Hematopoietic cell transplantation (HCT), dependent on hematopoietic stem cells (HSC), is widely used for treatment of many hematological and non-hematological disorders1. Successful clinical outcome of HCT predominantly relies on adequate homing and long-term engraftment of HSC into the bone marrow (BM). Enhancing the efficacy of HSC homing and engraftment could have a relevant impact on improving HCT and patient survival, especially when limiting numbers of HSCs are available, as in using umbilical cord blood (CB) or poorly mobilized peripheral blood2–4. Although efforts have been devoted to developing potential means to enhance HSC engraftment, including boosting HSC expansion5, 6, enhancing HSC homing7, 8, mitigating Extra Physiologic Oxygen Shock/Stress (EPHOSS)9 or generating HSCs by reprogramming10, 11, it is not yet clear which procedures will have the most clinical efficacy, and there is still an urgent need in clinical practice for simple and efficacious methods to enhance HSC engraftment.
Nitric oxide (NO) is a gaseous molecule which acts as a signal molecule in mammalian cells, and plays important roles in a variety of physiological regulations, such as synaptic plasticity, endothelial cell relaxation, and immune responses12, 13. NO can freely diffuse across the cellular membrane and activate a cytoplasm enzyme, soluble guanylyl cyclase (sGC), to produce cyclic guanosine monophosphate (cGMP) from guanosine triphosphate (GTP). cGMP acts as an important secondary messenger and activates cGMP dependent protein kinase (PKG) to regulate a broad spectrum of downstream processes. In hematopoietic system, NO signaling is necessary for proper differentiation of HSC and lineage commitment involving multiple signaling pathways14. Besides, NO signaling is required in vascular niche for HSC generation and production during embryogenesis15, implicating potential beneficial effects of NO signaling for HSC biology and HCT. However, potential roles of NO and cGMP in regulating HSC homing and engraftment remain to be determined.
To explore the role of NO signaling in regulating HSC engraftment, effects of NO signaling activators on human CB HSC engraftment in NSG (NOD.Cg-PrkdcscidIL2rgtm1Wjl/Sz) mice was evaluated, which is the widely accepted golden standard for in vivo determination of human HSC functionality. We first used Sodium NitroPrusside (SNP, an NO donor), and Riociguat (a sGC stimulator) to activate NO signaling. CD34+ cells were isolated from fresh human CB, and treated with SNP or Riociguat for 16 hours in medium containing 100ng/mL SCF, TPO and FL cytokines (Figure 1A). Treatment of human CB CD34+ cells with SNP or Riociguat resulted in significantly more human cell chimerism in primary NSG mouse recipients 16 weeks after transplantation, compared with vehicle control treatment (Figures 1B, C and S1A). Donor derived lymphoid/myeloid ratio in BM at 16 weeks showed no discernible changes between vehicle-treated, SNP-treated or Riociguat-treated groups (Figure S1B). In addition, limiting dilution assay (LDA) was conducted to quantitatively compare engraftment and calculate frequencies of SCID-repopulating cells (SRCs) in vehicle and Riociguat treated CB CD34+ cells (Table S1). Poisson distribution analysis revealed an SRC frequency of 1/2977 for the vehicle control treated group and 1/512 for the Riociguat treated group (Figure 1D and Table S2). We calculated the presence of 336 SRCs in vehicle control group and 1953 SRCs in the Riociguat treated group, resulting in a 5.8-fold increase in numbers of SRCs (Figures 1E and Tables S2).
Figure 1. Pharmacological activation of NO signaling pathway promotes CB HSC engraftment.
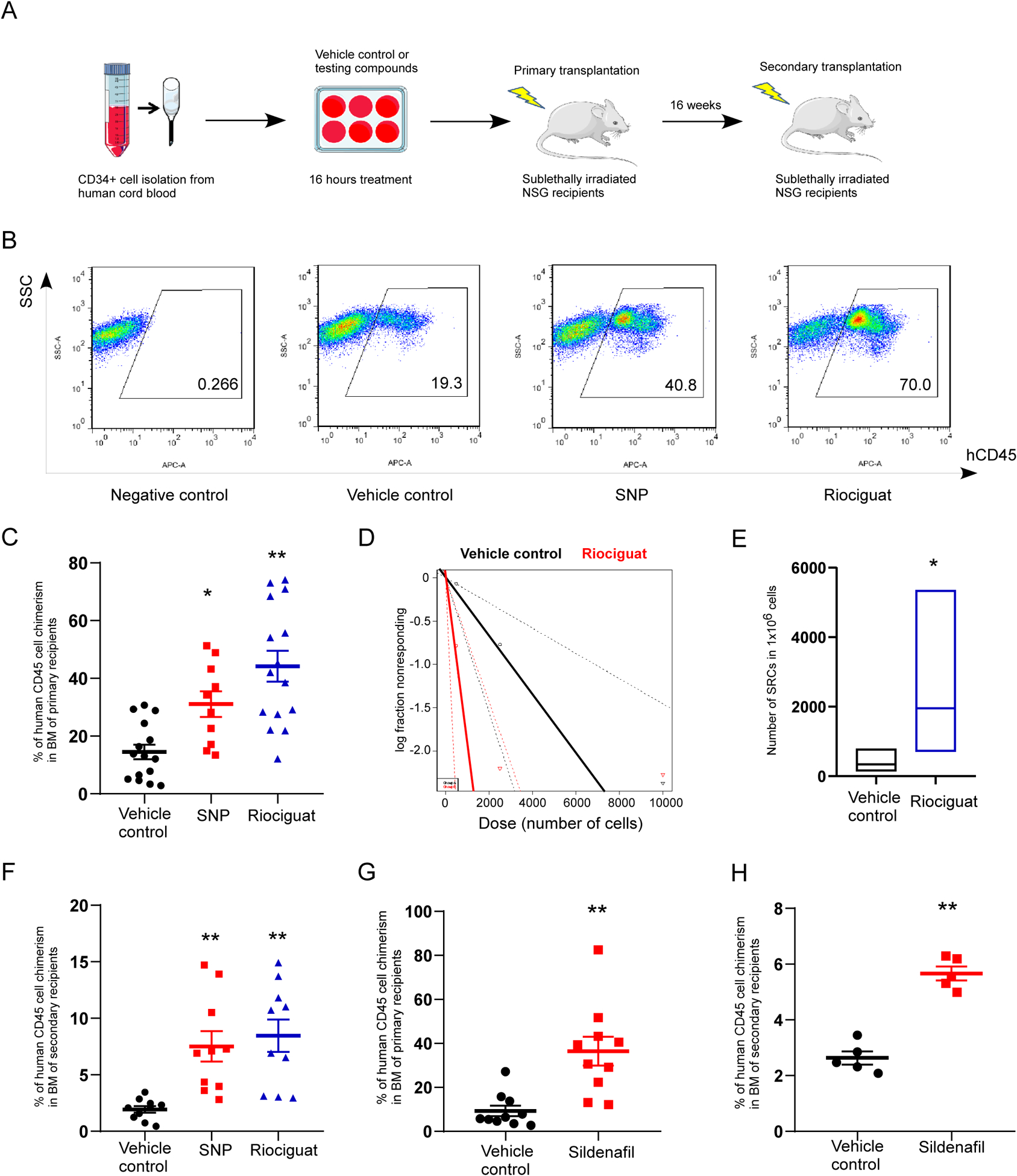
A. Experimental scheme for evaluating the role of NO signaling in HSC engraftment.
B. Representative flow cytometry plots of human CD45+ cell chimerism in the BM of primary NSG recipient mice.
C. Percentage of human CD45+ cell chimerism in the BM of primary NSG recipient mice 16 weeks after transplantation with 10,000 CB CD34+ cells that have been treated with vehicle control, SNP or Riociguat. The data were pooled from three independent experiments (n=15 for vehicle control group, n=10 for SNP group, n=15 for Riociguat group; One-way ANOVA, *p<0.05, **p<0.01).
D. Poisson statistical analysis of transplantation data from Supplementary Table 1. n=30 mice in total. Solid lines represent the best-fit linear model for each group, dotted lines indicate 95% confidence intervals.
E. HSC frequencies (line in the box) and 95% confidence intervals (box) of vehicle control and Riociguat treated groups represent as the numbers of SRCs in one million CD34+ cells. Poisson statistical analysis, *p<0.05.
F. Percentage of human CD45+ cell chimerism in the BM of secondary NSG recipient mice at 16 weeks. The data were pooled from two independent experiments (n=10 for vehicle control, SNP and Riociguat groups; One-way ANOVA, **p<0.01).
G. Percentage of human CD45+ cell chimerism in the BM of primary NSG recipient mice 16 weeks after transplantation with 10,000 CB CD34+ cells that have been treated with vehicle control or Sildenafil. The data were pooled from two independent experiments (n=10 mice per group; t test, **p<0.01).
H. Percentage of human CD45+ cell chimerism in the BM of secondary NSG recipient mice at 16 weeks (n=5 mice per group; t test, **p<0.01).
For all panels, data are shown as mean±s.e.m..
To determine long-term repopulation capability of donor human CB CD34+ cells pretreated with vehicle control, SNP or Riociguat, BM cells derived from primary transplant recipient animals were transplanted into secondary sublethally-irradiated NSG recipient mice. SNP or Riociguat treated CB CD34+ cells had significantly increased human cell engraftment in secondary NSG recipients 16 weeks after transplantation, compared to the vehicle control (Figures 1F and S1D). Donor lymphoid/myeloid ratio remained unchanged among different treatment groups (Figure S1C). These data indicate that there is an increased in the engraftment of long-term repopulating human CB HSC after activating the NO signaling pathway.
Inside the cell cGMP is degraded by phosphodiesterase 5 (PDE5). PDE5 inhibitor can suppress cGMP breakdown and activate cGMP signaling. Therefore, we next conducted a transplantation assay with CB CD34+ cells pretreated with Sildenafil, a PDE5 inhibitor. Sildenafil treated CB CD34+ cells exhibited significant higher human cell chimerism in both primary and secondary NSG recipient mice 16 weeks after transplantation (Figures 1G, H, S1E). Donor derived lymphoid/myeloid ratio in BM of NSG recipients showed no discernible difference between vehicle control and Sildenafil group (Figures S1F, G). Taken together, activation of NO signaling by short term treatment of CB CD34+ cells with SNP, Riociguat or Sildenafil enhances human CB HSC long-term engraftment in vivo.
To reveal underlying mechanisms regarding how activation of NO signaling promotes HSC engraftment, we performed RNA sequencing analysis, comparing the transcriptome of vehicle control and Riociguat treated CB CD34+ cells. A number of differentially expressed genes (DEGs) were identified (Figure 2A and Supplementary Table S3). Many Gene Ontology (GO) biological processes were significantly over-represented in DEGs upregulated by Riociguat treatment, including cell migration, chemotaxis, cell motility (Figure 2B). Particularly, expression levels of a number of genes linked with cell migration were elevated, including CXCR4 (Figure 2C). These results support the role of NO signaling in regulating HSC engraftment through cell migration.
Figure 2. Activation of NO signaling pathway increases surface CXCR4 expression and HSC homing.
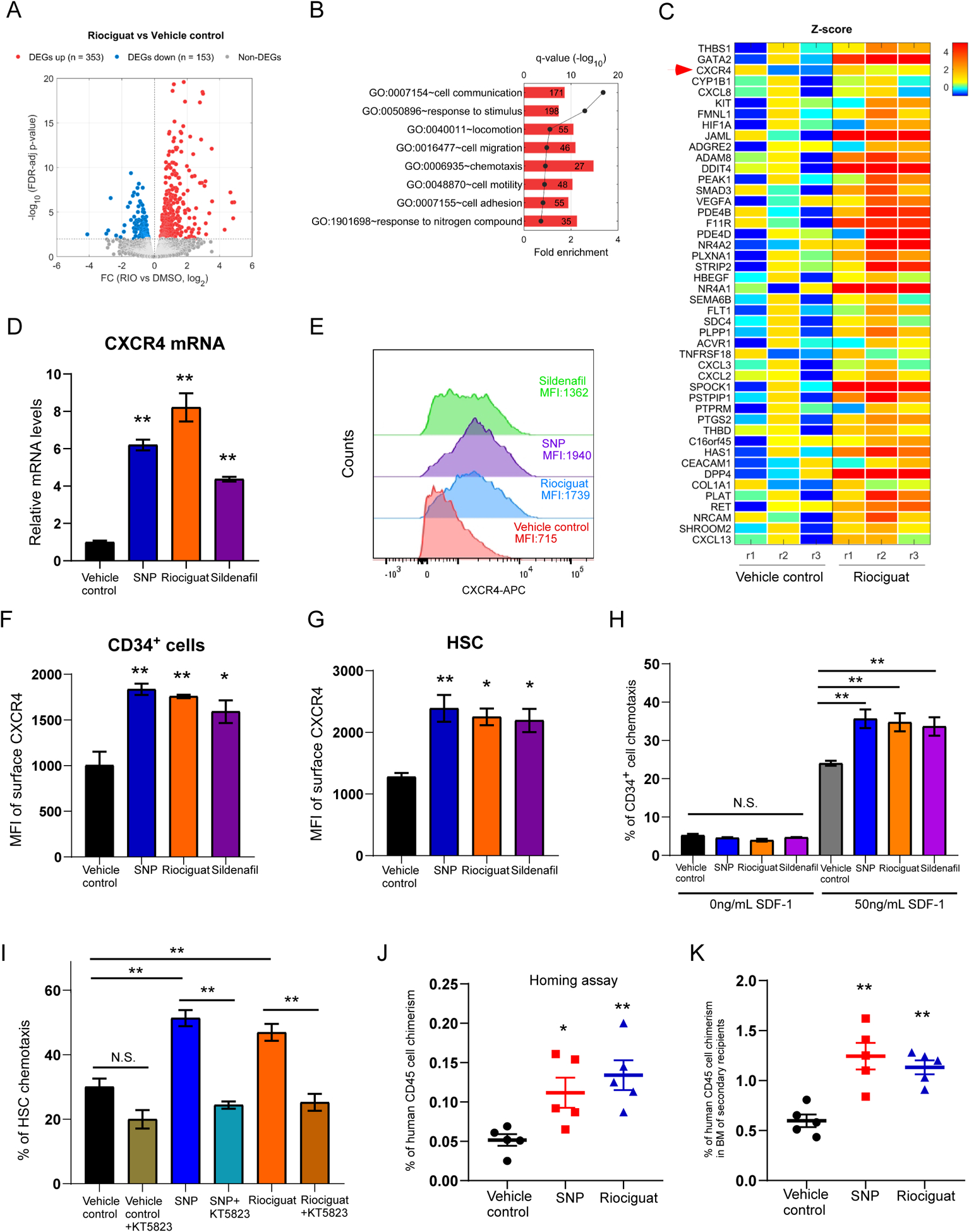
A. Volcano plot of differential expressed genes (DEGs) in Riociguat and vehicle control treated CB CD34+ cells. The list of DEGs can be found in Supplementary Table 3.
B. Selected GO biological processes significantly enriched in DEGs upregulated by Riociguat treatment compared to vehicle control treated group. The number in the box represents the number of activated DEGs involved in the biological process.
C. Heat map of Z-scores (see Supplementary Methods for details) of DEGs associated with “cell migration” upregulated by Riociguat treatment. Each treatment condition has three replicates (r1, r2, r3).
D. Quantitative RT-PCR analysis of CXCR4 expression in vehicle control, SNP, Riociguat or Sildenafil treated CB CD34+ cells. n=3, One-way ANOVA, **p<0.01.
E. Representative histogram of surface CXCR4 expression of human CB CD34+ cells treated with vehicle control, SNP, Riociguat or Sildenafil.
F. Quantification of Mean Fluorescence Intensity (MFI) of membrane CXCR4 expression on CB CD34+ cells treated with vehicle control, SNP, Riociguat or Sildenafil. n=3 CB samples, One-way ANOVA, *p<0.05, **p<0.01.
G. Quantification of Mean Fluorescence Intensity (MFI) of membrane CXCR4 expression on HSCs (CD34+CD45RA−CD38−CD90+CD49f+) treated with vehicle control, SNP, Riociguat or Sildenafil. n=3 CB samples, One-way ANOVA, *p<0.05, **p<0.01.
H. Percentage of human CB CD34+ cells migrated towards human SDF-1, as quantified by flow cytometry. Data pooled from three independent experiments (n=3 CB samples, One-way ANOVA, **p<0.01, N.S. indicates p>0.05).
I. Percentage of human CB HSCs (CD34+CD45RA−CD38−CD90+CD49f+) migrated towards human SDF-1, as quantified by flow cytometry. Data pooled from three independent experiments (n=3 CB samples, One-way ANOVA, **p<0.01, N.S. indicates p>0.05).
J. Percentage of human CD45+ cell chimerism in the BM of NSG mice 24 hours after transplantation with 500,000 CB CD34+ cells treated with vehicle control, SNP or Riociguat. Data pooled from five independent experiments (n=5 mice per group, One-way ANOVA, *p<0.05, **p<0.01).
K. Percentage of human CD45+ cell chimerism in the BM of secondary NSG recipient mice at 16 weeks. n=5 for vehicle control, SNP and Riociguat groups; One-way ANOVA, **p<0.01.
For all panels, data are shown as mean±s.e.m..
Stromal cell-derived factor-1 (SDF-1/CXCL12)/CXCR4 interactions play a crucial role in regulating HSC trafficking and homing, so we next determine whether CXCR4 expression on the HSCs can be regulated by NO signaling pathway. Quantitative RT-PCR analysis further confirmed that mRNA levels of CXCR4 in SNP, Riociguat and Sildenafil treated CB CD34+ cells were notably increased (Figure 2D). Consistently, SNP, Riociguat or Sildenafil treatment strongly increased expression of membrane CXCR4 on CB CD34+ cells compared to vehicle control (Figures 2E, F). Increase surface CXCR4 expression was also observed in rigorously defined HSC (CD34+CD45RA−CD38−CD90+CD49f+), multipotent progenitors (CD34+CD45RA−CD38−CD90−CD49f−) and CD34+CD90+ cells (Figures 2G, S2A, B).
In vitro, HSCs and progenitor cells selectively migrate towards CXCL12, a process believed to reflect their trafficking and homing capability. To determine whether NO/cGMP activation-mediated CXCR4 upregulation enhance HSC migration, we used an in vitro transwell migration assay to measure cell motility towards the chemo-attractant CXCL12. While there was no significant difference in CD34+ cell migration without SDF-1, chemotaxis in the presence of SDF-1 was significantly higher in SNP, Riociguat or Sildenafil treated CD34+ cells compared to the vehicle control group (Figure 2H). Of interest, enhanced migration towards SDF-1 by SNP and Riociguat was also observed in the HSC population, an effect suppressed by the PKG inhibitor KT5823 (Figure 2I). This suggests that enhanced HSC migration was mediated through cGMP and PKG.
To directly evaluate in vivo homing, SNP and Riociguat treated CB CD34+ cells were transplanted into sublethally irradiated NSG mice and human cells homing to the mouse BM were analyzed 24 hours after transplantation. SNP or Riociguat treated CB CD34+ cells manifested significantly increased human cell homing compared to vehicle control treatment (Figure 2J). To demonstrate that the homed cells contain HSCs, we harvested BM cells from primary NSG recipients used in the homing assay and transplanted them into secondary NSG recipients. We observed significant higher human cell engraftment in transplanted secondary NSG recipients in the SNP or Riociguat treated groups (Figure 2K), suggesting we have increased homing of HSCs by activating the NO signaling pathway.
To test if numbers of HSCs could be ex vivo expanded by activating NO signaling, CB CD34+ cells were cultured with vehicle control, SNP, or Riociguat for 4 days. We did not observe significant changes in numbers of phenotypic HSCs, indicating that activation of NO signaling did not promote expansion of phenotypic populations of HSC (Figures S2C, D).
Recent studies have highlighted the importance of homing process during HSC transplantation. A major mechanism of HSC homing so far defined has been the interaction of CXCR4 on HSC surface and CXCL12 in the BM. Here we define a new regulation of HSC homing by activating NO/cGMP signaling, involving upregulation of CXCR4 surface expression and facilitation of chemotaxis towards CXCL12. Compounds tested in our study, such as SNP, Riociguat, and Sildenafil are FDA approved medications. Therefore, evaluations of these drugs in HCT should be practical. Our work offers another unique and simple approach to bolster effectiveness of HCT.
Supplementary Material
Acknowledgements
We are indebted to all members of Broxmeyer lab for helpful discussions and assistance. We thank personnel from the In Vivo Therapeutics Core and Flow Cytometry Core of the Indiana University School of Medicine for assistance during experiments, which was supported in part by U54 DK106846. The bioinformatics analysis was performed by the Collaborative Core for Cancer Bioinformatics (C3B) shared by IU Simon Cancer Center ((P30CA082709) and Purdue University Center for Cancer Research (P30CA023168) with support from the Walther Cancer Foundation. This work was supported by US Public Health Service Grants to HEB from the NIH (R35HL139599, R01HL056416, R01HL112669, R01DK109188, U54 DK106846), Indiana University CCEH Pilot and Feasibility Grant and Fudan University Start-up Research Grant to XH, the National Key R&D Program of China Stem Cell and Translation Research (2019YFA0111800) to BG. We also thank the support from Indiana University Precision Health Initiative (PHI).
Footnotes
The authors declare that they have no competing financial interests.
References
- 1.Doulatov S, Notta F, Laurenti E, Dick JE. Hematopoiesis: a human perspective. Cell Stem Cell 2012. February 3; 10(2): 120–136. [DOI] [PubMed] [Google Scholar]
- 2.Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 2013. July 25; 122(4): 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamiak M, Abdelbaset-Ismail A, Kucia M, Ratajczak J, Ratajczak MZ. Toll-like receptor signaling-deficient mice are easy mobilizers: evidence that TLR signaling prevents mobilization of hematopoietic stem/progenitor cells in HO-1-dependent manner. Leukemia 2016. December; 30(12): 2416–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 2005. April 18; 201(8): 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo B, Huang X, Lee MR, Lee SA, Broxmeyer HE. Antagonism of PPAR-gamma signaling expands human hematopoietic stem and progenitor cells by enhancing glycolysis. Nat Med 2018. March; 24(3): 360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fares I, Chagraoui J, Gareau Y, Gingras S, Ruel R, Mayotte N, et al. Cord blood expansion. Pyrimidoindole derivatives are agonists of human hematopoietic stem cell self-renewal. Science 2014. September 19; 345(6203): 1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang X, Guo B, Liu S, Wan J, Broxmeyer HE. Neutralizing negative epigenetic regulation by HDAC5 enhances human haematopoietic stem cell homing and engraftment. Nat Commun 2018. July 16; 9(1): 2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo B, Huang X, Cooper S, Broxmeyer HE. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat Med 2017. April; 23(4): 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell 2015. June 18; 161(7): 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimura R, Jha DK, Han A, Soria-Valles C, da Rocha EL, Lu YF, et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature 2017. May 25; 545(7655): 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandler VM, Lis R, Liu Y, Kedem A, James D, Elemento O, et al. Reprogramming human endothelial cells to haematopoietic cells requires vascular induction. Nature 2014. July 17; 511(7509): 312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buono R, Vantaggiato C, Pisa V, Azzoni E, Bassi MT, Brunelli S, et al. Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells 2012. February; 30(2): 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardingham N, Dachtler J, Fox K. The role of nitric oxide in pre-synaptic plasticity and homeostasis. Front Cell Neurosci 2013. October 31; 7: 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nogueira-Pedro A, Dias CC, Regina H, Segreto C, Addios PC, Lungato L, et al. Nitric oxide-induced murine hematopoietic stem cell fate involves multiple signaling proteins, gene expression, and redox modulation. Stem Cells 2014. November; 32(11): 2949–2960. [DOI] [PubMed] [Google Scholar]
- 15.North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, et al. Hematopoietic stem cell development is dependent on blood flow. Cell 2009. May 15; 137(4): 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


