Abstract
This study investigates the effect of 2 laparoscopic methods on ovarian reserve in patients of reproductive age with endometriomas.
This was a retrospective study performed at a tertiary medical center from Jan 1st to Dec 31st, 2016. Laparoscopic cystectomy (group 1, 46 patients) and laparoscopic ovarian drainage and ablation with bipolar coagulation at low power (group 2, 30 patients) were performed to treat endometriomas larger than 3 cm. Anti-Müllerian hormone was used to assess ovarian reserve before and after surgery.
There were no statistically significant differences in patients’ baseline clinical characteristics, endometriotic stage, operative time, and follow-up time between the groups. The mean serum anti-Müllerian hormone concentration decreased significantly from 4.25 ng/ml to 3.40 ng/ml in group 1 compared with 4.47 ng/ml to 3.95 ng/ml in group 2 (P = .04). Pregnancy rates were 71.05% in group 1 and 73.08% in group 2, with a mean follow-up of 30.40 months and 32.35 months (P > .99), respectively. Although there was no statistical significance, the recurrence rate in group 1 was lower than that in group 2 (4.35% vs 16.67%, respectively; P = .11). The mean diameter of recurrent cysts was 1.75 cm in group 1 and 1.54 cm in group 2 (P = .13).
Appropriate laparoscopic electrocautery of the endometrioma wall with a bipolar instrument may be a valid alternative to traditional laparoscopic cystectomy, with less effects on ovarian reserve.
Keywords: anti-Müllerian hormone, endometrioma, laparoscopic ablation, laparoscopic cystectomy, ovarian reserve
1. Introduction
Endometriosis is a common but still enigmatic gynecological disease affecting women of reproductive age that is usually characterized by dysmenorrhea, pelvic pain, and infertility in symptomatic patients. Indeed, approximately 30% to 70% of infertile women have been reported to have endometriosis.[1] It is now widely accepted that laparoscopy is the gold standard in the diagnosis and treatment of endometriosis.[2] However, there are several controversies regarding the most appropriate method to treat endometrioma for patients with pregnancy intention.[2]
Evidence suggests that excisional surgery for endometrioma provides better results than drainage and ablation by bipolar coagulation regarding cyst recurrence and pain symptoms, and subsequent spontaneous pregnancy in patients who were previously subfertile. This recommendation appears in a Cochrane systematic review based on the results of 3 randomized controlled trials (RCTs).[3] Unfortunately, the studies were insufficient to determine the most effective treatment for subsequent ovarian function after surgery.[3]
The ability to release ova is closely associated with ovarian reserve. Several studies indicated that ovarian function would be compromised by endometrioma cystectomy.[2,4–8] According to Muzii et al,[9] ovarian tissue was inadvertently excised together with the endometriotic cyst wall in most patients during endometrioma excision. In particular, close to the ovarian hilus, ovarian tissue removed along the endometrioma wall contained primordial, primary, and secondary follicles in 69% of cases. There is obviously an absence of a clear plane of cleavage, especially close to the ovarian hilus. Hence, laparoscopic drainage of endometrioma followed by ablation or coagulation of the cyst wall may be an alternative treatment for patients with pregnancy intention, to reduce the afore-mentioned risk.
Currently, ablation of the endometrioma wall can be achieved by carbon dioxide (CO2) laser, and plasma energy and bipolar instruments. Recently, ablative surgery with a CO2 laser or plasma energy device is considered to preserve more ovarian tissue because the heat damage is more superficial.[10–13] Bipolar instruments are widely used for hemostasis during laparoscopic surgery and have distinct thermal spread depending on the power setting and application time. To the best of our knowledge, few studies have provided detailed information describing the power and duration of bipolar coagulation to destroy the internal wall of endometriomas, even in the RCTs mentioned in the Cochrane systematic review and in a recent multicenter RCT.[3,9] Therefore, this study aimed to compare the effect of laparoscopic cystectomy and ablative surgery with bipolar coagulation at low power on ovarian reserve in patients with endometriomas and conception requirements.
2. Materials and methods
2.1. Patients
In this retrospective study, patients undergoing conservative laparoscopy for endometriomas were consecutively enrolled at the Department of Obstetrics and Gynecology from Jan 1st to Dec 31st, 2016. This study was approved by the Medical Ethics Committee of Sir Run Run Shaw Hospital. The inclusion criteria were as follows: aged 18 to 40 years, regular menstrual cycle, uni- or bi-lateral symptomatic endometriomas ≥3 cm in diameter, and no contraindication for the use of gonadotropin-releasing hormone agonists or oral contraceptives. Continuous contraceptive pill intake was proposed preoperatively for all patients to prevent menstruation before and after surgery. The exclusion criteria were as follows: body mass index >30 kg/m2, previous pelvic surgery for benign ovarian cysts, history of cancer, suspected malignancy, presurgical suspicion, or evidence of decline in ovarian reserve (anti-Müllerian hormone [AMH] <2 ng/ml), presurgical suspicion or evidence of deep infiltrating endometriosis or adenomyosis, concurrent other types of ovarian cyst, pregnancy, thin endometrium, and male factor infertility.
All patients underwent transvaginal ultrasonography in the early proliferative phase of the cycle preoperatively, and the mean diameter of the 3 perpendicular dimensions of the cyst was calculated. The number and depth of the location (superficial or endogenetic) were also assessed by transvaginal ultrasonography before surgery. Patients with multiple endometriomas, endogenetic cysts, or bilateral endometriomas, especially those with pregnancy intent or infertility, tended to choose ablative surgery. The selection of operative method was also considered according to intraoperative findings. When the boundary between the cyst wall and the ovarian cortex was unclear, and/or ovarian adhesions were extensive, ablative surgery was preferred to minimize iatrogenic injury to the ovarian reserve. Patients with single, exogenous, or unilateral cysts tended to choose laparoscopic cystectomy. All surgical options were discussed with all patients in detail preoperatively, and informed consent was obtained from each subject. A total of 46 patients who underwent laparoscopic cystectomy comprised group 1, and 30 patients who underwent laparoscopic ovarian drainage and ablation with bipolar coagulation at low power comprised group 2.
2.2. Operative procedure
All surgeries were performed by the same team of experienced minimally invasive reproductive surgeons who used similar techniques developed in our unit. Laparoscopy was performed by inserting a 10-mm umbilical trocar and three 5-mm trocars in the lower abdomen, and included inspecting the pelvic and peritoneal organs, staging the endometriosis in accordance with the revised American Society for Reproductive Medicine classification, and adhesiolysis to fully release the ovaries from the surrounding structures. In addition, electrocision of peritoneal endometriotic lesions was performed using a monopolar instrument.
Laparscopic endometrioma cystectomy in group 1: First, diluted vasopressin was injected into the space between the ovarian tissue and the cyst wall to facilitate dissecting the cyst wall of the ovarian stroma. Then, the endometrioma was punctured to drain the cyst contents. Further irrigation and extension of the incision facilitated meticulous inspection of the internal wall to exclude suspicious areas. After identifying a clear cleavage plane, the ovarian cyst wall was stripped carefully from the adjacent normal ovarian tissue. The surgeon was able to completely remove the endometrioma with minimal bleeding, resulting in minimal damage to the ovary and no use of cautery if the correct plane was distinguished. Hemostasis was achieved with as few applications as possible using the tip of a bipolar forceps. The specimens were then removed from the abdomen in a specimen bag. The pelvic cavity was copiously irrigated with physiological saline solution and then suctioned (Fig. 1).
Figure 1.
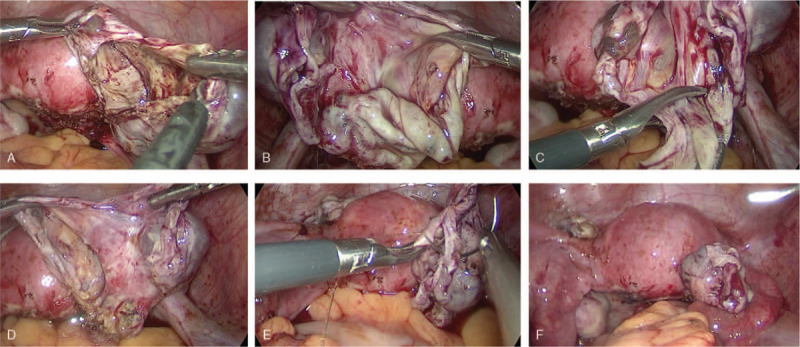
Laparoscopic endometrioma cystectomy. (A) The endometrioma is opened. (B) Identifying a clear cleavage plane between the endometrioma wall and the ovarian cortex. (C) The ovarian cyst wall is stripped carefully from the adjacent normal ovarian tissue. (D) Removing the endometrioma with minimal bleeding resulting in minimal damage to the ovary. (E) Suturing and closing the ovarian incision. (F) Final pelvic view, and removing the specimen from the abdomen in a specimen bag.
Laparoscopic endometrioma drainage and ablation with bipolar coagulation at low power in group 2: After drainage, irrigation, and inspection of the internal wall, a 1-cm × 1-cm biopsy of the cyst wall was obtained and sent for histological examination to confirm the diagnosis. Subsequently, we used the tip of a bipolar forceps to sweep the whole internal wall meticulously and sequentially at 30 W until the color of the cyst changed to yellowish-white. This was performed without damaging the adjacent ovarian tissue, and the ovary was cooled frequently with irrigation fluid. The average duration of contact between the forceps and the lesion was approximately 1 second. When mild redness of the internal wall returned shortly after ablation, the appropriate depth of endometrioma destruction was achieved. All laparoscopic operations were performed by the first author who had diverse experience performing both techniques (Fig. 2).
Figure 2.
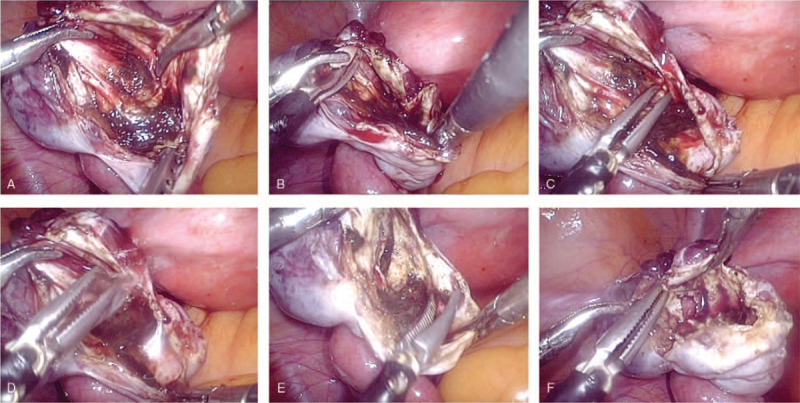
Laparoscopic endometrioma drainage and ablation with bipolar coagulation. (A) Drainage, irrigation, and inspection of the internal wall. (B) A 1-cm × 1-cm biopsy of the cyst wall is obtained for histological examination. (C) Coagulating the cyst lining systematically using bipolar forceps at 30 W. (D) The ovary is cooled frequently using irrigation fluid. (E) Coagulating the whole internal wall with very short-duration ablation times until the color of the cyst wall changes to yellowish-white without damaging the adjacent ovarian tissue. (F) When mild redness of the internal wall returns shortly after ablation, the appropriate depth of destruction of the endometrioma has been achieved.
2.3. Postoperative follow-up
In all patients, AMH was assayed during a spontaneous cycle before and 3 to 6 months after surgery, except for 1 patient who conceived within 3 months postoperatively. Premature ovarian insufficiency was diagnosed as amenorrhea or oligomenorrhea with baseline follicle-stimulating hormone >25 U/L at least twice or AMH <1.1 ng/ml.
In our practice, patients were followed with a gynecological examination and transvaginal pelvic ultrasonography 3, 6, 12, 18, and 24 months after operation, or earlier, if symptoms associated with possible recurrence were observed. Recurrence was defined as an endometrioma in the operated ovary observed by repeated transvaginal ultrasonography, or by subsequent surgery, such as cesarean section.
2.4. Statistical analysis
Data analysis was performed using SPSS 15.0 software (IBM Corp, Armonk, NY). The chi-square or Fisher exact test was used to compare categorical variables, and Student t test and the Wilcoxon–Mann–Whitney test were used to compare continuous variables. Results are presented as mean ± standard deviation. P < .05 was considered statistically significant.
3. Results
Group 1 constituted 46 patients who underwent laparoscopic cystectomy, and group 2 constituted 30 patients who underwent laparoscopic cyst wall ablation. The number of patients with infertility was 30 (65.22%) in group 1 and 22 (73.33%) in group 2 (P > .99). Patients’ demographic data, baseline clinical characteristics, and ultrasonographic findings for each group (Table 1) were comparable for age, body mass index, nulliparity, presenting symptoms, mean cyst diameter, and laterality. The baseline operative findings and follow-up results of each group are summarized in Table 2. The endometriotic stage according to the revised American Society for Reproductive Medicine classification, operative time, length of postoperative hospitalization, and follow-up time were similar between the groups. Conversions to laparotomy or perioperative complications were not observed in either group.
Table 1.
Baseline clinical characteristics and ultrasonographic findings of 2 groups of patients with ovarian endometriomas.
| Group 1 (n = 46) | Group 2 (n = 30) | P | |
| Age (yrs) (mean ±SD) | 28.65 ± 3.66 | 30.23 ± 3.94 | .66 |
| BMI (kg/m2) (mean ±SD) | 20.14 ± 2.25 | 21.17 ± 2.25 | .65 |
| Nulliparous | 34 (73.91%) | 21 (70.00%) | .80 |
| Infertility | 30 (65.22%) | 22 (73.33%) | .61 |
| Dysmenorrhea | 18 (39.13%) | 12 (40.00%) | >.99 |
| Chronic pelvic pain | 4 (8.70%) | 2 (6.67%) | >.99 |
| Diameter of endometrioma (cm) (mean ±SD) | 4.81 ± 1.48 | 3.95 ± 1.15 | .09 |
| Unilateral | 37 (80.43%) | 22 (73.33%) | .58 |
| Bilateral | 9 (19.57%) | 8 (26.67%) |
Table 2.
Surgical characteristics, follow-up results of 2 groups of patients with ovarian endometriomas.
| Group 1 (n = 46) | Group 2 (n = 30) | P | |
| Median rAFS score (mean ±SD) | 35.91 ± 20.02 | 43.93 ± 21.73 | .42 |
| Percentage of patients with stage III | (33/46) 71.74% | (18/30) 60.00% | .33 |
| Percentage of patients with stage IV | (13/46) 28.26% | (12/30) 40.00% | |
| Mean operating time (min) (mean ±SD) | 95.92 ± 31.03 | 91.61 ± 37.21 | .60 |
| Salpingectomy | 0 | 1 | .40 |
| Postoperative hospital stay (d) (mean ±SD) | 2.78 ± 0.85 | 2.76 ± 0.89 | .87 |
| Follow-up (m) (mean ±SD) | 30.40 ± 3.83 | 32.35 ± 4.14 | .54 |
| Preoperative AMH (ng/ml) (mean ±SD) | 4.25 ± 1.49 | 4.47 ± 1.56 | .65 |
| Postoperative AMH (ng/ml) (mean ±SD) | 3.40 ± 1.35 | 3.95 ± 1.79 | .04 |
| Decrease of AMH (ng/ml) (mean ±SD) | 0.85 ± 0.64 | 0.52 ± 0.58 | .04 |
| Diminished ovarian reserve | (1/46) 2.17% | (1/30) 3.33% | >.99 |
| Conception plan | 38 | 26 | .75 |
| Pregnancy rate | (27/38) 71.05% | (19/26) 73.08% | >.99 |
| Spontaneous | 16 | 10 | .77 |
| IVF | 11 | 9 | |
| Abortion | 1 | 3 | 0.29 |
| Ectopic pregnancy | 1 | 0 | >.99 |
| Time of recurrence (m) (25th–75th) | 17.00 ± 2.83 (15–19) | 12.20 ± 7.40 (3–22) | .23 |
| Diameter of recurrent cyst (cm) (mean ±SD) | 1.75 ± 0.35 | 1.54 ± 0.68 | .13 |
| Recurrence at 12 m | |||
| Per patient | (0/46) 0.00% | (2/30) 6.67% | .15 |
| Per endometrioma | (0/55) 0.00% | (2/38) 5.26% | .16 |
| Recurrence at 24 m | |||
| Per patient | (2/46) 4.35% | (5/30) 16.67% | .11 |
| Per endometrioma | (2/55) 3.64% | (5/38) 13.16% | .12 |
A significant difference regarding the decrease in AMH concentrations was observed in group 1 (from 4.25 ng/ml to 3.40 ng/ml) compared with group 2 (from 4.47 ng/mL to 3.95 ng/ml). Additionally, diminished ovarian reserve was observed in 1 of 46 patients in group 1 and 1 of 30 patients in group 2 (P > .99); however, premature ovarian insufficiency or premature ovarian failure was not observed in either group.
Of the 64 women with pregnancy intention, 38 were in group 1, and 26 were in group 2 (P = .75). The number of pregnancies was 27 in group 1 and 19 in group 2 (71.05% vs 73.08%, respectively; P > .99) with a mean follow-up of 30.40 months and 32.35 months, respectively. The number of spontaneous pregnancies was 16 in group 1 and 10 in group 2 (P = .77).
Two recurrences were observed in group 1 by transvaginal ultrasonography, whereas 5 were observed in group 2 by transvaginal ultrasonography or subsequent surgery. Although there was no statistically significant difference, the recurrence rate in group 1 was lower than that in group 2 (4.35% vs 16.67%, respectively; P = .11). The mean recurrence time was 17.00 months in group 1 and 12.20 months in group 2 (P = .23). The mean diameter of recurrent cysts was 1.75 cm in group 1 and 1.54 cm in group 2 (P = .13). Two recurrences in group 2 were found unexpectedly during cesarean section, and the cysts had a mean diameter of 1 cm; these were not detected by preoperative ultrasonography.
4. Discussion
4.1. Endometrioma cystectomy reduces ovarian function
Currently, the appropriate management of endometriomas remains a subject of debate and discussion.[2] Accumulating evidence indicates that endometrioma cystectomy reduces ovarian function, lowers the success rate of in vitro fertilization, and does not prevent recurrences in patients wishing to conceive.[2,4–8,14] Raffi et al conducted a meta-analysis to investigate the impact of surgery for endometriomas on ovarian reserve, and the report included 8 prospective cohort studies.[15] The results showed a negative impact of endometrioma excision on ovarian reserve as evidenced by a significant decrease in serum AMH concentration after cystectomy (weighted mean difference −1.13 ng/ml; 95% confidence interval: −0.37 to −1.88).
Due to the absence of a cleavage plane caused by endometriosis-induced fibrosis, stripping of the endometriotic cyst often leads to inadvertent removal of some surrounding ovarian cortex, especially when the lesion is near the hilus, which was confirmed by histological studies.[7,16] In addition, hemostasis of the ovarian tissue after excision requires using energy equipment, which has a negative impact on the ovarian blood supply, especially when the bleeding is close to the hilus.[6,17]
4.2. Endometrioma ablation minimizes ovarian function damage
Minimal loss of ovarian cortex was confirmed in patients undergoing endometrioma ablation.[18] Currently, cauterization can be achieved using bipolar instruments, CO2 lasers, and plasma energy devices. Bipolar instruments are widely used for hemostasis during laparoscopic surgery and have distinct thermal spreads depending on the power setting and application time. However, there are few studies reporting detailed information for bipolar coagulation in ablative surgery. Muzii et al conducted a multicenter RCT to compare the stripping technique and the combined excisional/ablative technique to treat bilateral ovarian endometriomas, in which ablative surgery was completed with bipolar coagulation at 30 to 40 W.[9] However, the study included no description of duration.
Hefermehl et al found that bipolar instruments exhibited a mean critical thermal spread in the musculofascial tissues ranging from 1.5 (0.8) to 2.1 (1.2) mm at 30 W and 60 W for 1 second, respectively.[19] Because the thickness of ectopic epithelium and stroma is usually thinner than 1.0 to 1.5 mm, it is suggested that only the internal layer with a thickness not exceeding 1.5 mm should be cauterized.[20] In our practice, ablative surgery was performed at 30 W for 1 second, which may not destroy the adjacent normal ovarian tissue.
Although there was no statistical significance, the median revised American Fertility Society (rAFS) score in group 2 was higher than that in group 1, which reflected the more serious adhesions in group 2. Adhesiolysis can injure the ovarian cortex and has a negative impact on ovarian reserve. In the present study, the adhesions in group 2 were more severe, but the decrease in AMH concentration was less that than in group 1. This result indicates that the ovarian reserve was impacted significantly less after ablation of the cyst wall by bipolar coagulation at low power, as reflected by the decrease in AMH (0.85 ng/ml vs 0.52 ng/ml, group 1 vs group 2, respectively; P = .04). Our favorable result could be explained as follows: the removal of ovarian tissue was avoided, and thermal damage was minimized.
The postoperative pregnancy rate varies and is related to several factors, such as ovarian function, endometriotic stage, and follow-up period. In our study, there was no significant difference in age, cyst diameter, endometriotic stage, and rAFS score between the 2 groups. In a prospective study comparing patients who underwent cystectomy and cyst ablation with plasma energy, comparable pregnancy rates of 69.3% and 61.3%, respectively, were found 24 months after surgery.[21] In the present study, the pregnancy rates in the 2 groups were similar and over 70%, which was reassuring.
4.3. Recurrence of endometriomas
The discrepancy in recurrence rates between the rates in our study and those in the literature could be attributed to several factors, such as different criteria for defining recurrence, different stages, different surgeon skill levels, and different follow-up intervals. Generally, endometrioma recurrence rates vary between 10% and 50% 2 to 5 years after operation.[22,23] In our study, the recurrence rate was 16.67%, and the mean diameter of recurrent cysts was 1.54 cm after 2 years of follow-up after ablative surgery, which seems acceptable for moderately to severely affected patients. Transvaginal ultrasonography is an effective technique for confirming or excluding the diagnosis of endometriomas when lesions are typical. However, it should be noted that 2 (2/5) recurrences after ablative surgery were found unexpectedly in this study during subsequent cesarean section; cysts had a mean diameter of 1 cm. Therefore, the real recurrence rates in the 2 groups require further evaluation.
4.4. Limitations
The limitations of our study are its retrospective design, small sample size, and short follow-up time. Therefore, long-term follow-up studies with larger sample sizes are warranted to prospectively analyze the postoperative results in patients treated using different surgical techniques.
5. Conclusions
In summary, our study indicated that appropriate laparoscopic electrocautery of the endometrioma wall with a bipolar instrument may be a valid alternative for traditional laparoscopic cystectomy, with less effects on ovarian reserve, especially in patients with pregnancy intention.
Acknowledgments
We thank Jane Charbonneau, DVM, from Liwen Bianji, Edanz Group China, for editing the English text of a draft of this manuscript.
Author contributions
Conceptualization: Dong Huang, Songying Zhang.
Data curation: Dong Huang, Jiaren Zhang, Jing Li.
Formal analysis: Jiaren Zhang, Libing Shi.
Investigation: Jianmin Chen, Libing Shi.
Supervision: Songying Zhang.
Writing – original draft: Jianmin Chen.
Writing – review & editing: Jianmin Chen, Songying Zhang.
Footnotes
Abbreviations: AMH = anti-Müllerian hormone, CO2= carbon dioxide, rAFS = revised American Fertility Society, RCT = randomized controlled trial.
How to cite this article: Chen J, Huang D, Zhang J, Shi L, Li J, Zhang S. The effect of laparoscopic excisional and ablative surgery on ovarian reserve in patients with endometriomas: a retrospective study. Medicine. 2021;100:7(e24362).
JC and DH contributed equally to this work.
This study was supported by the National Natural Science Foundation of China (81671435 and 81871135), Zhejiang Provincial Natural Science Foundation of China (LQ19H040014), and the Medical Science and Technology Project Foundation of Zhejiang Province (2018KY465, 2018KY109, and 2018KY486).
The authors have no conflicts of interest to disclose.
The data analyzed in the present study are available at the Assisted Reproduction Unit, Department of Obstetrics and Gynecology, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine.
The datasets generated during and/or analyzed during the current study are publicly available.
Values are n, (%), unless otherwise defined. P < .05 indicates a significant difference.
BMI = body mass index, SD = standard deviation.
Values are n, or n/N, (%), unless otherwise defined. P < .05 indicates a significant difference.
AMH = anti-Müllerian hormone, IVF = in vitro fertilization, rAFS = revised American Fertility Society, SD = standard deviation.
References
- [1].Nisolle M, Donnez J. Peritoneal endometriosis, ovarian endometriosis and adenomyotic nodules of the rectovaginal septum are three distinct entities. Fertil Steril 1997;68:585–96. [DOI] [PubMed] [Google Scholar]
- [2].Kaponis A, Taniguchi F, Azuma Y, et al. Current treatment of endometrioma. Obstet Gynecol Surv 2015;70:183–95. [DOI] [PubMed] [Google Scholar]
- [3].Hart RJ, Hickey M, Maouris P, et al. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev 2008;2:1–31. [DOI] [PubMed] [Google Scholar]
- [4].Hachisuga T, Kawarabayashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod 2002;17:432–5. [DOI] [PubMed] [Google Scholar]
- [5].Somigliana E, Ragni G, Benedetti F, et al. Does laparoscopic excision of endometriotic ovarian cysts significantly affect ovarian reserve? Insights from IVF cycles. Hum Reprod 2003;18:2450–3. [DOI] [PubMed] [Google Scholar]
- [6].Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol 2004;191:68–72. [DOI] [PubMed] [Google Scholar]
- [7].Muzii L, Bellati F, Bianchi A, et al. Laparoscopic stripping of endometriomas: a randomized trial on different surgical techniques. Part II. Pathological results Hum Reprod 2005;20:1987–92. [DOI] [PubMed] [Google Scholar]
- [8].Busacca M, Vignali M. Endometrioma excision and ovarian reserve: a dangerous relation. J Minim Invasive Gynecol 2009;16:142–8. [DOI] [PubMed] [Google Scholar]
- [9].Muzii L, Achilli C, Bergamini V, et al. Comparison between the stripping technique and the combined excisional/ablative technique for the treatment of bilateral ovarian endometriomas: a multicentre RCT. Hum Reprod 2016;31:339–44. [DOI] [PubMed] [Google Scholar]
- [10].Roman H, Pura I, Tarta O, et al. Vaporization of ovarian endometrioma using plasma energy: histological findings of a pilot study. Fertil Steril 2011;95:1853–6. [DOI] [PubMed] [Google Scholar]
- [11].Roman H, Auber M, Mokdad C, et al. Ovarian endometrioma ablation using plasma energy versus cystectomy: a step toward better preservation of the ovarian parenchyma in women wishing to conceive. Fertil Steril 2011;96:1396–400. [DOI] [PubMed] [Google Scholar]
- [12].Roman H, Auber M, Bourdel N, et al. Postoperative recurrences and fertility after endometrioma ablation using plasma energy: retrospective assessment of a 3-year experience. J Minim Invasive Gynecol 2013;20:573–82. [DOI] [PubMed] [Google Scholar]
- [13].Mircea O, Puscasiu L, Resch B, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: A multicentric comparative study. J Minim Invasive Gynecol 2016;23:1138–45. [DOI] [PubMed] [Google Scholar]
- [14].Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometrioma recurrence: a randomized controlled trial. Fertil Steril 2010;93:52–6. [DOI] [PubMed] [Google Scholar]
- [15].Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab 2012;97:3146–54. [DOI] [PubMed] [Google Scholar]
- [16].Muzii L, Bellati F, Palaia I, et al. Laparoscopic stripping of endometriomas: a randomized trial on different surgical techniques. Part I: clinical results. Hum Reprod 2005;20: 1981-1186. [DOI] [PubMed] [Google Scholar]
- [17].Reich H, Abrao MS. Post-surgical ovarian failure after laparoscopic excision of bilateral endometriomas: is this rare problem preventable? Am J Obstet Gynecol 2006;195:339–40. [DOI] [PubMed] [Google Scholar]
- [18].Wyns C, Donnez J. Laser vaporization of ovarian endometriomas: the impact on the response to gonadotrophin stimulation. Gynecol Obstet Fertil 2003;32:337–42. [DOI] [PubMed] [Google Scholar]
- [19].Hefermehl LJ, Largo RA, Hermanns T, et al. Lateral temperature spread of monopolar, bipolar and ultrasonic instruments for robot-assisted laparoscopic surgery. BJU Int 2014;114:245–52. [DOI] [PubMed] [Google Scholar]
- [20].Donnez J, Wyns C, Nissole M. Does ovarian surgery for endometriomas impair the ovarian response to gonadotropin? Fertil Steril 2001;76:662–5. [DOI] [PubMed] [Google Scholar]
- [21].Mircea O, Puscasiu L, Resch BP, et al. Fertility outcomes after ablation using plasma energy versus cystectomy in infertile women with ovarian endometrioma: a multicentric comparative study. J Minim Invasive Gynecol 2016;23:1138–45. [DOI] [PubMed] [Google Scholar]
- [22].Vercellini P, Somigliana E, Vigano P, et al. Post-operative endometriosis recurrence: a plea for prevention based on pathogenetic, epidemiological and clinical evidence. Reprod Biomed Online 2010;21:259–65. [DOI] [PubMed] [Google Scholar]
- [23].Porpora MG, Pallante D, Ferro A, et al. Pain and ovarian endometrioma recurrence after laparoscopic treatment of endometriosis: a long-term prospective study. Fertil Steril 2010;93:716–21. [DOI] [PubMed] [Google Scholar]


