Abstract
An increasing amount of research effort is being directed toward investigating the neural bases of social cognition from a systems neuroscience perspective. Evidence from multiple animal species is beginning to provide a mechanistic understanding of the substrates of social behaviors at multiple levels of neurobiology, ranging from those underlying high-level social constructs in humans and their more rudimentary underpinnings in monkeys to circuit-level and cell-type specific instantiations of social behaviors in rodents. Here, we review literature examining the neural mechanisms of social decision-making in humans, nonhuman primates, and rodents, focusing on the amygdala, medial and orbital prefrontal cortical regions and their functional interactions. We also discuss how the neuropeptide oxytocin impacts these circuits and their downstream effects on social behaviors. Overall, we conclude that regulated interactions of neuronal activity in the prefrontal-amygdala pathways critically contribute to social decision-making in the brains of primates and rodents.
Introduction
Recent years have seen an increased interest in investigating the neural bases of social cognition from a systems neuroscience perspective1–7, moving away from phenomenologically mapping social functions to brain areas and toward parsing out mechanisms at the level of neural codes, interregional coordination, connections, and cell types involved. This approach has advanced our knowledge beyond descriptive labels of areas belonging to the ‘social brain’.
Research has focused on examining the neurobiology of social behaviors in several model species including humans, nonhuman primates, and rodents, each with their own advantages and disadvantages (Fig. 1). While the social repertoires of rodents largely involve grooming, sniffing, mating, and aggression, this model allows dissection of the molecular and genetic contributions to social behaviors. For example, optogenetics in rodents has highlighted the roles of cell types and neuronal pathways in vivo. This technology is not currently optimized for primates, though significant advances are being made8. The social repertoires of nonhuman primates are more complex than rodents, and primate studies provide an opportunity to investigate human-like social cognition in individual neurons. Finally, through functional magnetic resonance imaging (fMRI) in humans blood oxygenation-level dependent (BOLD) signals can be examined along with self-reported thoughts and feelings.
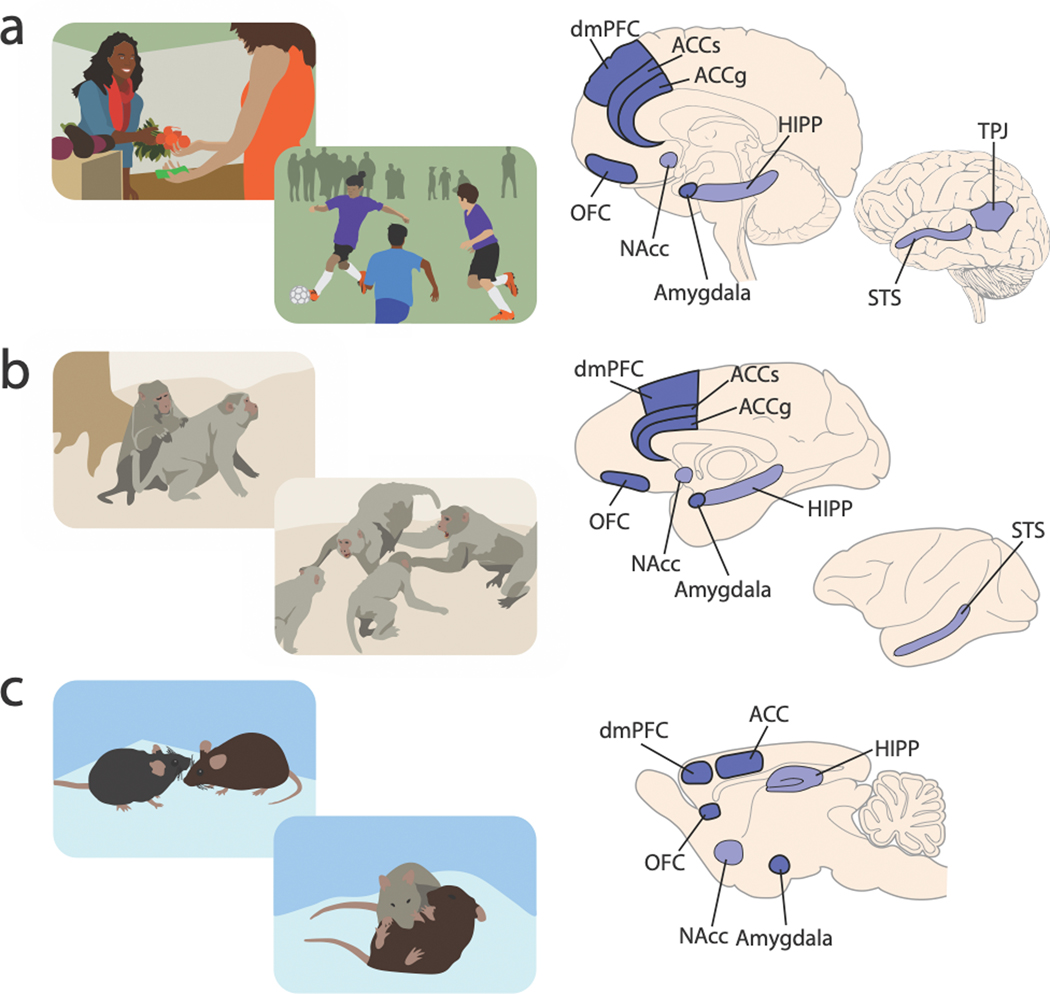
Figure 1. Behavioral ecology of social interaction and brain regions commonly recruited by social behaviors in humans, nonhuman primates, and rodents.
Behavioral illustrations in the left column depict selected social interaction scenarios for humans (a), rhesus macaques (b), and mice (c), exhibiting different levels of complexity in social interactions. Brain illustrations on the right column depict key brain regions that are discussed in this review (darker contrast) and other related regions briefly mentioned in connection (lighter contrast) that are implicated in various social behaviors in each species. (ACCg, anterior cingulate gyrus; ACCs, anterior cingulate sulcus; dmPFC, dorsomedial prefrontal cortex; OFC, orbitofrontal cortex; NAcc, nucleus accumbens; STS, superior temporal sulcus; TPJ, temporal parietal junction; HIPP, hippocampus). Social operations in these brain regions are being actively investigated at multiple neurobiological levels across humans, nonhuman primates, and rodents.
An integrative approach considering research from distinct model systems can be invaluable for understanding the neurobiology of social cognition. While there are vast interspecies differences in behavioral repertoires and investigative methodologies (Box 1), there are also significant commonalities in fundamental processes. In social decision-making, these processes can be broadly divided into the following stages: i) social perception, ii) social learning, valuation, and reward, and iii) social action or response. Of course, social processes are complex, and these stages are iterative and continuous. Still, organized by these themes, this review integrates literature across humans (accompanied by Figs. 1a, 2a, 3a, 4a-b), nonhuman primates (Figs. 1b, 2b, 3b), and rodents (Figs. 1c, 2c, 3c, 4c-e) to discuss the neurobiological substrates in these broadly defined aspects of social decision-making. We focus on the amygdala and prefrontal cortex (PFC) subregions, and the functional interactions in the PFC-amygdala pathways (see Box 2 for an anatomical overview). We also examine the contribution of the neuropeptide oxytocin (OT) in the PFC-amygdala pathways under an integrative framework of OT in multiple stages of social decision-making9 (Figs. 4, 5).
Box 1: Important considerations when comparing across species and different methodologies.
Comparing across different species for investigating the neural bases of species-typical social behaviors can be extremely valuable. However, one must carefully account for distinct ethology and common methodologies used in different species. Each methodology and model system comes with its own advantages and disadvantages. The differences in social repertoire between species vary greatly, complicating cross-species comparisons. For example, while macaques and humans primarily use facial features for visual recognition of conspecifics, rodents predominantly use odor. This difference in principal sensory modality involved in social perception limit direct comparisons of neurobiological processes during social recognition between primates and rodents.
In studies of nonhuman primates, the sample size of data is derived from the number of cells recorded while the number of animals studied is usually small (typically two), so correlations between neural activity and behavior need to be replicated over multiple studies to learn about any neural effects on social relationships and individual differences. The field of functional magnetic resonance imaging (fMRI), most commonly used in humans, has been criticized for low sample sizes in earlier studies, lack of replicability, inflated false positives130, and dependence on particular analytic frameworks131, though constant progress is being made toward higher field-wide standards. For example, a newer approach involves scanning individuals at multiple timepoints, rather than just once, in order to establish more robust databases albeit having a smaller number of unique brains132,133. Even still, fMRI provides a coarse image of a proxy for neural activity, and the difference in temporal scale of the blood oxygenation-level dependent (BOLD) response and neuronal firing rates is considerable. Moreover, signal-to-noise ratio in fMRI signals are highly sensitive to geometric distortion and nearby draining veins, complicating the interpretation and comparison of BOLD responses in different cortical and subcortical regions. For example, because of the anatomical locations of the orbitofrontal cortex (OFC) and the amygdala, the BOLD signals obtained from these regions are particularly susceptible to such problems and may lead to biased interpretations in favor of large-scale signal changes. Similar concerns also exist for functional near-infrared spectroscopy (fNIRS), which also utilizes BOLD signals, though fNIRS presents opportunities to easily study face-to-face or group-based interactions coupled with easily wearable headcaps. While other techniques such as electroencephalography (EEG) and magnetoencephalography (MEG) are also employed, with the advantages of much higher temporal resolution than fMRI or fNIRS, these techniques come with significantly poorer spatial resolution. Neuronal recording in epileptic patients provides the unique opportunity to obtain single-neuron electrophysiological data in humans but comes with the inherent confound of a biased sample population and difficulty controlling desired experimental variables.
Cortical and subcortical lesions have also been frequently used in animal models for testing causal social functions of specific brain regions. However, critical caveats of lesion studies are that the downstream effects of lesioning are unknown; lesions induce adaptation and plasticity; and directly comparing studies is challenging due to differences in surgical techniques used to create focal lesions (excitotoxic vs. aspiration). Indeed, most early lesions studies have employed aspiration lesions, which is prone to affecting fibers of passage, making it difficult to assign region-specific effects (see ref134). Moreover, lesion studies have found factors like age at the time of lesioning, familiarity with conspecifics, and the social structure at the time of experiments to influence the directionality and extent of changes in certain social behaviors24–26. Still, lesion studies provide valuable evidence testing the necessity and sufficiency of a neural region’s involvement in cognitive and behavioral processes135.
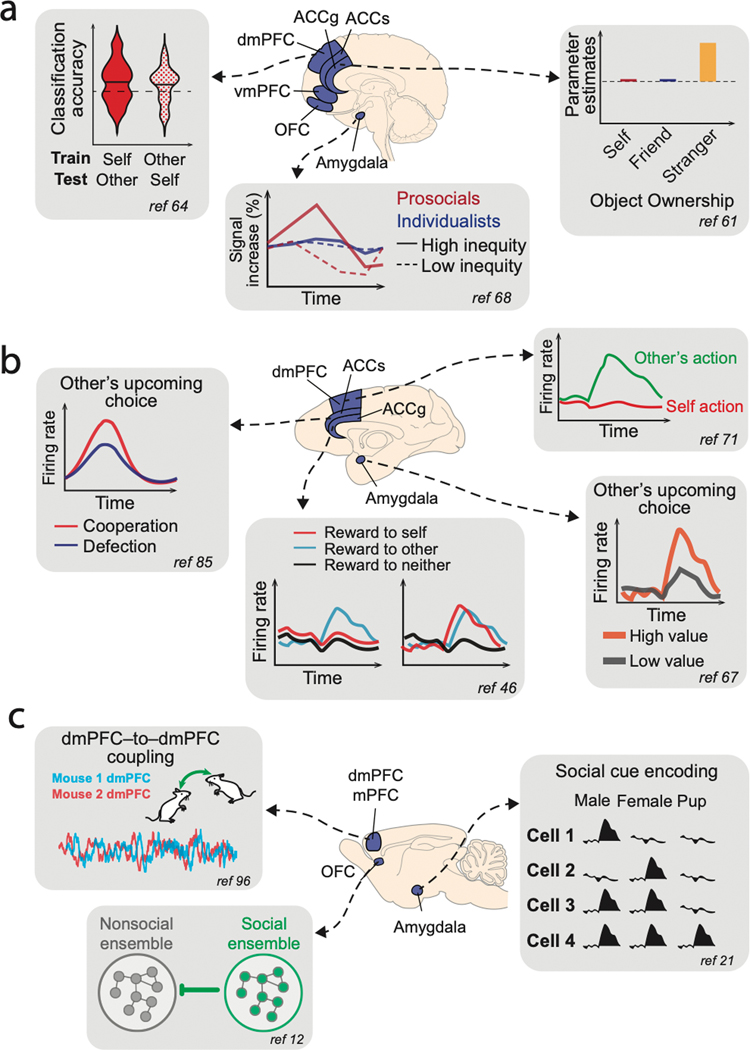
Figure 2. Illustrations of selected results demonstrating the importance of PFC and amygdala in social behaviors.
Across humans (a), macaques (b), and mice (c), summary diagrams illustrating selected findings from particular studies are shown with corresponding references. (a) (left) In humans, dmPFC BOLD activations tracked subjective value for making decisions for self as well as on behalf of another individual in a shared manner64. (middle) BOLD signals in the human amygdala showed different response patterns to high versus low inequity in reward outcomes between self and another individual for prosocial individuals68. (right) BOLD activations in ACCg in the human brain signaled objects belonging to strangers but not to self or friends61. (b) (left) Neuronal activity in a population of ACC neurons in monkeys was found to predict if a partner monkey was going to cooperate or defect in a prisoner’s dilemma task85. (middle) Neuronal activity in a population of ACCg neurons either exclusively signaled conspecific’s reward outcome or signaled the reward outcome of self or other in a comparable fashion46. (top right) Many neurons in dmPFC selectively increased activity to encode other’s action in a turn-taking task between two monkeys71. (bottom right) Activity of a group of amygdala neurons in monkeys was found to encode conspecific’s upcoming choice when observing other’s value-guided actions67. (c) (top left) During social interactions, mice exhibited dmPFC-to-dmPFC neuronal synchrony in behaviorally relevant manners96. (bottom left) In the mouse OFC, neuronal ensembles selective for social behavior were shown to inhibit neuronal ensembles selective for nonsocial behavior12. (right) Neurons in the mouse amygdala were shown to discriminate social cues21.
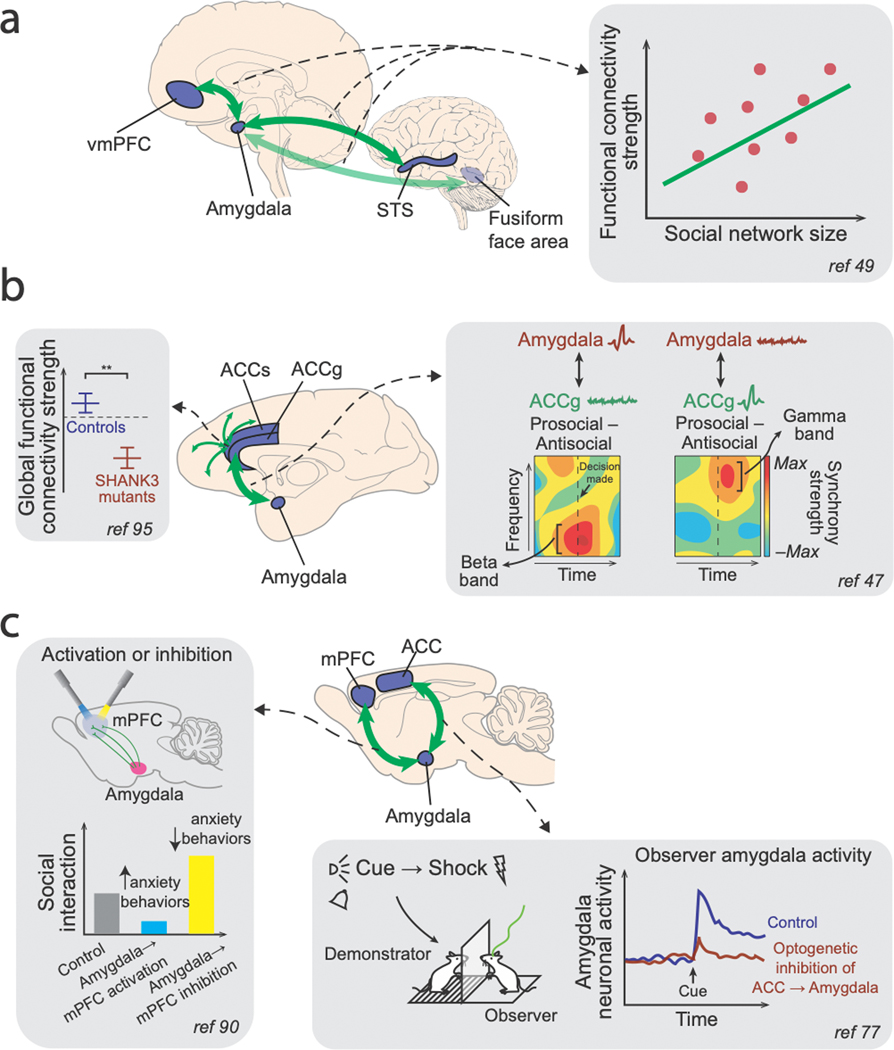
Figure 3. Illustrations of selected results demonstrating the importance of PFC-amygdala interactions in social behaviors.
(a) In humans, resting-state functional connectivity measures between the amygdala and vmPFC, between the amygdala and STS, as well as between the amygdala and the fusiform face area were shown to index individual participants’ social network size49. (b) Macaques with SHANK3 mutation, a model system frequently used to study autism spectrum disorder based on disrupted synaptic communication, exhibit abnormal global functional connectivity patterns involving ACC, among other regions95. In addition, coherence between spiking activity and LFP signals between ACCg and the basolateral amygdala was enhanced during prosocial decisions compared to antisocial decisions in distinct frequency channels47. (c) In mice, optogenetic activation of basolateral amygdala (BLA) neurons innervating mPFC reduce social interaction (but increased anxiety behaviors), whereas inhibiting the same projection neurons enhanced social interaction (but decreased anxiety behaviors)90. Moreover, ACC input to BLA neurons was found to be necessary for BLA neurons to signal observational fear cues, and optogenetically inhibiting these BLA-projecting ACC neurons prevented observational fear learning in mice77.
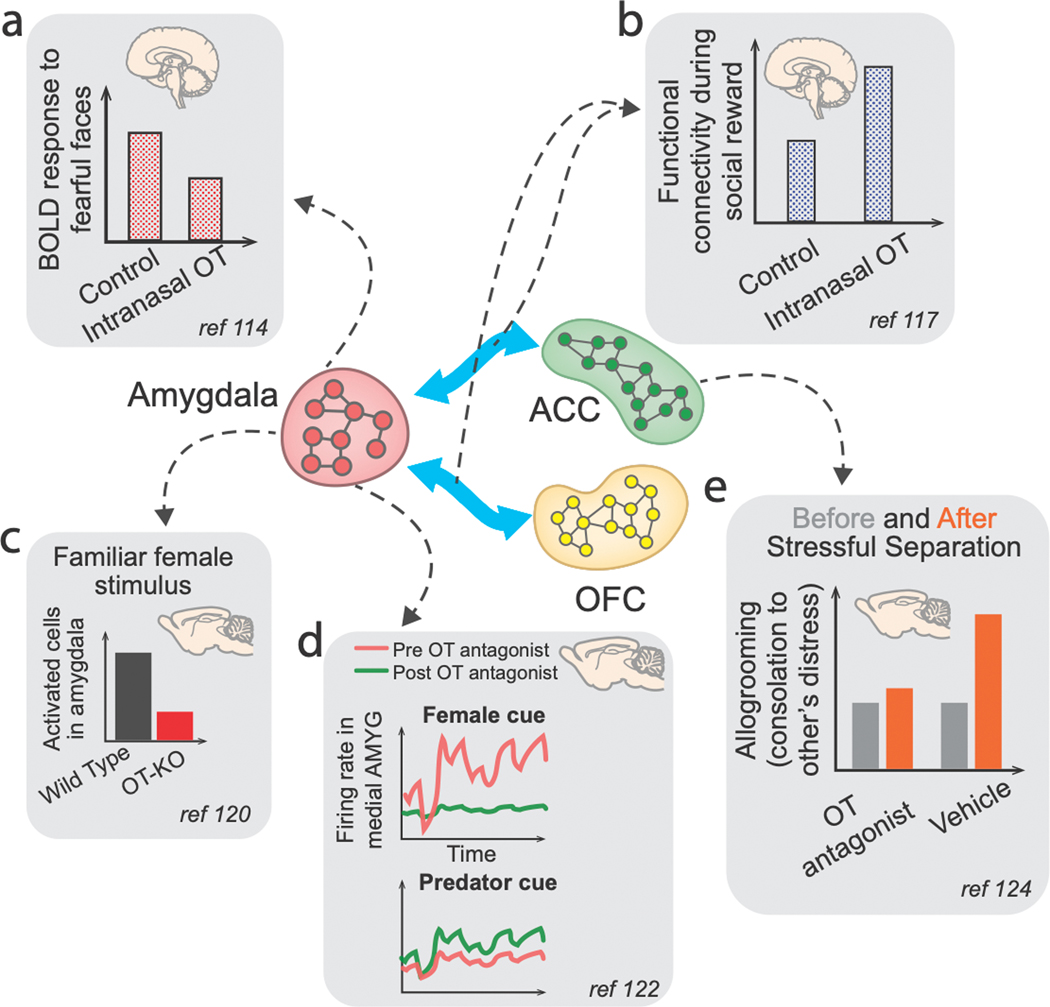
Figure 4. Illustrations of selected findings showing neuromodulation by OT in PFC–amygdala pathways.
(a) Intranasally administered OT in humans was shown to attenuate amygdala BOLD responses to fearful faces114 and (b) modulate OFC–amygdala and ACC–amygdala functional connectivity strength when perceiving a socially rewarding stimulus (infant laughter)117. (c) OT function in the medial amygdala is required for social recognition and for sex discrimination of social cues in mice120. (d) Blocking OT function in the medial amygdala in male mice reduced the processing of female cues but increased processing of predator cues122. (e) In mice, OT processing in ACC is involved in partner-directed grooming behaviors to a conspecific under distress124.
Box 2: Anatomical substrates of PFC-amygdala interactions.
Neurons in the prefrontal cortex (PFC), especially in the medial prefrontal cortex (mPFC) and the orbitofrontal cortex (OFC), strongly project to the amygdala, and also receive substantial projections from the amygdala136,137. These pathways are evolutionarily conserved, as they are found in humans, nonhuman primates, and rodents1,2,6,7,138. The bidirectional communications between the amygdala and the PFC areas are theorized to mediate synergistic interactions to enable goal-directed behaviors based on affective and reward-related information139. In addition to the direct amygdalocortical pathway, a distinct population of neurons in the amygdala also influences PFC through an indirect, amygdalothalamic pathway through the mediodorsal thalamus in both nonhuman primates and rodents140. However, it remains to be elucidated how the amygdalocortical and amygdalothalamic pathways differentially contribute functionally to behaviors.
Projections from PFC areas to the amygdala predominantly originate from layer 5, and the amygdala reciprocally projects to layers 1–2 and 5–6 of PFC areas137,141. In the amygdala, the basolateral subdivision consisting of the lateral, basal, and accessory-basal nuclei, is predominantly involved in bidirectional communications with PFC areas136. The greatest amount of projections from the amygdala to PFC areas are present in the orbital and medial PFC regions including the rostral ACC137,141. Furthermore, among the projections between the amygdala and PFC, there are differentiated anatomical projection patterns depending on PFC subregions, possibly laying the grounds for, or reflecting, functional differences of these connections. ACC neurons project more substantially to amygdala than vice versa, while amygdala neurons have larger and denser projections to OFC than to ACC137. Among different PFC areas, mPFC and the medial aspects of OFC are inter-connected with all known limbic brain structures136. These subregions of PFC receive much denser and more widespread anatomical connections from the amygdala, whereas lateral PFC areas and lateral and posterior aspects of the OFC instead receive most strong projections from parietal and temporal areas142. These anatomical characteristics suggest that coordination of neural activity in the limbic network involving the amygdala, mPFC, and OFC integrate affective and reward information from the amygdala143 (and from other subcortical limbic structures, such as the nucleus accumbens) with goal-directed and principally agent-specific processes by medial and orbital PFC regions5 to guide learning and decision-making in social contexts.
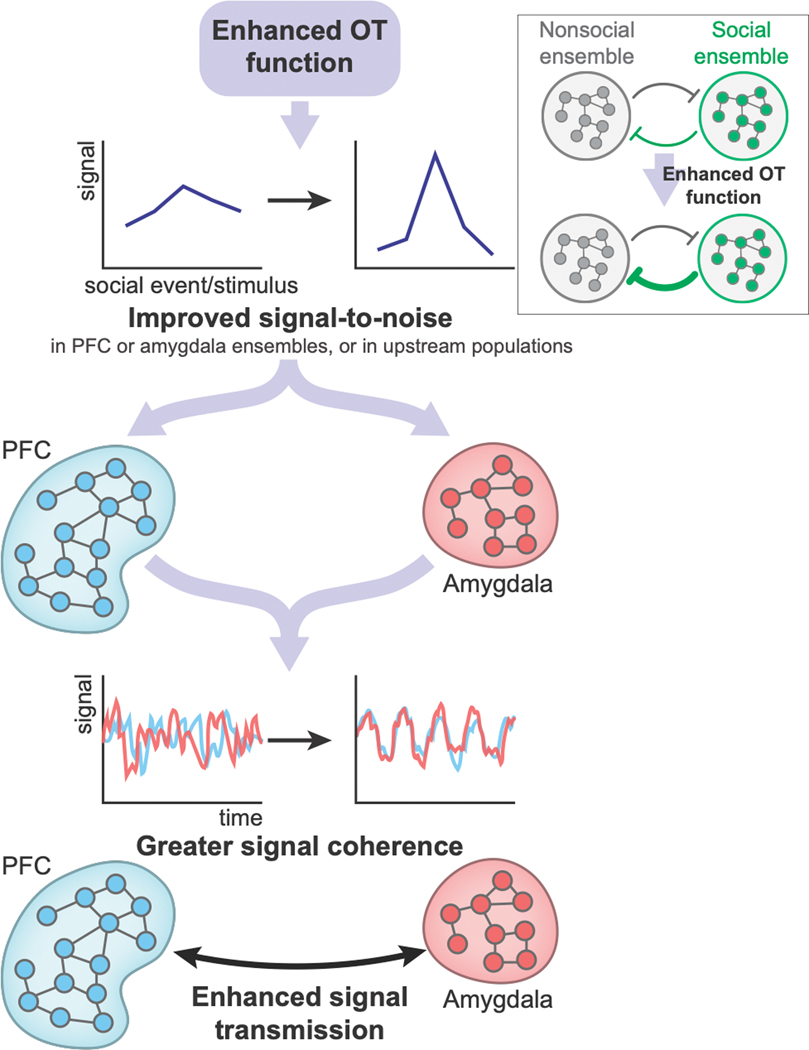
Figure 5. A hypothesized mechanism by which OT may enhance social functions in the PFC–amygdala pathways.
OT may improve signal-to-noise ratio of neural signals125 either in amygdala and PFC neural populations or in neural populations upstream to amygdala or PFC. Moreover, when there are mutual inhibition processes between neural ensembles linked to nonsocial behaviors and neural ensembles linked to social behaviors, OT may strengthen the inhibition of nonsocial ensembles by social ensembles (inset). As a result, OT would enhance neural signal transmission and possibly strengthen synchrony across neural ensembles between amygdala and PFC subregions. According to this hypothesis, OT would therefore enhance social functions that critically depend on PFC-amygdala interactions. Note that this mechanism is likely to be a general means by which many types of neuromodulators modulate various cognitive functions in multiple neural circuits.
Social Perception
The identification and recognition of a conspecific is critical for contextualizing social decisions. Depending upon an animal’s ethology, the process of social perception varies drastically. Social perception in primates relies heavily on vision, whereas in rodents it is primarily achieved by olfaction. Particularly, the amygdala and PFC subregions including the medial PFC (mPFC) and orbitofrontal cortex (OFC) have been found to play major roles in social perceptual processes in the primate and rodent brains10–14.
Recognizing and perceiving social information
Faces and facial expressions are central to social recognition in primates15 and impact social decisions. Evidence supporting face selectivity exists in the hierarchical network of ‘face patches’ in the inferior temporal cortex and OFC of macaques, where the overwhelming majority of neurons within each patch has been shown to fire preferentially to faces and to modulate firing based on facial features and identity16–18. In humans, the fusiform face area, in addition to face-selective prefrontal and temporal regions, is purported to be specialized for face perception15,19 (also see ref20). In rodents, conspecific odors are often used to study social perception, and neurons in the medial amygdala have been reported to fire differentially to males, females, pups, or nonsocial controls21,22 (Fig. 2c). Intriguingly, sexual experience and steroid signaling enhanced the neural discriminability of these stimuli22, suggesting an increased need to identify and discriminate between sexual partners with increased mating. In monkeys, many amygdala neurons have been shown to alter activity in response to faces13 but also to other variables like reward amount and stimulus category14, suggesting that these individual neurons process social and nonsocial information in concert23.
Lesion studies have been informative for testing the necessity of the amygdala and PFC subregions in social functions24–26. For instance, monkeys with excitotoxic bilateral amygdala lesions spent less time looking at other’s eyes compared to controls27, suggesting a causal role of the amygdala in social attention. In the human brain, it has been reported that the patient SM with bilateral amygdala lesions performed poorly on recognizing emotion, particularly fearful expressions28. Later, it was found that an instruction to attend to stimuli’s eyes restored her ability to judge emotion, suggesting that the amygdala is necessary for acquiring appropriate social information for emotion recognition29. The amygdala also seems to process perception of personal space, as amygdala BOLD activity in humans was shown to covary with perceived interpersonal distance, in which the patient SM exhibited impairments30.
Categorizing and inferring from social information
Differentiating others by social status, familiarity, identity, or other individual-level information helps constrain social decisions. In Capgras syndrome, subjects exhibit delusions in which familiar individuals have been replaced by imposters, possibly resulting from disrupted interactions between the amygdala and the inferior temporal cortex linked to face processing31. In mice, immediate early gene-based connectivity revealed that protein synthesis in the basolateral amygdala, mPFC, the anterior cingulate cortex (ACC), and hippocampus, mediates remembering conspecifics32. Emotional discrimination is another important function mediated by the amygdala and mPFC. In mice, OT-induced neuromodulation in the central amygdala underlies the ability to discriminate emotional states of conspecifics33. In macaques13,34 and neurosurgical patients35,36, activity of neurons in the amygdala (and additionally ACC in macaques) was shown to categorize facial expressions, even when expressions were ambiguous35,36. Human fMRI studies have extended the amygdala’s role in categorizing socioemotional variables to categorizing individuals. BOLD responses in the amygdala were found to differentiate direct-gaze faces of racial in-group versus outgroup members37. Further, amygdala BOLD responses were found to index subjective and implicit trustworthiness of faces38,39. Evidence thus indicates a role for the amygdala in categorizing others, even based on incomplete information, implicating this region in facilitating social bias.
Inferring an individual’s rank in the social hierarchy is another essential function of social perception, as quickly incorporating this information into decision-making can be critical for survival. The amygdala and mPFC have been shown to track the ranks of both oneself and others. BOLD signals from the human amygdala were found to correlate with the social rank of faces40, even when the hierarchy was unstable41. Moreover, when learning the ranks of self and others, BOLD activity in the human mPFC correlated with hierarchy-updating learning40. Further, psychophysiological interaction revealed increased functional connectivity between mPFC and the amygdala for updating estimates about one’s hierarchical position40, suggesting that mPFC-amygdala coordination might facilitate social inference. The link between the amygdala and social status perception has also been found in nonhuman primates42,43 and rodents44. For example, as a consequence of altered social hierarchies in macaques, the amygdala exhibited an increase in gray matter with increasing social status42.
Another important consideration is the size of social network, as larger groups require greater ‘neural bandwidth’ to recognize and identify others, track increasingly complex information about social ranks, and infer meaningful information from frequent social encounters. In macaques, gray matter in the mid-superior temporal sulcus (STS) and rostral PFC was found to increase in accordance with increased social network size45. Increasing group size also enhanced resting-state BOLD correlations between mid-STS and the gyrus region of ACC (ACCg) in monkeys45, suggesting that larger social networks recruit communications between social perceptual processing in mid-STS and social valuation/reward processing in ACCg46–48. Changes in functional connectivity in these regions have similarly been found in humans for social network size49 (Fig. 3a) and may be linked to socioemotional understanding50,51.
Overall, PFC subregions and the amygdala in humans, nonhuman primates, and rodents constitute crucial nodes in the networks that enable social perception and support the initial stages of social decision-making.
Social Learning, Valuation, and Reward
Social information leads to value judgements about potential rewards and punishments for self or others, which then are used to calculate future actions. These processes can be modeled in a value-based decision-making framework and serve as broad heuristics for social decision-making. However, definitional boundaries between these constructs are ambiguous, as they are often interdependent. Still, considerable anatomical, functional, and genetic evidence suggests that many neural processes are largely conserved across species1,7,52,53.
There are close conceptual and theoretical ties between social and nonsocial decision-making at the levels of value and reward processing. While social and nonsocial stimuli are perceptually distinct by definition, value and reward related processes arising from such social and nonsocial stimuli are both eventually guided by goal-directed and internal representations. One theory proposes that neural valuation processes are co-opted in social contexts, arguing against social specializations (discussed in ref1,2). In this framework, social stimuli are themselves rewarding and recruit neurons that otherwise engage in nonsocial valuation and decision-making. For instance, a recent study found that the activity of the same amygdala neurons covaried with both reward value and the hierarchical rank or facial expressions of conspecifics, lending support to a common-currency valuation hypothesis43. An alternative theory postulates that the brain developed socially specialized substrates, suggesting that the neural mechanisms underlying social and nonsocial decision-making are distinct (discussed in ref1,2). For example, the gyrus of ACC (ACCg), compared to the sulcus of ACC (ACCs), is more specialized in encoding the reward outcome of a conspecific following prosocial decisions, compared to encoding one’s own reward outcome4,46. Additionally, in the aforementioned social hierarchy study, neither in OFC nor in ACC did neurons exhibit shared responses between social and nonsocial stimuli43, which is supported by another study reporting that non-overlapping OFC neurons were modulated by juice value or the hierarchical rank of conspecific54. Evidence for both hypotheses exists, but the answer may vary by brain region. To consolidate different views, a recent effort provided a novel framework regarding the ‘social brain’ by proposing that a process can be socially specific at different levels of explanation. That is, social specificity can be found at the algorithmic level for encoding a specific algorithm or rule (e.g., reinforcement learning) that is similar or different between social and nonsocial domains55. Social specificity can also be found at the implementational level where the same or different brain areas, circuits, or cells perform social and nonsocial functions55. Therefore, it is important to consider different levels of explanation at which social specificity operates in the brain.
Agent specificity of decision variables
Learning from other’s actions and outcomes is imperative for mastering one’s environment, and in social species it is crucial for survival to balance information obtained by monitoring others with exploiting nonsocial resources or information56. Integrating other’s valuation and choice helps to predict other’s future action and this provides valuable information for adapting one’s own future action. Therefore, in social decision-making, the brain must track both self and other’s decision variables, including reward probability, choices, and outcomes. Considerable evidence implicates the amygdala, mPFC, and OFC in value-based computations for social decision-making (see ref2). Moreover, PFC subregions involved in learning and decision-making – dorsomedial prefrontal cortex (dmPFC), ACC, and OFC – display consistent intrinsic functional connectivity with the amygdala in both humans and nonhuman primates57, suggesting evolutionarily well-conserved decision-making networks in primates.
The primate ACCg is a key node in processing other-referenced decision variables4. Single-neuron evidence for this was obtained in spike recordings from ACCg, ACCs and OFC during a social reward allocation task in which an actor monkey chose to deliver rewards to himself, a conspecific, or no one. A greater proportion of ACCg neurons, compared to ACCs or OFC, signaled the reward outcome of the conspecific either exclusively or together with the reward outcome of self46 (Fig. 2b), suggesting a role of ACCg in other-referenced reward processing. The necessity of ACCg for other-referenced reward processing and social valuation has also been examined. Upon measuring response time to reach for a reward in the presence of social and nonsocial stimuli, lesions to ACCg, but not ACCs or OFC, resulted in behaviors consistent with abnormal social valuation48. A recent lesion study using a modified social reward allocation task found that excitotoxic lesions to the whole ACC in monkeys led to a specific disruption in learning a stimulus-reward association when the reward was for a conspecific monkey but not when it was for self58. However, it remains to be tested if the lesion to ACCg, but not ACCs, was the reason for this other-referenced learning deficit. Research in humans also supports a role of ACCg in other-referenced processing. BOLD responses in ACCg were found to correlate with the value of others’ rewards and prediction errors, and this relationship was moderated by trait-level empathy59,60. Further, when participants learned about ownership of picture stimuli, ACCg signaled stranger’s ownership, while ACCs exhibited greater activations to stimuli owned by self rather than others61 (Fig. 2a). Such findings (see ref1,4,55) support the notion that ACCg is specialized for other-referenced valuation and reward.
Self–other processing also engages other PFC subregions. In the social reward allocation task, the majority of OFC neurons were found to exclusively signal self reward46, suggesting a role for OFC in self-referenced reward and modulations by social context. In line with this, activity of neurons in the monkey OFC was found to covary with reward size for self and was modulated by the identity and rank of the monkey with whom the reward was shared62, documenting one way by which reward signals in OFC are modulated by social context. Moreover, in dmPFC, ‘self-type’ and ‘other-type’ neurons were found to scale activity according to reward magnitudes exclusively for self and a conspecific, respectively63. The human dmPFC and the ventromedial prefrontal cortex (vmPFC) are also implicated in self–other processing. When human participants made decisions on behalf of another, BOLD signals in dmPFC and vmPFC covaried with the decision value for the other64,65. However, dmPFC, but not vmPFC, generalized subjective value representations between self and other based on classification accuracy64 (Fig. 2a). Therefore, both dmPFC and vmPFC seem to compute other-referenced decision values, but these regions may differ on how they relate self and other.
The amygdala also signals reward variables for self and other. When monkeys made decisions for self or other, activity in the basolateral amygdala neurons tracked reward value for both agents66,67. However, it remains unknown how individual differences in social preference would affect such shared value tuning. Indeed, individual differences in social preference in humans might be mediated by the amygdala. For example, amygdala BOLD responses of individuals with prosocial orientation, but not individualists, were found to covary with the reward inequity between self and other68 (Fig. 2a) (also ref 37–39). Future research should examine if self–other processing in the amygdala is shaped through learning.
Learning from others
Monitoring and learning from others is essential for navigating social life69. When monitoring other’s choices, a subset of amygdala neurons in monkeys predictively tracked upcoming value-based choices of another monkey67 (Fig. 2b). In humans, patients with amygdala lesions were unable to learn whom to trust from observing other’s decisions in a trust game70. Evidence also supports a role of dmPFC in social monitoring: during turn-taking interactions that required monitoring other’s choices, many neurons in the monkey dmPFC encoded other’s actions or errors71,72 (Fig. 2b), and even when monitoring the actions of a human experimenter73.
Across species, ACC’s role in observational learning is well-conserved74,75. In observational fear learning in rodents, an observer learns an association between a cue and electric shocks by observing the freezing of a demonstrator. After observation, the observer exhibits freezing to the same cue without ever experiencing the shocks. Inactivating ACC in mice or genetically reducing ACC activity was shown to impair this learning76. A subsequent study found that a population of ACC neurons projecting to the basolateral amygdala causally contributed to acquisition, but not recall, of observational fear learning77 (Fig. 3c). It remains to be tested, however, if this pathway also mediates learning from other’s positive outcomes, e.g. observational reward learning. The primate ACC is similarly implicated in social learning. The primate ACC is similarly implicated in social learning. As mentioned earlier, ACC lesions ACC in monkeys led to a deficit in other-referenced reward learning58. In humans, BOLD signals in ACCg were shown to be correlated with learning from the perspective of another individual. When evaluating another’s advice for making reward-maximizing decisions, the volatility associated with learning from confederate was signaled by ACCg78. In teaching, ACCg BOLD activity in teachers also signaled prediction errors of student’s learning59. Therefore, ACC in both primates and rodents seems to be important for learning from, and perhaps also about, others.
Social behaviors require integrations of multiple cognitive and affective operations that necessitate interareal coordination. Functionally relevant regions exhibit correlated activity at temporal scales ranging from milliseconds to several seconds79. Oscillatory coupling is proposed to facilitate cognitive functions by enabling interactions within and across local circuits80,81. A study in prairie voles provides an example of how interregional coordination promotes social decision-making. This study found that cross-frequency coupling between mPFC and the nucleus accumbens (NAcc), a region implicated in pair-bonding in monogamous voles82, facilitated affiliative behaviors in females and promoted social bonding83, indicating a role of interregional coordination involving mPFC in species-typical social functions. In addition, reciprocally connected pathways between the amygdala and mPFC/OFC regions have been implicated in fundamental aspects of valuation, learning, and decision-making84. In concert with the significance of mPFC and the amygdala in social decision-making, a recent study found that oscillatory interactions between these areas guided prosocial decision-making. When monkeys made decisions whether or not to deliver juice rewards to a conspecific, neuronal synchrony between the amygdala and ACCg was enhanced for making prosocial decisions but suppressed for antisocial decisions47 (Fig. 3b). This interaction was frequency-specific, occurring in beta and gamma frequency bands depending on the area contributing the spikes (Fig. 3b), and exhibited increased directionality from the amygdala to ACCg for prosocial decisions47. Although there is still much to learn, existing evidence supports a specialized role of interaction dynamics between mPFC and subcortical regions, such as the amygdala and NAcc, in facilitating social decision-making.
Social Action or Response
The final step in social decision-making involves selecting an action or response that maximizes reward or minimizes harmful consequences. Strategic social decision-making has been studied using interactive games. During a prisoner’s dilemma task, spiking activity in the primate ACC predicted whether the opponent’s upcoming decision was to cooperate or defect85 (Fig. 2b). Microstimulation of these neurons reduced the number of cooperative choices following a cooperative choice from the other player, suggesting a causal role of ACC in reciprocal cooperative interactions85. In a prisoner’s dilemma task in humans, mutual cooperation activated OFC, which could suggest that OFC has a role in processing reinforcing aspects of cooperation86,87. NAcc and the caudate nucleus were also co-activated with OFC, highlighting how mutual cooperation recruits reward-related networks87.
In rodents, social responses typically involve affiliative or aggressive behaviors, in which both the amygdala and PFC are implicated. For example, aromatase-expressing88, as well as GABAergic and glutamatergic neurons89 in the mouse medial amygdala regulate aggression, potentially by moderating anxiety-like behaviors. Interestingly, GABAergic and glutamatergic populations opposingly regulated social and repetitive nonsocial behaviors89, suggesting an antagonistic mechanism of social and nonsocial behaviors implemented via distinct cell types. As another example in mice, selective inactivation of amygdala neurons projecting to mPFC increased social interactions but decreased anxiety-like behaviors, whereas the reverse behavioral pattern was observed when this pathway was activated90 (Fig. 3c). Evidence for a regulatory association has also been found in the mouse OFC: activation of socially-selective OFC neuronal ensembles led to inhibition of feeding behavior12 (Fig. 2c), again suggesting that social and nonsocial behaviors might be interrelated in certain circuits. Taken together, these findings support the novel hypothesis that social and nonsocial behaviors might be tightly regulated in an antagonistic manner in the brain. Based on potential competition between functional signals from separate but spatially close population of neurons, we speculate that during evolution cells specific to social behavior were repurposed from nonsocial cells in the overlapping population, and that the resulting two populations developed a mutually inhibitory relationship because of functional tradeoff or behavioral conflicts. It is worthwhile to note that antagonistic implementations have also been found between two opposing social behaviors – that is, in the ventrolateral subdivision of the mouse ventromedial hypothalamus, a population of neurons that are activated during male aggression have been found to be inhibited during mating91. Therefore, such an antagonistic regulation may reflect a more general implementational principle in the brain between two functionally conflicting behaviors.
Evidence also suggests that intricate balance within neural activity, such as that mediated by excitation/inhibition balance, may critically regulate social functions. Increasing, but not decreasing, the excitation/inhibition balance in mPFC resulted in reduced exploration of a novel mouse over a wire mesh cup in a three-chamber task, suggesting impaired social preference92. Parvalbumin interneurons in the mouse mPFC seem to contribute to the excitation/inhibition balance as they selectively increased firing for interacting with a novel conspecific over a novel object93. Important insights into how social functions in the PFC-amygdala pathways are regulated by neural activity have also come from transgenic animal models with social deficits. Mice lacking the autism-linked gene CNTNAP2, which encodes a cell-adhesion protein, show cortical hyperactivity and impaired social behavior, and optogenetic stimulation of mPFC parvalbumin interneurons restored both the excitation/inhibition balance and the social exploration deficit93.
The importance of mPFC–amygdala interactions in social behaviors is also apparent in animal models of autism spectrum disorder. For example, Pten+/− mice, which lack one copy of an autism-linked gene important for neuronal arborization, show impaired social preference that is driven by both the anatomical hyper-connectivity between mPFC and the basolateral amygdala and the hyper-activity in these brain regions94. In nonhuman primates, transgenic cynomolgus macaques with a mutation in SHANK3, which encodes a synaptic protein, showed impairments in social interaction as well as dysregulated global BOLD connectivity involving dmPFC and rostral ACC95 (Fig. 3b). These findings further reinforce the notion that mPFC-amygdala interactions regulate social behaviors.
Finally, the impact of social interactions extends beyond affecting the neural activity of just one recorded individual. Exciting recent studies have reported that two socially interacting mice96 (Fig. 2c) or bats97 show inter-individual PFC synchronization that is further modulated by social context, a phenomenon also observed in human research98. These studies in mice and bats document the first direct evidence of inter-brain synchrony at the neuronal level in social interactions. Continued research into the contributions and necessity of brain-to-brain coordination will enhance our understanding of the neural substrates of social interaction.
Oxytocin modulations in amygdala and PFC functions
Multiple neuromodulators shape social behaviors, including OT, vasopressin, and testosterone, among others. OT and vasopressin have been studied extensively in the context of affiliative and prosocial behaviors99, whereas testosterone has long been regarded as a major contributor to aggression and competition, possibly for the purpose of seeking and maintaining social dominance100. However, it remains largely unclear at which stages or aspects of social decision-making these neuromodulators exert their effects. What is clear is that no single neuromodulator system works independently of others. In this section, we discuss how OT regulates social decision-making in the amygdala and mPFC.
Role of OT in different aspects of social decision-making
OT is an evolutionarily conserved neuropeptide with major functions in birth, parental, and non-parental behaviors101. OT was repurposed during evolution from nonsocial functions, such as water regulation and anxiolysis, to social functions, such as parenting and social bonding1,99,102, and the distributions of OT receptors in the mammalian brain generally support species-typical social functions103 (Box 3).
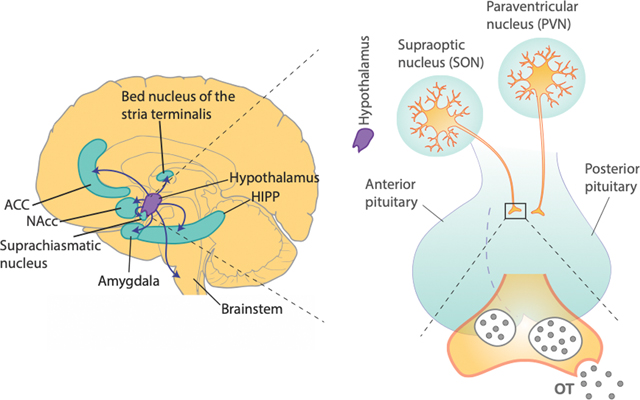
Box 3: Species difference in OT receptor distribution.
The neuropeptide OT is primarily released from the hypothalamus-posterior pituitary pathway102, and its receptors are predominantly localized in limbic regions of the brain (See the figure). There are notable species differences in the brain regions that are modulated by OT103. The most well-documented evidence comes from pair-bonding literature in voles. Monogamous prairie voles express abundant OT receptors in NAcc, mPFC, and caudate nucleus, whereas non-monogamous montane voles do not82. Indeed, it was demonstrated that OT action in NAcc is required for social bonding in voles82 and social preference formation in mice107. These findings not only show the importance of OT in mediating social reward in certain species, but also the role of OT in enhancing reward value of social stimuli or agents, a process that is important for guiding decisions concerning conspecifics in multiple species.
Our knowledge of OT receptor distributions in humans and nonhuman primates are generally limited compared to those in rodents. Moreover, OT also binds to arginine vasopressin receptors, which is more widely expressed in the primate brain than OT receptors144, making it challenging to elucidate OT-specific functions in the brain. Importantly, based on existing literature, OT receptor distributions in different species seem to critically depend on the dominant sensory modality of different species that guides social interaction. In fact, strong OT receptor expression in mice is found in brain areas involved in olfactory processing, the main sensory modality in guiding social behaviors in this species103. In rhesus macaques, OT receptor expression is particularly high in the nucleus basalis of Meynert, superior colliculus, ventromedial hypothalamus, among other regions, that are all implicated in visual orienting behavior144, which is critical for macaques in navigating their social environments. Likewise, in humans and marmosets, OT receptors are robustly present in these brain regions involved in visual orienting, such as the superior colliculus and the nucleus basalis of Meynert145,146. The OT fibers from brain regions with high levels of OT receptors often innervate several brain regions involved in multiple aspects of social decision-making. In the primate brain, for example, OT cells in the nucleus basalis of Meynert project to the amygdala, and these innervations are thought to directly regulate social functions in the amygdala1,147. Taken together, the anatomical distributions of OT receptors generally correspond to the dominant social modality (e.g., OT receptors are abundantly present in brain regions involved in visual orienting in primates) as well as ethology in different species (e.g., OT receptors are abundantly present in reward related regions in pair-bonding monogamous voles)103.
OT seems to act on multiple aspects of social decision-making9. For example, at a sensory stage, OT processing in mice sharpens the tuning of auditory cortical neurons to pup calls and promote pup-retrieval decisions, by increasing the saliency of acoustic stimuli triggered by the calls104. At a more perceptual stage, OT influences the representation of socioemotional stimuli in the human amygdala by attenuating the response to fearful expressions105,106 or increasing the response to happy expressions105. At the valuation stage, OT-mediated plasticity in NAcc is necessary for the development of reinforcing properties of social interaction in mice107, and OT in NAcc is necessary for pair-bonding in monogamous voles82. In macaques, intranasal OT was shown to amplify social decision preference and attention to others108–110. In humans, OT-induced changes in social attention were associated with increased functional connectivity between amygdala and the superior colliculus105. Thus, rather than conceptualizing OT as being specific to just one category of social function, it is more appropriate to consider OT as impacting different aspects of social decision-making processes9.
Influence of OT in social functions of amygdala and mPFC
Several studies have examined how intranasal OT affects BOLD signals in the amygdala and PFC subregions in humans. Although intranasal OT administration increases OT concentrations in the primate brain108, with this method it is difficult to control the amount of exogenous OT reaching the brain or to study region-specific effects. Concerns also exist with respect to replicability, effect size, confounding factors due to changes in peripheral OT levels, and lack of proper dose–response quantifications111,112. With these caveats in mind, studies combining intranasal OT and socioemotional tasks have found that OT alters BOLD responses in limbic regions implicated in social behaviors, including insula, OFC, ACC, mPFC, hippocampus, and hypothalamus113. Among those, the most consistent neural effect from intranasal OT seems to be its effect on altering amygdala BOLD signals to socioemotional stimuli105,106,114 (Fig. 4a).
Generally speaking, however, it remains unclear how OT affects a wide array of social behaviors. One likely possibility is that OT modulates neural activity in multiple brain areas that participate in processes precipitating social decision-making9 and facilitate interregional coordination. Indeed, OT has been shown to affect the strength of resting-state connectivity between mPFC/ACC and amygdala115,116, although the direction of this effect may depend on individuals. For example, OT increased amygdala-ACC/mPFC resting-state connectivity in participants with generalized anxiety, but decreased it in healthy individuals116. Moreover, when presented with socially rewarding stimuli, like infant laughter, functional connectivity of the amygdala with ACC and OFC was increased after intranasal OT117 (Fig. 4b). Although the mechanisms underlying OT-induced changes in interregional coordination remain unclear, these findings advance exciting avenues for future research.
Research in rodents has provided direct evidence that OT influences social functions in the PFC-amygdala pathways. It is well-established that OT in the amygdala is required for recognition and memory of conspecifics in mice118. For example, mice lacking the OXT gene failed to recall familiar conspecifics after repeated social exposures119,120. Importantly, this deficit was linked to reduced neuronal activity in the medial amygdala119,120 (Fig. 4c). Moreover, focal infusions of OT in the medial amygdala rescued social recognition in the OXT knockout mice, whereas focal infusions of an OT antagonist induced similar social deficits in wild-type mice120, supporting the notion that OT processing in this region is both necessary and sufficient for social recognition leading to social memory.
OT effects are often sexually dimorphic in rodents. For example, time spent interacting with juveniles correlated positively with OT receptor density in the medial amygdala of male rats but negatively in the central amygdala of females121. The presence of OT receptors in aromatase-expressing neurons of the medial amygdala was required for male mice to preferentially interact with a female over another male122. In the absence of OT receptors in aromatase neurons, however, female-evoked neural responses were reduced while activity to predator odor cue was enhanced122 (Fig. 4d), suggesting that OT tunes neural activity in the medial amygdala toward behaviorally preferred social stimuli (female) over other relevant stimuli. In addition to the medial amygdala, OT is involved in social learning in ACC. For example, when an observer mouse was exposed to either a familiar or unfamiliar conspecific under distress, intranasal OT in the observer acutely increased activity of ACC neurons during observational fear acquisition and caused the observer to better acquire fear from the unfamiliar conspecific123. Therefore, observational learning not only requires ACC and its functional interactions with the amygdala76,77, but also the regulation of ACC by OT. Moreover, in prairie voles, ACC activity was increased when a familiar conspecific was under distress, and focally infusing OT receptor antagonists into ACC abolished partner-directed grooming toward the distressed conspecific124 (Fig. 4e), supporting OT’s role in ACC for promoting empathetic responses to others.
Together, evidence from humans, nonhuman primates, and rodents suggests that OT is critically involved in multiple aspects of social decision-making in the mPFC-amygdala pathways. How OT impacts neuronal activity and local and global information transmission remains to be better understood. A study in mouse hippocampal slices showed that OT increases fast-spiking interneuron activity, improving the signal-to-noise ratio125. This mechanism may underlie certain social effects of OT by enhancing neural information transmission125 and facilitating interareal communications, including in the mPFC-amygdala pathways (Fig. 5).
Concluding remarks
We have focused on studies that took a systems neuroscience approach to social cognition. The evidence discussed here supports the notion that neural activity in medial and orbital PFC areas and the amygdala, as well as interactions between these areas, contribute to social decision-making. The topics covered here are by no means exhaustive; for example, corticostriatal circuits also importantly contribute to social learning and reward83,126 and likely contribute to simulating and understanding others3. Moreover, cell-type and projection-specific interactions within subcortical areas are known to regulate social functions127. Finally, although we have focused on medial and orbital PFC regions, socially relevant signals are certainly processed in lateral PFC regions128,129.
Social functions in the mPFC-amygdala pathways may be under oxytocinergic influence, although more research is needed to understand how OT modulates neuronal activity guiding social decision-making, especially in the primate brain. As existing studies in humans and nonhuman primates have mostly used intranasal OT, there remains a gap in understanding how the resulting changes in central OT concentration impacts functions in specific regions/circuits. Future efforts in primate OT research should examine causal changes in neuronal activity and interregional coordination in the mPFC-amygdala pathways following site-specific pharmacological or genetic manipulations. Together with the translational advantage of intranasal OT, region/circuit-specific approaches in nonhuman primates will provide novel knowledge toward understanding and treating social dysfunction.
Looking ahead, experiments in more naturalistic settings may reveal novel insights that might not be easily tractable in typical laboratory conditions. Indeed, the field is beginning to reflect this concern. Navigating the intricate tradeoff between rigorous control and naturalistic implementations undoubtedly presents a challenge. Understanding similarities and differences in neural functions under experimentally controlled behaviors versus natural, spontaneous, behaviors is an important topic for future research.
Acknowledgements
This work was supported by the National Institute of Mental Health (R01 MH120081, R01MH110750). We thank Philip Putnam, Siqi Fan, and Olivia Meisner for their helpful comments on the manuscript and Colin Stanton for help with the figure illustrations.
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Chang SWC et al. Neuroethology of primate social behavior. Proc. Natl. Acad. Sci. U. S. A. 110 Suppl 2, 10387–10394 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruff CC & Fehr E. The neurobiology of rewards and values in social decision making. Nat. Rev. Neurosci. 15, 549–562 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Giese MA & Rizzolatti G. Neural and computational mechanisms of action processing: interaction between visual and motor representations. Neuron 88, 167–180 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Apps MAJ, Rushworth MFS & Chang SWC The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron 90, 692–707 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang SWC An emerging field of primate social neurophysiology: current developments. eNeuro 4, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P & Hong W. Neural circuit mechanisms of social behavior. Neuron 98, 16–30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittmann MK, Lockwood PL & Rushworth MFS Neural mechanisms of social cognition in primates. Annu. Rev. Neurosci. 41, 99–118 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galvan A et al. Nonhuman primate optogenetics: Recent advances and future directions. J. Neurosci. 37, 10894–10903 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piva M & Chang SWC An integrated framework for the role of oxytocin in multistage social decision-making. Am. J. Primatol. 80, e22735 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliwa J & Freiwald WA A dedicated network for social interaction processing in the primate brain. Science 356, 745–749 (2017).This functional neuroimaging study in macaques uncovered a set of brain regions that are selectively recruited for perceiving scenes of dynamic social interactions over object interactions and non-interacting conspecifics, demonstrating how social interaction is processed uniquely in the primate brain.
- 11.Lee E et al. Enhanced neuronal activity in the medial prefrontal cortex during social approach behavior. J. Neurosci. Off. J. Soc. Neurosci. 36, 6926–6936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings JH et al. Interacting neural ensembles in orbitofrontal cortex for social and feeding behaviour. Nature 565, 645–649 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gothard KM, Battaglia FP, Erickson CA, Spitler KM & Amaral DG Neural responses to facial expression and face identity in the monkey amygdala. J. Neurophysiol. 97, 1671–1683 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Putnam PT & Gothard KM Multi-dimensional neural selectivity in the primate amygdala. eNeuro 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allison T, Puce A & McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 4, 267–278 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Chang L & Tsao DY The code for facial identity in the primate brain. Cell 169, 1013–1028 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freiwald W, Duchaine B & Yovel G. Face processing systems: from neurons to real-world social perception. Annu. Rev. Neurosci. 39, 325–346 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsao DY, Moeller S & Freiwald WA Comparing face patch systems in macaques and humans. Proc. Natl. Acad. Sci. 105, 19514–19519 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanwisher N, McDermott J & Chun MM The fusiform face area: A module in human extrastriate cortex specialized for face perception. J. Neurosci. 17, 4302–4311 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bilalić M, Langner R, Ulrich R & Grodd W. Many faces of expertise: Fusiform face area in chess experts and novices. J. Neurosci. 31, 10206–10214 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergan JF, Ben-Shaul Y & Dulac C. Sex-specific processing of social cues in the medial amygdala. eLife 3, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y et al. Neuronal representation of social information in the medial amygdala of awake behaving mice. Cell 171, 1176–1190 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gothard KM Multidimensional processing in the amygdala. Nat. Rev. Neurosci. 21, 565–575 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bliss-Moreau E, Moadab G, Bauman MD & Amaral DG The impact of early amygdala damage on juvenile rhesus macaque social behavior. J. Cogn. Neurosci. 25, 2124–2140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emery NJ et al. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 115, 515–544 (2001). [PubMed] [Google Scholar]
- 26.Machado CJ & Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav. Neurosci. 120, 761–786 (2006). [DOI] [PubMed] [Google Scholar]
- 27.Dal Monte O, Costa VD, Noble PL, Murray EA & Averbeck BB Amygdala lesions in rhesus macaques decrease attention to threat. Nat. Commun. 6, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adolphs R, Tranel D, Damasio H & Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature 372, 669–672 (1994). [DOI] [PubMed] [Google Scholar]
- 29.Adolphs R et al. A mechanism for impaired fear recognition after amygdala damage. Nature 433, 68–72 (2005). [DOI] [PubMed] [Google Scholar]
- 30.Kennedy DP, Gläscher J, Tyszka JM & Adolphs R. Personal space regulation by the human amygdala. Nat. Neurosci. 12, 1226–1227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirstein W & Ramachandran VS Capgras syndrome: a novel probe for understanding the neural representation of the identity and familiarity of persons. Proc. R. Soc. B Biol. Sci. 264, 437–444 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanimizu T et al. Functional connectivity of multiple brain regions required for the consolidation of social recognition memory. J. Neurosci. 37, 4103–4116 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferretti V et al. Oxytocin signaling in the central amygdala modulates emotion discrimination in mice. Curr. Biol. 29, 1938–1953 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Livneh U, Resnik J, Shohat Y & Paz R. Self-monitoring of social facial expressions in the primate amygdala and cingulate cortex. Proc. Natl. Acad. Sci. 109, 18956–18961 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang S et al. The human amygdala parametrically encodes the intensity of specific facial emotions and their categorical ambiguity. Nat. Commun. 8, 14821 (2017).This study uniquely combines multiple imaging methods, including fMRI, single-neuron recording, and lesions, finding evidence for the amygdala’s core role in perceiving affective facial expressions and their ambiguity.
- 36.Wang S et al. Neurons in the human amygdala selective for perceived emotion. Proc. Natl. Acad. Sci. 111, E3110–E3119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richeson JA, Todd AR, Trawalter S & Baird AA Eye-gaze direction modulates race-related amygdala activity. Group Process. Intergroup Relat. 11, 233–246 (2008). [Google Scholar]
- 38.Baron SG, Gobbini MI, Engell AD & Todorov A. Amygdala and dorsomedial prefrontal cortex responses to appearance-based and behavior-based person impressions. Soc. Cogn. Affect. Neurosci. 6, 572–581 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engell AD, Haxby JV & Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. J. Cogn. Neurosci. 19, 1508–1519 (2007). [DOI] [PubMed] [Google Scholar]
- 40.Kumaran D, Banino A, Blundell C, Hassabis D & Dayan P. Computations underlying social hierarchy learning: distinct neural mechanisms for updating and representing self-relevant information. Neuron 92, 1135–1147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zink CF et al. Know your place: neural processing of social hierarchy in humans. Neuron 58, 273–283 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noonan MP et al. A neural circuit covarying with social hierarchy in macaques. PLoS Biol 12, e1001940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Munuera J, Rigotti M & Salzman CD Shared neural coding for social hierarchy and reward value in primate amygdala. Nat. Neurosci. 21, 415–423 (2018).This macaque study found that the same neuronal ensembles in the amygdala encode both the value associated with juice rewards and the hierarchical rank of conspecifics, demonstrating a link between nonsocial and social value processing.
- 44.So N, Franks B, Lim S & Curley JP A social network approach reveals associations between mouse social dominance and brain gene expression. PloS One 10, e0134509 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sallet J et al. Social network size affects neural circuits in macaques. Science 334, 697–700 (2011).Utilizing neuroimaging in macaques, this work revealed how anatomical structures and intrinsic coupling between areas in the primate brain become altered as a function of changing one’s social network size, documenting neural adaptations to new social environments.
- 46.Chang SWC, Gariépy J-F & Platt ML Neuronal reference frames for social decisions in primate frontal cortex. Nat. Neurosci. 16, 243–250 (2013).This research capitalized on vicarious reward in macaques to reveal a specialized function of anterior cingulate gyrus neurons in signaling the reward received by a conspecific, demonstrating other-referenced reward processing in this brain region.
- 47.Dal Monte O, Chu CCJ, Fagan NA & Chang SWC Specialized medial prefrontal–amygdala coordination in other-regarding decision preference. Nat. Neurosci. 23, 565–574 (2020).This work examined how neurons in the basolateral amygdala and the anterior cingulate gyrus interact in social decision-making in macaques and found that enhanced neuronal synchrony between the two areas underlies prosocial decision-making, establishing a role of interregional synchrony in primate social behavior.
- 48.Rudebeck PH et al. A role for the macaque anterior cingulate gyrus in social valuation. Science 313, 1310–1312 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Bickart KC, Hollenbeck MC, Barrett LF & Dickerson BC Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J. Neurosci. 32, 14729–14741 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cremers HR et al. Neuroticism modulates amygdala-prefrontal connectivity in response to negative emotional facial expressions. NeuroImage 49, 963–970 (2010). [DOI] [PubMed] [Google Scholar]
- 51.Satterthwaite TD et al. Opposing amygdala and ventral striatum connectivity during emotion identification. Brain Cogn. 76, 353–363 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Connell LA & Hofmann HA Evolution of a vertebrate social decision-making network. Science 336, 1154–1157 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Tremblay S, Sharika KM & Platt ML Social decision-making and the brain: A comparative perspective. Trends Cogn. Sci. 21, 265–276 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson KK & Platt ML Social signals in primate orbitofrontal cortex. Curr. Biol. 22, 2268–2273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lockwood PL, Apps MAJ & Chang SWC Is there a ‘social brain’?: Algorithms and implementations. Trends Cogn. Sci. 20, 802–813 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinberg-Wolf H & Chang SWC Differences in how macaques monitor others: Does serotonin play a central role? Wiley Interdiscip. Rev. Cogn. Sci. 10, e1494 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Neubert F-X, Mars RB, Sallet J & Rushworth MFS Connectivity reveals relationship of brain areas for reward-guided learning and decision making in human and monkey frontal cortex. Proc. Natl. Acad. Sci. U. S. A. 112, E2695–2704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Basile BM, Schafroth JL, Karaskiewicz CL, Chang SWC & Murray EA The anterior cingulate cortex is necessary for forming prosocial preferences from vicarious reinforcement in monkeys. PLOS Biol. 18, e3000677 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Apps MAJ, Lesage E & Ramnani N. Vicarious reinforcement learning signals when instructing others. J. Neurosci. 35, 2904–2913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lockwood PL, Apps MAJ, Roiser JP & Viding E. Encoding of vicarious reward prediction in anterior cingulate cortex and relationship with trait empathy. J. Neurosci. 35, 13720–13727 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lockwood PL et al. Neural mechanisms for learning self and other ownership. Nat. Commun. 9, 4747 (2018).This human neuroimaging study found that BOLD signal from the anterior cingulate gyrus selectively track object ownership information for strangers over self or familiar others, demonstrating a role of this brain region in other-referenced stimulus processing.
- 62.Azzi JCB, Sirigu A & Duhamel J-R Modulation of value representation by social context in the primate orbitofrontal cortex. Proc. Natl. Acad. Sci. U. S. A. 109, 2126–2131 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noritake A, Ninomiya T & Isoda M. Social reward monitoring and valuation in the macaque brain. Nat. Neurosci. 21, 1452–1462 (2018).This study in macaques revealed that neurons in dmPFC signal juice reward values in an agent-specific manner, whereas dopaminergic midbrain neurons signal the integrated subjective value based on relative juice amounts between self and other, demonstrating a specialized function of this region in separating agency in reward processing.
- 64.Piva M et al. The dorsomedial prefrontal cortex computes task-invariant relative subjective value for self and other. eLife 8, e44939 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nicolle A et al. An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron 75, 1114–1121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chang SWC et al. Neural mechanisms of social decision-making in the primate amygdala. Proc. Natl. Acad. Sci. U. S. A. 112, 16012–16017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grabenhorst F, Báez-Mendoza R, Genest W, Deco G & Schultz W. Primate amygdala neurons simulate decision processes of social partners. Cell 177, 986–998 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haruno M & Frith CD Activity in the amygdala elicited by unfair divisions predicts social value orientation. Nat. Neurosci. 13, 160–161 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joiner J, Piva M, Turrin C & Chang SW Social learning through prediction error in the brain. Npj Sci. Learn. 2, 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenberger LA et al. The human basolateral amygdala is indispensable for social experiential learning. Curr. Biol. CB 29, 3532–3537 (2019). [DOI] [PubMed] [Google Scholar]
- 71.Yoshida K, Saito N, Iriki A & Isoda M. Representation of others’ action by neurons in monkey medial frontal cortex. Curr. Biol. 21, 249–253 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Yoshida K, Saito N, Iriki A & Isoda M. Social error monitoring in macaque frontal cortex. Nat. Neurosci. 15, 1307–1312 (2012). [DOI] [PubMed] [Google Scholar]
- 73.Falcone R, Cirillo R, Ferraina S & Genovesio A. Neural activity in macaque medial frontal cortex represents others’ choices. Sci. Rep. 7, 12663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burgos-Robles A, Gothard KM, Monfils MH, Morozov A & Vicentic A. Conserved features of anterior cingulate networks support observational learning across species. Neurosci. Biobehav. Rev. 107, 215–228 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Debiec J & Olsson A. Social fear learning: from animal models to human function. Trends Cogn. Sci. 21, 546–555 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeon D et al. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Allsop SA et al. Corticoamygdala transfer of socially derived information gates observational learning. Cell 173, 1329–1342 (2018).Using an observational fear learning paradigm in mice, this research revealed an important function of ACC neurons projecting to the basolateral amygdala in learning from others’ outcomes, demonstrating a circuit-specific mechanism of observational learning.
- 78.Behrens TEJ, Hunt LT, Woolrich MW & Rushworth MFS Associative learning of social value. Nature 456, 245–249 (2008).This human neuroimaging study revealed a specialized computational function of the anterior cingulate gyrus in evaluating another’s advice to guide one’s reward-maximizing decisions, demonstrating other-referenced learning signals in this brain region.
- 79.Buzsáki G, Logothetis N & Singer W. Scaling brain size, keeping timing: Evolutionary preservation of brain rhythms. Neuron 80, 751–764 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn. Sci. 9, 474–480 (2005). [DOI] [PubMed] [Google Scholar]
- 81.Friston KJ, Bastos AM, Pinotsis D & Litvak V. LFP and oscillations—what do they tell us? Curr. Opin. Neurobiol. 31, 1–6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young LJ & Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 7, 1048–1054 (2004). [DOI] [PubMed] [Google Scholar]
- 83.Amadei EA et al. Dynamic corticostriatal activity biases social bonding in monogamous female prairie voles. Nature 546, 297–301 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray EA & Izquierdo A. Orbitofrontal cortex and amygdala contributions to affect and action in primates. Ann. N. Y. Acad. Sci. 1121, 273–296 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Haroush K & Williams ZM Neuronal prediction of opponent’s behavior during cooperative social interchange in primates. Cell 160, 1233–1245 (2015).In pairs of macaques engaged in the prisoner’s dilemma task, this research discovered that neurons in the anterior cingulate cortex are involved in predictively signaling cooperative decisions based on cooperation history, and further that microstimulating these neurons reduced reciprocating cooperation, demonstrating a role of this region in reciprocal social interaction.
- 86.Decety J, Jackson PL, Sommerville JA, Chaminade T & Meltzoff AN The neural bases of cooperation and competition: an fMRI investigation. NeuroImage 23, 744–751 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rilling JK et al. A neural basis for social cooperation. Neuron 35, 395–405 (2002). [DOI] [PubMed] [Google Scholar]
- 88.Unger EK et al. Medial amygdalar aromatase neurons regulate aggression in both sexes. Cell Rep. 10, 453–462 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong W, Kim D-W & Anderson DJ Antagonistic control of social behaviors by inhibitory and excitatory neurons in the medial amygdala. Cell 158, 1348–1361 (2014).By comparing aggressive social behaviors and repetitive nonsocial behaviors in mice, this work revealed that excitatory and inhibitory neurons in the medial amygdala are involved in antagonistically regulating social and nonsocial behaviors, providing evidence for how these behaviors are regulated in the brain.
- 90.Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA & Tye KM Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209 (2016).This study in mice discovered that social behaviors and anxiety-like behaviors are regulated by projections from the amygdala to the medial prefrontal cortex, demonstrating how the same pathways can regulate social and nonsocial functions.
- 91.Lin D et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yizhar O et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Selimbeyoglu A et al. Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci. Transl. Med. 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang W-C, Chen Y & Page DT Hyperconnectivity of prefrontal cortex to amygdala projections in a mouse model of macrocephaly/autism syndrome. Nat. Commun. 7, 13421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou Y et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature 570, 326–331 (2019). [DOI] [PubMed] [Google Scholar]
- 96.Kingsbury L et al. Correlated neural activity and encoding of behavior across brains of socially interacting animals. Cell 178, 429–446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang W & Yartsev MM Correlated neural activity across the brains of socially interacting bats. Cell 178, 413–428 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hasson U, Ghazanfar AA, Galantucci B, Garrod S & Keysers C. Brain-to-brain coupling: a mechanism for creating and sharing a social world. Trends Cogn. Sci. 16, 114–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Donaldson ZR & Young LJ Oxytocin, vasopressin, and the neurogenetics of sociality. Science 322, 900–904 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Eisenegger C, Haushofer J & Fehr E. The role of testosterone in social interaction. Trends Cogn. Sci. 15, 263–271 (2011). [DOI] [PubMed] [Google Scholar]
- 101.Pedersen CA, Chang SWC & Williams CL Evolutionary perspectives on the role of oxytocin in human social behavior, social cognition and psychopathology. Brain Res. 1580, 1–7 (2014). [DOI] [PubMed] [Google Scholar]
- 102.Neumann ID Brain oxytocin: a key regulator of emotional and social behaviours in both females and males. J. Neuroendocrinol. 20, 858–865 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Freeman SM & Young LJ Comparative perspectives on oxytocin and vasopressin receptor research in rodents and primates: translational implications. J. Neuroendocrinol. 28, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marlin BJ, Mitre M, D’amour JA, Chao MV & Froemke RC Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520, 499–504 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gamer M, Zurowski B & Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc. Natl. Acad. Sci. 107, 9400–9405 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petrovic P, Kalisch R, Singer T & Dolan RJ Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. J. Neurosci. 28, 6607–6615 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dölen G, Darvishzadeh A, Huang KW & Malenka RC Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang SWC, Barter JW, Ebitz RB, Watson KK & Platt ML Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc. Natl. Acad. Sci. 109, 959–964 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dal Monte O, Noble PL, Costa VD & Averbeck BB Oxytocin enhances attention to the eye region in rhesus monkeys. Front. Neurosci. 8, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Putnam PT, Roman JM, Zimmerman PE & Gothard KM Oxytocin enhances gaze-following responses to videos of natural social behavior in adult male rhesus monkeys. Psychoneuroendocrinology 72, 47–53 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leng G & Ludwig M. Intranasal oxytocin: Myths and delusions. Biol. Psychiatry 79, 243–250 (2016). [DOI] [PubMed] [Google Scholar]
- 112.Walum H, Waldman ID & Young LJ Statistical and Methodological Considerations for the Interpretation of Intranasal Oxytocin Studies. Biol. Psychiatry 79, 251–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bethlehem RAI, van Honk J, Auyeung B & Baron-Cohen S. Oxytocin, brain physiology, and functional connectivity: A review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology 38, 962–974 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Kirsch P. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 25, 11489–11493 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ebner NC et al. Oxytocin’s Effect on Resting-State Functional Connectivity Varies by Age and Sex. Psychoneuroendocrinology 69, 50–59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dodhia S et al. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology 39, 2061–2069 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Riem MME et al. No laughing matter: Intranasal oxytocin administration changes functional brain connectivity during exposure to infant laughter. Neuropsychopharmacology 37, 1257–1266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lukas M, Toth I, Veenema AH & Neumann ID Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology 38, 916–926 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Ferguson JN et al. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 25, 284–288 (2000). [DOI] [PubMed] [Google Scholar]
- 120.Ferguson JN, Aldag JM, Insel TR & Young LJ Oxytocin in the medial amygdala is essential for social recognition in the mouse. J. Neurosci. 21, 8278–8285 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dumais KM, Bredewold R, Mayer TE & Veenema AH Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm. Behav. 64, 693–701 (2013). [DOI] [PubMed] [Google Scholar]
- 122.Yao S, Bergan J, Lanjuin A & Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife 6, e31373 (2017).By examining oxytocin signaling in the mouse medial amygdala, this work discovered that oxytocin receptors expressed on aromatase-expressing neurons in males mediate preferred social interaction with females, demonstrating how oxytocin regulates sex-specific behaviors in this region.
- 123.Pisansky MT, Hanson LR, Gottesman II & Gewirtz JC Oxytocin enhances observational fear in mice. Nat. Commun. 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burkett JP et al. Oxytocin-dependent consolation behavior in rodents. Science 351, 375–378 (2016).This work in prairie voles revealed that activity in the anterior cingulate cortex and oxytocin processing in this brain region enables partner-directed consolation behaviors toward conspecifics under distress, uncovering how empathy related behaviors are controlled by this brain region and oxytocin.
- 125.Owen SF et al. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500, 458–462 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murugan M et al. Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171, 1663–1677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anderson DJ Circuit modules linking internal states and social behaviour in flies and mice. Nat. Rev. Neurosci. 17, 692–704 (2016). [DOI] [PubMed] [Google Scholar]
- 128.Hosokawa T & Watanabe M. Prefrontal neurons represent winning and losing during competitive video shooting games between monkeys. J. Neurosci. 32, 7662–7671 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nummela SU, Jovanovic V, Mothe L de la & Miller CT Social context-dependent activity in marmoset frontal cortex populations during natural conversations. J. Neurosci. 37, 7036–7047 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Poldrack RA et al. Scanning the horizon: towards transparent and reproducible neuroimaging research. Nat. Rev. Neurosci. 18, 115–126 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Botvinik-Nezer R et al. Variability in the analysis of a single neuroimaging dataset by many teams. Nature 582, 84–88 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gordon EM et al. Precision functional mapping of individual human brains. Neuron 95, 791–807 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Poldrack RA et al. Long-term neural and physiological phenotyping of a single human. Nat. Commun. 6, 8885 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rudebeck PH, Saunders RC, Prescott AT, Chau LS & Murray EA Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat. Neurosci. 16, 1140–1145 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vaidya AR, Pujara MS, Petrides M, Murray EA & Fellows LK Lesion studies in contemporary neuroscience. Trends Cogn. Sci. 23, 653–671 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carmichael ST & Price JL Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J. Comp. Neurol. 363, 615–641 (1995). [DOI] [PubMed] [Google Scholar]
- 137.Ghashghaei HT, Hilgetag CC & Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage 34, 905–923 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Janak PH & Tye KM From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Barbas H. Connections underlying the synthesis of cognition, memory and emotion in primate prefrontal cortices. Brain Res. Bull. 52, 319–330 (2000). [DOI] [PubMed] [Google Scholar]
- 140.Miyashita T, Ichinohe N & Rockland KS Differential modes of termination of amygdalothalamic and amygdalocortical projections in the monkey. J. Comp. Neurol. 502, 309–324 (2007). [DOI] [PubMed] [Google Scholar]
- 141.Amaral DG & Price JL Amygdalo-cortical projections in the monkey (Macaca fascicularis). J. Comp. Neurol. 230, 465–496 (1984). [DOI] [PubMed] [Google Scholar]
- 142.Carmichael ST & Price JL Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 363, 642–664 (1995). [DOI] [PubMed] [Google Scholar]
- 143.Baxter MG & Murray EA The amygdala and reward. Nat. Rev. Neurosci. 3, 563–573 (2002). [DOI] [PubMed] [Google Scholar]
- 144.Freeman SM, Inoue K, Smith AL, Goodman MM & Young LJ The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology 45, 128–141 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Loup F, Tribollet E, Dubois-Dauphin M & Dreifuss JJ Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Res. 555, 220–232 (1991). [DOI] [PubMed] [Google Scholar]
- 146.Schorscher-Petcu A, Dupré A & Tribollet E. Distribution of vasopressin and oxytocin binding sites in the brain and upper spinal cord of the common marmoset. Neurosci. Lett. 461, 217–222 (2009). [DOI] [PubMed] [Google Scholar]
- 147.Putnam PT, Young LJ & Gothard KM Bridging the gap between rodents and humans: The role of non-human primates in oxytocin research. Am. J. Primatol. e22756 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]