2) | 11 (1.8) | 35 (2.0) | 0.0140* |
| Swell | 8 (1.3) | 9 (1.5) | 5 (0.8) | 22 (1.2) | 0.5543 |
| Induration | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.1) | 0.3326 |
| Systemic AEs, n (%) | 123 (20.5) | 151 (25.3) | 136 (22.7) | 410 (22.8) | 0.1478 |
| Fever | 94 (15.7) | 116 (19.4) | 108 (18.0) | 318 (17.7) | 0.2319 |
| Headache | 33 (5.5) | 37 (6.2) | 27 (4.5) | 97 (5.4) | 0.4146 |
| Fatigue | 20 (3.3) | 25 (4.2) | 22 (3.7) | 67 (3.7) | 0.7275 |
| Myalgia | 5 (0.8) | 11 (1.8) | 6 (1.0) | 22 (1.2) | 0.2621 |
| Arthralgia | 2 (0.3) | 7 (1.2) | 2 (0.3) | 11 (0.6) | 0.1077 |
| Nausea | 8 (1.3) | 8 (1.3) | 7 (1.2) | 23 (1.3) | 0.9660 |
| Allergy | 2 (0.3) | 6 (1.0) | 7 (1.2) | 15 (0.8) | 0.2286 |
| Unsolicited AEs, n (%) | 35 (5.8) | 45 (7.5) | 39 (6.5) | 119 (6.6) | 0.4982 |
*The incidence of itch: Essen group vs. control group p = 0.1440, Essen group vs. Zagreb group p = 0.0037.
The incidence of solicited AEs after each injection in Zagreb group, Essen group, and control group is compared in Table 3. The incidence of solicited AEs after each injection in Essen group was 19.9%, 11.3%, 9.5%, 6.4%, and 2.9%, respectively, and that in control group was 18.7%, 8.1%, 7.9%, 6.4%, and 1.7%, respectively. There was no significant difference between the two groups. The incidence of solicited AEs was 23.2%, 7.5%, and 3.2%, respectively, on d 0, d 7, and d 21 in Zagreb group. There was no significant difference in the incidence of solicited AEs after each injection between Zagreb group and the corresponding dose in Essen group. Adverse reactions in each group occurred mainly after the first injection and did not increase with the number of injections.
Table 3.
Incidence of solicited AEs of each vaccination
| Vaccination injection | Zagreb group (%) | Essen group (%) | control group (%) | p |
|---|---|---|---|---|
| D 0 | 23.2 (139/600) | 19.9 (119/598) | 18.7 (112/600) | 0.1387 |
| D 3 | - | 11.3 (65/576) | 8.1 (46/567) | 0.0729 |
| D 7 | 7.5 (43/573) | 9.5 (54/568) | 7.9 (44/556) | 0.4363 |
| D 14 | - | 6.4 (36/563) | 6.4 (35/550) | 1.0000 |
| D 21/28 | 3.2 (18/561) | 2.9 (16/559) | 1.7 (9/544) | 0.2322 |
Immunogenicity
The seroconversion rate of antibodies after vaccination in the three groups was compared in Table 4. On d 7, the seroconversion rates of antibodies in the subjects who were seronegative at baseline were 48.5%, 57.6%, and 42.2%, respectively, in Zagreb group, Essen group, and control group. The seroconversion rates of antibodies were significantly different among the three groups (p = 0.0015). The inter-group comparison indicated that the seroconversion rate of antibodies was higher in Zagreb group than that in Essen group (p = 0.0333), of which was no significant difference between Essen group and control group (p = 0.1433). On d 14 and d 35/42, the seroconversion rates of the three groups were all 100.0%. There was no significant difference among the three groups (p = 1.0000).
Table 4.
Comparing positive conversion rate of antibody after vaccination
| Zagreb group |
Essen group |
Control group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Time | N | % (n) | 95% CI | N | % (n) | 95% CI | N | % (n) | 95% CI | p |
| Seronegative before vaccination | D 7 | 276 | 57.6 (159) |
51.54–63.51 | 270 | 48.5 (131) |
42.42–54.65 | 263 | 42.2 (111) |
36.16–48.42 | 0.0015* |
| D 14 | 248 | 100.0 (248) |
98.52–100.0 | 241 | 100.0 (241) |
98.48–100.0 | 238 | 100.0 (238) |
98.46–100.0 | 1.0000 | |
| D 35/42 | 524 | 100.0 (524) |
99.30–100.0 | 511 | 100.0 (511) |
99.28–100.0 | 501 | 100.0 (501) |
99.27–100.0 | 1.0000 | |
| Seropositive before vaccination | D 7 | 17 | 94.1 (16) |
71.31–99.85 | 16 | 81.3 (13) |
54.35–95.95 | 19 | 79.0 (15) |
54.43–93.95 | 0.4491 |
| D 14 | 1 | 100.0 (1) |
2.50–100.0 | 6 | 66.7 (4) |
22.28–95.67 | 5 | 100.0 (5) |
47.82–100.0 | 0.5455 | |
| D 35/42 | 18 | 100.0 (18) |
81.47–100.0 | 22 | 90.9 (20) |
70.84–98.88 | 24 | 100.0 (24) |
85.75–100.0 | 0.1905 | |
| Total participants |
D 7 | 293 | 59.7 (175) |
53.87–65.39 | 286 | 50.4 (144) |
44.40–56.29 | 282 | 44.7 (126) |
38.78–50.69 | 0.0013** |
| D 14 | 249 | 100.0 (249) |
98.53–100.0 | 247 | 99.2 (245) |
97.11–99.90 | 243 | 100.0 (243) |
98.49–100.0 | 0.2192 | |
| D 35/42 | 542 | 100.0 (542) |
99.32–100.0 | 533 | 99.6 (531) |
98.65–99.95 | 525 | 100.0 (525) |
99.30–100.0 | 0.2184 | |
* Essen group vs. Zagreb group p = 0.0333, Essen group vs. control group p = 0.1433.
** Essen group vs. Zagreb group p = 0.0233, Essen group vs. control group p = 0.1762.
On d 14, the rate difference of antibody seroconversion rate between the Essen group and control group was 0.00% (97.5% CI, −1.80% to 1.82%), of which was both 0.00% (97.5% CI, −1.80% to 1.75%) between Zagreb group and Essen/control group. The results showed that Essen group was not inferior to control group, and Zagreb group was not inferior to Essen group on d 14.
The geometric mean concentration (GMC) of the three groups was compared in Table 5. The GMC before vaccination was low and similar between Zagreb group, Essen group, and control group. On d 7, the GMC in the subjects who were seronegative at baseline was 0.57, 0.48, and 0.40 IU/ml, respectively, and was significantly different among the three groups (p = 0.0105). However, the intergroup comparison found that there was no significant difference between Zagreb group and Essen group (p = 0.1179) and the same between Essen group and control group (p = 0.1520).
Table 5.
Comparing GMC before and after vaccination
| RVNA concentration before the first vaccination (IU/ml) (baseline) |
RVNA concentration after vaccination (IU/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group | N | GMC | 95% CI | p | GMC | 95% CI | p | |
| Site 1 (7-d blood collection group) | Zagreb group | 293 | 0.08 | 0.07–0.08 | 0.9653 | 0.70 | 0.59–0.83 | 0.0298* |
| Essen group | 286 | 0.08 | 0.07–0.08 | 0.57 | 0.48–0.68 | |||
| Control group | 282 | 0.08 | 0.07–0.08 | 0.50 | 0.42–0.60 | |||
| Site 2 (14-d blood collection group) | Zagreb group | 249 | 0.06 | 0.06–0.07 | 0.5900 | 42.92 | 39.36–46.81 | 0.3655 |
| Essen group | 247 | 0.07 | 0.06–0.07 | 40.06 | 36.44–44.04 | |||
| Control group | 243 | 0.07 | 0.06–0.07 | 43.78 | 39.91–48.02 | |||
| Total (D 35/42) | Zagreb group | 542 | 0.07 | 0.07–0.07 | 0.7707 | 25.70 | 24.22–27.27 | 0.4010 |
| Essen group | 533 | 0.07 | 0.07–0.08 | 25.11 | 23.61–26.71 | |||
| Control group | 525 | 0.07 | 0.07–0.08 | 26.63 | 25.05–28.32 | |||
*Zagreb group vs. Essen group p = 0.1150, Essen group vs. control group p = 0.2912.
Blood samples for immunogenicity were collected from each subject prior to the vaccination on d 0, 7, and 14.
On d 14, the GMC was 42.86, 39.82, and 43.29 IU/ml, respectively. On d 35/42, the GMC was 25.22, 24.71, and 25.89 IU/ml, respectively. Thus, on d 14 and d 35/42, the GMC of the three groups was much higher than the immune protection level of 0.5 IU/ml. The GMC was not significantly different among the three groups on d 14 and d 35/42 (p = 0.3866, p = 0.5750). All the three groups showed that d 14 showed the highest GMCs, and d 35/42 showed a decrease, but nodifferences among groups.
Discussion
Vaccination is an efficacious way of preventing rabies. In the current world, many approved rabies vaccines of different components (such as PVRV, PCECV, and HDCV13) and different vaccination programs (Zagreb, Essen11,12) are being widely used. This study evaluated the immunogenicity and safety of a new trial PVRV in healthy subjects comparing with the control PVRV (Speeda) following Essen regimen and contrasted the safety and immunogenicity of the trial vaccine between Zagreb regimen of 0, 7, and 21 d and Essen regimen.
The total incidence of AEs was not significantly different among Zagreb group, Essen group, and control group. However, from the incidence of solicited AEs, Essen group was higher than that in control group and Zagreb group. Adverse reactions in each group occurred mainly after the first injection and occurred mainly within 3 d after vaccination (96.1%). The incidence of local and systemic reactions was not different among the three groups. The main symptoms of local reaction were pain, and fever was the main symptoms of systemic reaction. For the safety analysis, pain of injection site and fever were the most general symptoms, respectively, in local and systemic reactions, which was in agreement with Hu.11 In addition, the incidence of solicited AEs was higher in Zagreb group than that in Essen and control groups after the first injection, possibly due to the two doses of the first injection in Zagreb group, and that was no significantly different among groups by statistical comparison, and also there was no intergroup difference after the 7th-day vaccination and last vaccination. Adverse reactions did not increase with the number of injections.
It has been reported internationally that rabies vaccine can rapidly produce protective antibodies by traditional Essen regimen, and the seroconversion rates of antibodies reached 100% on the 14th day after the first vaccination.14 As shown in the results of this study, in the subjects who were seronegative before vaccination, the seroconversion rates of antibodies of each group achieved 100% on d 14 and d 35/42 and at the same time attained RVNA concentrations ≥0.5 IU/ml, exceeding sufficient titers as specified by the WHO, which was in agreement with Wang.15 On d 14, the results of non-inferiority test showed that the Essen group was not inferior to the control group, and the Zagreb group was not inferior to the Essen group. On d 14 and d 35/42, the GMC was not significantly different between Essen group and control group, while it was not lower in Zagreb group than that in Essen group. However, a significant difference could be observed between Zagreb group and Essen group on d 7 in terms of seroconversion rates of antibodies, demonstrating that the antibody induced by Zagreb regimen is much earlier than that by Essen regimen, which is similar to other studies concerning 5-dose vaccination.6 All the three groups showed that the 14th day after the first vaccination showed the highest GMCs, and the 14th day after the last vaccination showed a decrease, but no differences among the groups.
Materials and methods
Study design and objectives
This study was designed by Hubei Provincial Center for Disease Control and Prevention (HBCDC) and Xianshengweike Biopharmaceutical Co., Ltd. (the study sponsor). The trial was performed at two research sites (site 1: Dangyang city and site 2: Tuanfeng county) from December 2014 to March 2017. The clinical trial protocol was approved by the independent institutional review board of the HBCDC. Before enrollment, the informed consent was signed by each subject or the subject’s parent/legal guardian. This clinical trial was undertaken in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice guidelines, and Chinese regulatory requirements.
Subjects
We recruited and enrolled 1800 of healthy Chinese children and adults who were aged 10–50 y of both genders. The subjects were in good health and received physical examination before entering the study. Exclusion criteria included: history of rabies vaccination; history of allergy, convulsion, epilepsy, and psychiatric or neurological, family history of epilepsy; hypersensitivity to any vaccine component; diagnosis of known immune impairment or deficiency; splenectomy; a fever higher than 38°C (axillary) in the past 3 d; history of congenital malformation; developmental disorder; severe chronic disease; severe cardiovascular disease; diabetes; uncontrollable hypertension; hepatopathy and malignant tumor; inoculation of any vaccine, antiserum, and immunoglobulin preparation in the past month; acute or chronic infectious disease, active infection, severe asthma, and infectious dermatosis; pregnant or breastfeeding women; and any situation that the researchers believe may affect trial evaluation.
Vaccines
The trial rabies vaccines (vero cell, Lot no. 20140301) used in the study were manufactured in Xianshengweike Biopharmaceutical Co., Ltd. (Jiangsu, China), and the control rabies vaccines (vero cell, Lot no. 201404081) were manufactured in Liaoning Chengda Biotechnology Co., Ltd. (Shenyang, China). All the vaccines were freeze-dried vero cell rabies vaccine. The potency of the trial vaccines and control vaccines was 4.3 IU and 5.7 IU per 0.5 ml-ampule, respectively, which tested by the National Institutes for Food and Drug Control (NIFDC).
Procedures
The central stratified block randomized method was used. The length of the block was 6. According to the number of treatment groups, centers, and the number of subjects in each center, the procedure plan of SAS statistical software was used to set up the block length and other parameters and generate the random coding table. 1800 subjects were randomly vaccinated with trial vaccine (Zagreb regimen, 2-1-1 or Essen regimen, 1-1-1-1-1) or control vaccine (Essen regimen, 1-1-1-1-1) according to 1:1:1 ratio. At present, there was no officially approved Zagreb regimen in China, so there was no Zagreb regimen group in the control group. The Essen group was set up with a control group and implement double-blinding, but the Zagreb group could not set up a control group, so it could only be double-blind before randomization. The three vaccine preparations had identical packaging and were blindly labeled with the sequential numbers.
The subjects were intramuscularly vaccinated at the deltoid muscle of the upper arms. In the Essen group, one dose of PVRV was given on d 0 (D0), d 3 (D3), d 7 (D7), d 14 (D14), and d 28 (D28), respectively. In the Zagreb group, two doses of PVRV were given on d 0 (D0) (one dose at each of the arms), followed by one dose of PVRV given on D7 and D21.
Safety assessment
After each dose of vaccination, the subjects were required to observe the immediate AEs for 30 min, and the frequency and severity of all solicited AEs were recorded for up to 7 d following each vaccination; unsolicited AEs were recorded throughout the study for 30 d. Solicited local AEs were erythema, itch, swell, induration, and pain at the site of injection; solicited systemic AEs included fever (defined as an axillary temperature ≥38°C), headache, fatigue, myalgia, arthralgia, nausea, and allergy. The relativity of local and systemic AEs to the investigational vaccine was judged by the investigator. AEs were graded according to the guiding principles of AE grading standards for clinical trials of preventive vaccines issued by the China Food and Drug Administration.16
Immunogenicity assessment
Blood samples for immunogenicity were collected from each subject immediately on d 0, 7/14, and 35/42 and prior to the vaccination on day 0, 7, and 14. For the ethical considerations, and reducing the blood drawing times as far as possible, we divided the Essen regimen into two subgroups: collecting blood samples on d 0, 7, and 42 and d 0, 14, and 42. The Zagreb regimen divided into two subgroups: collecting blood samples on d 0, 7, and 35 and d 0, 14, and 35. Among this, the objective of d 14 and 42 was to compare the immunogenicity of the Essen regimen group and the control group. D 7, 14, and 35/42 was to compare the immunogenicity of the Essen regimen group and the Zagreb regimen group. Blood samples were tested by the National Institutes for Food and Drug Control (Beijing, China), and RVNA concentration was measured by a Rapid Fluorescent Focus Inhibition Test (RFFIT). The rabies virus neutralizing antibody titer <0.5 IU/ml before immunization was considered negative, and the titer ≥0.5 IU/ml after immunization was positive.
Statistical analysis
We used SAS software (version 9.3) to perform statistical analysis. Statistical significance was defined as a p value ≤0.05. Continuous data were presented as mean ± standard deviation (SD) and median. Categorical data were tested with chi-square or Fisher’s exact test. ANOVA test for log-transformed GMC was used to compare the immunogenicity among these three groups. For the immunogenicity endpoint, PPS was used as the primary analysis set.
The non-inferiority test was confirmed by the following steps: on the 14th d after the fist vaccination, antibody positive rate of the Essen group and the Zagreb group was not less than 97%, and the Essen group was not inferior to the control group or the Zagreb group was not inferior to the Essen group. The adjusted one-sided test level α = 0.0125, and the non-inferiority lower limit was −5%, that is, 97.5% CI lower limit of the 14-d antibody positive rate (trial group–control group) was not lower than −5%, means that the trial group is not inferior to the control group.
Safety was analyzed based on safety set (SS) which for all subjects who had injected at least one dose of the vaccine. The incidence and the corresponding severity of AEs and adverse reactions in each group were calculated, and the rate of AEs (reactions) between groups was compared by Fisher’s exact probability method.
Funding Statement
The study was supported by the National Science and Technology Major Project of the Chinese Ministry of Science and Technology [Project No. 2018ZX09734004] and Health Commission of Hubei Province [Project No. WJ2017M135]. And Xianshengweike Biotechnology provided financial support.
Conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.World Health Organization . WHO Expert Consultation on Rabies. Third report. Geneva, Switzerland.: World Health Organization;2018. (WHO Technical Report Series, No.1012). [Google Scholar]
- 2.Shi N, Zhang Y, Zheng H, Zhu Z, Wang D, Li S, Li Y, Yang L, Zhang J, Bai Y, et al. Immunogenicity, safety and antibody persistence of a purified vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects. Hum Vaccin Immunother. 2017;13(6):1338–45. doi: 10.1080/21645515.2017.1279770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinese center for disease control and prevention . Technical guidelines for human rabies prevention and control. Chin J Viral Dis. 2016;6(3):161–88. [Google Scholar]
- 4.WHO Expert Committee on Rabies . Guide for post-exposure treatment. Eighth report. WHO technical report 824. Geneva Switzerland: World Health Organization; 1992. [Google Scholar]
- 5.Huazhuang L, Guihua H, Qing C, Huai P.. Adverse reaction and immune effect of domestic adjuvant-free rabies vaccine prepared with vero cells. Chin J Biol. 2008;21:327–28. [Google Scholar]
- 6.Li Z, Jun G, Jianying H, Zhumu B, Deming Y. Safety and immune effect of adjuvant-free rabies vaccine. Chin J Biol. 2006;19:206–07. [Google Scholar]
- 7.Ma J, Wang H, Li J, Chang L, Xie Y, Liu Z, Zhao Y, Malerczyk C. A randomized open-labeled study to demonstrate the non-inferiority of purified chick-embryo cell rabies vaccine administered in the zagreb regimen (2-1-1) compared with the essen regimen in chinese adults. Hum Vaccin Immunother. 2014;10:2805–12. PMID:25483635. doi: 10.4161/21645515.2014.972773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahendra BJ, Narayana DA, Agarkhedkar S, Ravish HS, Harish BR, Agarkhedkar S, Madhusudana SN, Belludi A, Ahmed K, Jonnalagedda R, et al. Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen). Hum Vaccin Immunother 2015;11:428–34. doi: 10.4161/21645515.2014.995059. PMID:25692792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Li Y, Wen S, Wen H, Nong Y, Mo Z, Xie F, Pellegrini M. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study. Hum Vaccin Immunother. 2015;11:435–42. PMID:25692350. doi: 10.4161/21645515.2014.994460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CL, Zhang XW, Yu YX. Study on the compliance and economic cost of rabies vaccination. Zhong Guo Yi Miao He Mian Yi. 2010;16:254–57. PMID:20726270. [PubMed] [Google Scholar]
- 11.Hu Q, Liu MQ, Zhu ZG, Zhu ZR, Lu S. Comparison of safety and immunogenicity of purified chick embryo cell vaccine using Zagreb and Essen regimens in patients with category II exposure in China. Hum Vaccin Immunother. 2014;10:1645–49. PMID:24632727. doi: 10.4161/hv.28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Huang G, Tang Q, Li J, Cao S, Fu C, Cao Q, Liu B, Pan H, Wang M, et al. The immunogenicity and safety of vaccination with purified vero cell rabies vaccine (PVRV) in China under a 2-1-1 regimen. Hum Vaccin Immunother 2011;7:220–24. doi: 10.4161/hv.7.2.14003. PMID:21311216. [DOI] [PubMed] [Google Scholar]
- 13.Verma R, Khanna P, Prinja S, Rajput M. Intra-dermal administration of rabies vaccines in developing countries: at an affordable cost. Hum Vaccin Immunother. 2011;7:792–94. doi: 10.4161/hv.7.7.15410. [DOI] [PubMed] [Google Scholar]
- 14.Schelermann N, Baer J, Hilfenhaus J, Marcus I, Zoulek G. Reactogenicity and immunogenicity of the newly developed purified chick embryo cell (PCEC) rabies vaccine in man. Zbl Bakt Hyg. 1987;265(3–4):439–50. [DOI] [PubMed] [Google Scholar]
- 15.Wang J, Luo F, Feng Z, Li L, Bai Y, Ai X, Ma J, Zhang Z, Shi N. Immunogenicity and safety of purified vero cell rabies vaccine (PVRV) produced by Liaoning Cheng Da Co. under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese adults aged 50 and above. Hum Vaccin Immunother. 2017;13(1):144–50. doi: 10.1080/21645515.2016.1230260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.China Food and Drug Administration . The standard guidelines for adverse reactions grading of vaccine clinical trials. 2005. October 14. http://www.nmpa.gov.cn/WS04/CL2138/373037.html


