Abstract
Premature mortality and increased physical comorbidity associated with bipolar disorder (BD) may be related to accelerated biological aging. Sleep disturbances and inflammation may be key mechanisms underlying accelerated aging in adults with BD. To our knowledge, these relationships have not been examined rigorously. This cross-sectional study included 50 adults with BD and 73 age- and sex-comparable non-psychiatric comparison (NC) subjects, age 26–65 years. Participants were assessed with wrist-worn actigraphy for total sleep time (TST), percent sleep (PS), and bed/wake times for 7 consecutive nights as well as completing scales for subjective sleep quality. Within-individual variability in sleep measures included intra-individual standard deviation (iSD) and atypicality of one evening’s sleep. Blood-based inflammatory biomarkers included interleukin (IL)-6, C-reactive protein (CRP), and tumor necrosis factor-alpha (TNF-α). Linear regression analyses tested relationships of mean and iSD sleep variables with inflammatory marker levels; time-lagged analyses tested the influence of the previous evening’s sleep on inflammation. BD participants had worse subjective sleep quality, as well as greater TST iSD and wake time iSD compared to the NC group. In all participants, higher TST iSD and lower mean PS were associated with higher IL-6 levels (p=.04, ηp2=.042; p=.05, ηp2=.039, respectively). Lower mean PS was associated with higher CRP levels (p=.05, ηp2=.039). Atypicality of the previous night’s TST predicted next day IL-6 levels (p = 0.05, ηp2 = 0.04). All of these relationships were present in both BD and NC groups and remained significant even after controlling for sleep medications. Overall, sleep measures and their variability may influence inflammatory markers in all adults. Thus, sleep may be linked to the inflammatory processes believed to underlie accelerated aging in BD.
Keywords: interleukin-6, total sleep time, serious mental illness
Introduction
Bipolar disorder (BD) is a serious mental illness that affects 1% of adults (Harvard Medical School, 2007; Merikangas et al., 2007) and is associated with serious functional impairment(Kessler et al., 2005) as well as premature and higher mortality due to age-related chronic diseases (Dev et al., 2017; Fries et al., 2017; Lomholt et al., 2019; Powell et al., 2018; van den Ameele et al., 2018; Yang et al., 2018). Inflammation is a key biological process that may underlie biological aging and increased mortality in BD (Rosenblat and McIntyre, 2017; Sayana et al., 2017); h wever, the exact links between inflammatory cytokines, behavior, and psycho athology have not been fully elucidated.
Sleep disturbance may be a potential mechanism of inflammaging in adults with BD. Sleep disruptions are integr to the primary psychopathology of BD (Harvey et al., 2009), as decreased need for sleep is a cardinal symptom of mania, and insomnia/hypersomnia are often associated with depressive episodes. Key sleep features occurring with manic episodes include reduced REM latency and shortened total sleep time (TST) while sleep features of depressive episodes include increased fragmentation of REM sleep and greater percentage of time awake (Gold and Sylvia, 2016). Even when the mood symptoms remit, persons with BD continue to report worse sleep quality and objectively can have more disturbed sleep (e.g., lower sleep efficiency) compared with the general population (Ng et al., 2015; Robillard et al., 2015). Furthermore, within the general population and medically ill patients, self-reported sleep disturbances and sleep deprivation have been associated with higher levels of commonly studied pro-inflammatory peripheral cytokines [e.g., higher Interleukin-(IL-)6, C-Reactive protein (CRP), and TNF-(Tumor Necrosis Factor-)α levels].(Irwin, 2015) Sleep deprivation for a single night can significantly impact inflammatory markers the following morning (Irwin et al., 2006). Therefore, both chronic and acute sleep disturbances may affect inflammatory measures and warrant examination.
Intra-individual variability and irregularity of sleep and circadian activity are also characteristic of persons with BD. Adults with BD have increased variability of actigraphic sleep measures (TST and sleep efficiency) (Ngetal.,2015; Robillard et al., 2015) as well as delayed (Robillard et al., 2015) and decreased circadian activity amplitude (Salvatore et al., 2008). Increased variability sleep measures were observed in persons with inter-episode BD (Jones et al., 2005; Millar et al., 2004; Ng et al., 2015). Increased variability of sleep and circadian disturbances has been associated with poor outcomes including more severe manic and depressive symptoms (Kaufmann et al., 2016), greater mood variability (Carr et al., 2018; Melo et al., 2017), medication adherence (Kaufmann et al., 2016), working memory and verbal learning performance (Kanady et al., 2017) among adults with BD. In the general population, sleep variability in objective measures (i.e., TST, Bed time, Wake time) has been linked with increased cytokine levels (most commonly IL-6, but also CRP, TNF- α in a few studies) (Okun et al., 2011; Park et al., 2016). Understanding the link between sleep variability and inflammation may be important for developing interventions that improve functioning and decrease the rate of aging in BD. Notably, one study found that sleep variability was modifiable and improvements in sleep variability post-treatment were associated with improved cognitive performance in adults with BD (Kanady et al., 2017). It is not known if these same findings are seen with regards to inflammation. To our knowledge, this is the first investigation of the links between inflammation and variability of objective sleep measures among persons with BD.
In the current study, we examined subjective and objective sleep measures in adults with BD and non-psychiatric comparison (NC) subjects. We hypothesized that relative to NCs, persons with BD would have higher levels of inflammatory markers (IL-6, CRP, and TNF-α), worse sleep measures (worse sleep quality, lower percent sleep [PS], shorter TST, later Bed time and later Wake time) and increased variability of objective sleep measures. Furthermore, based on the findings in the published literature, we hypothesized that worse mean and increased variability of objective sleep measures would be associated with higher IL-6 levels in BD and NC groups. We also explored the relationships of CRP and TNF-α levels with the subjective and objective sleep measures. In the BD group, we explored the role of mood symptoms and psychotropic medication load on the sleep-inflammation relationships. Last, we explored the relationship of single night’s sleep on the following morning’s inflammatory assessment, using time-lagged analyses.
Materials and Methods
Subjects
All subjects (adults with BD and age- and sex-comparable NCs, age 26–65 years) were participants in an ongoing longitudinal study of inflammaging in BD. Participants were recruited using sex- and age-binning to ensure similar distributions in the BD and NC groups. The protocol was approved by the University of California, San Diego (UCSD) Human Research Protections Program, and all participants provided written informed consent. All participants were English-speaking and lived in the greater San Diego area. BD diagnosis was based on the Structured Clinical Interview for the DSM-IV-TR (SCID).(First, November 2002) Subjects were excluded for the following: 1) other current DSM-IV-TR Axis I diagnoses; 2) acute illness or pregnancy; 3) recent vaccination; 4) history of neurodegenerative or a major neurological illness; 5) history of cancer treatment; and 6) uncontrolled medical illness affecting a subject’s ability to complete study procedures. All subjects were assessed annually, using a two-week burst design, during which they completed up to 14-days wrist-worn actigraphy assessments and three in-clinic assessments for inflammatory biomarker levels among other assessments. Subjects with at least 7 days/nights actigraphy data were included to ensure enough data for the intra-individual variability analyses.
Interviews
Subjects were interviewed by trained study staff and completed the following standardized assessments for: overall psychopathology (Brief Psychiatric Rating Scale or BPRS), manic symptoms (Young Mania Rating Scale or YMRS), depressive symptoms (Hamilt Depression Rating Scale or HAM and Patient Health Questionnaire-9 item or PHQ-9), mental well-being (Short Form Health Survey - Mental), and physical well-being (Short Form Health Survey - Physical) (Hamilton, 1960; Kroenke et al., 2001; Ventura et al., 2000; Ware and Sherbourne, 1992; Young et al., 1978). Subjects were interviewed about their current medications, and current medication load for all psychotropic medications was calculated based on dosages as previously described (Hassel et al., 2008). Specific sleep-related medication use was assessed using the item #7 of Pittsburgh Sleep Quality Index (Buysse et al., 1989): “During the past month, how often have you taken medicine (prescribed or “over the counter”) to help you sleep?” The responses were dichotomized as Never versus Ever use of sleep medications. BMI was calculated from the participant’s measured height and weight, which were assessed in the clinic.
Sleep assessments
Self-reported sleep quality was measured using the Pittsburgh Sleep Quality Index (Buysse et al., 1989). Objective sleep measures from wrist-worn actigraphy were assessed for at least seven 24-hour periods (days and nights) using the actisleep-BT (Actigraph, Pensacola, FL), a tri-axial accelerometer. F subjects with more than 7 days/nights of objective sleep data, only the first seven 24-hour periods were included in the analyses. The data were analyzed using validat d algorithms to determine PS, TST, Bed time and Wake time (Ancoli-Israel et al., 2003). PS is the percentage of time in bed that is spent sleeping. TST is the aggregated number of minutes spent sleeping during an overnight sleep bout. Bed time and Wake time reflect the times that overnight sleep is initiated and terminated, respectively. Participants completed a nightly sleep log with bed-time and wake-times which were used to edit the actigraphy data.
Sleep variability
Within-subject variability in sleep measures was assessed with intraindividual standard deviation (iSD) across the seven days/nights of actigraphy data (Taylor et al., 2016). We also studied more acute influences of variability by conducting time-lagged analyses of the prediction of next day inflammation by the previous night’s sleep atypicality. For these analyses, sleep atypicality (a measure of how atypical the previous evening’s sleep was relative to the mean values) was calculated by squaring the difference between each individual’s mean sleep value and their sleep value of the previous night.(Kaufmann et al., 2016). Larger atypicality values indicate an abnormal night of sleep for that individual and smaller atypicality values indicate a normal night of sleep for that individual. Atypicality was assesed for each sleep measure (TST, PS, BT, and WT).
Inflammatory assessments
All blood-based biomarkers were assessed at three timepoints during the two-week burst. The values in this work represent the average of the three assay results.
Plasma IL-6, TNF-α, and CRP levels were quantified using Meso Scale Discovery (MSD) MULTI-SPOT® Assay System and analyzed on a SECTOR Imager 2400 instrument (Rockville, MD, USA). Using MSD Discovery Workbench® analysis software, standard curves were formed by fitting the electrochemiluminescence signal from calibrators to4-parameter logistic model with a 1/y2 weighting. All samples were run in duplicates, using V-PLEX Hum n Biomarker panels (Catalog # K151A0H-2) to measure the cytokines, and V-PLEX Human kit (Catalog # K15198D-1) for CRP. V-PLEX kits are fully validated according to fit-for-purpose principles and the FDA’s analytical validation guidelines according to the manufacturer (MSD). The laboratory technician performing the assays was “blinded” to the subject’s diagnosis. Intra-assay variability was <10% and inter-assay variability was <5% for both cytokines. The lowest detected levels for cytokines were: 0.05 pg/mL (IL-6) and 0.06 pg/mL (TNF-α). No sample showed cytokine levels below the detection limits. Intra- and inter-assay variability coefficients for CRP were <5%.
Statistical analyses
Variables were assessed for violation of distribution assumptions. Mann-Whitney U test, independent sample t-tests and chi-square tests were used to assess differences in sociodemographic, mental and physical health, cytokine, and sleep variables between the BD and NC groups. Variables with non-normal distributions were log-transformed for the regression analyses. Relationships between sleep measures over the 7-day time period and mean inflammatory biomarker levels (over 3 blood draws) were examined. General linear models were conducted to examine the relationship of sleep variability to cytokine levels, controlling for age, diagnostic group, sex, sleep medication use, and mean sleep measure. Major assumptions of these models included 1) linear relationships between sleep variability and cytokine levels, homoscedasticity, independence of sleep assessments between individuals, and normal distributions of log-transformed sleep variables and cytokine levels. We included the mean sleep measures in the models because iSD is often correlated with the mean, and we desired to observe relationships of inflammation with variability over and above any relationships to the mean sleep measures. Models were also run to examine the relationship of sleep quality (PSQI) to cytokine levels, controlling for age, diagnostic group, sleep medication use, and sex. Linear models also included mood symptoms and psychotropic medication load in the BD group only. Follow-up linear models with interaction terms diagnosis × sleep measures were run if there was a significant relationship between sleep and inflammation in the first model.
Follow-up time-lagged analyses were performed to examine the acute relationship between one night’s sleep and the following morning’s inflammatory marker levels. For the time-lagged models, sleep data were selected for one evening and the inflammatory assessment data from the following morning. Most participants had one or two datapoints with both sleep and inflammatory data; thus, for participants with multiple assessments that met this criterion, the first timepoint was selected for the analyses. In these models, the dependent variables were the inflammatory biomarker levels and the independent variables were the sleep characteristics – the previous night’s measure and the atypicality of that night’s measure (described above). All models also included age, diagnostic group, sex, and sleep medication use.
Significance was defined as α < 0.05 (two-tailed) all analyses and False Discovery Rate (FDR) was used to account for multiple comparisons in the regression models to ensure overall Type 1 error at α = 0.05. All analyses were conducted in SPSS Statistics for Windows, Version 26 (IBM Corp., Armonk, N.Y., USA).
Results
The total sample included 50 dults with BD and 73 NC subjects. Of all assessed participants, 5.3% of participant-visits (similar number of BD and NC participants) had less than 7 nights of data and were excluded from the analyses. The BD and NC groups were comparable in mean age as well as distribution of sex and race (Table 1). The BD group had lower mean years of education compared with the NCs. The BD group also had greater depressive symptoms and higher levels of IL-6 and TNF-α. The BD group also had worse reported sleep quality on the PSQI and were more likely to have ever used sleep-inducing medications. Though mean objective sleep values did not differ between the groups, the BD group had increased variability in TST and Wake Time.
Table 1:
Demographic comparison of bipolar disorder and non-psychiatric comparison groups
| Non-psychiatric comparison subjects | Bipolar disorder subjects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean or % | SD | N | Mean or % | SD | t or X2 | df | p | Cohen’s d | |
| Sociodemographic Factors | ||||||||||
| Age (yrs) | 73 | 48.3 | 8.3 | 50 | 48.1 | 9.0 | 0.17 | 121 | 0.87 | 0.03 |
| Sex (% women) | 58 | 62 | 0.25 | 1 | 0.62 | |||||
| Race (% Caucasian) | 44 | 56 | 2.37 | 1 | 0.31 | |||||
| Education (yrs) | 72 | 15.5 | 2.1 | 49 | 14.4 | 2.1 | ||||
| Sleep medication use (% never) | 90 | 27 | 48.9 | 1 | <0.001 | |||||
| Psychopathology | ||||||||||
| Duration of Illness (yrs) | 40 | 33.9 | 9.8 | |||||||
| Antipsychotic dose (mg/d) | 50 | 0.33 | 0.50 | |||||||
| Medication load | 46 | 3.15 | 2.12 | |||||||
| Overall Psychopathology (BPRS) | 37 | 40.4 | 8.2 | |||||||
| Manic symptoms (YMRS) | 38 | 6.9 | 5.4 | |||||||
| Clinician rated depressive symptoms (HAM) | 49 | 14.6 | 7.4 | |||||||
| Self-rated depressive symptoms (PHQ-9) | 61 | 1.2 | 1.9 | 33 | 9.5 | 5.7 | 1835.0a | <0.001 | ||
| Physical Health | ||||||||||
| BMI (Kg/m2) | 73 | 28.5 | 6.7 | 47 | 30.0 | 6.1 | −1.44 | 118 | 0.15 | −0.27 |
| Inflammatory Biomarkers | ||||||||||
| Interleukin-6 (pg/mL) | 64 | 0.7 | 0.5 | 50 | 0.9 | 0.7 | −2.38 | 112 | 0.02 | −0.45 |
| C-Reactive Protein (mg/L) | 64 | 3.06 | 4.55 | 50 | 5.33 | 7.97 | −1.28 | 112 | 0.20 | −0.24 |
| Tumor necrosis factor-α (pg/mL) | 64 | 2.2 | 0.7 | 50 | 2.7 | 0.8 | −3.46 | 112 | 0.001 | −0.65 |
| Sleep Measures | ||||||||||
| Subjective Sleep Quality (PSQI) | 66 | 5.20 | 2.67 | 47 | 9.96 | 3.75 | −7.17 | 111 | <0.001 | −1.40 |
| Actigraphic Mean Total Sleep Time (min) | 73 | 398.8 | 53.3 | 50 | 396.5 | 79.9 | 0.59 | 121 | 0.56 | 0.10 |
| Mean Percent sleep (%) | 73 | 85.6 | 5.7 | 50 | 85.3 | 7.4 | 0.32 | 121 | 0.75 | 0.05 |
| Mean Bed time (24-hour clock) | 73 | 22:58 | 72.1b | 50 | 23:33 | 103.0b | −2.15 | 121 | 0.03 | −0.38 |
| Mean Wake time (24-hour clock) | 73 | 6:52 | 83.7b | 50 | 7:26 | 107.4b | −1.86 | 121 | 0.07 | −0.34 |
| Sleep Variability Measures | ||||||||||
| iSD Total Sleep Time (min) | 73 | 7.46 | 4.41 | 50 | 8.23 | 4.96 | −2.84 | 121 | 0.005 | −0.52 |
| iSD Percent sleep (%) | 73 | 5.30 | 2.82 | 50 | 6.07 | 3.60 | −0.75 | 121 | 0.45 | −0.14 |
| iSD Bed time (min) | 73 | 54.1 | 29.3 | 50 | 75.2 | 74.7 | −2.01 | 121 | 0.05 | −0.36 |
| iSD Wake time (min) | 73 | 66.5 | 52.8 | 50 | 87.9 | 69.8 | −2.60 | 121 | 0.01 | −0.48 |
BMI = Body Mass Index, BPRS = Brief Psychiatric Rating Scale, HAM = Hamilton Depression Rating Scale, iSD = intra-individual Standard Deviation, PHQ-9 = Patient Health Questionnaire-9 item, PSQI = Pittsburgh Sleep Quality Index, YMRS = Young Mania Rating Scale
compared using Mann-Whitney U
standard deviation in minutes
Sleep measures and IL-6 levels
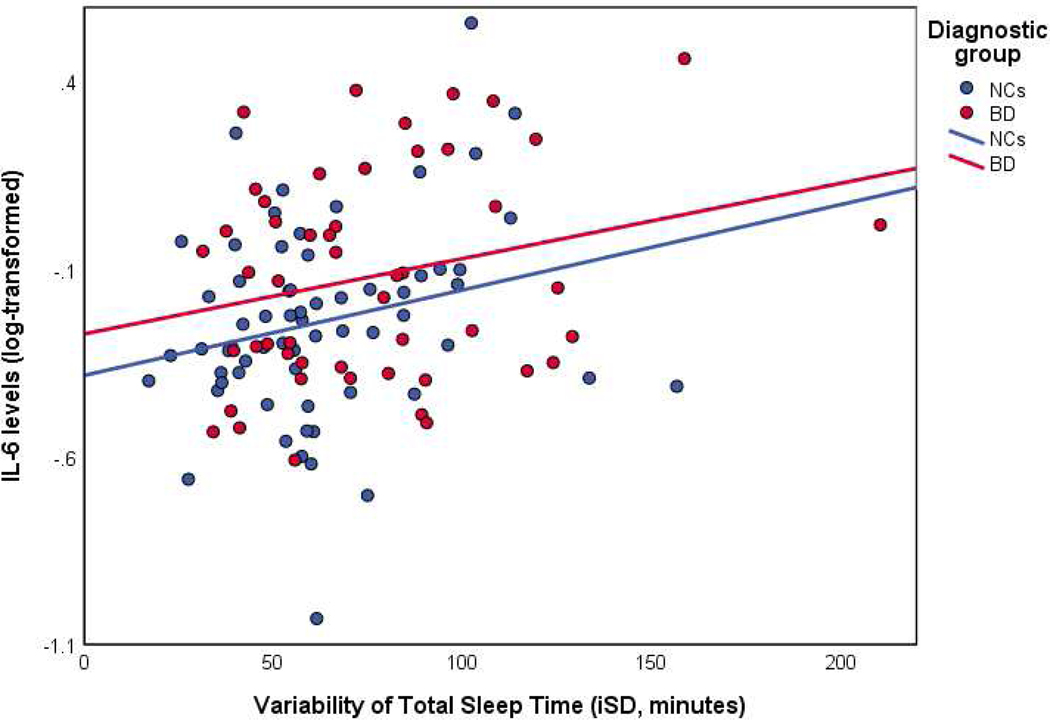
The general linear models found that variability of TST (iSD), but not mean TST, was significantly associated with IL-6 levels, such that greater variability of TST was linked with higher IL-6 levels, controlling for age, diagnostic group, sex, sleep medication use, and mean TST (Table 2). There was no significant main effect of diagnostic group in this model (B = −0.08, SE = 0.07, p = 0.27), although the general linear model of IL-6 without sleep variables (including only sex, diagnostic group and age as independent variables) did show a significant main effect diagnostic group (B = −0.12, SE = 0.05, p = 0.02, ηp2 = 0.05). A follow-up gene al linear model with the group × TST variability interaction found no significant interaction (B = −0.34, SE = 0.74, p = 0.65). The scatterplot of the relationship between TST and IL-6 levels showed similar relationships in the BD and NC groups (Figure 1). Due to high collinearity of depressive symptoms and psychotropic medication load with diagnostic group, these factors were examined within the BD group lo e, nd neither model found a change in the effect size of the association between IL-6 and TST variability after including the potential confounds.
Table 2:
General linear models for Interleukin-6 levels and objective sleep measures
| Interleukin-6 Levels | ||||
|---|---|---|---|---|
| B | SE | p* | ηp2 | |
| Total Sleep Time (TST) | ||||
| Sex (female) | 0.002 | 0.05 | 0.96 | <0.001 |
| Diagnostic Group (NC) | −0.08 | 0.07 | 0.27 | 0.012 |
| Sleep Medication (never) | −0.06 | 0.07 | 0.41 | 0.007 |
| Age | 0.003 | 0.003 | 0.40 | 0.007 |
| Mean TST | −0.45 | 0.35 | 0.20 | 0.016 |
| iSD TST | 0.30 | 0.14 | 0.04 | 0.042 |
| Percent Sleep (PS) | ||||
| Sex (female) | 0.01 | 0.05 | 0.79 | 0.001 |
| Diagnostic Group (NC) | −0.10 | 0.06 | 0.12 | 0.023 |
| Sleep Medication (never) | −0.06 | 0.07 | 0.33 | 0.009 |
| Age | 0.001 | 0.003 | 0.72 | 0.001 |
| Mean PS | −1.65 | 0.82 | 0.05 | 0.039 |
| iSD PS | 0.18 | 0.13 | 0.16 | 0.020 |
| Bed time | ||||
| Sex (female) | −0.03 | 0.05 | 0.58 | 0.003 |
| Diagnostic Group (NC) | −0.09 | 0.07 | 0.18 | 0.018 |
| Sleep Medication (never) | −0.05 | 0.07 | 0.46 | 0.005 |
| Age | 0.004 | 0.003 | 0.23 | 0.014 |
| Mean Bed time | 0.97 | 1.02 | 0.34 | 0.009 |
| iSD Bed time | 0.11 | 0.11 | 0.31 | 0.010 |
| Wake time | ||||
| Sex (female) | −0.03 | 0.05 | 0.61 | 0.003 |
| Diagnostic Group (NC) | −0.10 | 0.07 | 0.16 | 0.02 |
| Sleep Medication (never) | −0.06 | 0.07 | 0.40 | 0.007 |
| Age | 0.004 | 0.003 | 0.24 | 0.01 |
| Mean Wake time | 0.12 | 0.28 | 0.69 | 0.002 |
| iSD Wake time | 0.03 | 0.10 | 0.76 | 0.001 |
False discovery rate (FDR)-adjusted p-values
iSD = intra-individual standard deviation, NC = non-psychiatric comparison subjects
Figure 1: Scatterplot of total sleep time variability with Interleukin-6 (IL-6) levels in persons with bipolar disorder (BD) and non-psychiatric comparison subjects (NCs).
BD = bipolar disorder
IL-6 = interleukin-6
iSD = intra-individual standard deviation
NC = non-psychiatric comparison subjects
Lower mean PS was also significantly associated with higher IL-6 levels, with no significant group interaction (Table 2). No relationships of IL-6 with variability of PS, or mean and variability of Bed time and Wake time (controlling for sex, group, age, and sleep medication use) were observed.
Subjective sleep quality (PSQI) was not significantly associated with IL-6 levels (B = −0.10, SE = 0.07, p = 0.16), while controlling for sex, diagnostic group, age, and sleep medication use (data not shown).
Time-lagged analyses also found that greater atypicality of the previous evening’s TST (B = < 0.001, SE = < 0.001, p = 0.05, ηp2 = 0.04), but not the previous evening’s TST (B = < 0.001, SE = < 0.001, p = 0.56), was significantly associated with higher IL-6 levels, while controlling for sex, diagnostic group, age, and sleep medication use. The time-lagged analyses did not find a relationship between PS, Bed time, or Wake time and IL-6 levels (all p’s > 0.05). Sex, diagnostic group, age, psychotropic medication load, and sleep medication were not significantly associated with IL-6 levels in these models (all p’s > 0.05).
Sleep measures with CRP and TNF-α levels
The general linear models for CRP levels found that lower mean PS was associated with higher CRP levels (Table 3). Other mean and variability of sleep measures were not related to CRP levels when controlling for sex, diagnostic group, age, and sleep medication use. The time-lagged analysis did not find a significant relationship between previous night’s PS and CRP (B = −0.008, SE = 0.008, p = 0.28). However, time-lagged analysis did show small but significant relationships between greater TST atypicality and higher CRP levels (B <0.001, SE <0.001, p = 0.05) as well as between previous night’s Wake time and higher CRP levels (B = 0.002, SE = 0.001, p = 0.02).
Table 3:
General linear models for CRP and TNF-α levels with objective sleep measures
| CRP | TNF-α | |||||||
|---|---|---|---|---|---|---|---|---|
| B | SE | p | ηp2 | B | SE | p | ηp2 | |
| Total Sleep Time (TST) | ||||||||
| Sex (female) | 0.05 | 0.12 | 0.70 | 0.001 | −0.04 | 0.02 | 0.07 | 0.033 |
| Diagnostic Group (NC) | −0.16 | 0.15 | 0.29 | 0.011 | −0.08 | 0.03 | 0.009 | 0.065 |
| Sleep Medication (never) | 0.02 | 0.15 | 0.91 | <0.001 | −0.001 | 0.03 | 0.99 | <0.001 |
| Age | 0.007 | 0.007 | 0.31 | 0.010 | 0.006 | 0.001 | <0.001 | 0.149 |
| Mean TST | 0.05 | 0.81 | 0.95 | <0.001 | 0.03 | 0.16 | 0.87 | <0.001 |
| iSD TST | 0.15 | 0.33 | 0.65 | 0.002 | 0.07 | 0.06 | 0.31 | 0.010 |
| Percent Sleep (PS) | ||||||||
| Sex (female) | 0.11 | 0.12 | 0.37 | 0.008 | −0.04 | 0.02 | 0.09 | 0.029 |
| Diagnostic Group (NC) | −0.17 | 0.15 | 0.25 | 0.013 | −0.08 | 0.03 | 0.006 | 0.073 |
| Sleep Medication (never) | 0.007 | 0.15 | 0.96 | <0.001 | −0.004 | 0.03 | 0.91 | <0.001 |
| Age | 0.004 | 0.007 | 0.60 | 0.003 | 0.005 | 0.001 | <0.001 | 0.127 |
| Mean PS | −3.77 | 1.86 | 0.05 | 0.039 | −0.13 | 0.38 | 0.74 | 0.001 |
| iSD PS | 0.12 | 0.29 | 0.67 | 0.002 | 0.04 | 0.06 | 0.55 | 0.004 |
| Bed time | ||||||||
| Sex (female) | 0.03 | 0.12 | 0.79 | 0.001 | −0.05 | 0.02 | 0.03 | 0.048 |
| Diagnostic Group (NC) | −0.15 | 0.15 | 0.32 | 0.010 | −0.08 | 0.03 | 0.01 | 0.063 |
| Sleep Medication (never) | 0.02 | 0.15 | 0.89 | <0.001 | −0.005 | 0.03 | 0.88 | <0.001 |
| Age | 0.007 | 0.007 | 0.29 | 0.011 | 0.006 | 0.001 | <0.001 | 0.162 |
| Mean Bed time | 1.89 | 2.29 | 0.41 | 0.007 | 0.77 | 0.45 | 0.09 | 0.028 |
| iSD Bed time | 0.13 | 0.24 | 0.58 | 0.003 | −0.03 | 0.05 | 0.47 | 0.005 |
| Wake time | ||||||||
| Sex (female) | 0.03 | 0.12 | 0.81 | 0.001 | −0.05 | 0.02 | 0.04 | 0.042 |
| Diagnostic Group (NC) | −0.14 | 0.15 | 0.37 | 0.008 | −0.09 | 0.03 | 0.005 | 0.076 |
| Sleep Medication (never) | 0.01 | 0.15 | 0.94 | <0.001 | −0.003 | 0.03 | 0.92 | <0.001 |
| Age | 0.007 | 0.007 | 0.34 | 0.009 | 0.006 | 0.001 | <0.001 | 0.163 |
| Mean Wake time | 0.53 | 0.63 | 0.40 | 0.007 | 0.07 | 0.12 | 0.60 | 0.003 |
| iSD Wake time | 0.18 | 0.23 | 0.45 | 0.006 | −0.04 | 0.05 | 0.38 | 0.008 |
iSD = intra-individual standard deviation, NC = non-psychiatric comparison subjects
In all the sleep models, diagnostic group (BD) and age (older) were strongly associated with higher TNF-alpha levels, but there were no associations between sleep measures and TNF-alpha levels (Table 3). Time-lagged analyses did not find a significant relationship between sleep measures and TNF-α levels (p>0.05).
Subjective sleep quality (PSQI) was not significantly associated with CRP levels (B = −0.01, SE = 0.15, p = 0.94) or TNF-α levels (B = −0.002, SE = 0.03, p = 0.95).
Discussion
This study found associations of objective, but not subjective, measures of sleep with inflammatory biomarkers among adults with BD and NCs. While the BD group did have higher inflammatory marker levels and worse self-repoted sleep quality compared to NCs, the groups did not differ on mean objective sleep measures. The BD group did, however, show increased variability in TST and Wake time compared to the NC group. We found that greater TST variability and atypicality of the previous night’s TST were associated with higher IL-6 levels. Individuals with lower (worse) mean PS had higher IL-6 and, in exploratory analyses, CRP levels. However, PS on the previous night did not relate to levels of either of these inflammatory markers. Self-reported sleep quality, sleep medication, and psychotropic medication load were not related to inflammatory biomarker levels.
Similar to several other published studies, the current investigation found no difference in mean objective sleep values, but increased variability in TST and Wake time (Ankers and Jones, 2009; Gershon et al., 2012; Kaplan et al., 2012; Millar et al., 2004; Ng et al., 2015). While a few studies have demonstrated increased TST and decreased efficiency in patients with BD, these studies differed in sample population: a non-psychiatric comparison group of good sleepers (Harvey et al., 2005), adolescent subjects (age 11–17 years) (Mullin et al., 2011), and an older BD group compared to a younger NC group (Ritter et al., 2012). For the current study, our groups were comparable for age and there were no eligibility criteria for the NC group that required good sleep. Thus, the comparison group had mean sleep duration that was less than the recommended 7–9 hours per night. Similarly, since sleep duration among US adolescents likely differs from adults due to developmental changes in sleep (Bartel et al., 2015; Twenge et al., 2017), our findings may not be comparable to those from adolescent BD samples.
The relationship between IL-6 levels and TST variability was consistent with a 2011 study by Okun et al. that examined inflammatory biomarker levels and sleep variability in older adults (age 60+ years) (Okun t al., 2011). This study examined variability of self-reported time in bed for either 7 or 14 nights, comparing good sleepers vs. people with insomnia vs. bereaved adults vs. caregivers for spouses with dementia. Standard deviation, the most common measure of intra-individual variability (Ng et al., 2015), was used. Our finding was modestly supported with time-lagged analyses where greater sleep atypicality was associated with higher IL-6 levels. The effects of sleep appear to occur both on very short-term scales (the previous evening’s sleep affects the following morning’s inflammatory response) as well as over the timespan of a week, which may mirror both acute and chronic inflammatory changes. The effects of potential sleep interventions should consider both acute and longer-term effects on inflammatory responses.
The association between IL-6 levels and PS was consistent with the findings in the literature where sleep deprivation and poor sleep quality are associated with elevated IL-6 levels in studies of older adults (Irwin, 2006; Nowakowski et al., 2018; Vgontzas et al., 2002; von Kanel et al., 2006), but not with studies of younger adults (Floam et al., 2014). This discrepancy could reflect the impact of chronic low-grade inflammation associated with aging (“inflammaging”) in the studies of older adults. Furthermore, persons with BD have even higher inflammatory marker levels compared to the general population. Together, age and serious mental illness were found to be associated with TNF-α levels within the models of sleep factors. Depression is another important contributory factor to inflammation. While few studies have compared inflammation between major depressive disorder (MDD) and BD, one meta-analysis reported considerable overlap between biomarker findings for BD and MDD (Yuan et al., 2019). Future studies should consider the contribution symptom domains versus diagnostic domains on the sleep-inflammation elationship and include persons with BD, MDD, and schizophrenia.
Interestingly, the associations between sleep variables and inflammation did not differ significantly between study groups, despite higher inflammatory marker levels in the BD group. Two possible explanations include: 1) inadequate power in this study to detect differences in the associations in BD and HC groups or 2) individuals with BD are more vulnerable to sleep disturbances (i.e., have greater inflammatory response to sleep problems) than controls. Furthermore, sleep interventions might be helpful in reducing the excess inflammation seen in those with BD. Of note, we found that differences in IL-6 levels between BD and HC groups were diminished in their effect size and no longer significant when TST mean and variability as well as sleep medication were included in the statistical model. The same was not true for CRP or TNF-alpha, which may reflect the specific mechanistic pathways that link sleep and inflammation, chronicity of the inflammatory response, and immune cell response (Irwin, 2015; Morris et al., 2018). This finding suggests that IL-6 elevations shown frequently in BD, in particular, may be driven by poor sleep and thus might be most responsive to sleep-focused interventions.
Interestingly, in the present study, there were no significant relationships between self-reported sleep quality (PSQI scores) and the inflammatory assessments. This observation may reflect the potential inaccuracy self-reported sleep measures (Kaufmann et al., 2019) or a mismatch in the timescales of the PSQI and the inflammatory markers. For example, the PSQI assesses sleep disturbances over the past month; however, inflammatory biomarkers may respond more acutely to sleep problems, i.e., one evening of poor sleep may result in increased inflammation the following day. Self-reported sleep quality has been reported to be associated with negative mental and physical he lth outcomes. Thus, longitudinal daily assessment through modalities like ecological momentary assessment may relate more closely to outcomes like inflammatory marker levels.
This study had several limitations. Due to the cross-sectional design, we cannot infer causality. While actigraphy has the advantages of being deployed in free-living environments, due to the lack of polysomnography data, sleep staging was not possible. Peripheral cytokine measures may be limited in their assessment of the inflammatory cascade. In this study, TST was examined only as a continuous variable due to the small size of certain subgroups. However, short and long sleep may both be associated with increased inflammatory processes. Long sleep (>8 hours, by self-report) was associated with higher IL-6 levels the following morning in a study of over a thousand Taiwanese adults (53+ years old) (Dowd et al., 2011). Due to the high collinearity of depression scores with diagnostic group, the depression measures could not be included in the main sleep-inflammation models, although including depression severity in BD-only models did not influence effect sizes of the relationship between sleep and inflammation. Furthermore, the BD group in the current study included outpatients with a chronic and relatively stable course of illness and 14 years of education on average; thus, these results may not be generalizable to medication-naïve, acutely ill, and treatment-resistant patients. On the other hand, the fact that sleep-inflammation links could be found in this sample with relatively mild mood symptoms, such relationships appear to be persistent (Ng et al., 2015; Robillard t al., 2015). Sleep medication use was not assessed on a nightly basis, which limits the ability to assess the direct impact of sleep medications on the evening’s sleep. Furthermore, due to the low relative usage of medications (sleep and other types) in the control group, the interactions between medication use and the study variables could not be fully assessed. The role of BMI was not explicitly examined in this study, as mean BMI was similar between the two diagnostic groups. Obesity affects both sleep and inflammation, and warrants further examination in future work. The strengths of the study include the objective sleep measures, multiple cytokine biomarkers, and a patient sample that was not currently suffering from high levels of mania or depression.
Future studies of sleep in BD populations should explore the longitudinal relationships between sleep and inflammation. Objective sleep measures, specifically intra-individual variability of those measures, may provide an additional clinical measure to predict decline in mental well-being and physical functioning, beyond the mean sleep measures. On the other hand, self-reported sleep quality may not capture important characteristics of sleep that impact inflammation. In light of the strong associations between poor sleep and mental/physical health, further investigation is required to improve longevity and quality of life for persons with BD, a vulnerable high-risk population. Measurement burst designs can integrate both acute and chronic effects of sleep on inflammatory measures. Better understanding of the sleep-inflammation links in psychiatrically ill populations may present novel approaches to improving health and well-being in persons with serious mental illnesses.
Acknowledgments
Funding Support: This study was supported, in part, by the National Institutes of Health [NIMH R01MH103318 (PI: Lisa T. Eyler)], [NIMH K23MH119375-01 (PI: Ellen E. Lee)], [NIA K01AG061239 (PI: Christopher N. Kaufmann] and [NIMH T32 Geriatric Mental Health Program MH019934 (PI: Dilip V. Jeste), NARSAD Young Investigator grant from the Brain and Behavior Research Foundation (PI: Ellen E. Lee), and by the Stein Institute for Research on Aging (Director: Dilip V. Jeste, MD) at the University of California San Diego. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Potential conflicts of Interest: The authors declare no financial or other relationship relevant to the subject of this article. Dr. Malhotra is PI on NIH RO1 HL085188, K24 HL132105, T32 HL134632 and co-investigator on R21 HL121794, RO1 HL 119201, RO1 HL081823. As an Officer of the American Thoracic Society, Dr. Malhotra has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to the UC San Diego in support of a sleep center. Dr. Ancoli-Israel consults for Eisai, Merck. Biogen.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP, 2003. The role of actigraphy in the study of sleep and circadian rhythms. Sleep 26(3), 342–392. [DOI] [PubMed] [Google Scholar]
- Ankers D, Jones SH, 2009. Objective assessment of circadian activity and sleep patterns in individuals at behavioural risk of hypomania. Journal of clinical psychology 65(10), 1071–1086. [DOI] [PubMed] [Google Scholar]
- Bartel KA, Gradisar M, Williamson P, 2015. Protective and risk factors for adolescent sleep: a meta-analytic review. Sleep medicine reviews 21, 72–85. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ, 1989. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2), 193–213. [DOI] [PubMed] [Google Scholar]
- Carr O, Saunders KEA, Tsanas A, Bilderbeck AC, Palmius N, Geddes JR, Foster R, Goodwin GM, De Vos M, 2018. Variability in phase and amplitude of diurnal rhythms is related to variation of mood in bipolar and borderline personality disorder. Scientific reports 8(1), 1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev SI, Nguyen TT, McKenna BS, Sutherland AN, Bartsch H, Theilmann RJ, Eyler LT, 2017. Steeper Slope of Age-Related Changes in White Matter Microstructure and Processing Speed in Bipolar Disorder. The American geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry 25(7), 744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Goldman N, Weinstein M, 2011. Sleep Duration, Sleep Quality, and Biomarkers of Inflammation in a Taiwanese Population. Annals of pidemiology 21(11), 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Wiliams JBW, November 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, atient Edition (SCID-I/P). Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Floam S, Simpson N, Nemeth E, Scott - Sutherland J, Gautam S, Haack M, 2014. Sleep characteristics as predictor variables of stress systems markers in insomnia disorder. Journal of sleep research 24(3), 296–304. [DOI] [PubMed] [Google Scholar]
- Fries GR, Bauer IE, Scai i G, Wu MJ, Kazimi IF, Valvassori SS, Zunta-Soares G, Walss-Bass C, Soares JC, Quevedo J, 2017. Accelerated epigenetic aging and mitochondrial DNA copy n mber in bipolar disorder. Translational psychiatry 7(12), 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A, Thompson WK, Eidelman P, McGlinchey EL, Kaplan KA, Harvey AG, 2012. Restless pillow, ruffled mind: Sleep and affect coupling in interepisode bipolar disorder. Journal of abnormal psych l gy 121(4), 863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AK, Sylvia LG, 2016. The role of sleep in bipolar disorder. Nature and science of sleep 8, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvard Medical School, 2007. National Comorbidity Survey (NSC). https://www.hcp.med.harvard.edu/ncs/index.php. (Accessed June 17 2019). [Google Scholar]
- Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM, 2005. Sleep-Related Functioning in Euthymic Patients With Bipolar Disorder, Patients With Insomnia, and Subjects Without Sleep Problems. American Journal of Psychiatry 162(1), 50–57. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Talbot LS, Gershon A, 2009. Sleep Disturbance in Bipolar Disorder Across the Lifespan. Clin Psychol (New York) 16(2), 256–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML, 2008. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar disorders 10(8), 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, 2006. Sleep Deprivation and Activation of Morning Levels of Cellular and Genomic Markers of Inflammation. Archives of Internal Medicine 166(16), 1756. [DOI] [PubMed] [Google Scholar]
- Irwin MR, 2015. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol 66, 143–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S, 2006. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med 166(16), 1756–1762. [DOI] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K, 2005. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar disorders 7(2), 176–186. [DOI] [PubMed] [Google Scholar]
- Kanady JC, Soehner AM, Klein AB, Harvey AG, 2017. The association between insomnia-related sleep disruptions and cognitive dysfunction during the inter-episode phase of bipolar disorder. J Psychiatr Res 88, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan KA, Talbot LS, Gruber J, Harvey AG, 2012. Evaluating sleep in bipolar disorder: comparison between actigraphy, polysomnography, and sleep diary. Bipolar disorders 14(8), 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann CN, Gershon A, Eyler LT, Depp CA, 2016. Clinical signi icance of mobile health assessed sleep duration and variability in bipolar disorder. J Psychiatr Res 81, 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann CN, Nakhla MZ, Lee EE, Yoon HK, Wing D, Depp CA, Eyler LT, 2019. Inaccuracy between subjective reports and objective measu es sleep duration and clinical correlates in bipolar disorder. J Affect Disord 250, 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62(6), 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, Williams JB, 2001. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine 16(9), 606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomholt LH, Andersen DV, Sejrsgaard- Jacobsen C, Ozdemir CM, Graff C, Schjerning O, Jensen SE, Straszek SPV, Licht RW, Grontved S, Nielsen RE, 2019. Mortality rate trends in patients diagnosed with schizophrenia or bipolar disorder: a nationwide study with 20 years of follow-up. Int J Bipolar Disord 7(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo MCA, Abreu RLC, Linhares Neto VB, de Bruin PFC, de Bruin VMS, 2017. Chronotype and circadian hythm in bipolar disorder: A systematic review. Sleep medicine reviews 34, 46–58. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, Kessler RC, 2007. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64(5), 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar A, Espie CA,Scott J, 2004. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord 80(2–3), 145–153. [DOI] [PubMed] [Google Scholar]
- Morris G, Stubbs B, Kohler CA, Walder K, Slyepchenko A, Berk M, Carvalho AF, 2018. The putative role of oxidative stress and inflammation in the pathophysiology of sleep dysfunction across neuropsychiatric disorders: Focus on chronic fatigue syndrome, bipolar disorder and multiple sclerosis. Sleep medicine reviews. [DOI] [PubMed] [Google Scholar]
- Mullin BC, Harvey AG, Hinshaw SP, 2011. A preliminary study of sleep in adolescents with bipolar disorder, ADHD, and non-patient controls. Bipolar disorders 13(4), 425–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TH, Chung KF, Ho FY, Yeung WF, Yung KP, Lam TH, 2015. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep medicine reviews 20, 46–58. [DOI] [PubMed] [Google Scholar]
- Nowakowski S, Matthews KA, von Känel R, Hall MH, Thurston RC, 2018. Sleep characteristics and inflammatory biomarkers among midlife women. Sleep 41(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Reynolds CF 3rd, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M, 2011. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosomatic medicine 73(2), 142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Tsai KM, Dahl RE, Irwin MR, McCreath H, Seeman TE, Fuligni AJ, 2016Sleep and Inflammation During Adolescence. Psychosomatic medicine 78(6), 677–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell TR, Dima D, Frangou S, Breen G, 2018. Telomere Length and Bipolar Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43(2), 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter PS, Marx C, Lewtschenko N, Pfeiffer S, Leopold K, Bauer M, Pfennig A, 2012. The characteristics of sleep in patients with manifest bipolar disorder, subjects at high risk of developing the disease and healthy controls. Journal of Neural Transmission 119(10), 1173–1184. [DOI] [PubMed] [Google Scholar]
- Robillard R, Hermens DF, Naismith SL, White D, Rogers NL, Ip TK, Mullin SJ, Alvares GA, Guastella AJ, Smith KL, Rong Y, Whitwell B, Southan J, Glozier N, Scott EM, Hickie IB, 2015. Ambulatory sleep-wake patterns and variability in young people with emerging mental disorders. J Psychiatry Neurosci 40(1), 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblat JD, McIntyre RS, 2017. Bipolar Disorder and Immune Dysfunction: Epidemiological Findings, Proposed Pathophysiology and Clinical Implications. Brain sciences 7(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, Baldessarini RJ, 2008. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar disorders 10(2), 256–265. [DOI] [PubMed] [Google Scholar]
- Sayana P, Colpo GD, Simoes LR, Giridharan VV, Teixei a AL, Quevedo J, Barichello T, 2017. A systematic review of evidence for the role of inflammatory biomarkers in bipolar patients. J Psychiatr Res 92, 160–182. [DOI] [PubMed] [Google Scholar]
- Taylor BJ, Matthews KA, Hasler BP, Roeckl in KA, Kline CE, Buysse DJ, Kravitz HM, Tiani AG, Harlow SD, Hall MH, 2016. Bedtime Variability and Metabolic Health in Midlife Women: The SWAN Sleep Study. Sleep 39(2), 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge JM, Krizan Z, Hisler G, 2017. Decreases in self-reported sleep duration among U.S. adolescents 2009–2015 and association with new media screen time. Sleep medicine 39, 47–53. [DOI] [PubMed] [Google Scholar]
- van den Ameele S, Fuchs D, Coppens V, de Boer P, Timmers M, Sabbe B, Morrens M, 2018. Markers of Inflammation d Mono mine Metabolism Indicate Accelerated Aging in Bipolar Disorder. Frontiers in psychiatry 9, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA, 2000. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res 97(2–3), 129–135. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP, 2002. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism: clinical and experimental 51(7), 887–892. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Dimsdale JE, Ancoli-Israel S, Mills PJ, Patterson TL, McKibbin CL, Archuleta C, Grant I, 2006. Poor sleep is associated with higher plasma proinflammatory cytokine interleukin-6 and procoagulant marker fibrin D-dimer in older caregivers of people with Alzheimer’s disease. J Am Geriatr Soc 54(3), 431–437. [DOI] [PubMed] [Google Scholar]
- Ware JE Jr., Sherbourne CD, 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30(6), 473–483. [PubMed] [Google Scholar]
- Yang F, Barbosa IG, Vieira EL, Bauer ME, Rocha NP, Teixeira AL, 2018. Further Evidence of Accelerated Aging in Bipolar Disorder: Focus on GDF-15. Transl Neurosci 9, 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A Rating Scale for Mania: Reliability, Validity and Sensitivity. British Journal of Psychiatry 133(5), 429–435. [DOI] [PubMed] [Google Scholar]
- Yuan N, Chen Y, Xia Y, Dai J, Liu C, 2019. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Translational psychiatry 9(1), 233. [DOI] [PMC free article] [PubMed] [Google Scholar]