Abstract
Background
There has been a considerable rise in the number of musculoskeletal/orthopaedic oncology fellowships and subsequently, orthopaedic oncologists, in the nation. National societies have been concerned that the increasing number of orthopaedic oncologists, coupled with a limited number of patients with bone and soft-tissue sarcomas in the country, may have led to an unintended impact on the training spectrum and/or exposure of orthopaedic oncology fellows-in-training over time. Fellows who are unable to gain exposure by operating on varied cancer presentations during training may be less confident in dealing with a wide array of patients in their practice. Despite these concerns, the volume and variability of procedures performed by fellows-in-training remains unknown. Understanding these parameters will be helpful in establishing policies for standardizing training of prospective fellows to ensure they are well-equipped to care for patients with bone and/or soft-tissue sarcomas in the beginning of their career.
Questions/purposes
(1) Has the median surgical procedure volume per fellow changed over time? (2) How much variability in procedural volume exists between fellows, based on the most recent (2017) Accreditation Council on Graduate Medical Education (ACGME) procedure log data? (3) What proportion of fellows are meeting the minimum procedure volume thresholds, as recommended by the Musculoskeletal Tumor Society (MSTS)?
Methods
The 2010 to 2017 ACGME fellowship procedure logs for musculoskeletal oncology fellowships were retrieved from the council’s official website. All fellows enrolled in ACGME-accredited fellowships are mandated to complete case logs before graduation. This study did not include operative procedures performed by fellows in nonACGME-approved fellowship programs. The 2010 to 2016 anatomic site-based procedure log data were used to evaluate fellows’ overall and location-specific median operative or patient volume, using descriptive statistics. Linear regression analyses were used to assess changes in the median procedure volume over time. The 2017 categorized procedure log data were used to assess variability in procedure volume between the lowest (10th percentile) and highest (90th percentile) of all fellows. Using 2017 procedure logs, we compared the minimum procedure volume standards, as defined by the MSTS, against the number of procedures performed by fellows across the 10th, 30th, 50th (median), 70th, and 90th percentiles.
Results
There was no change in the median (range) procedural volume per fellow from 2010 (292 procedures [131 to 634]) to 2017 (312 procedures [174 to 479]; p = 0.58). Based on 2017 categorized procedure log data, there was considerable variability in procedural volume between the lowest (10th) percentile and highest (90th) percentile of fellows across programs: pediatric oncologic procedures (10-fold difference), surgical management of complications from limb-salvage surgery (sevenfold difference), soft-tissue resections or reconstructions (fourfold difference), bone sarcoma resections or limb-salvage surgery (fourfold difference), and spine, sacrum, and pelvis procedures (threefold difference). A fair proportion of fellows did not meet the minimum procedure volume standards, as recommended by the MSTS across certain categories. For the spine and pelvis (minimum = 10 procedures), fellows in the lowest 10th percentile performed only six procedures. For patients with bone sarcomas or limb salvage (minimum = 20 procedures), fellows in the lowest 10th percentile performed only 14 procedures. For pediatric patients with oncologic conditions (minimum = 15 procedures), fellows in the 50th percentile (13 procedures) and below failed to meet the thresholds. For surgical management of complications from limb-salvage procedures (minimum = five procedures), fellows in the lowest 10th percentile performed only three procedures.
Conclusion
Although we were encouraged to observe that the median number of procedures performed by musculoskeletal oncology fellows over this time has not changed, we observed wide variability in the procedure volume among fellows for pediatric sarcomas, soft-tissue resection and reconstruction, limb salvage procedures, and spine procedures. We do not know how this compares with fellows trained in nonaccredited fellowship programs.
Clinical Relevance
Although we recognize that the education of fellows entails much more than performing operations, national societies have recognized a need to bring about more uniformity or standardization of training in musculoskeletal oncology. Limiting the number of orthopaedic oncology fellowships to high-volume institutions, expanding the training time period, and/or introducing subspecialty certification may be possible avenues through which standardization of training can be defined.
Introduction
Due to improvements in surgical technology, a need for expanded knowledge, and greater surgical skill, orthopaedic surgery has become an increasingly subspecialized field of medicine. In the United States, more than 90% of orthopaedic residents complete a fellowship after residency, with the number of fellowship applicants and fellowship programs increasing every year [15]. Although an increasing number of subspecialized orthopaedic surgeons is required to meet the surgical demand of a rising elderly population seeking elective procedures (such as total joint arthroplasties), it may not be entirely suitable for branches of orthopaedics with limited practice spectrums, such as orthopaedic/musculoskeletal oncology. When the Musculoskeletal Tumor Society (MSTS; a national orthopaedic oncology society) was founded, there were only three musculoskeletal oncology fellowships [8]. As of 2019, there were 19 fellowships (accredited and nonaccredited) offered by different institutions across the United States. Current statistics show that there are more than 200 orthopaedic oncologists nationally, with each year bringing a “fresh” group of 12 to 19 newly graduated orthopaedic oncologists, surpassing the number of individuals retiring or leaving practice [4]. With a limited number of patients with bone and soft-tissue sarcomas in the nation, there have been discussions among current practicing surgeons at national society meetings as to whether the influx of newly trained operating surgeons may have an unintended consequence of reducing the number of surgical procedures each fellow performs in his or her training, which could lower the educational content and subsequent preparedness of fellows when they enter practice [4, 14].
Prior reports have already shown that early-career orthopaedic oncologists are not performing what is viewed to be a sufficient number tumor surgical procedures based from a recent survey that was published in Clinical Orthopaedics and Related Research®, and case variability in the beginning years of practice remains a matter of concern [7, 11]. The relatively low tumor procedures for early-career orthopaedic oncologists could be due to a decrease in the patient population, secondary to an increasing number of orthopaedic oncologists dividing up the volume among regions; consequently, newly-minted orthopaedic oncologists might not be comfortable doing certain procedures in which they have had limited exposure during their training. Although prior studies have not exactly estimated confidence/comfort level in doing procedures early on, in our personal experience it does impact a practice early on, particularly when cases need to be submitted for the board certification exams. Insufficient experience in training may also adversely affect patient care, if the operating surgeon does not have the surgical skills required to treat patients with complex cancers early-on in their practice. Despite the latter concerns, little is known about whether orthopaedic oncology fellows-in-training have experienced changes in surgical volume or how much variability in procedure volume exists between fellowship programs. Concurrent with the growth in the number of musculoskeletal oncology fellowships over the past decade, the MSTS has pushed to ensure more uniformity in training across different educational sites by recommending minimum surgical volumes and/or thresholds. The MSTS recommended that to demonstrate competence, fellows should complete a minimum of 150 oncologic procedures, with at least 20 soft-tissue resections or reconstructions; 20 bone sarcoma resections, reconstructions, or limb-salvage procedures; 20 operations for distant metastatic disease; 15 procedures to treat pediatric patients with tumors involving the spine, soft tissue, or bone; 10 procedures to treat spinal or pelvis tumors; and five procedures to treat complications associated with limb salvage surgery [12]. Despite the establishment of these recommendations, it is unknown what proportion of fellows are able to complete the minimum number of required cases before graduation.
We therefore asked the following questions: (1) Has the median surgical procedure volume per fellow changed over time? (2) How much variability in procedural volume exists between fellows, based on the most recent (2017) Accreditation Council on Graduate Medical Education (ACGME) procedure log data? (3) What proportion of fellows are meeting the minimum procedure volume thresholds, as recommended by the MSTS?
Materials and Methods
This retrospective cross-sectional study was performed using the ACGME fellowship procedure logs. In accordance with ACGME guidelines, fellows enrolled in accredited fellowships across the United States are required to log their operative experience by entering Current Procedural Terminology (CPT) codes into an online data portal system for quality improvement and analytical purposes. To help answer questions regarding operative experience and inter-institution variability, the ACGME compiles procedure log data in a de-identified format and makes it available publicly on their official website [1]. In addition to reporting the median procedure volume, the case logs also report procedure volume across the 10th, 30th, 50th (median), 70th, and 90th percentiles of fellows. Due to compliance with HIPAA laws, the ACGME do not report individual procedure volumes of each fellow. Readers should note that, even though residents and fellow enrolled in surgical residencies and fellowships are mandated to complete their case logs, the accuracy of case-logging remains questionable [9, 16]. Much of this inaccuracy appears to stem from an inadequate ability to properly code for a large variety of procedures [13]. Although no study has investigated the accuracy/consistency of case logs in musculoskeletal oncology, given the limited number of procedures and, subsequent, CPT codes present, we feel that these case logs would be pretty much reflective of the actual surgical procedure volume of fellows. From 2010 to 2016, ACGME procedure log data for musculoskeletal oncology fellowships were based on a standard deidentified format that described oncologic procedures based on the anatomic site (such as the hand or wrist, shoulder or elbow, femur or knee, pelvis or hip, or spine). Beginning in 2017, to establish uniformity and help societies set volume and procedure type-specific standards, the ACGME revised the categories into distinct groups (spine or pelvis; soft tissue resections and reconstructions; bone sarcoma resections and limb salvage operations; pediatric oncologic procedures involving the soft tissue, bone, and spine; surgical management of limb-salvage complications; and management of distant metastatic disease).
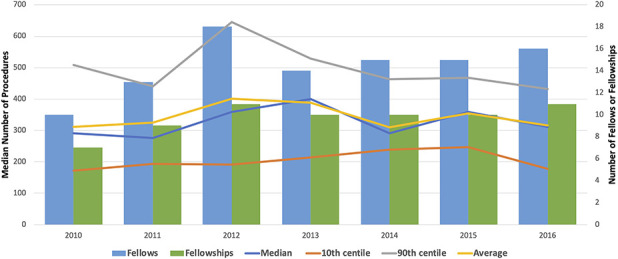
From 2010 to 2017, the number of ACGME-accredited musculoskeletal oncology fellowship programs increased from seven to 11, with the number of corresponding fellows increasing from 10 to 18. We used the 2010 to 2016 location-based procedure log data to evaluate the numbers of overall and anatomic site-specific median operative volume that fellows reported, using descriptive statistics. We used linear regression analyses to assess the presence of changes in the median procedure volume over time. The 2017 categorized procedure log data were used to assess variability in procedure volume between the lowest (10th percentile) and highest (90th percentile) of all fellows. We assessed variability using the following formula:
Minimum case volume standards, as recommended by the MSTS (Table 1), were compared against the number of procedures performed by fellows across the 10th, 30th, 50th (median), 70th, and 90th percentiles.
Table 1.
MSTS recommended minimum procedure volume for musculoskeletal oncology fellows
| Category | Minimum volume (number of procedures) |
| Soft-tissue resections or reconstructions | 20 |
| Bone sarcoma resections, reconstructions or limb-salvage procedures | 20 |
| Operations for distant metastatic disease | 20 |
| Pediatric sarcomas | 15 |
| Spine/pelvis tumors | 10 |
| Procedures involving management of complications associated with limb-salvage surgery | 5 |
Linear regression analyses for assessing the potential changes in median procedure volume were performed using Microsoft Excel. For all statistical purposes, a p value of less than 0.05 was considered statistically significant. Because data were derived from deidentified, publicly available reports, the study was exempt from institutional review board approval.
Results
Median Musculoskeletal Oncology Fellow Case Volume, 2010 to 2016
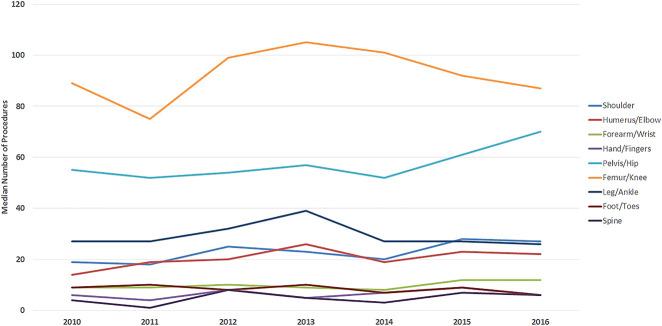
With the numbers we had, we found no difference in the median (range) procedure volume per fellow over time. The overall operative volume went from 292 procedures (131 to 634) in 2010 to 312 (174 to 479) procedures in 2016 (p = 0.58) (Fig. 1). Using anatomic site-based procedure log data (Table 2), we found there was a slight increase in the median number of shoulder procedures, from 19 procedures (10 to 40) in 2010 to 27 procedures (15 to 41) in 2016 (p = 0.049). With the numbers we had, we did not observe changes over time with regard to the median volume of procedures involving the humerus or elbow (p = 0.13), forearm or wrist (p = 0.12), hand or fingers (p = 0.37), pelvis or hip (p = 0.06), femur or knee (p = 0.62), leg or ankle (p = 0.78), foot and toes (p = 0.14), and spine (p = 0.35). The largest numbers of procedures were seen in the categories of femur/knee and pelvis/hip (Fig. 2).
Fig. 1.
This graph shows the number of fellows, fellowships, and median procedure volume from 2010 to 2016. A color image accompanies the online version of this article.
Table 2.
Median procedure volume as reported by fellows over time
| Category, number (range) | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | p valuea |
| Shoulder | 19 | 18 | 25 | 23 | 20 | 28 | 27 | 0.049 |
| (10 to 40) | (6 to 49) | (10 to 58) | (7 to 47) | (12 to 51) | (11 to 44) | (15 to 41) | ||
| Humerus/elbow | 14 | 19 | 20 | 26 | 19 | 23 | 22 | 0.127 |
| (9 to 38) | (8 to 34) | (5 to 51) | (9 to 41) | (6 to 32) | (8 to 32) | (10 to 45) | ||
| Forearm/wrist | 9 | 9 | 10 | 9 | 8 | 12 | 12 | 0.124 |
| (4 to 30) | (3 to 25) | (4 to 26) | (5 to 32) | (3 to 21) | (1 to 21) | (2 to 21) | ||
| Hand/fingers | 6 | 4 | 8 | 5 | 7 | 9 | 6 | 0.369 |
| (3 to 44) | (1 to 44) | (0 to 33) | (1 to 26) | (0 to 24) | (1 to 21) | (1 to 27) | ||
| Pelvis/hip | 55 | 52 | 54 | 57 | 52 | 61 | 70 | 0.061 |
| (27-84) | (25 to 122) | (35 to 134) | (24 to 136) | (34 to 89) | (40 to 83) | (24 to 85) | ||
| Femur/knee | 89 | 75 | 99 | 105 | 101 | 92 | 87 | 0.623 |
| (57 to 152) | (46 to 197) | (47 to 221) | (68 to 155) | (57 to 137) | (56 to 138) | (54 to 148) | ||
| Leg/ankle | 27 | 27 | 32 | 39 | 27 | 27 | 26 | 0.779 |
| (17 to 58) | (16 to 61) | (11 to 46) | (19 to 51) | (9 to 39) | (16 to 61) | (19 to 57) | ||
| Foot/toes | 9 | 10 | 8 | 10 | 7 | 9 | 6 | 0.144 |
| (5 to 13) | (3 to 28) | (2 to 30) | (3 to 24) | (0 to 15) | (2 to 20) | (2 to 19) | ||
| Spine | 4 | 1 | 8 | 5 | 3 | 7 | 6 | 0.353 |
| (0 to 45) | (0 to 54) | (0 to 52) | (0 to 65) | (0 to 32) | (0 to 143) | (0 to 41) | ||
| Total procedures | 292 | 276 | 360 | 400 | 290 | 358 | 312 | 0.578 |
| (164 to 634) | (169 to 832) | (169 to 732) | (189 to 628) | (185 to 497) | (217 to 498) | (174 to 479) |
p values retrieved from linear regression models assessing the presence of a trend in median procedure volume over time.
Fig. 2.
This graph shows the median number of procedures, per location, performed by fellows from 2010 to 2016. A color image accompanies the online version of this article.
Variability in Reported Procedures between Fellows in 2017
Based on 2017 categorized procedure log data, the greatest variability in procedures between the lowest (10th percentile) and highest (90th percentile) of fellows was seen for pediatric oncologic procedures (10-fold difference), procedures to treat complications from limb-salvage surgery (sevenfold difference), soft-tissue resections or reconstructions (fourfold difference), bone sarcoma resections or limb-salvage surgery (fourfold difference), and spine or pelvis procedures (threefold difference) (Table 3).
Table 3.
Variation in the procedure volume reported by fellows between the lowest (10th) and the highest (90th) percentile
| Procedure | Number of procedures per year |
| Spine/pelvis | |
| 10th | 6 |
| 90th | 18 |
| Variation | 3 |
| Soft-tissue resections and reconstruction | |
| 10th | 17 |
| 90th | 63 |
| Variation | 4 |
| Bone sarcoma resection or limb-salvage surgery | |
| 10th | 14 |
| 90th | 50 |
| Variation | 4 |
| Pediatric oncologic or sarcoma procedures | |
| 10th | 2 |
| 90th | 20 |
| Variation | 10 |
| Surgical management of complications from limb-salvage surgery | |
| 10th | 3 |
| 90th | 22 |
| Variation | 7 |
| Management of distant metastatic disease | |
| 10th | 25 |
| 90th | 67 |
| Variation | 3 |
Variation calculated by dividing the number of procedures performed by fellows in the 90th percentile by the number of procedures performed by fellows in the lowest 10th percentile.
Minimum MSTS Procedure Volume Thresholds
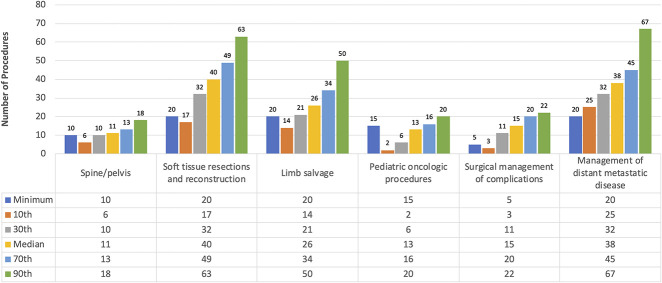
Based on the minimum procedure volume standards recommended by the MSTS, a number of fellows did not meet the thresholds across certain categories. For the spine and pelvis (minimum = 10 procedures), fellows in the lowest 10th percentile performed only six pelvic procedures. For bone sarcoma and limb-salvage procedures (minimum = 20 procedures), fellows in the lowest 10th percentile performed only 14 procedures. For pediatric oncologic procedures (minimum = 15 procedures), fellows in the 50th percentile (13 procedures) and below failed to meet the thresholds. For operations to manage complications from limb-salvage procedures (minimum = five procedures), fellows in the lowest 10th percentile performed only three procedures (Fig. 3).
Fig. 3.
This graph shows the number of procedures performed by fellows across all percentiles in 2017 compared with the minimum defined procedure volume standard. A color image accompanies the online version of this article.
Discussion
The number of accredited and nonaccredited musculoskeletal oncology fellowship and/or training positions has increased over time [18]. This has not only fueled concerns about the demand for and supply of orthopaedic oncologic procedures among prospective fellows and current practicing surgeons, but also has led to discussions among MSTS members on the need for standardization of training across institutions to ensure that fellows are appropriately exposed to all facets of this field and are adequately prepared to treat patients with sarcomas. Using national case logs from fellows enrolled in accredited musculoskeletal oncology fellowships in the United States, we found that although the median procedure volume of fellows has not changed over time, substantial variability exists in procedural exposure among fellows. Furthermore, a fair number of fellows fall short of meeting the MSTS-recommended surgical volume thresholds. These findings are concerning, and highlight the need for action by national societies (such as the MSTS and the American Academy of Orthopaedic Surgeons) to ensure that prospective fellows are being adequately trained on all aspects of orthopaedic oncology. There are a few plausible real-world approaches that can be adopted (for example, expanding fellowship time, limiting fellowships to high-volume institutions, and introducing subspecialty certification); the utility of will be discussed in the forthcoming sections.
The study is limited by the inclusion of fellowship procedure log data from ACGME-accredited fellowships only. Even though most orthopaedic oncology fellowships are ACGME-accredited, procedures performed by fellows in nonACGME fellowships may be different. Unfortunately, it is impossible to confirm the latter assumption because nonACGME fellowships are not required to submit procedure log data. However, given that most accredited and nonaccredited fellowships are at cancer institutes, we would anticipate their surgical volume to be largely similar to each other. The ACGME recently switched from anatomic site-based to category-specific procedure log data, and therefore we were only able to assess variability between different procedure types based on data from 2017 only. Future studies should be performed 5 to 10 years from now to evaluate changes in variability between different procedures over time using the category-specific procedure log data. The categorized definitions also combine spinal tumor surgeries and pelvic bone excisions (ilium, pubic rami, acetabulum, and ischial tuberosity) under one broad group, making it difficult to ascertain whether the variability observed was because of spine or pelvic procedures. The new log system may be an improvement on the previous system, but it currently has no minimums for shoulder endoprosthetic reconstructions or benign surgical tumor care. This may warrant a thorough reevaluation and reworking of the new category-specific log system to clearly identify and understand the full scope of musculoskeletal oncology training. Another important study limitation is that an orthopaedic oncology fellowship typically encompasses more than just surgical procedures, such as clinical assessment of patients, interpretation of imaging, delivering bad news to patients, research and shared decision-making, all of which cannot be captured in case logs. However, given that surgical skill remains the main “anchor” of a fellowship, there is still a need for driving standardization of training across different institutions. Lastly, our findings are based of small samples sizes (10 to 18 fellows), and therefore the confidence intervals of our estimates are likely wide. A more continuous evaluative approach by the MSTS is required, perhaps on a biannual basis, to ensure fellow training is being monitored across the nation. Lastly, as mentioned previously, we are uncertain of the accuracy of coding in these case logs. However, given that orthopaedic oncology fellows normally do a limited set of procedures, they subsequently have to deal with fewer CPT codes than fellows enrolled in other orthopaedic fellowships, such as trauma, where the diverse range of CPT codes may lead to errors during logging procedures.
Unlike other subspecialties (such as adult reconstruction/joints, shoulder, spine) where the ever-increasing demand of elective surgical care requires a continuous supply of more surgeons, orthopaedic oncology is a unique super-specialized branch of orthopaedics. Given the limited number of bone and soft tissue sarcomas in the nation, and the increasing number of orthopaedic oncologists, many surgeons have been concerned that this may inevitably affect the training of prospective fellows and practice spectrum of newly graduated orthopaedic oncologists by dividing up the case-load among institutions. Despite these concerns, we were unable to detect a change in the median procedure volume of fellows from 2010 to 2016. Although this finding may be surprising, most fellowships are offered at major cancer hospitals, where the generally high volume of sarcomas may not have influenced the training spectrum of fellows. However, our results may be limited by the inclusion of procedure log data from accredited fellowships only. Most procedures performed by fellows were limited to the lower extremity, with generally few procedures involving the upper extremity and/or vertebral column. This could be because most sarcomas tend to occur in the pelvis, hip, and knee region; surgeons in other subspecialties (such as hand and upper extremity and the spine) may be operating on patients with sarcomas in those locations. This is particularly evident in patients with spinal tumors or spine metastases. Based on our results, the median number of spine-only procedures performed by orthopaedic oncology fellows from 2010 to 2016 ranged from four to eight procedures only. Given their precarious location and the possible adjunct compression of the spinal cord, spine tumors (both primary and metastatic) may be cared for by neurologic surgeons, orthopaedic spine surgeons, or orthopaedic oncologists, which may dilute the experience of trainees in all of these specialties. Although this may be understandable, considering that surgeons in both specialties receive training in the spine and are well-suited to address patients’ surgical needs, most spine surgeons are not expert oncologic surgeons, and many orthopaedic oncologists are not educated or familiar with the complexities of current spinal operations. In addition, neurosurgical and orthopaedic spine surgeons may be suited to perform aspects of the patient’s surgical care but largely perform only the technical portions of it. In contrast, given their extensive training in tumor biology and knowledge regarding adjunct radiation and systemic treatments, orthopaedic oncologists may be better posed to provide care for spine tumors, provided they receive additional and adequate spine training. With an anticipated increase in the number of patients with cancer who are treated for metastatic spine disease, restructuring the training of orthopaedic oncology fellows by offering combined spine-oncology fellowships may be warranted. There are a few practicing orthopaedic oncologists in the nation who have completed both a spine and an oncology fellowship for this very reason, and the interest in dual fellowship appears to be growing. Conversely, ensuring that fellowship institutions have access to a multidisciplinary spine oncology team (spine surgeons, orthopaedic oncologists, and radiation oncologists) may be required before allowing them to implement a training program.
Adding to these concerns, we found a large amount of variation in the median procedure volume of pediatric sarcomas and soft-tissue sarcomas in our study. However, because the ACGME changed from using standardized anatomic site-based procedure log data to one that was categorized and more specific to musculoskeletal oncology in 2017, we only have 1 year of data on which to make observations. This should be monitored more closely in the future. Based on our findings, there was variation in pediatric sarcoma procedures, bone sarcoma resections and limb salvage, and spine and pelvis procedures between the upper and lower percentiles. For pediatric sarcomas, fellows in the highest (90th percentile) may have had a dedicated rotation in a pediatric hospital where they may have been exposed to a number of patients with sarcomas. Given that more than half of all fellows (below the 70th percentile) failed to meet the recommended minimum procedure volume to treat sarcomas in pediatric patients set by the MSTS [15], many of these procedures may have been performed by pediatric orthopaedic surgeons or trainees. Pediatric soft-tissue sarcomas are also treated by pediatric surgeons in some institutions, which may be another reason for the low number of these procedures reported by orthopaedic oncology fellows in this study. Although variability will inevitably exist across all orthopaedic fellowships (including spine, hand, sports), the effect of variability for orthopaedic oncology training remains concerning. We already know that early-career orthopaedic oncologists are not performing what is believed to be a sufficient number of tumor surgical procedures [11], and variability in procedures and/or exposure to sarcomas in the beginning years of practice remains a matter of concern. One may not see this for other subspecialties; for example, due to a growing elderly patient population, a recently trained arthroplasty or spine surgeon will, undoubtedly, have a practice that consists of 90% to 100% arthroplasty or 90% to 100% spine, respectively. Although surgeons have noted that the relatively low tumor procedure volume for early-career orthopaedic oncologists could be due to a decrease in the patient population, that is secondary to an increasing number of orthopaedic oncologists dividing up the volume among regions. It is also possible that newly-graduated orthopaedic oncologists might not be comfortable doing certain procedures in which they have had limited exposure during their training. Although prior studies have not exactly estimated confidence/comfort level in doing procedures early on, in our personal experience, it does influence a practice early on, particularly when procedures must be submitted for the American Board of Orthopaedic Surgery Part II exam. Future study investigating the confidence of newly-graduated orthopaedic oncologists is required to better answer these questions.
Possible Approaches Towards Standardization
There are a few plausible approaches which can be taken toward mitigating the variability and/or improving training of prospective fellows in the coming years. Limiting the number of orthopaedic oncology fellowships to very high-volume institutions will be one possible way of preventing an undue division of cases both upstream (adequate procedure exposure during training) and downstream (appropriate practice spectrum for newly-minted orthopaedic oncologists). However, legal concerns about the restraint of trade might prevent this from happening [17], which brings us to the option of allowing exchange of training between two high-volume institutions. Although the latter option is useful, it is hard to ignore the financial (cost of travelling and living temporarily in another state) and emotional (staying away from family and children) challenges associated with such travelling fellowships. Institutions could also consider expanding fellowship to 2 years, and adding rotations with spine surgeons and pediatric orthopaedic surgeons. This may be an unpopular opinion because the expanded time that keeps a trainee from earning salary to pay off accrued medical school debt; however, it might be a necessary step to ensure adequate procedure exposure before starting independently. Our general surgical oncology counterparts have adopted a similar approach in the training of their fellows. Having recognized that there is a need for assessing the qualifications/expertise of trainees, they developed a comprehensive 2-year curriculum that involved core tumor knowledge, and clinical expertise that would allow an individual to gain appropriate experience for dealing with procedures in their practice [10]. Another example of cross-specialty sharing of knowledge from our own orthopaedic community is that of spine surgery. Recent studies have shown that even though both orthopaedic surgeons and neurosurgeons regularly perform spine surgery, their training spectrums are variable [6]: Orthopaedic spine surgeons get more experience with instrumentation and deformity procedures, whereas our neurosurgery counterparts have more exposure to minimally invasive spine and tumor surgeries. Although individuals have advocated for a spine surgery training track, it has yet to be implemented [5]. In recognition of this problem, orthopaedic and neurosurgeons are advocating exchange of information, by encouraging trainees to do cross-fellowships (that is, orthopaedic residents do a neurosurgery fellowship, and neurosurgery residents do an orthopaedic spine fellowship). Last but not the least, a Certificate of Added qualification (CAQ) might be another way forward for tackling this issue. Our surgical oncology counterparts have introduced something similar, wherein newly graduated surgical oncologists have a period of 7 years to apply/pass the exam, and gain subspecialty certification [2, 3]. By setting requirements of a healthy practice through assessment of individual procedure volumes, we might be able to offer these CAQs as a reflection of appropriate experience/exposure. Although the purpose of a CAQ would not be to identify “the best in business”, it would be a way of monitoring the credentials of the oncology workforce in the coming decade. The certification is not intended to stop people from practicing orthopaedic oncology if their procedure volume is low, but it will facilitate/encourage individuals on adopting best-referral practices. If an orthopaedic oncologist does not feel comfortable doing a complex procedure due to limited exposure, he or she can refer patients to another high-volume surgeon. If an orthopaedic oncologist develops a healthy practice over time, and subsequently gains experience, they can reapply for the CAQ, submit their case logs and outcomes, give an exam and subsequently gain subspecialty certification.
Conclusion
Despite an increasing number of fellowships, the median number of procedures performed by musculoskeletal oncology fellows has not changed over time. However, variability in the types of procedures performed by ACGME-accredited oncology fellows exists, with a number of fellows reporting procedure numbers that do not meet the recommended minimum threshold before completing their training. Discussion on ways of improving the current state of affairs, either through restricting the number of fellowships and/or introducing CAQs is suggested by these findings. The future of the field and patient outcomes depends on honesty as the next generation is trained to take care of complex bone/soft tissue sarcomas.
Footnotes
Each author certifies that neither he, nor any member of his immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Accreditation Council for Graduate Medical Education. Case Log Graduate Statistics. Available at: https://www.acgme.org/Data-Collection-Systems/Case-Log-Graduate-Statistics. Accessed May 25, 2020.
- 2.American Board of Surgery. Complex General Surgical Oncology. Available at: http://www.absurgery.org/default.jsp?examoffered_surgonc. Accessed May 25, 2020.
- 3.American Board of Surgery. ABS Announces New Certificate in Complex General Surgical Oncology. 2011. Available at: https://www.absurgery.org/default.jsp?newssurgonc. Accessed May 25, 2020.
- 4.Biermann JS. CORR Insights(R): how much tumor surgery do early-career orthopaedic oncologists perform? Clin Orthop Relat Res. 2015;473:703-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels AH, Ames CP, Garfin SR, Shaffrey CI, Riew KD, Smith JS, Anderson PA, Hart RA. Spine surgery training: is it time to consider categorical spine surgery residency? Spine J. 2015;15:1513-1518. [DOI] [PubMed] [Google Scholar]
- 6.Daniels AH, Ames CP, Smith JS, Hart RA. Variability in spine surgery procedures performed during orthopaedic and neurological surgery residency training: an analysis of ACGME case log data. J Bone Joint Surg Am. 2014;96:e196. [DOI] [PubMed] [Google Scholar]
- 7.Duchman KR, Miller BJ. Are Recently Trained Tumor Fellows Performing Less Tumor Surgery? An Analysis of 10 Years of the ABOS Part II Database. Clin Orthop Relat Res. 2017;475:221-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enneking WF. An abbreviated history of orthopaedic oncology in North America. Clin Orthop Relat Res. 2000:115-124. [DOI] [PubMed] [Google Scholar]
- 9.McClure PK, Woiczik M, Karol L, Sankar WN. Variation in National ACGME Case Log Data for Pediatric Orthopaedic Fellowships: Are Fellow Coding Practices Responsible? J Pediatr Orthop. 2017;37:e329-e334. [DOI] [PubMed] [Google Scholar]
- 10.Michelassi F. 2010 SSO Presidential Address: Subspecialty Certificate in Advanced Surgical Oncology. Annals of Surgical Oncology. 2010;17:3094-3103. [DOI] [PubMed] [Google Scholar]
- 11.Miller BJ, Rajani R, Leddy L, Carmody Soni EE, White JR, Musculoskeletal Oncology Research I. How much tumor surgery do early-career orthopaedic oncologists perform? Clin Orthop Relat Res. 2015;473:695-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumor Society Musculoskeletal. MSTS Fellowship Surgical Guidelines (RRC Requirements). Available at: http://www.msts.org/view/download.php/fellowships/surgical-guidelines. Accessed May 25, 2020.
- 13.Okike K, Berger PZ, Schoonover C, OT RV. Do Orthopaedic Resident and Fellow Case Logs Accurately Reflect Surgical Case Volume? J Surg Educ. 2018;75:1052-1057. [DOI] [PubMed] [Google Scholar]
- 14.Pratt CB, Champion JE, Fleming ID, Rao B, Kumar AP, Evans WE, Green AA, George S. Adjuvant chemotherapy for osteosarcoma of the extremity. Long-term results of two consecutive prospective protocol studies. Cancer. 1990;65:439-445. [DOI] [PubMed] [Google Scholar]
- 15.Ruddell JH, Eltorai AEM, DePasse JM, Kuris EO, Gil JA, Cho DK, Paxton ES, Green A, Daniels AH. Trends in the Orthopaedic Surgery Subspecialty Fellowship Match: Assessment of 2010 to 2017 Applicant and Program Data. JBJS. 2018;100:e139. [DOI] [PubMed] [Google Scholar]
- 16.Salazar D, Schiff A, Mitchell E, Hopkinson W. Variability in Accreditation Council for Graduate Medical Education Resident Case Log System practices among orthopaedic surgery residents. J Bone Joint Surg Am. 2014;96:e22. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz HS. CORR Insights(R): Are Recently Trained Tumor Fellows Performing Less Tumor Surgery? An Analysis of 10 Years of the ABOS Part II Database. Clin Orthop Relat Res. 2017;475:229-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White J, Toy P, Gibbs P, Enneking W, Scarborough M. The current practice of orthopaedic oncology in North America. Clin Orthop Relat Res. 2010;468:2840-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]