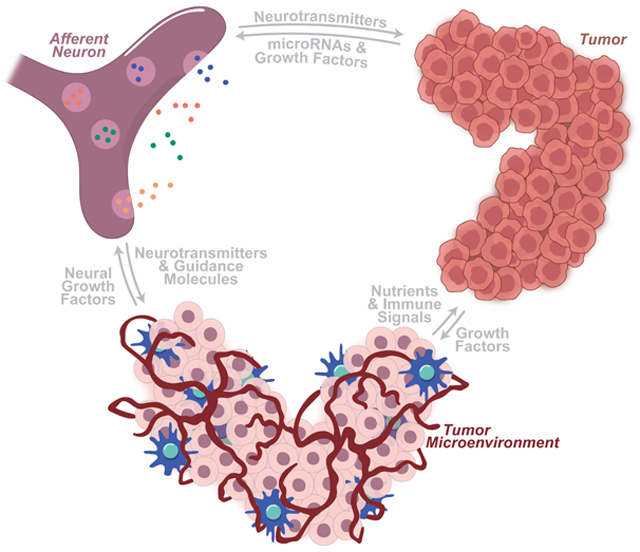
Abstract
Cancer neurobiology is an emerging discipline that inevitably unfurls new perspectives in oncology. The role that nerves play in cancer progression resonates with the long-reported dependency of tumors on neuro-molecular mechanisms that remain insufficiently elucidated. Whereas interactions between neurotrophic growth factors and receptors have been heavily studied in the nervous system, their expression in cancers and their impact on tumor cell growth and metastasis through their corresponding signaling pathways has been undervalued. Accumulating evidence suggests that trophic factors released by nerves strongly influence tumor development and that this neural contribution appears to not only play a stimulatory role but also function as an essential part of the tumor’s microenvironment. This bidirectional communication between proliferating cells and tumor-infiltrating nerves drives axonogenesis and tumor growth and migration. Acquiring a better understanding of the trophic interactions between primary afferent neurons and invading tumors will guide clinically actionable strategies to prevent tumor-associated axonogenesis, disrupting the chemical crosstalk between neurons and tumors and ultimately decreasing tumor growth and spread.
Keywords: neurotrophic growth, cancer progression, microRNA, tumor microenvironment
Graphical Abstract
INTRODUCTION
Cancer cells exploit the abundance of growth signals in their local microenvironment to further their survival and spread. Although stromal fibroblasts and immune infiltrate have gained much attention for their modulatory role in the tumor microenvironment, neurons are becoming an increasingly appreciated actor in this local environment, providing neurotransmitters (NTs), angiogenic signals, immunogenic compounds, and growth factors that are co-opted for use by these cancer cells. Innervation is essential for the proper growth and maintenance of stem cell niches and developing tissues [1–3]. In the same way, innervation of tumor microenvironments drives the growth of various cancers, including gastric [4–6], hematopoietic [7], oral [8], pancreatic [9], prostatic [10,11], and non-melanoma skin [12,13] cancers. These neuro-cancer interactions provide evidence of choreographed communication between tumor cells and their neural neighbors, resulting in concerted neuritogenesis and tumor growth.
A growing number of solid tumor types have been found to interact with a wide array of neuronal types, including autonomic and sensory neurons. Through transcriptional alterations and other shared cellular mechanisms, cancerous cells form manipulative relationships with local neurons, exploiting them toward a tumor-promoting state. The microRNAs, secreted growth factors, membrane-bound receptors, and associated molecular pathways that mediate this crosstalk illustrate previously unknown dependencies of tumors on neural relationships. The blockage of such relationships may herald more efficacious cancer-starving therapies.
In this review, we discuss the roles that tumors, their microenvironments, local neurons, and the signaling between them play in the molecular dance that drives neural-induced tumorigenesis. Here, we focus on the relationship between primary afferent neurons and invading tumors. Finally, we turn our attention to the clinical relevance of these neuron-tumor associations, focusing on current and potential therapies.
LOCAL NEURONAL ACTIVITY ALTERS THE TUMOR IMMUNE MICROENVIRONMENT
Neurons transmit messages over long distances via a combination of saltatory conduction and chemical neurotransmission. These chemical transmitters include catecholamines, acetylcholine, and neuropeptides, all of which can stimulate the growth of neurons. However, these transmitters also play a trophic role within the tumor microenvironment, driving tumor growth [10,14]. This tumor microenvironment often overlaps and coincides with the perineural niche—a collection of immune cells and structural cells that support and interact with neurons. The cells of the perineural niche are variable and dependent on the nerve type and region of the body. However, endoneurial macrophages are commonly found in this space. Since the majority of neuron-tumor trophic interactions occur between the tumor microenvironment and the perineural space, it is the cells found within these regions that constitute the main actors in this concerted dance between neurons and cancer [14].
It has been long known that inflammation can drive tumor growth and dissemination, making it possible for the neuroimmune interactions regulating inflammatory responses to also regulate tumor growth and progression [7,15]. Cholinergic innervation to viscera from the vagus nerve—connections that are well established in cancer progression and growth—have been shown to regulate the cytokine-mediated inflammatory reflex by activation of CREB and the JAK-STAT pathways within resident macrophages [16]. Similarly, sympathetic innervation can activate endoneurial macrophage infiltration of the tumor microenvironment, a process that is linked to metastasis in various types of cancer [17]. This is visibly evident in the transition of healthy pancreatic tissue to pancreatic intraepithelial neoplasia (PanIN) and in the subsequent transition to invasive pancreatic ductal adenocarcinoma (PDAC), which is characterized by a progressive infiltration of macrophages [18]. Although the initial invasion of the tumor microenvironment by these immune cells may be a response to tumor presence, endoneurial macrophages induce the release of a collection of factors, including IL-1, HIF-2α, MMP-9, uPA, NOS, FGF, HGF, EGFR-ligands, PDGF, TGF-β, TNF-α, and VEGF, which the tumor uses to promote angiogenesis, tumor cell invasion, and tumor growth and survival [19]. Furthermore, when endoneurial macrophage recruitment to the tumor bed in mice was ablated via Ccr2 knockout, a marked decrease in prostate and PDAC cancer cell invasion of the perineural niche was demonstrated [9,20]. Thus, endoneurial macrophages are important immune cells of the perineural niche that clearly mediate neuron-cancer trophic interactions.
LOCAL NEURONAL ACTIVITY ALTERS TUMOR ANGIOGENESIS
Until recently, the mechanism linking tumor and microenvironment innervation to increased growth and survival of the tumors remained unknown. Early reports implicated blood vessel growth as a method of increasing nutrient and growth factor delivery to the metabolically hyperactive tumor cells. Subsequently, tumor cells were described as releasing angiogenic growth factors, such as vascular endothelial growth factor (VEGF). However, therapeutic strategies aimed at inhibiting these secreted growth factors have shown only limited effect, suggesting that other factors are at play [21].
It is becoming increasingly recognized that the nerves infiltrating the tumor microenvironment, rather than the tumors themselves, drive the observed angiogenesis. These innervating neurons interact with the blood vessels that supply the tumor cells, providing neuropilins, netrins, Slit family proteins, the receptors for these tumor components, and other growth stimuli to these vessels, thus indirectly providing the tumor with oxygen, metabolic substrates, and other factors necessary for survival, growth, and spread. Indeed, it is widely known that sympathetic innervation is essential for the proper growth and maintenance of blood vessels throughout the body [22].
Sympathetic nerves within the tumor microenvironment remodel resident endothelial cells toward an angiogenic state, thereby driving tumorigenic angiogenesis. These infiltrating adrenergic neurons increase noradrenaline release from local axons, and induce increased beta 2 adrenergic receptor (ADRβ2) expression in the local population of endothelial cells. Continued stimulation of endothelial ADRβ2s results in a metabolic switch from oxidative phosphorylation to aerobic glycolysis in resident endothelial cells, which is necessary for proper angiogenesis [23].
In this way, local neuronal activity manipulates macrophages and endothelial vasculature cells within the tumor microenvironment to promote a tumorigenic state. However, cells of the tumor microenvironment, in concert with nearby cancer cells, reciprocally manipulate innervating neurons in elegant crosstalk to promote the growth of both neurons and tumors alike.
THE TUMOR MICROENVIRONMENT SHAPES LOCAL NEURONAL ACTIVITY
Neural development of primary afferent neurons is characterized by meandering processes that wind through the body in pursuit of semaphorins, netrins, Slits, and ephrins, which serve as guidance molecules by binding receptors on the neural growth cone [24–26]. Although neurogenesis is restricted after childhood, neurons remain exquisitely sensitive to signals in their surrounding tissues and continuously remodel distal processes to alter innervation patterns in response to these signals. By subverting this neuronal sensitivity and ability to remodel, tumor cells and other cells within the tumor microenvironment drive transcriptional and morphological changes that increase intratumoral innervation thereby driving tumor growth. In mouse models of p53-deficient oral cavity squamous cell carcinoma (OCSCC), extensive adrenergic neurite outgrowth is observable within the tumor microenvironment, which is recapitulated in p53-deficient OCSCC cells co-cultured with dorsal root ganglia (DRGs). Similarly, in patient OCSCC samples from The Cancer Genome Atlas, p53-mutated cancers demonstrate significantly increased neurite growth. In searching for the signal that drives this contextual neuritogenesis, extracellular vesicles (EVs) isolated from p53-deficient cell cultures were found to be sufficient to drive this increased DRG neurite growth. When microRNA miR-34a is added to these EVs derived from p53-deficient cells, neuritogenesis is inhibited, thus identifying miR-34a as an important antineuritogenic miRNA. However, introduction of miR-21 and miR-324, which are transported in these EVs, is sufficient to drive the observed neuritogenesis in trigeminal neurons. Not only could these tumor-generated miRNAs drive neuritogenesis, but they could also reprogram primary afferent neurons into adrenergic neurons prior to this induced neurite growth. In agreement with a body of work demonstrating adrenergic drive of tumor growth, these neoadrenergic neurons increase p53-deficient cell survival and growth. Thus, p53-deficient OCSCC cells transport specific miRNAs, including miR-21 and miR-324, to nearby primary afferent neurons via EV release, resulting in the reprogramming of these tumor-associated sensory neurons to adrenergic neurons that subsequently act to promote further tumor growth [8] (Fig. 1). This striking example illustrates the power of the tumor microenvironment in manipulating neurons for the benefit of the tumor.
Fig. 1. Tumors alter neuronal activity to drive tumor growth and spread.
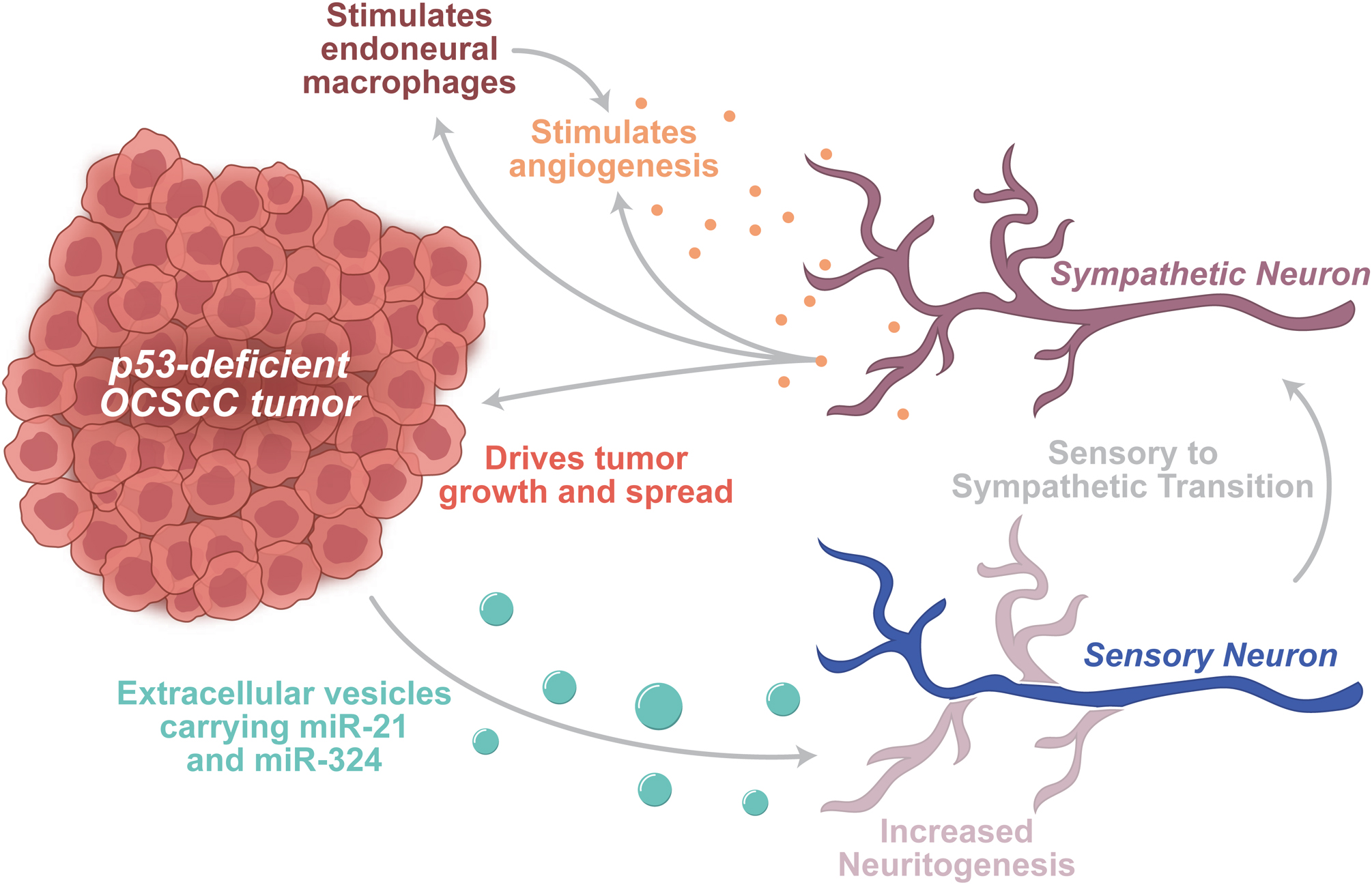
Depiction of a p53-deficient OCSCC tumor releasing EVs onto nearby trigeminal sensory neuron. These EVs carry pro-neuritogenic miRNAs, including miR-21 and miR-324. Along with driving neuritogenesis, these miRNAs also drive a sensory-to-sympathetic transition in these neurons. Sympathetic innervation of tumors and tumor microenvironments drives growth and spread of the tumor. Sympathetic release (adrenaline, noradrenaline) directly stimulates growth in certain tumors. In addition, sympathetic release stimulates endoneurial macrophages, which facilitate tumor growth and angiogenesis. In concert, sympathetic release stimulates ADRβ2 receptors, thereby driving a glycolytic switch in endothelial cells to spur angiogenesis.
Prostate tumors are another well studied cancer in which potent neuron-microenvironment crosstalk alters the growth patterns of both tumors and their surrounding neurons. Within the prostate tumor microenvironment, granulocyte colony-stimulated factor (G-CSF) and proNGF (the precursor of nerve growth factor, or NGF) are two neurotrophic factors whose expression drives aberrant neurite growth into these prostate tumors [27,28]. Similarly, gastric cancers directly release NGF into their microenvironment, thus promoting nearby neural growth through binding and activation of its cognate tyrosine kinase receptor (TrkA) and the corresponding downstream pathway [4]. Breast cancers also directly release NGF, and levels of microenvironmental NGF directly correlate with both the amount of tumor innervation and the aggression of the cancer in human samples [29]. Pancreatic tumors release NGF directly onto nearby neurons, thus promoting neuritogenesis. This neuronal growth is bolstered by the concomitant release of brain-derived neurotrophic factor from pancreatic tumor cells, which acts by binding its own cognate TrkB receptor. In combination, these factors drive sympathetic nerve growth into pancreatic tumors, thereby fueling growth of the tumor [30].
These examples illustrate the ability of tumors and their microenvironments to target neuronal sensitivities to not only increase the growth of nearby neural tissue but also direct the growth of these neurons to maximize the benefit of this growth toward their own survival. SLIT/ROBO are neural developmental proteins that determine the direction of neurite growth during development [24–26]. However, these proteins can also be hijacked to direct neurite growth into tumoral or stromal tissue. Pancreatic tumors have been found to redirect SLIT and ROBO expression for this purpose [31]. Thus, in concert with microenvironmental cells and factors, tumors control neural growth and activity to maximize tumor survival and growth [32]. This neuronal manipulation, however, is all based on the striking ability of neuronal activity to directly drive tumor survival, growth, and spread.
NEURONAL ACTIVITY DRIVES CANCER GROWTH AND METASTASIS
Neuronal activity drives cancer growth and metastasis, resulting in many cancers depending on local neuronal signaling to facilitate or drive their growth. The signals that mediate neurotrophic tumor growth are varied, as are the tumors in which these relationships are found. Indeed, this neural-driven cancer growth has been documented between primary afferent neurons and breast [33], gastric [5], prostatic [10], and pancreatic cancers [34]. However, some of the most clearly defined mechanisms of neuron-tumor crosstalk were discovered in tumors that invade the brain.
Metastatic breast cancers commonly spread to the brain, driven in large part by the misexpression of N-methyl-D-aspartate receptor (NMDAR) components in breast cancer cells. NMDAR binding by glutamate activates the cytoplasmic adaptor protein GKAP, which in turn alters translational regulatory pathways via its downstream effectors FMRP and HSF1 [35]. This broad regulatory change results in increased aggression and invasion into glutamate-rich regions such as the brain. To increase their access to glutamate, invading breast tumor cells form tripartite synapses with presynaptic and postsynaptic neurons [33]. These oncogenic pathways downstream of NMDAR activation also drive the trophic interactions between neurons and peripheral tumors, evidenced by overexpression of NMDAR in pancreatic tumors [35–37]. Moreover, increases in oxidative byproducts from bone tumors, reflected by increased expression of the detoxification enzymes glutathione peroxidase and superoxide dismutase, increase extracellular glutamate from intraosseal sympathetic neurons. This increase in glutamate release drives tumor growth, thus illustrating the dependence of peripheral bone tumors on glutamate release [38]. Whereas these glutamate-driven trophic relationships are found throughout the periphery, primary tumors of the brain serve as ideal models for uncovering mechanistic details that govern neuron-tumor trophic interactions.
Tripartite synapses are most commonly shared between presynaptic neurons, postsynaptic neurons, and glia, such as astrocytes [39]. Similarly, gliomas have been found to form tripartite synapses in communication with neurons in their surrounding microenvironment. However, more recently, human glioma samples have been shown to form bona fide neuroglial synapses. These synapses are dependent on GPC3—a potent tumorigenic protein [40]. These neurogliomal connections are functionally active and are driven by glutamate release from neurons and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor activation in the glioma cells. The activation of this AMPAR axis drives both invasion and growth of these gliomas in vivo via calcium-dependent and tumor microtube-related signaling pathways within the tumor. The mass effect of gliomas often manifests as seizures in patients with progressed disease. In light of these new data, these episodes of increased glutamatergic activity, believed to be caused by the tumor, are also driving the growth of the tumor [41,42]. This potent and succinct example of neural manipulation by tumor cells illustrates a powerful means of neuron-mediated growth for these cancers.
Neural activity has also been linked to glioma growth through signaling pathways activated by the synaptic protein, neuroligin-3 (NLGN3). Expression and secretion of this mitogen from neurons is increased in response to neural activity, and the presence of NLGN3 is sufficient to drive tumor growth in patient-derived xenograft models and growth of in vitro glioma cells. The increased presence of NLGN3 activates and powers a feedforward loop that increases NLGN3 expression in glioma cells. NLGN3 overexpression in glioma drives further proliferation of the tumor cells, thus leading to growth and spread of the cancer. Crucially, NLGN3 expression levels inversely correlate with survival in human patients with glioblastoma [43]. Thus, NLGN3 is one of many important trophic factors passed between neurons and tumors that is responsible for the malignancy of certain tumors; in the future, NLGN3 may be targeted to fight these cancers.
Peripheral tumors also demonstrate neuronally driven cancer growth. However, these relationships appear to be more complicated. In pancreatic adenocarcinoma, ablation of sensory neurons has resulted in inhibition of both the initiation and progression of tumor growth. These data demonstrate that primary afferent visceral neurons are necessary for pancreatic tumor survival and progression [34,44,45]. However, paradoxically, removal of pancreatic projections from the vagus nerve, which is primarily made up of sensory afferents, results in increased pancreatic tumor growth [30]. Thus, the neural regulation of pancreatic tumors and their microenvironments is complicated, since these cancer tissues appear to respond differently to the diversity of visceral sensory neurons.
We also know that pancreatic cancers are simultaneously innervated by adrenergic sympathetic and cholinergic parasympathetic neurons and by sensory afferents. Selective removal of cholinergic receptors from pancreatic tumors stimulates pancreatic tumor growth, mirroring the results found after removal of vagal innervation. Further work found that this cholinergic signaling suppresses the pancreatic stem cell compartment via inhibition of the MAPK/EGFR and PI3K/AKT pathways [30]. Alternatively, however, sympathetic innervation of pancreatic stem cells drives tumor growth, matching data previously discussed regarding sensory innervation of these cells [34,44–46]. Thus, in total, it appears that pancreatic stem cells lie in the balance between acetylcholine and other inhibitory signals released by cholinergic parasympathetic neurons, and the stimulatory NTs and growth factors released by sympathetic and sensory neurons. This neuronal balance lies at the heart of pancreatic cancer growth dynamics [32].
This relationship, in which neuronal activity plays a vital role in cancer survival and growth, is seen in a variety of cancers found throughout the body. Basal cell carcinoma is another tumor type in which surgical removal of sensory afferent neurons results in complete inhibition of tumor formation and growth [12]. Thus, it is evident that many tumor types take advantage of common neuronal activity to drive their own growth through various growth pathways (Fig. 2). Tumors also leverage pathways that allow them to turn neuronal activity into means of spreading throughout the body.
Fig 2. Fast NTs drive tumor growth and aggression.
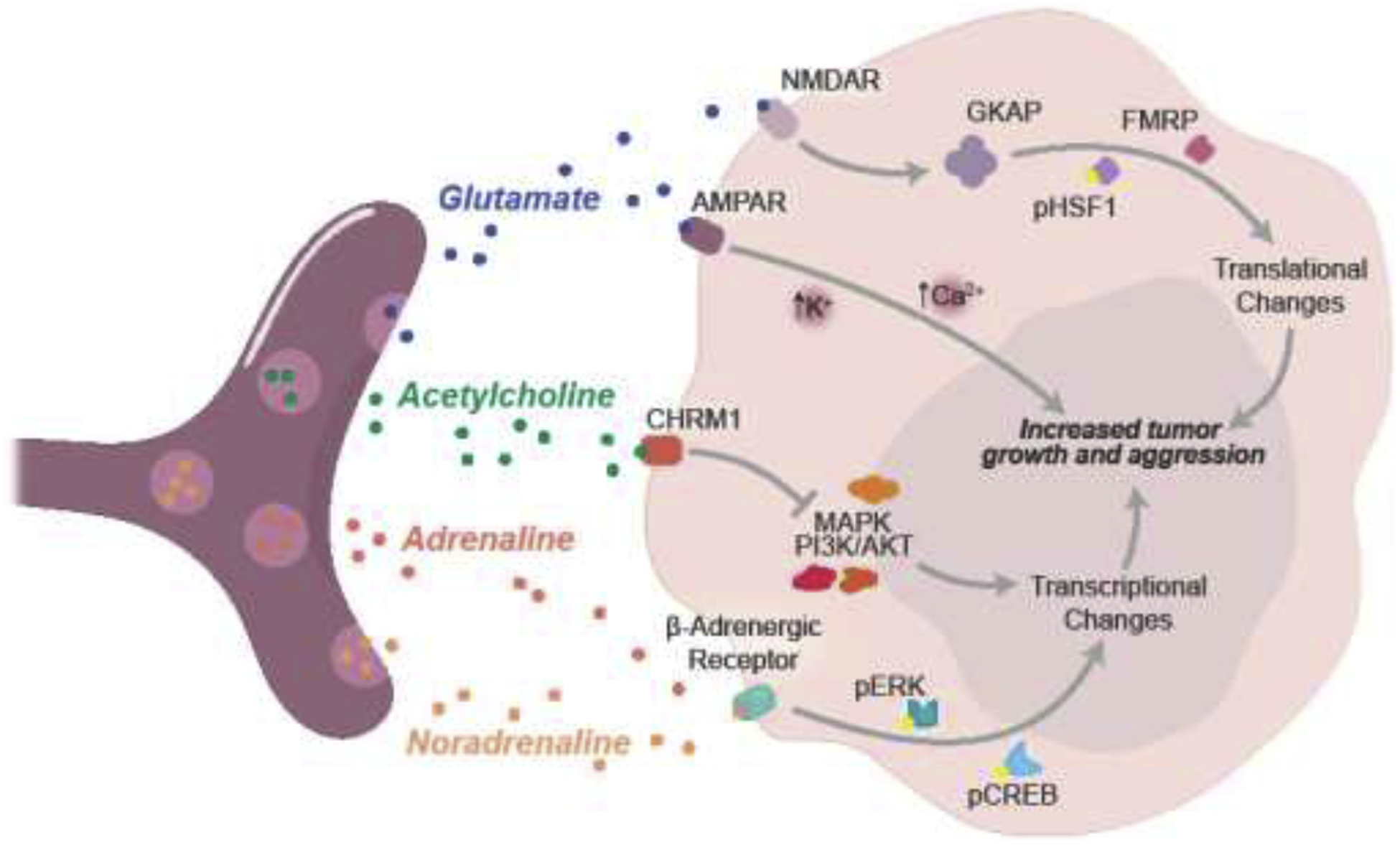
Tumor cells express NT receptors on their cell surface, thereby taking advantage of the abundance of these signals in the tumor microenvironment. Activation of NT receptors induces downstream regulatory pathways, resulting in transcriptional and translational changes that drive increased tumor growth and aggression. All four fast NTs depicted here typically come from different neuron types. The relationships between NT receptors and regulatory pathways differ in different tumor types.
NEURALLY RELEASED TROPHIC FACTORS DRIVE PERINEURAL INVASION
Traditionally, neuronal activity was understood to drive tumor metastasis via direct extension of the tumor body, or passage along lymphatic or hematological pathways. However, an important fourth method of spread that potently illustrates the tight trophic communications between nerves and tumors is the dissemination of tumor cells along neurons. This perineural invasion is dependent on the release of NTs, chemokines, and other growth factors, as well as the expression of membrane-anchored signals by both the neurons and the other cells within the perineural niche [14].
Neurotransmitter release is the primary mode of cell-to-cell communication for the majority of peripheral nervous system neurons. Although neuron-to-neuron signaling is most often discussed, NTs play an important role in stimulating the activity and growth of various cell types including immune cells, fibroblasts, and endothelial cells. Thus, NTs are able to modulate common players in the tumor microenvironment, which, combined with their widespread presence, makes them an important target for tumor use [47].
As previously described, cholinergic signaling from parasympathetic neurons stimulates the growth of certain cancers [32]. However, experiments in which cholinergic signaling is blocked via disruption of the cholinergic muscarinic receptor CHRM1 have shown that cholinergic signaling is also important in the perineural invasion of cholangiocarcinoma [48] and prostate cancer [10]. Similarly, sympathetic innervation can drive perineural invasion. This is supported by both in vitro experiments in which PDAC cells are continuously exposed to noradrenaline, as well as in vivo injections of noradrenaline into mouse PDAC models. In these experiments, the PDAC cells demonstrated increased perineural invasion into cultured DRGs and nearby nerves in vivo, delineating a clear link between this NT and perineural invasion [49].
Chemokines (or chemotactic cytokines) are a group of signaling molecules used to attract white blood cells and other immune cells to a site of interest. Neurons release chemokines to support a perineural inflammatory response in various settings, and this release may also drive perineural invasion. Tumor cells expressing the chemokine (C-X-C motif) receptor 5 (CXCR5) respond to stimulation by the stromally released ligand, CXCL13, thus increasing cancer cell invasion into the stroma during in vitro experiments [50]. In addition, CXCR5 overexpression is found in prostate and colorectal cancer cells associated with perineural invasion, further linking this chemokinetic release to perineural invasion [51,52]. Similarly, the release of C-X3-C motif chemokine ligand 1 (CX3CL1) from neurons induces migration of PDAC cells that express the corresponding receptor, CX3CR1. During in vivo experiments, PDAC xenografts expressing CX3CR1 are found to robustly invade nearby neurons. However, PDAC xenografts lacking CX3CR1 expression never demonstrate perineural invasion. Thus, neuronally released chemokines such as CX3CL1 may be necessary for certain perineural invasion events [53].
Other soluble neural growth factors that are released into the perineural space can be co-opted for use by the tumor. These include glial cell–derived neurotrophic factor (GDNF), which binds to the GDNF family receptor (GFRα1), thereby activating its cognate tyrosine kinase, RET [14]. Expression levels and concentration of RET and GDNF positively correlate with the magnitude of perineural invasion in several cancers, including breast cancer [54], cholangiocarcinoma [55], and pancreatic cancer [56]. Interestingly, damage to neurons, which may result from local tumor invasion, induces the release of soluble GFRα1 from these neurons, which, when taken up by nearby tumors, is able to drive perineural invasion by both GFRα1-positive and GFRα1-negative tumors [57]. Inhibiting RET activity through injections of the RET inhibitor pyrazolopyrimidine (PP1) is able to abate perineural invasion in mouse models of pancreatic cancer, thus decreasing neural symptoms in these mice [56].
NGF is another secreted neurogenic molecule that is co-opted by neurotrophic tumors. The perineural niche surrounding these tumors often expresses high amounts of NGF receptors, NGFR, and neurotrophic receptor tyrosine kinase 1 (NTRK1), which mediate the role of NGF in driving neurite outgrowth and perineural invasion [58,59]. During in vitro studies, inhibiting NGF activity with use of the small-molecule inhibitor Ro 08-2750 results in a dramatic decrease in cancer cell invasion of DRGs [60]. Thus, these experiments illustrate how these particular secreted factors, along with other secreted and membrane-bound factors, are important regulators of both the initiation and progression of perineural invasion.
In contrast to secreted factors, neurons and tumors express a multitude of membrane-bound factors that when activated, facilitate perineural invasion of tumor cells. The L1 cell adhesion molecule (L1CAM) is a widely expressed transmembrane neuronal glycoprotein of the immunoglobulin superfamily that regulates axonogenesis. Tumors and stromal tissue often overexpress L1CAM, and this overexpression is associated with both perineural invasion in human PDAC tumors, as well as poor outcomes in patients [61]. Inhibiting L1CAM activity with monoclonal antibodies is sufficient to markedly reduce neural invasion of pancreatic tumors both in vitro and in vivo [62]. These and additional data identify L1CAM as a potent regulator of neuronal axonogenesis and perineural invasion of tumor cells [14]. Although L1CAM serves as only one example, these data illustrate the important role that membrane-bound proteins serve in neuron-tumor trophic interactions. In combination with the growing literature on secreted growth factors, chemokines, and NTs, these results help us understand the chemical signaling that allows for perineural invasion. Furthermore, these data allow us to identify potential therapeutic targets that sever cancer-supporting trophic interactions between neurons and tumors, thereby preventing the growth and spread of cancers.
EMERGING DEVELOPMENTS AND THERAPEUTICS
Recent publications have revolutionized our understanding of the trophic relationships between tumors and the body, uncovering novel factors and pathways that serve as potential therapeutic targets. Therapeutic efforts are complicated by the tendency of tumors to hijack essential neuronal pathways to stimulate their own growth, thus making it difficult to remove the neuronally released stimulus without affecting the healthy neurons and surrounding tissue. However, because tumors are dependent on these neurotrophic relationships for survival, growth, and spread, specifically interrupting the interactions between tumors and neurons remains a promising therapeutic option, since this interruption would leave tumors vulnerable to endogenous and exogenous anticancer responses.
As previously described, sympathetic innervation of tumors drives growth in a variety of tumor types. In line with this, beta-adrenergic receptor blockers have shown promise for inhibiting tumor growth and spread in many cancers. These drugs are currently approved to treat cardiovascular diseases and anxiety disorders. However, they have also been found effective in treating breast [63], ovarian [64], pancreatic [30], and prostate [65] cancers. It is likely that these drugs interrupt adrenergic signaling from tumor-infiltrating sympathetic neurons, thus inhibiting the angiogenic and tumorigenic effects of sympathetic stimulation. Several open clinical trials are testing the efficacy of this class of drugs in treating various cancers (NCT02596867, NCT01308944, NCT03838029, NCT00502684, NCT01847001). The results of these trials will guide decisions to repurpose beta-blockers as an anticancer therapy.
Similar to the effects of pharmacological inhibition of autonomic innervation, surgical and chemical denervation of the prostate gland, which removes both adrenergic sympathetic and cholinergic parasympathetic signaling, results in complete inhibition of prostate cancer growth and dissemination. These findings are bolstered by knockout experiments of ADRβ2, ADRβ3, and CHRM1 receptors, in which stromal and prostate cancer cell growth is inhibited. These data thereby point to surgical, chemical, and potentially radiation-based interventions that aim to denervate the tumor bed as a means of severing neuron-tumor trophic interactions [10,66].
Neurons are sensitive to a number of compounds that may be used in chemically denervating cancer tissue. These might be effective in functionally decreasing tumorigenic signaling from these innervating neurons. One such compound that might prove useful is botulinum toxin, which inhibits release of acetylcholine from cholinergic neurons that bind and take up the toxin. Targeting tumor-innervating neurons with botulinum toxin has been used successfully in abating gastric cancer growth and enhancing survival in mice [5]. In addition, at least one clinical trial has successfully used botulinum toxin to decrease innervation of prostate cancers, thus driving apoptosis of tumor cells [67].
Ablating or modifying neural activity, however, may have off-target side effects, since these approaches indiscriminately alter pathogenic, as well as healthy, neural function. Thus, another possible therapeutic option is to target factors that are selectively neuritogenic or tumorigenic. Many of these signals, including growth factors, neuron guidance molecules, and miRNAs, are necessary during development but are not necessary for the maintenance of mature tissue. Anti-NGF antibodies have been effective in inhibiting the feedforward loop between gastric cancers and innervating cholinergic neurons, thereby stemming tumorigenesis in mice [4]. A similar approach used anti-NGF siRNA-coated gold nanoparticles to inhibit pancreatic tumor growth in mice [68].
In the microenvironment of p53-deficient OCSCCs, increased miR-34a expression decreases peritumoral neuritogenesis. Conversely, decreased expression of miR-21 and miR-324 results in decreased peritumoral neuritogenesis. Together, these findings indicate that regulating expression of these miRNAs may decrease aggression of this form of cancer [8]. Increased expression of miR-744 can be used to inhibit both proliferation and invasion of gastric cancers by regulation of brain-derived neurotrophic factor, thus demonstrating the therapeutic potential of this miRNA [69]. Reflecting the tumorigenic activity of Trk [70], inhibitors of Trk signaling, including entrectinib, merestinib, and larotrectinib, have proven to be effective in promoting regression and apoptosis in several cancer types [71].
Due to the tight link between the magnitude of tumor innervation and tumor aggression, innervation of the tumor and the microenvironment can be used as a diagnostic or predictive factor for outcome in cancer patients [29]. By extension, neurotrophic factors, including proNGF and NGF, might be detectable in the blood, thus potentially serving as valuable biomarkers of tumor innervation [71]. In addition, exosomes are easily isolated from multiple bodily fluids (including blood, saliva, urine, semen, sputum, breast milk, and cerebrospinal fluid) [72] and are increasingly recognized as an important messaging medium in neuron-tumor relationships [8]. Thus, it is possible that sampling exosomes and their contents from peripheral tissue could provide a source of disease biomarkers as well [73].
These therapies show promise for future use in stemming the growth and spread of cancers. Their efficacy and widespread use will depend on future work dissecting their mechanisms and describing their underlying role in mediating neuron-cancer trophic relationships.
CONCLUSIONS
Continued investigations into the trophic interactions between neurons and tumors have found that these collections of cells not only speak, but use their communicative ability to negotiate, fight, and manipulate each other toward their own benefit. In the past several decades, we have witnessed a surge of information about the signaling molecules, NTs, miRNAs, growth factors, and corresponding pathways that govern trophic interactions between neurons, tumors, and their microenvironments. This work has also uncovered the role of vascular endothelial cells, macrophages, and other immune cells within the perineural niche and tumor microenvironments. The pattern emerging from these interactions is that communication between neurons, tumors, and their microenvironment is shared in all directions. Future experimentation is needed to further untangle this complex web of cell-to-cell interactions, while exploring the mechanisms behind neuron-tumor relationships. These studies are also necessary for the development of novel therapies that can control these trophic interactions for the benefit of the patient.
Highlights.
Neurons alter members of the tumor microenvironment, including immune cells and vascular cells, to promote tumorigenesis.
Tumors use microRNAs and small molecule messengers to transform surrounding neurons.
Altered neuronal activity results in increased perineural invasion of tumor cells.
Methods of disrupting neuron-cancer communication provide clinical benefit.
ACKNOWLEDGEMENTS
The authors would like to kindly thank the Scientific Publications, Research Medical Library at MD Anderson for their help in reviewing the manuscript.
Financial support
Supported by the American Heart Association under award number 20PRE35040011, and BRASS: Baylor Research Advocates for Student Scientists (PJH). Supported by the NIH/NCI under award number P30CA016672.
Abbreviations:
- EV
extracellular vesicle
- DRG
dorsal root ganglion
- NT
neurotransmitter
- OCSCC
oral cavity squamous cell carcinoma
- PanIN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF COMPETING INTEREST
The authors have no competing interests to declare.
REFERENCES
- [1].Ge Y, Miao Y, Gur-Cohen S, Gomez N, Yang H, Nikolova M, Polak L, Hu Y, Verma A, Elemento O, Krueger JG, Fuchs E, The aging skin microenvironment dictates stem cell behavior, Proc. Natl. Acad. Sci 117 (2020) 5339–5350. 10.1073/pnas.1901720117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G, Quante M, Three-Dimensional Gastrointestinal Organoid Culture in Combination with Nerves or Fibroblasts: A Method to Characterize the Gastrointestinal Stem Cell Niche, Stem Cells Int. 2016 (2016). 10.1155/2016/3710836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kumar A, Brockes JP, Nerve dependence in tissue, organ, and appendage regeneration, Trends Neurosci. 35 (2012) 691–699. 10.1016/j.tins.2012.08.003. [DOI] [PubMed] [Google Scholar]
- [4].Hayakawa Y, Sakitani K, Konishi M, Asfaha S, Niikura R, Tomita H, Renz BW, Tailor Y, Macchini M, Middelhoff M, Jiang Z, Tanaka T, Dubeykovskaya ZA, Kim W, Chen X, Urbanska AM, Nagar K, Westphalen CB, Quante M, Lin CS, Gershon MD, Hara A, Zhao CM, Chen D, Worthley DL, Koike K, Wang TC, Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling, Cancer Cell. 31 (2017) 21–34. 10.1016/j.ccell.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhao CM, Hayakawa Y, Kodama Y, Muthupalani S, Westphalen CB, Andersen GT, Flatberg A, Johannessen H, Friedman RA, Renz BW, Sandvik AK, Beisvag V, Tomita H, Hara A, Quante M, Li Z, Gershon MD, Kaneko K, Fox JG, Wang TC, Chen D, Denervation suppresses gastric tumorigenesis, Sci. Transl. Med 6 (2014) 1–13. 10.1126/scitranslmed.3009569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Polli-Lopes AC, Zucoloto S, De Queirós Cunha F, Alves L Da Silva Figueiredo, S.B. Garcia, Myenteric denervation reduces the incidence of gastric tumors in rats, Cancer Lett. 190 (2003) 45–50. 10.1016/S0304-3835(02)00584-0. [DOI] [PubMed] [Google Scholar]
- [7].Hanoun M, Maryanovich M, Arnal-Estapé A, Frenette PS, Neural regulation of hematopoiesis, inflammation, and cancer, Neuron. 86 (2015) 360–373. 10.1016/j.neuron.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Amit M, Takahashi H, Dragomir MP, Lindemann A, Gleber-Netto FO, Pickering CR, Anfossi S, Osman AA, Cai Y, Wang R, Knutsen E, Shimizu M, Ivan C, Rao X, Wang J, Silverman DA, Tam S, Zhao M, Caulin C, Zinger A, Tasciotti E, Dougherty PM, El-Naggar A, Calin GA, Myers JN, Loss of p53 drives neuron reprogramming in head and neck cancer, Nature. 578 (2020) 449–454. 10.1038/s41586-020-1996-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cavel O, Shomron O, Shabtay A, Vital J, Trejo-Leider L, Weizman N, Krelin Y, Fong Y, Wong RJ, Amit M, Gil Z, Endoneurial macrophages induce perineural invasion of pancreatic cancer cells by secretion of GDNF and activation of RET tyrosine kinase receptor, Cancer Res. 72 (2012) 5733–5743. 10.1158/0008-5472.CAN-12-0764. [DOI] [PubMed] [Google Scholar]
- [10].Magnon C, Hall SJ, Lin J, Zue X, Gerber L, Freedland SJ, Frenette PS, Autonomic nerve development contributes to prostate cancer progression, Science (80-. ). 341 (2013) 12363611–12363619. 10.1038/aja.2013.113. [DOI] [PubMed] [Google Scholar]
- [11].Ayala GE, Wheeler TM, David Shine H, Schmelz M, Frolov A, Chakraborty S, Rowley D, In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer, Prostate. 49 (2001) 213–223. 10.1002/pros.1137. [DOI] [PubMed] [Google Scholar]
- [12].Peterson SC, Eberl M, Vagnozzi AN, Belkadi A, Veniaminova NA, Verhaegen ME, Bichakjian CK, Ward NL, Dlugosz AA, Wong SY, Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches, Cell Stem Cell. 16 (2015) 400–412. 10.1016/j.stem.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keskinov AA, Tapias V, Watkins SC, Ma Y, Shurin MR, Shurin GV, Impact of the sensory neurons on melanoma growth in vivo, PLoS One. 11 (2016). 10.1371/journal.pone.0156095. [DOI] [Google Scholar]
- [14].Amit M, Na’Ara S, Gil Z, Mechanisms of cancer dissemination along nerves, Nat. Rev. Cancer 16 (2016) 399–408. 10.1038/nrc.2016.38. [DOI] [PubMed] [Google Scholar]
- [15].Tuveson DA, Shaw AT, Willis NA, Silver DP, Jackson EL, Chang S, Mercer KL, Grochow R, Hock H, Crowley D, Hingorani SR, Zaks T, King C, Jacobetz MA, Wang L, Bronson RT, Orkin SH, DePinho RA, Jacks T, Endogenous oncogenic K-rasG12D stimulates proliferation and widespread neoplastic and developmental defects, Cancer Cell. 5 (2004) 375–387. 10.1016/S1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- [16].Chavan SS, Tracey KJ, Essential Neuroscience in Immunology, J. Immunol 198 (2017) 3389–3397. 10.4049/jimmunol.1601613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, Arevalo JMG, Morizono K, Karanikolas BDW, Wu L, Sood AK, Cole SW, The sympathetic nervous system induces a metastatic switch in primary breast cancer, Cancer Res. 70 (2010) 7042–7052. 10.1158/0008-5472.CAN-10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH, Dynamics of the immune reaction to pancreatic cancer from inception to invasion, Cancer Res. 67 (2007) 9518–9527. 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- [19].Pollard J, Tumour-educated macrophages promote tumour progression and metastasis, Nat. Rev. Cancer 4 (2004) 71–78. [DOI] [PubMed] [Google Scholar]
- [20].He S, He S, Chen CH, Deborde S, Bakst RL, Chernichenko N, McNamara WF, Lee SY, Barajas F, Yu Z, Al-Ahmadie HA, Wong RJ, The chemokine (CCL2-CCR2) signaling axis mediates perineural invasion, Mol. Cancer Res 13 (2015) 380–390. 10.1158/1541-7786.MCR-14-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Vasudev NS, Reynolds AR, Anti-angiogenic therapy for cancer: Current progress, unresolved questions and future directions, Angiogenesis. 17 (2014) 471–494. 10.1007/s10456-014-9420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A, Guidance of vascular development: Lessons from the nervous system, Circ. Res 104 (2009) 428–441. 10.1161/CIRCRESAHA.108.188144. [DOI] [PubMed] [Google Scholar]
- [23].Zahalka AH, Arnal-Estape A, Maryanovich M, Nakahara F, Cruz CD, Finley LWS, Frenette PS, Adrenergic nerves activate an angio-metabolic switch in prostate cancer, Science (80-. ). 358 (2017) 321–326. 10.1016/B978-1-4557-2865-7.00084-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S, slit: An EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system, Cell. 55 (1988) 1047–1059. 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- [25].Boyer NP, Gupton SL, Revisiting netrin-1: One who guides (Axons), Front. Cell. Neurosci 12 (2018) 1–18. 10.3389/fncel.2018.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].O’Donnell M, Chance RK, Bashaw GJ, Axon Growth and Guidance: Receptor Regulation and Signal Transduction, Annu. Rev. Neurosci 32 (2009) 383–412. 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dobrenis K, Gauthier LR, Barroca V, Magnon C, Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development, Int. J. Cancer 136 (2015) 982–988. 10.1002/ijc.29046. [DOI] [PubMed] [Google Scholar]
- [28].Pundavela J, Demont Y, Jobling P, Lincz LF, Roselli S, Thorne RF, Bond D, Bradshaw RA, Walker MM, Hondermarck H, ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer, Am. J. Pathol 184 (2014) 3156–3162. 10.1016/j.ajpath.2014.08.009. [DOI] [PubMed] [Google Scholar]
- [29].Pundavela J, Roselli S, Faulkner S, Attia J, Scott RJ, Thorne RF, Forbes JF, Bradshaw RA, Walker MM, Jobling P, Hondermarck H, Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer, Mol. Oncol 9 (2015) 1626–1635. 10.1016/j.molonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Renz BW, Tanaka T, Sunagawa M, Takahashi R, Jiang Z, Macchini M, Dantes Z, Valenti G, White RA, Middelhoff MA, Ilmer M, Oberstein PE, Angele MK, Deng H, Hayakawa Y, Westphalen CB, Werner J, Remotti H, Reichert M, Tailor YH, Nagar K, Friedman RA, Iuga AC, Olive KP, Wang TC, Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness, Cancer Discov. 8 (2018) 1458–1473. 10.1158/2159-8290.CD-18-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch AM, Wu J, Chang DK, Cowley MJ, Gardiner BB, Song S, Harliwong I, Idrisoglu S, Nourse C, Nourbakhsh E, Manning S, Wani S, Gongora M, Pajic M, Scarlett CJ, Gill AJ, Pinho AV, Rooman I, Anderson M, Holmes O, Leonard C, Taylor D, Wood S, Xu Q, Nones K, Fink JL, Christ A, Bruxner T, Cloonan N, Kolle G, Newell F, Pinese M, Mead RS, Humphris JL, Kaplan W, Jones MD, Colvin EK, Nagrial AM, Humphrey ES, Chou A, Chin VT, Chantrill LA, Mawson A, Samra JS, Kench JG, Lovell JA, Daly RJ, Merrett ND, Toon C, Epari K, Nguyen NQ, Barbour A, Zeps N, Kakkar N, Zhao F, Wu YQ, Wang M, Muzny DM, Fisher WE, Brunicardi FC, Hodges SE, Reid JG, Drummond J, Chang K, Han Y, Lewis LR, Dinh H, Buhay CJ, Beck T, Timms L, Sam M, Begley K, Brown A, Pai D, Panchal A, Buchner N, De Borja R, Denroche RE, Yung CK, Serra S, Onetto N, Mukhopadhyay D, Tsao MS, Shaw PA, Petersen GM, Gallinger S, Hruban RH, Maitra A, Iacobuzio-Donahue CA, Schulick RD, Wolfgang CL, Morgan RA, Lawlor RT, Capelli P, Corbo V, Scardoni M, Tortora G, Tempero MA, Mann KM, Jenkins NA, Perez-Mancera PA, Adams DJ, Largaespada DA, Wessels LFA, Rust AG, Stein LD, Tuveson DA, Copeland NG, Musgrove EA, Scarpa A, Eshleman JR, Hudson TJ, Sutherland RL, Wheeler DA, Pearson JV, McPherson JD, Gibbs RA, Grimmond SM, Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes, Nature. 491 (2012) 399–405. 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Faulkner S, Jobling P, March B, Jiang CC, Hondermarck H, Tumor neurobiology and the war of nerves in cancer, Cancer Discov. 9 (2019) 702–710. 10.1158/2159-8290.CD-18-1398. [DOI] [PubMed] [Google Scholar]
- [33].Zeng Q, Michael IP, Zhang P, Saghafinia S, Knott G, Jiao W, McCabe BD, Galván JA, Robinson HPC, Zlobec I, Ciriello G, Hanahan D, Synaptic proximity enables NMDAR signalling to promote brain metastasis, Nature. 573 (2019) 526–531. 10.1038/s41586-019-1576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Saloman JL, Albers KM, Li D, Hartman DJ, Crawford HC, Muha EA, Rhim AD, Davis BM, Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer, Proc. Natl. Acad. Sci. U. S. A 113 (2016) 3078–3083. 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li L, Zeng Q, Bhutkar A, Galván JA, Karamitopoulou E, Noordermeer D, Peng MW, Piersigilli A, Perren A, Zlobec I, Robinson H, Iruela-Arispe ML, Hanahan D, GKAP Acts as a Genetic Modulator of NMDAR Signaling to Govern Invasive Tumor Growth, Cancer Cell. 33 (2018) 736–751.e5. 10.1016/j.ccell.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li L, Hanahan D, Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion, Cell. 153 (2013) 86–100. 10.1016/j.cell.2013.02.051. [DOI] [PubMed] [Google Scholar]
- [37].Robinson HPC, Li L, Autocrine, paracrine and necrotic NMDA receptor signalling in mouse pancreatic neuroendocrine tumour cells, Open Biol. 7 (2017). 10.1098/rsob.170221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lozano-Ondoua AN, Symons-Liguori AM, Vanderah TW, Cancer-induced bone pain: Mechanisms and models, Neurosci. Lett 557 (2013) 52–59. 10.1016/j.neulet.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Araque A, Parpura V, Sanzgiri RP, Haydon PG, Tripartite synapses: Glia, the unacknowledged partner, Trends Neurosci. 22 (1999) 208–215. 10.1016/S0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- [40].Yu K, Lin CCJ, Hatcher A, Lozzi B, Kong K, Huang-Hobbs E, Cheng YT, Beechar VB, Zhu W, Zhang Y, Chen F, Mills GB, Mohila CA, Creighton CJ, Noebels JL, Scott KL, Deneen B, PIK3CA variants selectively initiate brain hyperactivity during gliomagenesis, Nature. 578 (2020) 166–171. 10.1038/s41586-020-1952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Venkataramani V, Tanev DI, Strahle C, Studier-Fischer A, Fankhauser L, Kessler T, Körber C, Kardorff M, Ratliff M, Xie R, Horstmann H, Messer M, Paik SP, Knabbe J, Sahm F, Kurz FT, Acikgöz AA, Herrmannsdörfer F, Agarwal A, Bergles DE, Chalmers A, Miletic H, Turcan S, Mawrin C, Hänggi D, Liu HK, Wick W, Winkler F, Kuner T, Glutamatergic synaptic input to glioma cells drives brain tumour progression, Nature. 573 (2019) 532–538. 10.1038/s41586-019-1564-x. [DOI] [PubMed] [Google Scholar]
- [42].Venkatesh HS, Morishita W, Geraghty AC, Silverbush D, Gillespie SM, Arzt M, Tam LT, Espenel C, Ponnuswami A, Ni L, Woo PJ, Taylor KR, Agarwal A, Regev A, Brang D, Vogel H, Hervey-Jumper S, Bergles DE, Suvà ML, Malenka RC, Monje M, Electrical and synaptic integration of glioma into neural circuits, Nature. 573 (2019) 539–545. 10.1038/s41586-019-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, Woo PJ, Malenka RC, Vogel H, Bredel M, Mallick P, Monje M, Neuronal activity promotes glioma growth through neuroligin-3 secretion, Cell. 161 (2015) 803–816. 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sinha S, Fu Y-Y, Grimont A, Ketcham M, Lafaro K, Saglimbeni JA, Askan G, Bailey JM, Melchor JP, Zhong Y, Joo MG, Grbovic-Huezo O, Yang I-H, Basturk O, Baker L, Park Y, Kurtz RC, Tuveson D, Leach SD, Pasricha PJ, PanIN Neuroendocrine Cells Promote Tumorigenesis via Neuronal Cross-talk, Cancer Res. 77 (2017) 1868–1879. 10.1158/0008-5472.can-16-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stopczynski RE, Normolle DP, Hartman DJ, Ying H, DeBerry JJ, Bielefeldt K, Rhim AD, DePinho RA, Albers KM, Davis BM, Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma, Cancer Res. 74 (2014) 1718–1727. 10.1158/0008-5472.CAN-13-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Renz BW, Takahashi R, Tanaka T, Macchini M, Hayakawa Y, Dantes Z, Maurer HC, Chen X, Jiang Z, Westphalen CB, Ilmer M, Valenti G, Mohanta SK, Habenicht AJR, Middelhoff M, Chu T, Nagar K, Tailor Y, Casadei R, Di Marco M, Kleespies A, Friedman RA, Remotti H, Reichert M, Worthley DL, Neumann J, Werner J, Iuga AC, Olive KP, Wang TC, β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer, Cancer Cell. 33 (2018) 75–90.e7. 10.1016/j.ccell.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Li ZJ, Cho CH, Neurotransmitters, more than meets the eye - Neurotransmitters and their perspectives in cancer development and therapy, Eur. J. Pharmacol 667 (2011) 17–22. 10.1016/j.ejphar.2011.05.077. [DOI] [PubMed] [Google Scholar]
- [48].Feng YJ, Zhang BY, Yao RY, Lu Y, Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells, Hepatobiliary Pancreat. Dis. Int 11 (2012) 418–423. 10.1016/S1499-3872(12)60201-X. [DOI] [PubMed] [Google Scholar]
- [49].Guo K, Ma Q, Li J, Wang Z, Shan T, Li W, Xu Q, Xie K, Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling, Mol. Cancer Ther 12 (2013) 264–273. 10.1158/1535-7163.MCT-12-0809. [DOI] [PubMed] [Google Scholar]
- [50].Zhu Z, Zhang X, Guo H, Fu L, Pan G, Sun Y, CXCL13-CXCR5 axis promotes the growth and invasion of colon cancer cells via PI3K/AKT pathway, Mol. Cell. Biochem 400 (2014) 287–295. 10.1007/s11010-014-2285-y. [DOI] [PubMed] [Google Scholar]
- [51].Singh S, Singh R, Singh UP, Rai SN, Novakovic KR, Chung LWK, Didier PJ, Grizzle WE, Lillard JW, Clinical and biological significance of CXCR5 expressed by prostate cancer specimens and cell lines, Int. J. Cancer 125 (2009) 2288–2295. 10.1002/ijc.24574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Qi XW, Xia SH, Yin Y, Jin LF, Pu Y, Hua D, Wu HR, Expression features of CXCR5 and its ligand, CXCL13 associated with poor prognosis of advanced colorectal cancer, Eur. Rev. Med. Pharmacol. Sci 18 (2014) 1916–1924. [PubMed] [Google Scholar]
- [53].Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P, Laghi L, Malesci A, Cervo L, Malosio ML, Reni M, Zerbi A, Di Carlo V, Mantovani A, Allavena P, The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma, Cancer Res. 68 (2008) 9060–9069. 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- [54].Esseghir S, Todd SK, Hunt T, Poulsom R, Plaza-Menacho I, Reis-Filho JS, Isacke CM, A role for glial cell-derived neurotrophic factor-induced expression by inflammatory cytokines and RET/GFRα1 receptor up-regulation in breast cancer, Cancer Res. 67 (2007) 11732–11741. 10.1158/0008-5472.CAN-07-2343. [DOI] [PubMed] [Google Scholar]
- [55].Iwahashi N, Nagasaka T, Tezel G, Iwashita T, Asai N, Murakumo Y, Kiuchi K, Sakata K, Nimura Y, Takahashi M, Expression of glial cell line-derived neurotrophic factor correlates with perineural invasion of bile duct carcinoma, Cancer. 94 (2002) 167–174. 10.1002/cncr.10169. [DOI] [PubMed] [Google Scholar]
- [56].Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, Carlson DL, Shah JP, Fong Y, Wong RJ, Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves, J. Natl. Cancer Inst 102 (2010) 107–118. 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].He S, Chen CH, Chernichenko N, He S, Bakst RL, Barajas F, Deborde S, Allen PJ, Vakiani E, Yu Z, Wong RJ, GFRα1 released by nerves enhances cancer cell perineural invasion through GDNF-RET signaling, Proc. Natl. Acad. Sci. U. S. A 111 (2014). 10.1073/pnas.1402944111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhu BZ, Friess H, Fabio F, Zimmermann A, Graber HU, Korc M, Bu MW, Nerve Growth Factor Expression Correlates With Perineural Invasion and Pain in Human Pancreatic Cancer, J. Clin. Oncol 17 (1999) 2419–2428. [DOI] [PubMed] [Google Scholar]
- [59].Geldof AA, De Kleijn MAT, Rao BR, Newling DWW, Nerve growth factor stimulates in vitro invasive capacity of DU145 human prostatic cancer cells, J. Cancer Res. Clin. Oncol 123 (1997) 107–112. 10.1007/BF01269888. [DOI] [PubMed] [Google Scholar]
- [60].Demir IE, Boldis A, Pfitzinger PL, Teller S, Brunner E, Klose N, Kehl T, Maak M, Lesina M, Laschinger M, Janssen KP, Algül H, Friess H, Ceyhan GO, Investigation of schwann cells at neoplastic cell sites before the onset of cancer invasion, J. Natl. Cancer Inst 106 (2014). 10.1093/jnci/dju184. [DOI] [PubMed] [Google Scholar]
- [61].Ben QW, Wang JC, Liu J, Zhu Y, Yuan F, Yao WY, Yuan YZ, Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma, Ann. Surg. Oncol 17 (2010) 2213–2221. 10.1245/s10434-010-0955-x. [DOI] [PubMed] [Google Scholar]
- [62].Na’ara S, Amit M, Gil Z, L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression, Oncogene. 38 (2019) 596–608. 10.1038/s41388-018-0458-y. [DOI] [PubMed] [Google Scholar]
- [63].Melhem-Bertrandt A, Chavez-MacGregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, Sood AK, Conzen SD, Hortobagyi GN, Gonzalez-Angulo AM, Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer, J. Clin. Oncol 29 (2011) 2645–2652. 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Watkins JL, Thaker PH, Nick AM, Ramondetta LM, Kumar S, Urbauer DL, Matsuo K, Squires KC, Coleman RL, Lutgendorf SK, Ramirez PT, Sood AK, Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer, Cancer. 121 (2015) 3444–3451. 10.1002/cncr.29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Grytli HH, Fagerland MW, Fosså SD, Taskén KA, Association between use of β-blockers and prostate cancer-specific survival: A cohort study of 3561 prostate cancer patients with high-risk or metastatic disease, Eur. Urol 65 (2014) 635–641. 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- [66].Demir IE, Reyes CM, Alrawashdeh W, Ceyhan GO, Deborde S, Friess H, Görgülü K, Istvanffy R, Jungwirth D, Kuner R, Maryanovich M, Na’ara S, Renders S, Saloman JL, Scheff NN, Steenfadt H, Stupakov P, Thiel V, Verma D, Yilmaz BS, White RA, Wang TC, Wong RJ, Frenette PS, Gil Z, Davis BM, Clinically Actional Strategies for Studying Neural Influences in Cancer, Cancer Cell. (2020) 1–4. 10.1016/j.ccell.2020.05.023. [DOI] [PubMed] [Google Scholar]
- [67].Coarfa C, Florentin D, Putluri NR, Ding Y, Au J, He D, Ragheb A, Frolov A, Michailidis G, Lee MJ, Kadmon D, Miles B, Smith C, Ittmann M, Rowley D, Sreekumar A, Creighton CJ, Ayala G, Influence of the neural microenvironment on prostate cancer, Prostate. 78 (2018) 128–139. 10.1002/pros.23454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lei Y, Tang L, Xie Y, Xianyu Y, Zhang L, Wang P, Hamada Y, Jiang K, Zheng W, Jiang X, Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer, Nat. Commun 8 (2017). 10.1038/ncomms15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Xu AJ, Fu LN, Wu HX, Yao XL, Meng R, MicroRNA-744 inhibits tumor cell proliferation and invasion of gastric cancer via targeting brain-derived neurotrophic factor, Mol. Med. Rep 16 (2017) 5055–5061. 10.3892/mmr.2017.7167. [DOI] [PubMed] [Google Scholar]
- [70].Demir IE, Tieftrunk E, Schorn S, Friess H, Ceyhan GO, Nerve growth factor & TrkA as novel therapeutic targets in cancer, Biochim. Biophys. Acta - Rev. Cancer 1866 (2016) 37–50. 10.1016/j.bbcan.2016.05.003. [DOI] [PubMed] [Google Scholar]
- [71].Griffin N, Faulkner S, Jobling P, Hondermarck H, Targeting neurotrophin signaling in cancer: The renaissance, Pharmacol. Res 135 (2018) 12–17. 10.1016/j.phrs.2018.07.019. [DOI] [PubMed] [Google Scholar]
- [72].Wu M, Ouyang Y, Wang Z, Zhang R, Huang PH, Chen C, Li H, Li P, Quinn D, Dao M, Suresh S, Sadovsky Y, Huang TJ, Isolation of exosomes from whole blood by integrating acoustics and microfluidics, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 10584–10589. 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Murillo OD, Thistlethwaite W, Rozowsky J, Subramanian SL, Lucero R, Shah N, Jackson AR, Srinivasan S, Chung A, Laurent CD, Kitchen RR, Galeev T, Warrell J, Diao JA, Welsh JA, Hanspers K, Riutta A, Burgstaller-Muehlbacher S, Shah RV, Yeri A, Jenkins LM, Ahsen ME, Cordon-Cardo C, Dogra N, Gifford SM, Smith JT, Stolovitzky G, Tewari AK, Wunsch BH, Yadav KK, Danielson KM, Filant J, Moeller C, Nejad P, Paul A, Simonson B, Wong DK, Zhang X, Balaj L, Gandhi R, Sood AK, Alexander RP, Wang L, Wu C, Wong DTW, Galas DJ, Van Keuren-Jensen K, Patel T, Jones JC, Das S, Cheung KH, Pico AR, Su AI, Raffai RL, Laurent LC, Roth ME, Gerstein MB, Milosavljevic A, exRNA Atlas Analysis Reveals Distinct Extracellular RNA Cargo Types and Their Carriers Present across Human Biofluids, Cell. 177 (2019) 463–477.e15. 10.1016/j.cell.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


