Abstract
Background
It was reported that systemic immune inflammation index (SII) was related to poor prognosis in a variety of cancers. We aimed to investigate the ability of the prognostic predictors of SII in patients with intrahepatic cholangiocarcinoma (iCCA) undergoing liver transplantation (LT).
Methods
The 28 iCCA patients who underwent LT at our hospital between 2013 and 2018 were reviewed. Kaplan–Meier survival curves and Cox regression analyses were used to evaluate the prognostic significance of SII. Patients were divided into the high and low SII groups according to the cut-off value.
Results
The 1-, 3-, and 5-year OS rates were significantly lower in the high SII group (85.7%, 28.6%, and 21.4%, respectively) than in the low SII group (92.9%, 71.4%, and 57.2%, respectively; P = 0.009). The 1-, 3-, and 5-year RFS rates were, respectively, 57.1%, 32.7%, and 21.8% in the high SII group and 85.7%, 61.1%, and 61.1% in the low SII group (P = 0.021). SII ≥ 447.48 × 109/L (HR 0.273, 95% CI 0.082–0.908; P = 0.034) was an independent prognostic factor for OS.
Conclusions
Our results showed that SII can be used to predict the survival of patients with iCCA who undergo LT.
1. Introduction
Cholangiocarcinoma (CCA) is a rare malignant tumor with an incidence of less than 2/100,000 persons [1]. CCA is classified into several subtypes. iCCA accounts for 8–10% of biliary tract cancers [2]. The incidence of iCCA has been increasing worldwide over the last 3 decades, which may be related to primary sclerosing cholangitis, viral hepatitis, or chemical exposure [3]. Because of the poor long-term outcomes, iCCA is usually a contraindication for LT [4]. However, data from several studies have reported poor outcomes in patients with iCCA after transplantation [5]. Thus, many centers consider iCCA to be a contraindication to liver transplantation typically [6]. Despite the controversy, several studies have proposed that LT may provide acceptable long-term survival in selected patients with iCCA [7, 8]. Therefore, it is necessary to establish appropriate criteria to select the right patients for liver transplantation. The current criteria for evaluating liver transplantation, such as the Milan criteria and Hangzhou criteria, that are effective for hepatocellular carcinoma (HCC) patients are not useful for evaluating patients with iCCA.
There is sufficient evidence that inflammation is related to tumor progression [9, 10]. It was reported that inflammatory cells such as lymphocytes and platelets change the tumor microenvironment play an important role in promoting the proliferation, invasion, and migration of tumors. Inflammation-based scores, including PLR, PNI, and SII, have been reported to be useful prognostic biomarkers for various cancers [11–14]. SII has been proved to be a prognostic predictor for several cancers. However, it remains unclear whether there is a correlation between preoperative SII and prognosis in patients with iCCA undergoing LT. The purpose of this study was to explore the prognostic value of SII in patients with iCCA undergoing LT.
2. Methods
The 28 patients who received liver transplantation for iCCA at the First Affiliated Hospital, Sun Yat-Sen University (Guangzhou, China), from 2013 to 2018 were retrospectively reviewed. The diagnosis was confirmed by medical imaging and pathological examination of tissue specimens. Clinical characteristics extracted from the medical records. Patients were followed monthly for the first 6 months. This study only included patients with iCCA at the explant. Patients with mixed iCCA + HCC (in the same or different nodule) were excluded from the study.
All tumor patients including iCCA on the waiting list evaluated for extrahepatic metastasis were evaluated. Patients with an expected waiting list time of over 6 months could have been treated with transarterial chemoembolization (TACE), ablation as a bridge to LT. In addition to TACE and ablation, patients with iCCA diagnosed preoperatively received chemotherapy based on gemcitabine and cisplatin.
Independent χ2 tests were used to compare categorical variables. Continuous variables were compared using t-tests. Survival curves were analyzed using the Kaplan-Meier method. The Cox regression analysis was used for univariate and multivariate analyses. The area under the receiver operating characteristic (ROC) curve (AUC) was calculated to examine the predictive value of the proposed model. All statistical analyses were performed using SPSS version 19.0 statistical software (SPSS, Chicago, IL, USA). P values < 0.05 were considered statistically significant.
All organs came from voluntary donations from citizens; no organs from executed prisoners (even with his/her consent) were used involved. The study was approved by the Institutional Review Board of the First Affiliated Hospital of Sun Yat-sen University and was performed in accordance with the Declaration of Istanbul. All protocols conformed to the ethical guidelines of the 1975 Helsinki Declaration.
3. Results
A total of 28 consecutive adult liver transplant patients with iCCA were included in the analysis. Clinical characteristics are summarized in Table 1. Patients diagnosed with iCCA received adjuvant therapy, with gemcitabine and cisplatin, and only a subset of patients received TACE and ablation for pretransplant locoregional therapy.
Table 1.
Baseline characteristics in iCCA patients.
| Characteristic | Values |
|---|---|
| Gender | |
| Male | 25 (89.3) |
| Female | 3 (10.7) |
| Age (years) | 51.5 (46.8-60.0) |
| Child-Pugh Class | |
| A | 7 (25.0) |
| B | 13 (46.4) |
| C | 8 (28.6) |
| BMI | 23.2 (20.4-23.9) |
| MELD score | 11.0 (7.8-16.5) |
| CEA (μg/L) | 3.2 (2.5-8.6) |
| CA19-9 (U/L) | 125.9 (28.1-1436.6) |
| AFP (ng/L) | 4.2 (2.6-5.1) |
| Tumor number | |
| Single | 19 (67.9) |
| Multiple | 9 (32.1) |
| Largest tumor size (cm) | 5.8 (2.4-7.5) |
| HBsAg | |
| Positive | 17 (60.7) |
| Negative | 11 (39.3) |
| Pretransplant locoregional therapy | 21 (75.0) |
| Differentiation | |
| Well | 2 (7.1) |
| Moderate | 18 (64.3) |
| Poor | 8 (28.6) |
| Vascular invasion | 9 (32.1) |
| Tumor recurrence | 15 (53.6) |
| SII | 447.5 (289.9-930.7) |
| PLR | 125.8 (98.6-184.1) |
| NLR | 2.9 (1.8-4.1) |
| Follow-up (months) | 33.5 (18.8-50.8) |
Data are presented as n (%) or median (IQR).
The 28 patients in the study were 25 (%) male and 3 (%) females. The median age was 51.5 (interquartile range (IQR) 46.8–60.0) years. The median follow-up duration was 33.5 months. The 5-year OS rate was 39.3%, and the 5-year RFS rate was 43.0%, respectively.
The ROC curves of SII, NLR, and PLR indicated that 447.48, 2.92, and 106.62 were the optimal cut-off values. According to the cut-off values, patients were divided into the low (<447.48 × 109/L, n = 14) and high (≥447.48 × 109/L, n = 14) SII groups. The demographic and clinicopathological characteristics of the two groups were compared (Table 2).
Table 2.
Comparison of characteristics between the high SII group and low SII group of patients with iCCA who underwent LT.
| Variables | SII < 447.48 (n = 14) | SII ≥ 447.48 (n = 14) | P value |
|---|---|---|---|
| Gender | P = 0.541 | ||
| Male | 13 (92.9) | 12 (85.7) | |
| Female | 1 (7.1) | 2 (14.3) | |
| Age (years) | 50.0 (45.3-60.0) | 54 (47.8-59.3) | P = 0.380 |
| Child-Pugh Class | P = 0.242 | ||
| A | 4 (28.6) | 3 (21.4) | |
| B | 8 (57.1) | 5 (35.7) | |
| C | 2 (14.3) | 6 (42.9) | |
| BMI | 23.5 (22.4-25.0) | 22.0 (19.9-23.5) | P = 0.423 |
| MELD score | 10 (7-14) | 12 (8-18) | P = 0.592 |
| CEA (μg/L) | 2.6 (1.4-4.2) | 8.2 (3.1-19.3) | P = 0.321 |
| CA19-9 (U/L) | 59.6 (12.0-235.1) | 576.5 (9.2-3874.4) | P = 0.264 |
| AFP (ng/L) | 3.2 (2.6-5.6) | 4.3 (2.8-5.5) | P = 0.624 |
| Tumor number | P = 0.686 | ||
| Single | 10 (71.4) | 9 (64.3) | |
| Multiple | 4 (28.6) | 5 (35.7) | |
| Largest tumor size (cm) | 2.9 (2.0-7.6) | 6.1 (4.3-7.0) | P = 0.428 |
| HBsAg | P = 0.699 | ||
| Positive | 8 (57.1) | 9 (64.3) | |
| Negative | 6 (42.9) | 5 (35.7) | |
| Pretransplant locoregional therapy | 11 (78.6) | 10 (71.4) | P = 0.663 |
| Differentiation | P = 0.329 | ||
| Well | 2 (14.3) | 0 (0.0) | |
| Moderate | 8 (57.1) | 10 (71.4) | |
| Poor | 4 (28.6) | 4 (28.6) | |
| Vascular invasion | 3 (21.4) | 5 (35.7) | P = 0.403 |
| Tumor recurrence | 6 (42.9) | 9 (64.3) | P = 0.256 |
Data are presented as n (%) or median (IQR).
The 5-year OS rates were significantly lower in the high SII group than in the low SII group (21.4% vs. 57.2%, P = 0.009) (Figure 1(a)). The 5-year RFS rates were 21.8% in the high SII group and 61.1% in the low SII group (P = 0.021) (Figure 1(d)). High PLR and NLR scores were also associated with poor OS (P = 0.001 and P = 0.006; Figures 1(b) and 1(c)) and poor RFS (P = 0.011 and P = 0.027; Figures 1(e) and 1(f)).
Figure 1.
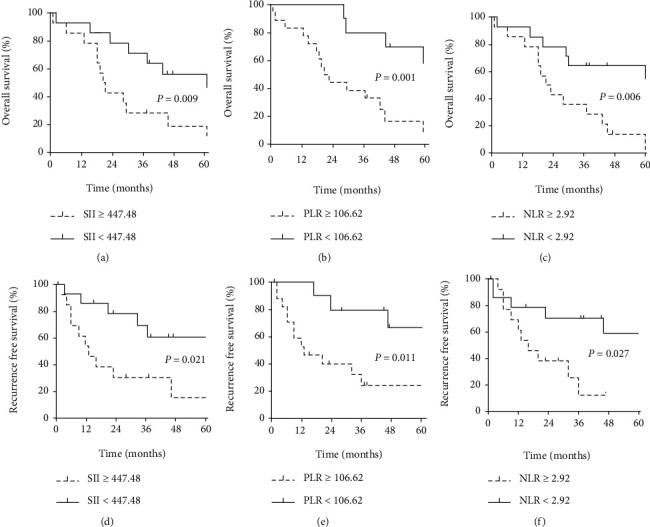
Overall survival curves after LT for iCCA patients classified by (a) SII, (b) PLR, and (c) NLR; recurrence-free survival curves after LT for iCCA patients classified by (d) SII, (e) PLR, and (f) NLR.
Univariate analysis revealed CEA level, tumor recurrence, SII, PLR, and NLR to be significant prognostic factors for OS. Results of multivariate analysis showed that SII ≥ 447.48 × 109/L was revealed to be an independent predictor of OS after LT in patients with iCCA (hazard ratio (HR) 0.273, 95% confidence interval (CI) 0.082–0.908; P = 0.034) (Table 3).
Table 3.
Univariate and multivariate analyses of factors related to overall survival in patients with iCCA who underwent LT.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender (male vs. female) | 1.452 | 0.422-4.997 | 0.554 | |||
| Age (years) | 1.035 | 0.989-1.082 | 0.142 | |||
| Child-Pugh Class (A or B vs. C) | 1.579 | 0.629-3.964 | 0.331 | |||
| BMI | 0.960 | 0.823-1.120 | 0.605 | |||
| MELD score | 1.014 | 0.974-1.056 | 0.508 | |||
| HBsAg (positive vs. negative) | 1.336 | 0.531-3.359 | 0.538 | |||
| Differentiation (well or moderate vs. poor) | 1.724 | 0.711-4.180 | 0.228 | |||
| AFP (ng/mL) (>20 vs. ≤20) | 1.656 | 0.217-12.66 | 0.627 | |||
| CEA (μg/L) (>10 vs. ≤10) | 0.292 | 0.091-0.937 | 0.038 | 0.713 | 0.170-2.997 | 0.645 |
| CA19-9 (U/L) (>37 vs. ≤37) | 0.742 | 0.295-1.868 | 0.526 | 1.202 | 0.406-3.564 | 0.740 |
| Largest tumor size (cm) (>5 vs. ≤5) | 0.399 | 0.157-1.011 | 0.053 | 0.689 | 0.192-2.470 | 0.567 |
| Tumor number (multiple vs. single) | 1.309 | 0.519-3.299 | 0.569 | 1.653 | 0.493-5.550 | 0.416 |
| Pretransplant locoregional therapy (yes vs. no) | 1.218 | 0.467-3.177 | 0.687 | 0.760 | 0.214-2.698 | 0.671 |
| Vascular invasion (yes vs. no) | 0.543 | 0.197-1.496 | 0.237 | 0.547 | 0.164-1.823 | 0.326 |
| Tumor recurrence (yes vs. no) | 5.101 | 1.672-15.569 | 0.004 | 3.106 | 0.723-13.338 | 0.127 |
| SII (≥447.48) | 0.311 | 0.122-0.792 | 0.014 | 0.273 | 0.082-0.908 | 0.034 |
| PLR (≥106.62) | 0.188 | 0.061-0.582 | 0.004 | 0.313 | 0.075-1.314 | 0.113 |
| NLR (≥2.92) | 0.269 | 0.099-0.729 | 0.01 | 0.496 | 0.146-1.689 | 0.262 |
4. Discussion
Compared with HCC, iCCA has a higher recurrence rate and a worse prognosis. As such, liver transplantation for iCCA is highly controversial. Because of high recurrence rates and poor long-term survival, liver transplantation for iCCA has been abandoned in most transplantation centers. While the indications for liver transplantation for iCCA are controversial. In 2016, Sapisochin et al. [15] conducted a multicenter study that the advanced group had a higher 5-year recurrence rate than the very early iCCA group (61% vs. 15%, respectively) and lower 5-year OS (45% vs. 65%, respectively). Therefore, appropriate selection criteria are required to ensure a better prognosis of patients undergoing liver transplantation for iCCA. At present, there is no relevant study to explore the predictive value of SII in patients with iCCA for LT. In this study, patients who underwent LT for iCCA and demonstrated that a high SII (≥447.48 × 109/L) significantly correlated with poorer prognosis.
As mentioned earlier, some studies have shown that inflammation factors are associated with prognosis in patients with cancer [16–18]. SII is widely accepted to be a new predictive marker to predict the prognosis of several types of cancer [19–21]. However, there are few studies on the prognosis of SII and iCCA. Although SII has been confirmed to be related to the prognosis of iCCA, the mechanism is not clear. SII is a systemic inflammatory marker, which can predict the prognosis of tumor from the level of inflammatory and immune. It has been reported that poor prognosis are concomitant with some inflammatory markers, such as NLR and PLR [22, 23]. Gomez et al. and Chen et al. reported that iCCA patients with a high preoperative NLR are related to poor prognosis [22, 24]. Chen et al. also confirmed that high PLR was related to poor prognosis [25].
The number of neutrophils in patients with malignant tumors increases plays an important role in the development of tumors [26–28]. It is reported that lymphocytes can mediate tumor regression effectively. The mechanism of which was realized by secreting cytokines and inducing cytotoxic cell death [29, 30]. In patients with intrahepatic cholangiocarcinoma, elevated NLR was independently associated with poor prognosis [31]. Platelets and neutrophils can secrete vascular endothelial growth factor (VEGF), which is important in tumor progression [32]. It has been shown that tumors are infiltrated by various lymphocytes, which is related to the progress of tumor [33, 34]. Immunooncology has become a promising approach in the field of new anticancer drug development [35, 36]. PD-L1 and HHLA2 are potential immunotherapeutic targets for iCCA patients [37, 38].
This study has several limitations. First, this was a retrospective, single-center analysis with a small number of patients. Second, SII was a dynamic index in the process of treatment and could be affected by unidentified infection and hepatitis B infection and so on.
In conclusion, our study suggests that preoperative SII is a simple and useful predictor of prognosis, which will help to select more suitable iCCA patients for liver transplantation and improve the prognosis of patients with cholangiocarcinoma after liver transplantation.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81873591 and 81670591); the Guangdong Natural Science Foundation (2016A030311028); the Science and Technology Planning Project of Guangdong Province (2018A050506030); the Science and Technology Program of Guangzhou (201704020073); the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007 and 2017B030314018); and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002).
Data Availability
The data used to support the findings of this study have not been made available because of local ethical guidelines.
Conflicts of Interest
The authors have declared no conflict of interest.
Authors' Contributions
A Ren and Y Ma are responsible for the conception and design. Y Ma obtained administrative support. A Ren and Z Li are involved in the provision of study materials or patients. Z Li, X Zhang, and P Cheng collected and assembled the data. A Ren, Z Li, P Cheng, and X Zhang are responsible for the data analysis and interpretation. All authors are involved in the manuscript writing. All authors approved the final manuscript.
References
- 1.Bergquist A., von Seth E. Epidemiology of cholangiocarcinoma. Best Practice & Research. Clinical Gastroenterology. 2015;29(2):221–232. doi: 10.1016/j.bpg.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A., Dixon E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. Hepatobiliary Surgery and Nutrition. 2017;6(2):101–104. doi: 10.21037/hbsn.2017.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vogel A., Saborowski A. Cholangiocellular carcinoma. Digestion. 2017;95(3):181–185. doi: 10.1159/000454763. [DOI] [PubMed] [Google Scholar]
- 4.Mazzaferro V., Gorgen A., Roayaie S., Droz dit Busset M., Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. Journal of Hepatology. 2020;72(2):364–377. doi: 10.1016/j.jhep.2019.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Laurent S., Verhelst X., Geerts A., et al. Update on liver transplantation for cholangiocarcinoma : a review of the recent literature. Acta Gastroenterologica Belgica. 2019;82(3):417–420. [PubMed] [Google Scholar]
- 6.Bridgewater J., Galle P. R., Khan S. A., et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. Journal of Hepatology. 2014;60(6):1268–1289. doi: 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Goldaracena N., Gorgen A., Sapisochin G. Current status of liver transplantation for cholangiocarcinoma. Liver Transplantation. 2018;24(2):294–303. doi: 10.1002/lt.24955. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K., Obeid J., Burmeister C. S., et al. Intrahepatic cholangiocarcinoma in the liver explant after liver transplantation: histological differentiation and prognosis. Annals of Transplantation. 2016;21:208–215. doi: 10.12659/AOT.895936. [DOI] [PubMed] [Google Scholar]
- 9.Grivennikov S. I., Greten F. R., Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diakos C. I., Charles K. A., McMillan D. C., Clarke S. J. Cancer-related inflammation and treatment effectiveness. The Lancet Oncology. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 11.Mowbray N. G., Griffith D., Hammoda M., Shingler G., Kambal A., al-Sarireh B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB: The Official Journal of the International Hepato Pancreato Biliary Association. 2018;20(5):379–384. doi: 10.1016/j.hpb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Pang Q., Zhang L. Q., Wang R. T., et al. Platelet to lymphocyte ratio as a novel prognostic tool for gallbladder carcinoma. World Journal of Gastroenterology. 2015;21(21):6675–6683. doi: 10.3748/wjg.v21.i21.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi X., Li J., Deng H., Li H., Su C., Guo X. Neutrophil-to-lymphocyte ratio for the prognostic assessment of hepatocellular carcinoma: a systematic review and meta-analysis of observational studies. Oncotarget. 2016;7(29):45283–45301. doi: 10.18632/oncotarget.9942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sellers C. M., Uhlig J., Ludwig J. M., Stein S. M., Kim H. S. Inflammatory markers in intrahepatic cholangiocarcinoma: effects of advanced liver disease. Cancer Medicine. 2019;8(13):5916–5929. doi: 10.1002/cam4.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sapisochin G., Facciuto M., Rubbia-Brandt L., et al. Liver transplantation for “very early” intrahepatic cholangiocarcinoma: international retrospective study supporting a prospective assessment. Hepatology. 2016;64(4):1178–1188. doi: 10.1002/hep.28744. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y., Okabayashi K., Hasegawa H., et al. Comparison of preoperative inflammation-based prognostic scores in patients with colorectal cancer. Annals of Surgery. 2018;267(3):527–531. doi: 10.1097/SLA.0000000000002115. [DOI] [PubMed] [Google Scholar]
- 17.Nakagawa K., Sho M., Akahori T., et al. Significance of the inflammation-based prognostic score in recurrent pancreatic cancer. Pancreatology. 2019;19(5):722–728. doi: 10.1016/j.pan.2019.05.461. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M., Kobayashi T., Kuroda S., et al. Verification of inflammation-based prognostic marker as a prognostic indicator in hepatocellular carcinoma. Annals of Gastroenterological Surgery. 2019;3(6):667–675. doi: 10.1002/ags3.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holub K., Biete A. Impact of systemic inflammation biomarkers on the survival outcomes of cervical cancer patients. Clinical & Translational Oncology. 2019;21(7):836–844. doi: 10.1007/s12094-018-1991-4. [DOI] [PubMed] [Google Scholar]
- 20.Murthy P., Zenati M. S., al Abbas A. I., et al. Prognostic value of the systemic immune-inflammation index (SII) after neoadjuvant therapy for patients with resected pancreatic cancer. Annals of Surgical Oncology. 2020;27(3):898–906. doi: 10.1245/s10434-019-08094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berardi R., Santoni M., Rinaldi S., et al. Pre-treatment systemic immune-inflammation represents a prognostic factor in patients with advanced non-small cell lung cancer. Annals of Translational Medicine. 2019;7(20):p. 572. doi: 10.21037/atm.2019.09.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q., Yang L. X., Li X. D., et al. The elevated preoperative neutrophil-to-lymphocyte ratio predicts poor prognosis in intrahepatic cholangiocarcinoma patients undergoing hepatectomy. Tumour Biology. 2015;36(7):5283–5289. doi: 10.1007/s13277-015-3188-6. [DOI] [PubMed] [Google Scholar]
- 23.Lin J., Fang T., Zhu M., et al. Comparative performance of inflammation-based prognostic scores in patients operated for intrahepatic cholangiocarcinoma. Cancer Management and Research. 2019;Volume 11:9107–9119. doi: 10.2147/CMAR.S198959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez D., Morris-Stiff G., Toogood G. J., Lodge J. P. A., Prasad K. R. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. Journal of Surgical Oncology. 2008;97(6):513–518. doi: 10.1002/jso.21001. [DOI] [PubMed] [Google Scholar]
- 25.Chen Q., Dai Z., Yin D., et al. Negative impact of preoperative platelet-lymphocyte ratio on outcome after hepatic resection for intrahepatic cholangiocarcinoma. Medicine (Baltimore) 2015;94(13, article e574) doi: 10.1097/MD.0000000000000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elinav E., Nowarski R., Thaiss C. A., Hu B., Jin C., Flavell R. A. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nature Reviews. Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 27.Coffelt S. B., Wellenstein M. D., de Visser K. E. Neutrophils in cancer: neutral no more. Nature Reviews. Cancer. 2016;16(7):431–446. doi: 10.1038/nrc.2016.52. [DOI] [PubMed] [Google Scholar]
- 28.Petrie H. T., Klassen L. W., Kay H. D. Inhibition of human cytotoxic T lymphocyte activity in vitro by autologous peripheral blood granulocytes. Journal of Immunology. 1985;134(1):230–234. [PubMed] [Google Scholar]
- 29.Robbins P. F. Tumor-infiltrating lymphocyte therapy and neoantigens. Cancer Journal. 2017;23(2):138–143. doi: 10.1097/PPO.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 30.Ferrone C., Dranoff G. Dual roles for immunity in gastrointestinal cancers. Journal of Clinical Oncology. 2010;28(26):4045–4051. doi: 10.1200/JCO.2010.27.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buettner S., Spolverato G., Kimbrough C. W., et al. The impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio among patients with intrahepatic cholangiocarcinoma. Surgery. 2018;164(3):411–418. doi: 10.1016/j.surg.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Bambace N. M., Holmes C. E. The platelet contribution to cancer progression. Journal of Thrombosis and Haemostasis. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 33.Chew V., Chen J., Lee D., et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61(3):427–438. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West N. R., Kost S. E., Martin S. D., et al. Tumour-infiltrating FOXP3(+) lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. British Journal of Cancer. 2013;108(1):155–162. doi: 10.1038/bjc.2012.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert C., Schachter J., Long G. V., et al. Pembrolizumab versus ipilimumab in advanced melanoma. The New England Journal of Medicine. 2015;372(26):2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 36.Garon E. B., Rizvi N. A., Hui R., et al. Pembrolizumab for the treatment of non-small-cell lung cancer. The New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 37.Jing C. Y., Fu Y. P., Yi Y., et al. HHLA2 in intrahepatic cholangiocarcinoma: an immune checkpoint with prognostic significance and wider expression compared with PD-L1. Journal for Immunotherapy of Cancer. 2019;7(1):p. 77. doi: 10.1186/s40425-019-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mody K., Starr J., Saul M., et al. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. Journal of Gastrointestinal Oncology. 2019;10(6):1099–1109. doi: 10.21037/jgo.2019.08.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study have not been made available because of local ethical guidelines.


