Abstract
Feto-maternal communication helps to maintain pregnancy and contributes to parturition at term and preterm. Endocrine and immune factor are well reported communication mediators. Recent advances in extracellular vesicle (EV) biology has introduced them as major communication channels between the mother and fetus. EVs are round structures with a lipid bilayer membrane. EVs are generally categorized based on their size and mode of biogenesis. The most commonly reported EVs are exosomes with a size range of 30 −160 nm that are formed inside the intraluminal vesicles of multivesicular body. Microvesicles (MVs) are larger than > 200nm and formed by outward budding of plasma membrane. Vesicles are released from all cells and carry various factors that reflect the physiologic state of cell at the time of their release. Analysis of vesicle provides a snapshot of origin cell. Recent studies in perinatal medicine has shown that exosomes are key communicators between feto-maternal units, and they can cross placenta. Fetal derived exosomes released under term labor associated conditions can cause parturition associated changes in maternal uterine tissues. Exosomes carrying inflammatory cargo can cause preterm birth in animal models suggesting their functional role in parturition. A few reports have profiled differences between exosome cargos from term and preterm pregnancies and indicated their biomarker potential to predict high risk pregnancy status. There are hardly any reports on MVs and their functional roles in reproduction. Herein, we review of EVs and MVs, their characteristics, function, and usefulness predicting adverse pregnancy complications such as preterm birth.
Keywords: amniotic fluid, exosomes, biomarkers, signaling, microvesicles
Preterm birth (birth before the 37th completed week of pregnancy) is a major pregnancy complication that affects ~ 10–12 % of all pregnancies worldwide.1 The most common phenotype (60%) of preterm birth occurs spontaneously (referred as PTB in this review) with 30–40% being preceded by preterm premature rupture of the fetal membranes (pPROM).1 Current interventions to reduce the risk of preterm labor have been designed primarily based on our understanding of signaling at the maternal myometrium, specifically minimizing contractions to prolong gestation. A higher rate of PTB around the globe warrants a better understanding of signals and their mechanisms that initiate normal term pregnancies, which can provide more insights into pathologic activation of such signals associated with preterm parturitions.2 Besides, these signals may also function as potential biomarkers of an underlying pathology. To address the problem of PTB, a clear understanding of the maternal-fetal signals that induce labor is needed. Significant knowledge gaps exist in this area, especially in our understanding of how signals from the fetus may affect the contractile state of the uterus and induce its transition from a relaxed to laboring state to facilitate birth.3 Current advancements in the field of extracellular vesicles (EVs) reveal novel concepts on intercellular communication pathways.4,5 This review is focused on EVs and their role in PTB.
The International Society for Extracellular Vesicles (ISEV) defines “extracellular vesicle” as the generic term for naturally released particles from the cell. EVs are membranous lipid bilayer structures without nuclei that are released from different cells as a part of physiologic and metabolic changes and cannot replicate.6 EVs include exosomes, microvesicles, microparticles, ectosomes, oncosomes, and apoptotic bodies.6 Particle name is often assessed based on their size, biogenesis, cargo contents, tissue tropism, and function.5,7,8 They carry a variety of molecules including nucleic acids, proteins, lipids, and other metabolites. The classification of EVs is still being debated6 and recent reports have referred EVs primarily as ectosomes and exosomes based on their size and biogensis.9,10 Exosomes have a size range of ~40 to 160 nm with an endosomal origin. Ectosomes pinch off the surface of the plasma membrane via outward budding and include microvesicles (MVs, discussed later in this review) and other large vesicles in the size range of ~50 nm to 1 μm. Apoptotic bodies are released by cells undergoing apoptosis and have a minimum size of 1μM or higher. A subclass of exosomes, named as exomeres, size range ~ 35 nm, were also isolated and reported by Zhang et al by using asymmetric flow field-flow fractionation.11 Differences in biogenesis, size, and cellular physiology can produce distinct EV population with unique cargo that can target specific recipient cell(s) to exert biological functions.10,12,13
One of the key contributors to heterogeneities in EV definition and classification is their isolation protocols.14 Several approaches are employed to isolate EVs from various biological fluids, cell culture media, and cell and tissues that often fail to separate particles into distinct size category groups.15 Heterogeneity in EV population can contribute to erroneous data and interpretation of EV’s role.15 To avoid any confounding effects of various particles in the preparation, stringent and specific approaches to segregate particles into different categories and characterize their properties are needed. The heterogeneity issue is introduced here so readers can interpret data with caution when reviewing articles related to as EV, specifically in reproductive biology. Lack of stringent criteria in various steps of EV research has hampered the reproducibility of data. This review will focus on the role of EVs in parturition signaling, their usefulness in understanding PTB pathology, mechanisms, and more importantly their usefulness as a biomarker in identifying women at high risk for adverse pregnancy outcomes.
EVs in the reproductive biology literature are often referred to as exosomes with a size range between 30 – 175 nm (EVs will be referred to as ‘exosomes’ in the rest of this review). 16–21 Several studies and reviews discuss the role of exosomes to embryogenesis22–24, implantation25–28, pregnancy and parturition.19,29–32 Exosomes’ functional role in preeclampsia, gestational diabetes pathogenesis, placental dysfunctions, and their biomarker potentials have been well reported.33–40 This was made possible partly due to the presence of placental alkaline phosphatase (PLAP), an enzyme present in placental trophoblast cells41 and expressed on the membrane of trophoblast-derived exosomes. Tagging PLAP has been used as a method of isolating and characterizing exosomes which facilitated studying them as a snapshot of placental function. To note, PLAP antibodies used from commercial sources have been seen to cross react with other reproductive cellular targets as well as in exosomes from these cells including fetal membranes, decidua, and myometrium. 29
The number of reports on exosomes on spontaneous PTB is far fewer than those seen in indicated PTB. A literature review using extracellular vesicles, and preterm birth, or exosomes and preterm birth yielded only 39 articles from the past decade. This indicates that studies on the role of EVs in preterm birth is still limited. The rest of this review will highlight the functional aspects of exosomes as a signaler in parturition at term and preterm and biomarker potential of exosomes and other vesicles. An untouched area of research is microvesicles and their research potential. We will provide a brief overview of microvesicles and an update on some of the ongoing work in this area.
Exosomes as a functional contributor of parturition:
This review introduced the need to better understand the signaling between feto-maternal tissues in both term and preterm parturitions. Although fetal endocrine factors are reported in the literature,2,42–44 paracrine signals are hardly explained.45 Our recent findings support the core hypothesis that oxidative stress (OS) and cellular senescence of the fetal membranes (amniochorion) trigger human parturition by affecting uterine tissue inflammatory status through paracrine pathways.46–49,50 In pursuit of this hypothesis, we were able to demonstrate a functional role for exosomes in promoting parturition using both in vitro and in vivo animal model experiments.
Exosomes produced in response to oxidative stress (OS), infection, and inflammation carry inflammatory mediators:
Exosomes are released from fetal membrane cells and their cargo contents differ in response to the type of stimulant. OS (induced by cigarette smoke extract – CSE), inflammation (induced by TNF-α), and infection (induced by lipopolysaccharide [LPS]) released exosomes from fetal membrane cells with distinct cargo content (Figure 2). Bioinformatics analysis showed that the proteomic cargo represented “inflammation” as the mechanistic function encoded in the exosomes. The pathways of inflammation were distinct in response to different stimuli.51 This is supportive of distinct pathways of inflammatory preterm labor signals activated by various risk factors.52,53 Exosomes released from different layers of the membranes are expected to cause paracrine changes and modify local environment. In normal term birth, localized inflammation and OS can generate distinct cargo enriched exosomes that can traverse through tissue layers, or through amniotic fluid or blood. Released exosomes can reach feto-maternal interface tissues and other destinations to cause parturition associate changes.32,54 Animal models have shown that exosomes can traffic between feto-maternal tissues (fetus→mother and mother →fetus).55,56 During PTB, risk factors such as OS, infection, and inflammation can prematurely generate exosomal signals to generate preterm contractions leading to delivery. Similarly, we have also reported the role of environmental pollutants like polybrominated diphenyl ethers (PBDEs) and bisphenols (A [BPA], tetrabromobisphenol A [TBBPA], and 2,4,6-Tribromophenol [TBP]) in causing unique proteomic cargo generation in exosomes derived from fetal amnion cells that may have functional implication as well as suggest their potential to be developed as a biomarker.57 These pollutants also produced inflammatory cargo but they were all distinct suggesting different pathways of inflammation initiated by separate exposures.
Figure 2.
Exosomes as a functional contributor to parturition and preterm birth. Exosomes released from fetal cells under stimulatory conditions such as oxidative stress, inflammation, and infection are characterized by higher number and altered contents. Fetal exosomes traffic to maternal side and initiate parturition signaling through disturbing immune-regulatory balance, activation of several inflammatory pathways, and the production of pro-inflammatory mediators as cytokines resulting in contraction of uterus and parturition.
Fetal exosomes cause uterine cell inflammation:
In vitro model studies showed that oxidatively stressed exosomes derived from human fetal cells can cause uterine inflammatory changes. Myometrial and decidual inflammation (cytokine and prostaglandin increase) was seen in response to exosomes from OS induced cells compared to control. Fetal exosomes were also increased in myometrial tissue segments in term labor samples compared to term not in labor myometrial sections supporting the hypothesis that exosomal signaling is one of the key mechanisms triggering parturition along with other feto-maternal signals.54
Inflammatory cargo enriched exosomes cause PTB in animal models:
The above hypothesis was further supported by evidence from animal model studies. During mouse pregnancy, inflammatory cargo enriched exosomes increase in maternal blood as gestation progress.58 The increase reached maximum on day 18, penultimate day before labor and delivery in this model. Isolation of day 18 exosomes and their subsequent injection to animal on day 15 resulted in PTB.58 This PTB was associated with increased inflammation (NF-kB activation and pro-inflammatory cytokine production) in the amniotic fluid, uterine decidua, and cervix. PTB was not associated with a systemic progesterone withdrawal or systemic maternal inflammation, suggesting that exosomes along can trigger parturition.59 Along with other endocrine mediators, immune system components, and mechanical mediators, exosomes can play a role in promoting parturition. In addition, exosomes under specific conditions may promote labor even in the absence of endocrine and physiologic changes or stimulants, suggesting their use as biomarkers for PTB.
Exosomes as biomarkers of PTB:
The first report on exosomes as biomarkers was by Cantonwine and McElrath’s group60. In this study, first-trimester maternal plasma samples from a prospective PTB cohort were used to analyze proteomic cargo in microparticles. The term ‘circulating microparticles’ was used for vesicles that are enriched from plasma by size exclusion chromatography and isocratically eluted using an elution reagent.60 Proteomic analysis of particle cargo contents revealed an inflammatory signature as early as in first-trimester in women who delivered preterm at < 34 weeks of gestation. McElrath et al further validated this study using plasma samples from 3 additional cohorts and showed that circulating microparticle cargo protein combination of F13A, FBLN1, IC1, ITIH2, and LCAT had revealed an area under the curve (AUC) of 0.74 (95% CI, 0.63–0.81).61 AUC was slightly better 0.77 (95% CI, 0.61–0.90) with IC1, LCAT, TRFE, and ITIH4 as markers within the cargo to predict high-risk status in nulliparous subjects at a very early stage of pregnancy. Additional validation of this cohort may provide an early stage biomarker for PTB. The particles analyzed may include both exosomes and other smaller microparticles, thus, the isolation of that unique cluster of particles maybe necessary to determine the biomarker potential of these particles. A third study by Fallen et al studied RNA in EV and reported them as a potential biomarker for PTB.62 This study used maternal plasma samples from women with spontaneous preterm labor between 24 and 34 weeks of gestation. The term EV was applied in this study to describe particles isolated from plasma using size exclusion chromatography.62
We have reported biomarker potential of EV using a prospective cohort from India where maternal plasma samples from 3 trimesters and at the time of labor ending with normal term birth or spontaneous PTB. In two separate studies, we analyzed for miRNA cargo63 or proteomic cargo30. In this longitudinal study design, particles were defined as ‘exosomes’ with a size range between 30 – 150 nm. In the first study, miRNA cargo was analyzed in the total exosome population in maternal plasma. A total of 167 and 153 miRNAs were found to significantly change as a function of the gestational age across term and PTB pregnancies, respectively. Interestingly, a comparison analysis between the exosomal miRNA profile between term and PTB reveals a total of 173 miRNAs that significantly change across gestation. The data obtained identified specific trends of changes (i.e., increase, decrease, and both) in exosomal miRNA specific to term and PTB as a function of the gestational age.
In a second study, PLAP+ve exosomes (referred to as EVs) were further isolated from the same samples and subjected to cargo protein analysis using proteomics. Several interesting findings based on size were observed between normal term and PTB groups in PLAP+ EVs. The gestational age variation of different populations of EVs based on their size (ie, <50 nm, 50–150 nm, 150–200 nm, and >200 nm) were seen in our analysis. Placental EV markers and morphology were not different between gestational ages in both term and PTB; however, a significant effect by subject (ie, patient variation indicating that the levels of EVs were different across the women) was identified in all EV subpopulations except for vesicles >200 nm. A significant effect as a function of the gestational age was also identified in EV subpopulations between 50 and 150 nm and 150 and 200 nm. This heterogeneity of particle size might be a function of an underlying condition and may impact cargo signature. A comparative analysis between the PLAP+ EV proteome from term and PTB revealed 96 proteins differing significantly across gestation. Bioinformatics analysis of differentially expressed proteins revealed consistent upregulation of inflammatory pathways in both upregulation of epithelial mesenchymal transition pathways at term and downregulation of coagulation/complement activation in preterm. As noted above, readers are cautioned about PLAP+ EVs as their isolation depends on the quality of antibody used and their specificity. In addition, the three studies described above use different nomenclature to describe the particle analyzed including our own (EV vs exosomes). We strongly urge researchers to define the methods used for isolation and particle size used for assay analysis.
In the last segment of this review, we introduce microvesicles that are hardly described in PTB literature.
Microvesicles:
As mentioned earlier, one of the major challenges in studying EVs is to establish a standard isolation method that could efficiently distinguish between exosomes and MVs.64 Most of our knowledge about EVs mainly comes from studies on exosomes or heterogeneous samples containing both exosomes and microvesicles. However, recent studies focusing on MVs only is becoming an area of intense research. Microvesicles, also known as microparticles, ectosomes, or shedding vesicles are considered a heterogeneous population of EVs. Their size, morphology, density, and composition overlap with exosomes. The absence of specific surface markers or a standard isolation method for MVs to obtain a clear distinction from exosomes is still an ongoing limitation.10
Unlike exosomes, microvesicles are released by outward budding and shedding from the plasma membrane. The biogenesis of microvesicles is not fully understood, but it depends on dynamic synchronization between the redistribution of phospholipids rafts on the cell surface and contraction of cytoskeletal protein mediated by actin-myosin interaction.65 Microvesicles formation and budding is induced by translocation and externalization of phosphatidylserine to the cell’s outer membrane leaflet.65,66 The interaction between actin and myosin results in the shedding of MVs through the outward budding phenomenon. MVs membrane can be stained by annexin-V that has a high affinity to phosphatidylserine, which can be used to differentiate them from exosomes.67,68
Because of their unique biogenesis, MVs share many characteristics with their parent cells such as surface markers. Some studies have reported some common surface markers such as integrins, selectins, flotillin-2, and CD40 that could be specific to most MVs.69,70
Like EVs, the MVs cargo represents information about the state of their origin cells that is specific to the time of their expression.71 Thus, MVs can be a tool to understand the cells and even organ activities in a real-time manner, especially in pregnancy complications such as pre-eclampsia, recurrent miscarriages, and PTB. Not only MVs can be a result of disease or complication, but also it might be a contributor. Therefore, MVs can be considered a mediator for several pregnancy complications but also a potential biomarker for the early diagnosis of several pathological conditions. However, all the reported studies focus on circulating microparticles and placental-derived MVs only. MVs have been also known for their immunomodulatory effects where they could act as pro-inflammatory or anti-inflammatory based on their cargo and surface receptors.72 MVs have been implicated in immune modulation during normal and complicated pregnancy.66,73 MVs derived from syncytiotrophoblast cells (STBM) showed a pro-inflammatory effect through stimulation of the release of inflammatory cytokines from circulating leukocytes in normal pregnancy and pre-eclampsia.74 STBM has been also reported to activate circulating monocytes.75
The association of MVs with preeclampsia has been widely reported. Excessive shedding of syncytiotrophoblast-derived EVs is associated with preeclampsia.76 It can be detected in the early-onset disease. The exact role of MVs in preeclampsia is not clear but the high amount of circulating syncytiotrophoblast-derived MVs in maternal blood may contribute to inflammation and endothelial injury leading to gestational vascular complications, monocyte stimulation, and an excessive maternal inflammatory reaction.75 Syncytin-1 has been found on trophoblast-derived MVs, which mediate the activation of peripheral blood mononuclear cells. Syncytin 1 and 2 are highly expressed from the placenta and are known for their role in trophoblast cell to cell fusion. The presence of such proteins on MVs might contribute to their fusion with target cells.77 It is suggested that PTB can result from the irregularities in the maternal-fetal communication caused by modulations in EVs signaling that lead to crashing the established network and thus initiating parturition.
The complete role of MVs in pregnancy is still not clear. Most of the current studies focused on placental-derived MVs only. However, no study has reported the functional role of MVs derived from the fetal membranes or other feto-maternal uterine tissues. Our laboratory is focused on understanding EVs and MVs from fetal membranes and thus, insights into fetal-membrane derived EVs open a novel approach in understanding some of the major pregnancy-related complications such as PTB. Since we have reported the role of exosomes released by amnion epithelial cells in pregnancy and PTB.54,78 In this context, our group has been working on studying microvesicles released from fetal membrane at term. We aim to fully characterize isolated MVs from cell culture through the identification of their protein cargo and specific biomarkers, morphology, the uptake of fetal-derived MVs by maternal cells, functional role in feto-maternal communication, and potential pro-inflammatory effect on maternal cells.
In summary, exosomes and MV research is still in its infancy in the field of PTB. Several functional models and biomarker trials are needed to understand how these particles are used for communication between the mother and the fetus and vice versa and how they contribute to pregnancy success and parturition both at term and preterm.
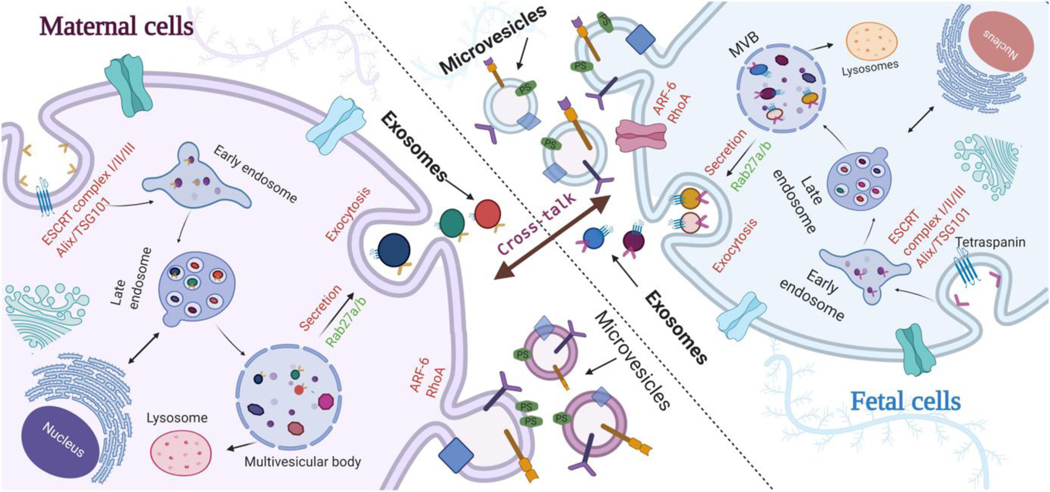
Figure 1.
Biogenesis of extracellular vesicles and trafficking between feto-maternal cells. Exosomes are formed inside the intraluminal vesicles of multivesicular body (MVBs). Exosomes get released by exocytosis from cell membrane into the extracellular space upon the fusion of MVBs with the cell surface. Microvesicles are formed by outward budding of plasma membrane through re-arrangement of lipid rafts leading to the exposure of phosphatidyl serine (PS) on the outer leaflets of the membrane. Exosomes and possibly microvesicles play a key role in feto-maternal cross-talk during pregnancy under normal and pathological conditions and contribute to parturition.
Figure 3.
ransmission Electron Microscopy (TEM) showing the morphology and size of exosomes and microvesicles isolated from amnion epithelial cells. (A) TEM for exosomes showing multiple circular vesicles with a size distribution between 30 nm and 120 nm. (B) TEM for MVs with oval shape and size distribution of 250 nm. Arrows indicate extracellular vesicles.
Acknowledgments
Funding: This study is funded by NIH/NIAD (1R21AI140249-01A1), NIH/NICHD (5 R03 HD098469 02 and 1R01HD084532-01A1) to R Menon
Footnotes
Conflicts of interest: Authors report no conflict of interest
REFERENCES
- 1.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon R, Bonney EA, Condon J, Mesiano S, Taylor RN. Novel concepts on pregnancy clocks and alarms: redundancy and synergy in human parturition. Hum Reprod Update. 2016;22(5):535–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maia J, Caja S, Strano Moraes MC, Couto N, Costa-Silva B. Exosome-Based Cell-Cell Communication in the Tumor Microenvironment. Front Cell Dev Biol. 2018;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. [DOI] [PubMed] [Google Scholar]
- 6.Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3(5):321–330. [DOI] [PubMed] [Google Scholar]
- 8.Rojalin T, Phong B, Koster HJ, Carney RP. Nanoplasmonic Approaches for Sensitive Detection and Molecular Characterization of Extracellular Vesicles. Front Chem. 2019;7:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–372. [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. [DOI] [PubMed] [Google Scholar]
- 13.van NG, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21. [DOI] [PubMed] [Google Scholar]
- 14.Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Shoaie N, Jahanpeyma F, Zhao J, Azimzadeh M, Al Jamal KT. Optical, electrochemical and electrical (nano)biosensors for detection of exosomes: A comprehensive overview. Biosens Bioelectron. 2020;161:112222. [DOI] [PubMed] [Google Scholar]
- 16.Mincheva-Nilsson L, Baranov V. The role of placental exosomes in reproduction. Am J Reprod Immunol. 2010;63(6):520–533. [DOI] [PubMed] [Google Scholar]
- 17.Jin J, Menon R. Placental exosomes: A proxy to understand pregnancy complications. Am J Reprod Immunol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Redman CW, Tannetta DS, Dragovic RA, et al. Review: Does size matter? Placental debris and the pathophysiology of pre-eclampsia 38. Placenta. 2012;33 Suppl:S48–S54. [DOI] [PubMed] [Google Scholar]
- 19.Salomon C, Nuzhat Z, Dixon CL, Menon R. Placental Exosomes During Gestation: Liquid Biopsies Carrying Signals for the Regulation of Human Parturition. Curr Pharm Des. 2018;24(9):974–982. [DOI] [PubMed] [Google Scholar]
- 20.Ilekis JV, Tsilou E, Fisher S, et al. Placental origins of adverse pregnancy outcomes: potential molecular targets: an Executive Workshop Summary of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Am J Obstet Gynecol. 2016;215(1 Suppl):S1–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell MD, Peiris HN, Kobayashi M, et al. Placental exosomes in normal and complicated pregnancy 1. Am J Obstet Gynecol. 2015;213(4 Suppl):S173–S181. [DOI] [PubMed] [Google Scholar]
- 22.Machtinger R, Rodosthenous RS, Adir M, et al. Extracellular microRNAs in follicular fluid and their potential association with oocyte fertilization and embryo quality: an exploratory study. J Assist Reprod Genet. 2017;34(4):525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saadeldin IM, Oh HJ, Lee BC. Embryonic-maternal cross-talk via exosomes: potential implications. Stem Cells Cloning. 2015;8:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross N, Kropp J, Khatib H. MicroRNA Signaling in Embryo Development. Biology (Basel). 2017;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbluth EM, Shelton DN, Wells LM, Sparks AE, Van Voorhis BJ. Human embryos secrete microRNAs into culture media--a potential biomarker for implantation. Fertil Steril. 2014;101(5):1493–1500. [DOI] [PubMed] [Google Scholar]
- 26.Andronico F, Battaglia R, Ragusa M, Barbagallo D, Purrello M, Di Pietro C. Extracellular Vesicles in Human Oogenesis and Implantation. Int J Mol Sci. 2019;20(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurung S, Greening DW, Catt S, Salamonsen L, Evans J. Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol Hum Reprod. 2020. [DOI] [PubMed] [Google Scholar]
- 28.Kaminski VL, Ellwanger JH, Chies JAB. Extracellular vesicles in host-pathogen interactions and immune regulation - exosomes as emerging actors in the immunological theater of pregnancy. Heliyon. 2019;5(8):e02355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Li H, Fan B, Xu W, Zhang X. Extracellular vesicles in normal pregnancy and pregnancy-related diseases. J Cell Mol Med. 2020;24(8):4377–4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon R, Debnath C, Lai A, et al. Protein Profile Changes in Circulating Placental Extracellular Vesicles in Term and Preterm Births: A Longitudinal Study. Endocrinology. 2020;161(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon R. Initiation of human parturition: signaling from senescent fetal tissues via extracellular vesicle mediated paracrine mechanism. Obstet Gynecol Sci. 2019;62(4):199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menon R, Mesiano S, Taylor RN. Programmed Fetal Membrane Senescence and Exosome-Mediated Signaling: A Mechanism Associated With Timing of Human Parturition. Front Endocrinol (Lausanne). 2017;8:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice GE, Scholz-Romero K, Sweeney E, et al. The Effect of Glucose on the Release and Bioactivity of Exosomes From First Trimester Trophoblast Cells 2. J Clin Endocrinol Metab. 2015;100(10):E1280–E1288. [DOI] [PubMed] [Google Scholar]
- 34.Salomon C, Guanzon D, Scholz-Romero K, et al. Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation. J Clin Endocrinol Metab. 2017;102(9):3182–3194. [DOI] [PubMed] [Google Scholar]
- 35.Vargas A, Zhou S, Ethier-Chiasson M, et al. Syncytin proteins incorporated in placenta exosomes are important for cell uptake and show variation in abundance in serum exosomes from patients with preeclampsia 21. FASEB J. 2014;28(8):3703–3719. [DOI] [PubMed] [Google Scholar]
- 36.Gill M, Motta-Mejia C, Kandzija N, et al. Placental Syncytiotrophoblast-Derived Extracellular Vesicles Carry Active NEP (Neprilysin) and Are Increased in Preeclampsia. Hypertension. 2019;73(5):1112–1119. [DOI] [PubMed] [Google Scholar]
- 37.Pillay P, Moodley K, Vatish M, Moodley J, Duarte R, Mackraj I. Exosomal Th1/Th2 cytokines in preeclampsia and HIV-positive preeclamptic women on highly active anti-retroviral therapy. Cytokine. 2020;125:154795. [DOI] [PubMed] [Google Scholar]
- 38.Pillay P, Vatish M, Duarte R, Moodley J, Mackraj I. Exosomal microRNA profiling in early and late onset preeclamptic pregnant women reflects pathophysiology. Int J Nanomedicine. 2019;14:5637–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clemente L, Boeldt DS, Grummer MA, et al. Adenoviral transduction of EGFR into pregnancy-adapted uterine artery endothelial cells remaps growth factor induction of endothelial dysfunction. Mol Cell Endocrinol. 2020;499:110590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sammar M, Dragovic R, Meiri H, et al. Reduced placental protein 13 (PP13) in placental derived syncytiotrophoblast extracellular vesicles in preeclampsia - A novel tool to study the impaired cargo transmission of the placenta to the maternal organs. Placenta. 2018;66:17–25. [DOI] [PubMed] [Google Scholar]
- 41.Novelli G, Mannello F, Cosmi EV, Biagioni S, Dallapiccola B. Alkaline phosphatase expression in human chorionic villi. Exp Cell Biol. 1987;55(1):34–41. [DOI] [PubMed] [Google Scholar]
- 42.Smith R, Mesiano S, McGrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108(2–3):159–164. [DOI] [PubMed] [Google Scholar]
- 43.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1(5):460–463. [DOI] [PubMed] [Google Scholar]
- 44.Petraglia F, Imperatore A, Challis JR. Neuroendocrine mechanisms in pregnancy and parturition. Endocr Rev. 2010;31(6):783–816. [DOI] [PubMed] [Google Scholar]
- 45.Iliodromiti Z, Antonakopoulos N, Sifakis S, et al. Endocrine, paracrine, and autocrine placental mediators in labor. Hormones (Athens). 2012;11(4):397–409. [DOI] [PubMed] [Google Scholar]
- 46.Menon R, Behnia F, Polettini J, Saade GR, Campisi J, Velarde M. Placental membrane aging and HMGB1 signaling associated with human parturition. Aging (Albany NY). 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menon R. Human fetal membranes at term: Dead tissue or signalers of parturition? Placenta. 2016;44:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polettini J, Behnia F, Taylor BD, Saade GR, Taylor RN, Menon R. Telomere Fragment Induced Amnion Cell Senescence: A Contributor to Parturition? PLoS One. 2015;10(9):e0137188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Behnia F, Taylor BD, Woodson M, et al. Chorioamniotic Membrane Senescence: A Signal for Parturition? Am J Obstet Gynecol. 2015. [DOI] [PubMed] [Google Scholar]
- 50.Bonney EA, Krebs K, Saade G, et al. Differential senescence in feto-maternal tissues during mouse pregnancy. Placenta. 2016;43:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monsivais LA, Sheller Miller S, Russell W, et al. Fetal Membrane Extracellular Vesicle Profiling Reveals Distinct Pathways Induced by Infection and Inflammation In Vitro. Am J Reprod Immunol. 2020:e13282. [DOI] [PubMed] [Google Scholar]
- 52.Behnia F, Sheller S, Menon R. Mechanistic Differences Leading to Infectious and Sterile Inflammation 3. Am J Reprod Immunol. 2016. [DOI] [PubMed] [Google Scholar]
- 53.Sheller-Miller S, Urrabaz-Garza R, Saade G, Menon R. Damage-Associated molecular pattern markers HMGB1 and cell-Free fetal telomere fragments in oxidative-Stressed amnion epithelial cell-Derived exosomes. J Reprod Immunol. 2017;123:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hadley EE, Sheller-Miller S, Saade G, et al. Amnion Epithelial Cell Derived Exosomes Induce Inflammatory Changes in Uterine Cells. Am J Obstet Gynecol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sheller-Miller S, Choi K, Choi C, Menon R. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am J Obstet Gynecol. 2019;221(5):502 e501–502 e512. [DOI] [PubMed] [Google Scholar]
- 56.Sheller-Miller S, Lei J, Saade G, Salomon C, Burd I, Menon R. Feto-Maternal Trafficking of Exosomes in Murine Pregnancy Models. Front Pharmacol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sheller-Miller S, Radnaa E, Arita Y, et al. Environmental pollutant induced cellular injury is reflected in exosomes from placental explants. Placenta. 2020;89:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheller-Miller S, Trivedi J, Yellon SM, Menon R. Exosomes Cause Preterm Birth in Mice: Evidence for Paracrine Signaling in Pregnancy. Sci Rep. 2019;9(1):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schander JA, Correa F, Bariani MV, et al. A role for the endocannabinoid system in premature luteal regression and progesterone withdrawal in lipopolysaccharide-induced early pregnancy loss model. Mol Hum Reprod. 2016;22(11):800–808. [DOI] [PubMed] [Google Scholar]
- 60.Cantonwine DE, Zhang Z, Rosenblatt K, et al. Evaluation of proteomic biomarkers associated with circulating microparticles as an effective means to stratify the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2016;214(5):631 e631–631 e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McElrath TF, Cantonwine DE, Jeyabalan A, et al. Circulating microparticle proteins obtained in the late first trimester predict spontaneous preterm birth at less than 35 weeks’ gestation: a panel validation with specific characterization by parity. Am J Obstet Gynecol. 2019;220(5):488 e481–488 e411. [DOI] [PubMed] [Google Scholar]
- 62.Fallen S, Baxter D, Wu X, et al. Extracellular vesicle RNAs reflect placenta dysfunction and are a biomarker source for preterm labour. J Cell Mol Med. 2018;22(5):2760–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menon R, Debnath C, Lai A, et al. Circulating Exosomal miRNA Profile During Term and Preterm Birth Pregnancies: A Longitudinal Study. Endocrinology. 2019;160(2):249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. Journal of Cell Biology. 2013;200(4):373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. Journal of Neuro-Oncology. 2013;113(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Neil EV, Burns GW, Spencer TE. Extracellular vesicles: Novel regulators of conceptus-uterine interactions? Theriogenology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanada M, Bachmann MH, Hardy JW, et al. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proceedings of the National Academy of Sciences. 2015;112(12):E1433–E1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Latham SL, Tiberti N, Gokoolparsadh N, et al. Immuno-analysis of microparticles: probing at the limits of detection. Scientific Reports. 2015;5(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jayachandran M, Miller VM, Heit JA, Owen WG. Methodology for isolation, identification and characterization of microvesicles in peripheral blood. Journal of Immunological Methods. 2012;375(1–2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giacomini E, Alleva E, Fornelli G, et al. Embryonic extracellular vesicles as informers to the immune cells at the maternal–fetal interface. Clinical and Experimental Immunology. 2019;198(1):15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cantonwine DE, Zhang Z, Rosenblatt K, et al. Evaluation of proteomic biomarkers associated with circulating microparticles as an effective means to stratify the risk of spontaneous preterm birth. Am J Obstet Gynecol. 2016;214(5):631. e631–631. e611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Van Hezel ME, Nieuwland R, Van Bruggen R, Juffermans NP. The ability of extracellular vesicles to induce a pro-inflammatory host response. International journal of molecular sciences. 2017;18(6):1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aharon A, Brenner B. Microparticles and pregnancy complications. Thrombosis Research. 2011;127:S67–S71. [DOI] [PubMed] [Google Scholar]
- 74.Germain SJ, Sacks GP, Soorana SR, Sargent IL, Redman CW. Systemic inflammatory priming in normal pregnancy and preeclampsia: the role of circulating syncytiotrophoblast microparticles. The Journal of Immunology. 2007;178(9):5949–5956. [DOI] [PubMed] [Google Scholar]
- 75.Messerli M, May K, Hansson S, et al. Feto-maternal interactions in pregnancies: placental microparticles activate peripheral blood monocytes. Placenta. 2010;31(2):106–112. [DOI] [PubMed] [Google Scholar]
- 76.Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I. Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. Journal of Reproductive Immunology. 2017;119:98–106. [DOI] [PubMed] [Google Scholar]
- 77.Holder BS, Tower CL, Forbes K, Mulla MJ, Aplin JD, Abrahams VM. Immune cell activation by trophoblast-derived microvesicles is mediated by syncytin 1. Immunology. 2012;136(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sheller S, Papaconstantinou J, Urrabaz-Garza R, et al. Amnion-epithelial-cell-derived exosomes demonstrate physiologic state of cell under oxidative stress. PloS one. 2016;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]