Abstract
Fine crackles are frequently heard in patients with interstitial lung diseases (ILDs) and are known as the sensitive indicator for ILDs, although the objective method for analyzing respiratory sounds including fine crackles is not clinically available. We have previously developed a machine-learning-based algorithm which can promptly analyze and quantify the respiratory sounds including fine crackles. In the present proof-of-concept study, we assessed the usefulness of fine crackles quantified by this algorithm in the diagnosis of ILDs.
We evaluated the fine crackles quantitative values (FCQVs) in 60 participants who underwent high-resolution computed tomography (HRCT) and chest X-ray in our hospital. Right and left lung fields were evaluated separately.
In sixty-seven lung fields with ILDs in HRCT, the mean FCQVs (0.121 ± 0.090) were significantly higher than those in the lung fields without ILDs (0.032 ± 0.023, P < .001). Among those with ILDs in HRCT, the mean FCQVs were significantly higher in those with idiopathic pulmonary fibrosis than in those with other types of ILDs (P = .002). In addition, the increased mean FCQV was associated with the presence of traction bronchiectasis (P = .003) and honeycombing (P = .004) in HRCT. Furthermore, in discriminating ILDs in HRCT, an FCQV-based determination of the presence or absence of fine crackles indicated a higher sensitivity compared to a chest X-ray-based determination of the presence or absence of ILDs.
We herein report that the machine-learning-based quantification of fine crackles can predict the HRCT findings of lung fibrosis and can support the prompt and sensitive diagnosis of ILDs.
Keywords: auscultation, fine crackles, machine learning, pulmonary fibrosis, stethoscopes
1. Introduction
Auscultation of respiratory sounds is a basic physical examination technique originating from the Hippocrates era. Among the various respiratory sounds that can be detected through auscultation, fine crackles are short and explosive sounds heard during mid-to-late inspiration and are frequently recognized through the auscultation for patients with interstitial lung diseases (ILDs).[1] The clinical utility of detecting fine crackles have been well discussed and the following findings have been reported: Fine crackles were heard in 60% of patients with interstitial pneumonia[2]; they are more sensitive than chest X-ray for detecting patients with mild interstitial pneumonia[2]; usual interstitial pneumonia patterns in high-resolution computed tomography (HRCT) were associated with fine crackles.[3,4]
Auscultation is completely non-invasive, less expensive, and much easier to perform repeatedly, as compared to all other various examination techniques including blood testing, HRCT, and pulmonary function tests (PFTs). However, the assessment of respiratory sounds has been performed subjectively, namely physicians’ determination, owing to the absence of a popularized objective analyzing method. Therefore, if we can quantify respiratory sounds promptly, it should be a convenient and useful indicator for respiratory diseases.
Recently, machine-learning-based analysis has gradually penetrated various medical settings including radiologic diagnosis, endoscopic examination, and histopathological diagnosis. Additionally, also in the field of respiratory sound analysis, machine-learning techniques are gradually being applied.[5,6] We have previously developed a machine-learning-based algorithm which can promptly analyze the respiratory sounds to quantify fine crackles, coarse crackles, wheezes, and rhonchi.[7]
Using this algorithm, we conducted a proof-of-concept study to investigate the usefulness of machine-learning-based quantification of fine crackles in the diagnosis of ILDs and to assess its sensitivity or specificity in comparison with chest X-ray.
2. Material and methods
2.1. Developing the analyzing algorithm using machine-learning methods
Supplementary Figure 1 shows the schematic diagram of the machine-learning process. Three professional pulmonologists independently listened to the respiratory sound data and labeled them as “normal,” “fine crackles,” “coarse crackles,” “wheezes” or “rhonchi.” The sound data which was labeled identically by all 3 pulmonologists were adopted either as the training dataset or as the validation dataset. The training dataset, which includes 55, 50, 26, 38, and 36 sound data labeled as normal, fine crackles, coarse crackles, wheezes, and rhonchi, respectively, was employed in the supervised machine-learning procedure to develop the polynomials for calculating the respiratory sound quantitative parameters. The polynomials consist of a hundred or more of feature quantities extracted through various analysis process including frequency analysis, local variance analysis, cepstrum analysis and liftering process. The type of feature quantities as well as their appropriate coefficients were determined through machine-learning. Subsequently, the polynomials’ performance was validated using the validation dataset including 30, 39, 31, 50 and 33 data labeled as normal, fine crackles, coarse crackles, wheezes, and rhonchi, respectively. Using these polynomials, “fine crackles quantitative value (FCQV),” “coarse crackles quantitative value (CCQV),” “wheezes quantitative value (WHQV),” and “rhonchi quantitative value (RHQV)” were calculated. Based on these quantitative values, the presence or absence of each respiratory sound was promptly judged.
2.2. Participants
Between May and August 2018, we recruited 60 patients who underwent chest X-ray and HRCT in our hospital for the evaluation of pulmonary diseases. Patients with apparent parenchymal lung diseases other than ILDs, for example bacterial pneumonia, were excluded. Differential diagnosis of ILD was made in accordance with the relevant guidelines.[8–11] This study was approved by the Ethics Committee of Hiroshima University Hospital (approval number E-784) and conducted in accordance with the ethical standards established in the Helsinki Declaration of 1975. All participants’ consent for study participation was obtained through the opt-out method.
2.3. HRCT and chest X-ray
The HRCTs, chest X-rays, and respiratory sounds of all participants were evaluated for right and left lung field, separately. For each lung field, two or more trained radiologists interpreted the findings in HRCT and determined the presence or absence of “ILD in HRCT”, which was defined as the diffuse distribution of linear and reticular shadows including some of the followings; irregular interface sign, bronchovascular bundle thickening, traction bronchiectasis, interlobular septal thickening, intralobular interstitial thickening, and honeycombing. Meanwhile, chest X-rays were interpreted by two trained pulmonologists who were blind to the patients’ clinical information and they independently determined the presence or absence of “ILD in X-ray” for each lung field. In case of a discordance, the third pulmonologist made the final determination.
2.4. Recording and analyzing the respiratory sounds
Participants were instructed to keep sitting position and breathe deeply. As the analyzing algorithm cannot eliminate background noise automatically, we repeated the recording procedure again in case of an interference by external sound such as someone else's voice to minimize the influence of background noise. Auscultations were performed by pulmonologists with five or more years-experiences at six points covering the basal part and the auscultation triangle at the back of each participant (Supplementary Figure 2a), and the respiratory sound data recorded by using the dedicated electronic stethoscopes developed by Pioneer Corp. (Tokyo, Japan) were simultaneously transferred by Bluetooth to the analyzing software running on tablet computers (Supplementary Figure 2b). The analyzing software outputs the FCQV, CCQV, WHQV, RHQV and the presence or absence of each four type of respiratory sound based on the algorithm described above. The means of the FCQVs, CCQVs, WHQVs, and RHQVs obtained from unilateral three auscultation points were deemed as the representative parameters of each type of respiratory sound for each lung field. In addition, if fine crackles were determined to be present by the analyzing software in at least one of three auscultation points, they were deemed to be present in that lung field. To evaluate the correlations between the FCQVs and the results of PFTs, the representative FCQVs for each participant with ILD in HRCT were calculated by dividing the sum of mean FCQVs in the bilateral lung fields by two.
2.5. Measuring the pulmonary function
For patients with ILDs, the results from PFTs including forced vital capacity (FVC), forced expiratory volume in one second, and diffusion capacity for carbon monoxide were collected from their medical records. Each PFT procedure was performed by specialized technicians in accordance with the recommendations of the American Thoracic Society.[12]
2.6. Statistical analysis
All statistical analyses were performed using SPSS for Windows, version 18.0 (SPSS Inc., Chicago, IL). To determine the sample size, we estimated the standard deviation of FCQVs as 0.10 and also estimated the difference in mean FCQVs between those with and without ILDs as 0.10, based on the distribution of FCQVs in the validation dataset used in the developing process of the analyzing algorithm. As a result, the minimum sample size was calculated as 16 for each group. To recruite more than 16 patients with ILDs in HRCT, we determined the recruitment period as 4 months. Numerical values were presented as mean ± standard deviation. The difference between two groups was tested by the Mann–Whitney U test, and that among three groups was tested by the Kruskal–Wallis test followed by multiple comparisons with the Bonferroni correction. To test the utility of FCQV in discriminating the lung fields with ILDs from those without, the receiver operating characteristic (ROC) analysis was performed. To investigate the associations between binary dependent variable and independent variables, logistic regression analyses were performed. To investigate the correlations between two variables, Pearson's correlation tests or Chi-square tests were used as appropriate. Except in the case of Bonferroni correction, P < .05 was considered to be statistically significant.
3. Results
3.1. FCQVs are useful for discriminating lung fields with ILDs
The characteristics of all participants are shown in Table 1. Among 120 lung fields of 60 participants, ILD in HRCT was present in 67 lung fields of 34 participants (bilaterally in 33 participants and unilaterally in 1). In Table 1, one participant in whom ILD in HRCT was present only in the left lung field was included in those with ILD in HRCT. The body mass index and smoking status were similar between those with ILD in HRCT and those without, although those with ILD in HRCT tended to be older and more male dominant. Among those with ILD in HRCT, 12 patients were with collagen vascular disease related ILD, and 10 were with idiopathic interstitial pneumonia (IIPs). As shown in Figure 1A, the mean FCQVs, CCQVs and WHQVs in the lung fields with ILD in HRCT were significantly higher than those in the lung fields without. However, the ROC analysis demonstrated that the area under the curve (AUC) of FCQV for discriminating the lung fields with ILD in HRCT from those without was sufficiently high (Fig. 1B), whereas the AUC of CCQV and WHQV were lower than the acceptable levels; furthermore, the AUC of RHQV was lower than the level of significance.
Table 1.
Characteristics of the subjects.
| ILD in HRCT | |||
| Total | Yes∗ | No | |
| Number of subjects | 60 | 34 | 26 |
| Age (yr) | 68.9 ± 13.0 | 72.2 ± 11.4 | 64.5 ± 13.5 |
| Gender (male/female) | 39 / 21 | 24 / 10 | 15 / 11 |
| BMI | 22.1 ± 4.0 | 21.8 ± 3.5 | 22.5 ± 4.6 |
| Smoking (Yes / No) | 36 / 24 | 21 / 13 | 15 / 11 |
| FVC (percent predicted) | 88.1 ± 21.6 | NA | |
| DLco (percent predicted) | 57.2 ± 20.2 | NA | |
| Subjects with ILD | 34 | 34 | 0 |
| CVD-ILD | 12 | 12 | 0 |
| IIPs | 10 | 10 | 0 |
| HP | 4 | 4 | 0 |
| Other ILD | 8 | 8 | 0 |
| Subjects without ILD | 26 | 0 | 26 |
| COPD or asthma | 8 | 0 | 8 |
| Lung tumor | 7 | 0 | 7 |
| Lung nodule | 5 | 0 | 5 |
| Other | 6 | 0 | 6 |
Figure 1.
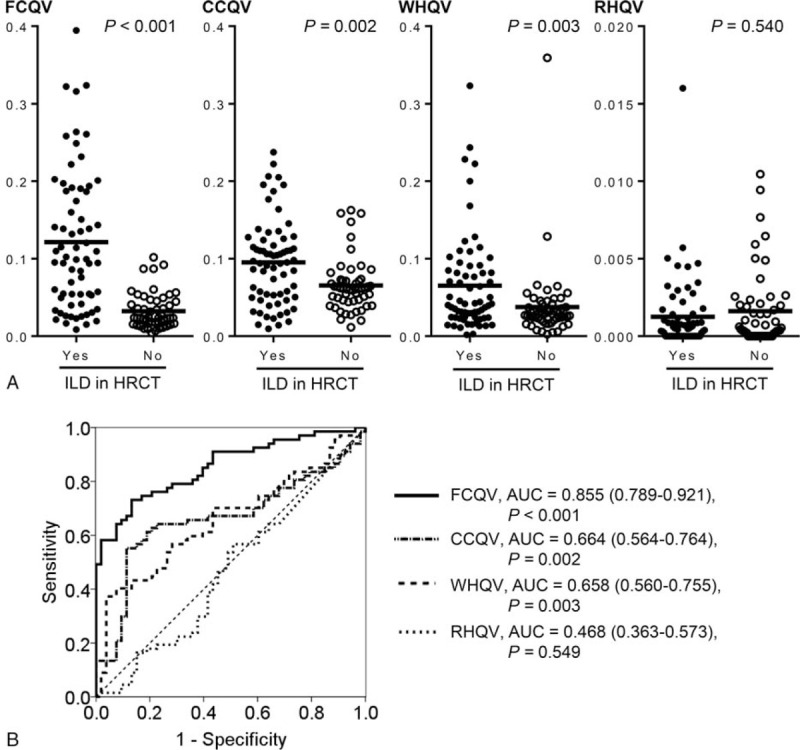
Comparisons of the four types of quantitative parameters for respiratory sounds between the lung fields with and without ILD in HRCT. (A) Mean FCQVs in the lung fields with and without ILD in HRCT were 0.121 ± 0.090 and 0.032 ± 0.023, respectively. Mean CCQVs in the lung fields with and without ILD in HRCT were 0.095 ± 0.055 and 0.065 ± 0.034, respectively. Mean WHQVs in the lung fields with and without ILD in HRCT were 0.065 ± 0.063 and 0.038 ± 0.049, respectively. Mean RHQVs in the lung fields with and without ILD in HRCT were 0.013 ± 0.0023 and 0.0016 ± 0.025, respectively. (B) AUC of ROC curve for FCQV, CCQV, WHQV, and RHQV were 0.855 (95% CI. = 0.789–0.921), 0.664 (95% CI. = 0.564–0.764), 0.658 (95% CI. = 0.560–0.755) and 0.468 (95% CI. = 0.363–0.573), respectively. AUC = area under the curve, CCQV = coarse crackles quantitative value, CI = confidence interval, FCQV = fine crackles quantitative value, RHQV = rhonchi quantitative value, receiver operating characteristic, WHQV = wheezes quantitative value.
3.2. Associations between FCQVs and ILD in HRCT are statistically independent
As shown in Supplementary Figures 3a, b and c, neither age, body mass index nor smoking status indicated significant correlations with the FCQVs. Although there seem to be a weak correlation between age and FCQVs (Supplementary Figures 3A), when we stratify the subjects according to the presence or absence of ILD in HRCT, no significant correlation between age and FCQVs was observed (P = .788 and P = .455, for those with and without ILD in HRCT, respectively). Additionally, in the lung fields with ILD in HRCT, the presence or absence of emphysema, defined as the areas of abnormally low attenuation delimited by a very thin or no wall, was investigated. We found no significant difference in mean FCQVs among those with emphysema versus those without, although emphysema have been reported to decrease transmission of respiratory sounds (Supplementary Figure 3d).[13] Furthermore, logistic regression analysis confirmed that the associations between mean FCQVs and ILD in HRCT were statistically independent from other potential confounding factors (Table 2).
Table 2.
Logistic regression analysis of the relative odds of interstitial lung disease.
| Odds ratio | 95% CI. | P value | |
| Univariate models | |||
| Age, numerical | 1.05 | 1.01–1.08 | .005 |
| BMI, numerical | 0.959 | 0.875–1.05 | .377 |
| Smoking, yes | 1.288 | 0.618–2.69 | .500 |
| FCQV, numerical | 6.14 × 1015 | 1.53 × 10^9–2.46 × 1022 | <.001 |
| Multivariate model | |||
| Age, numerical | 1.04 | 0.994–1.08 | .089 |
| BMI, numerical | 0.953 | 0.837–1.09 | .470 |
| Smoking, yes | 0.929 | 0.353–2.45 | .881 |
| FCQV, numerical | 3.30 × 1015 | 4.60 × 108–2.36 × 1022 | <.001 |
3.3. FCQVs correlate with the fibrosis-related findings in HRCT
Among the lung fields with ILD in HRCT, the mean FCQVs were significantly higher in the lung fields with IIPs than in those with other ILDs (Fig. 2A). Furthermore, among those with IIPs, the mean FCQVs were significantly higher in those with idiopathic pulmonary fibrosis (IPF) than in those with other IIPs (Fig. 2B). In addition, the presence of traction bronchiectasis or honeycombing was associated with significantly high mean FCQVs (Fig. 2C and D). In this context, the mean FCQVs were the highest in the lung fields with both traction bronchiectasis and honeycombing, and the lowest in those with neither (Fig. 2E). The difference between these two groups was statistically significant even after the Bonferroni's correction (Fig. 2E). To evaluate the correlations between the FCQVs and the results of PFTs, the representative FCQVs for each participant with ILD in HRCT were calculated by dividing the sum of mean FCQVs in the bilateral lung fields by 2. Consequently, higher representative FCQV significantly correlated with a lower FVC, although no significant correlation was indicated between the FCQV and forced expiratory volume in 1 second or diffusion capacity for carbon monoxide (Fig. 3).
Figure 2.
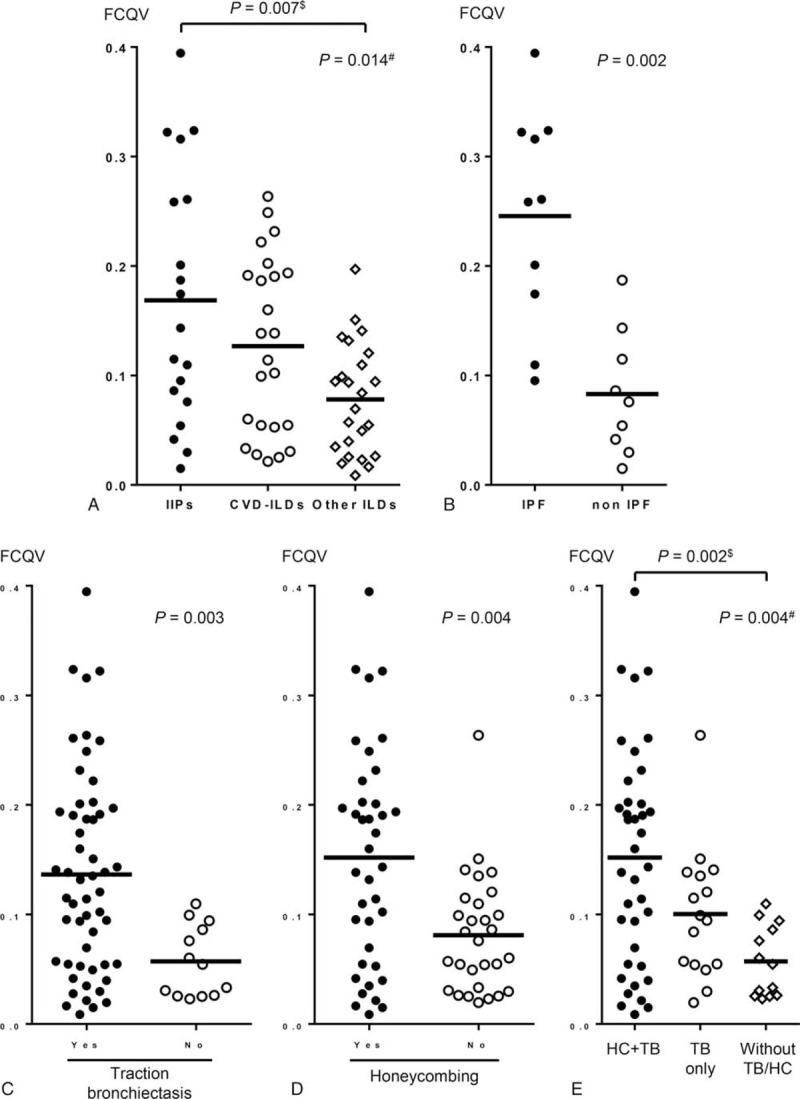
FCQVs were associated with the differential diagnosis of ILDs and with the fibrotic changes in HRCT. (A) Among the lung fields with ILD in HRCT, mean FCQVs in those with IIPs, CVD-ILDs and other ILDs were 0.169 ± 0.026, 0.127 ± 0.016 and 0.078 ± 0.010, respectively. Multiple comparisons with Bonferroni correction (significance level was set at P = .017) revealed the difference in FCQVs between those with IIPs and other ILDs was significant. (B) Among the lung fields with IIPs, mean FCQVs in those with IPF and with other IIPs were 0.246 ± 0.031 and 0.083 ± 0.019, respectively. (C) Mean FCQVs in the lung fields with and without TB were 0.137 ± 0.093 and 0.057 ± 0.032, respectively. (D) Mean FCQVs in the lung fields with and without HC were 0.152 ± 0.100 and 0.081 ± 0.054, respectively. (E) Mean FCQVs in the lung fields with both TB and HC, in those with TB only and in those with neither were 0.152 ± 0.100, 0.100 ± 0.060 and 0.057 ± 0.032, respectively. Multiple comparisons with Bonferroni correction (significance level was set at P = .017) revealed the difference in FCQVs between the lung fields with both TB and HC and those with neither was significant. In (A) and (E), P value with # was calculated by Kruskal-Wallis test, and that with $ was calculated by Mann-Whitney U test. CVD-ILD = collagen vascular disease related interstitial lung disease, FCQV = fine crackles quantitative value, HC = honeycombing, HRCT = high-resolution computed tomography, IIPs = idiopathic interstitial pneumonia, ILD = interstitial lung disease, IPF = idiopathic pulmonary fibrosis, TB = traction bronchiectasis.
Figure 3.
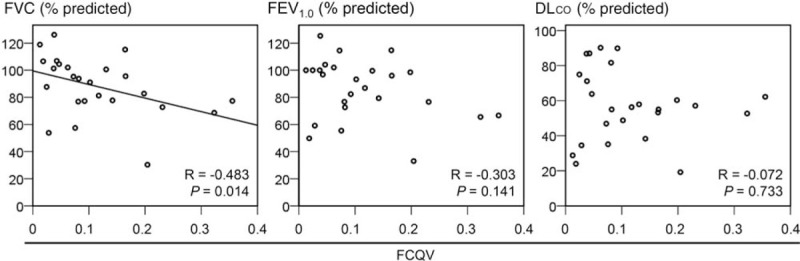
Correlations between mean FCQVs and pulmonary functions. DLCO = diffusion capacity for carbon monoxide, FCQV = fine crackles quantitative value, FEV1.0 = forced expiratory volume in one second, FVC = forced vital capacity.
3.4. Fine crackles demonstrated higher sensitivity for discriminating ILDs in HRCT than chest X-ray
Next, we compare the abilities of discriminating ILDs in HRCT based on the following two parameters: first, presence or absence of fine crackles determined by the analyzing software; second, ILD in X-ray determined by pulmonologists. Based on the threshold value derived from the ROC analysis of the FCQV, the analyzing software determined 65 lung fields as having fine crackles, whereas 55 lung fields as not. Meanwhile, the pulmonologists determined that ILD in X-ray was evident in 42 lung fields, whereas not in 78 lung fields (Table 3). Both fine crackles determined by the analyzing software and ILD in X-ray determined by pulmonologists demonstrated significant correlations with the presence or absence of ILD in HRCT (Table 3).
Table 3.
Cross-tabulation between fine crackles or X-ray and presence or absence of interstitial lung disease in high-resolution computed tomography.
| ILD in HRCT | ||||
| Yes | No | Total | P value | |
| With fine crackles | 51 | 14 | 65 | <.001 |
| Without fine crackles | 16 | 39 | 55 | |
| Total | 67 | 53 | 120 | |
| With ILD in X-ray | 42 | 0 | 42 | <.001 |
| Without ILD in X-ray | 25 | 53 | 78 | |
| Total | 67 | 53 | 120 | |
As shown in Table 3, the sensitivity of fine crackles was higher than that of chest X-ray in discriminating ILDs in HRCT, whereas the specificity of X-ray was higher than that of fine crackles. The diagnostic accuracy was almost similar for both the cases (Table 4).
Table 4.
Diagnostic accuracy for fine crackles and chest X-ray.
| fine crackles | X-ray | |
| Sensitivity | 0.761 | 0.627 |
| Specificity | 0.736 | 1.00 |
| PLR | 2.883 | ∞ |
| NLR | 0.325 | 0.373 |
| Accuracy | 0.750 | 0.792 |
Supplementary Figure 4 shows the chest X-rays and HRCT scans of three patients that were determined as exhibiting ILD in HRCT, and fine crackles were present according to the analyzing software, whereas not exhibiting ILD in X-ray. In Supplementary Figure 4a and b, interstitial abnormalities were present almost within the area lower than the diaphragm domes, and in Supplementary Figure 4c, pure ground-glass attenuation without reticulation or honeycombing were present. These relatively limited and/or mild interstitial abnormalities may cause a false negative in the chest X-rays.
4. Discussion
In this study, we demonstrated that the FCQV, calculated based on the machine-learning-based analyzing algorithm, can help discriminate the lung fields with ILD in HRCT. Especially, the high FCQV is significantly associated with the diagnosis of IPF, fibrosis-related findings in HRCT, namely traction bronchiectasis and/or honeycombing, and low FVC. Furthermore, the sensitivity of fine crackles for discriminating ILDs in HRCT was higher than that of chest X-ray, and the diagnostic accuracy of fine crackles was almost comparable to that of chest X-ray.
The most important finding of the present study is that the high FCQVs were significantly associated with the fibrosis-related findings in HRCT and the diagnosis of IPF. Usual interstitial pneumonia, pattern in HRCT was reported to be associated with fine crackles determined by physicians’ auscultations.[3,4] In addition, Fukumitsu and coworkers reported that the acoustic characteristics detected by a sound spectrometer can predict honeycombing in HRCT.[14] These previous reports are in concordance with our results. Crackles are considered to be produced when abnormally closed small airways are suddenly opened.[15] As the number of abnormally closed small airways may increase in a lung with advanced fibrosis, crackles may be heard more strongly and frequently, resulting in the high FCQV in the severely fibrotic lung.
Another important finding of the present study is that the sensitivity of fine crackles was higher than that of chest X-ray in discriminating ILDs in HRCT (Table 4). Epler and coworkers reported that 15 out of 37 patients in whom fine crackles were heard presented normal chest X-rays.[2] In agreement with their report, our findings may indicate that the quantification of fine crackles enables more sensitive discrimination of ILDs than chest X-rays. Meanwhile, chest X-rays presented excellently high specificity (Table 4). However, it is noteworthy that patients with apparent parenchymal lung diseases other than ILDs, including bacterial pneumonia, were excluded from this study and this selection bias may increase the specificity of chest X-ray. Based on these results, although the diagnostic capacity of the FCQV is promising, further investigations including patients with various kind of diseases are required.
In addition to the FCQV, the CCQV and WHQV were significantly higher in the lung fields with ILDs in HRCT than those in without ILD. We can speculate that the feature quantities for each of four types of respiratory sounds overlap in part, thus resulting in nonspecific mild elevations of the CCQV and WHQV in the lung fields with ILD in HRCT. Further investigations including patients with bacterial pneumonia and those with asthma or COPD are required to clarify whether the elevations of the CCQV and WHQV in the lung fields with ILD are truly nonspecific. In any case, it is important that only the FCQV indicated a sufficiently high AUC, that is, higher than 0.8, whereas the AUCs for both the CCQV and WHQV were lower than the acceptable level, that is, under 0.7.
In the present study, machine-learning-based analyzing algorithm could simultaneously quantify the different types of respiratory sounds and output the FCQV, CCQV, WHQV, and RHQV promptly after auscultation. Auscultation is non-invasive diagnostic procedure, but it requires well trained medical staff, and moreover, there could be an interobserver disagreement in the classification of respiratory sounds.[16] To overcome these problems of auscultation, several efforts to quantify fine crackles focusing on the waveforms and/or frequency structures of respiratory sounds have been reported.[17,18] However, only a limited number of sound parameters were investigated in most of these previous systems. For example, Munakata and coworkers reported that four parameters expressing waveforms and two expressing frequency structures were different between fine and coarse crackles.[17] Ono and coworkers reported that 2 parameters expressing frequency structures were associated with pulmonary functions.[18] In contrast, a hundred or more of feature quantities expressing the frequency structure and time series change characteristics are handled in our machine-learning-based algorithm. As a result, this algorithm can quantify the different types of respiratory sounds simultaneously and it can be especially useful in the screening of diseases or in telemedicine. Additional prospective study with larger sample sizes are required to confirm the results of this study.
Some limitations are present in this study. The most important limitation is that this is a single-center study with a limited number of selected subjects, and those with parenchymal lung diseases other than ILDs were excluded from this study. This selection bias may lead to an overestimation of discriminating ability of FCQVs. Thus, further investigations including patients with unselected various kind of diseases are required. Besides, combination with other methods to provide more accurate diagnosis is not tested. Therefore, additional utility of FCQVs to the conventional diagnostic examinations should be evaluated in the future study. Furthermore, the analyzing algorithm cannot eliminate background noise automatically. However, the significant correlations between FCQVs and the fibrosis-related findings in HRCT indicate that FCQVs, despite the background noise, can reflect the pathological changes in lung.
We believe that the present study, despite several limitations, is still important in that it demonstrated a potential usefulness of fine crackles, quantified through our machine-learning-based analyzing algorithm, as an indicator for ILDs.
5. Conclusions
In conclusion, we demonstrate that the fine crackles quantified by the machine-learning-based analyzing algorithm was associated with the progression of lung fibrosis and can be an important diagnostic indicator for ILDs. Our results suggest that machine-learning-based discrimination of respiratory sound could be a useful tool for screening and monitoring of interstitial pneumonitis, but further validation study in various clinical settings would be warranted.
Acknowledgments
We are grateful to Drs. Sachiko Shioya, Wakako Daido, and Kiyofumi Shimoji in our department for their support in recording the respiratory sounds. We also appreciate the doctors and staff of the Department of Diagnostic Radiology in our hospital for their contribution in radiological assessments. The electronic stethoscope and the analyzing software were developed by Pioneer Corp. (Tokyo, Japan).
Author contributions
Conceptualization: Yasushi Horimasu, Shinichiro Ohshimo, Hiroshi Iwamoto, Nobuaki Shime.
Data curation: Yasushi Horimasu, Kakuhiro Yamaguchi, Takeshi Masuda, Taku Nakashima, Shintaro Miyamoto, Kazunori Fujitaka, Hironobu Hamada.
Formal analysis: Yasushi Horimasu.
Funding acquisition: Shinichiro Ohshimo, Takuma Sadamori, Nobuaki Shime.
Investigation: Yasushi Horimasu, Kakuhiro Yamaguchi, Shinjiro Sakamoto, Takeshi Masuda, Taku Nakashima, Shintaro Miyamoto, Hiroshi Iwamoto, Hironobu Hamada.
Methodology: Yasushi Horimasu, Shinichiro Ohshimo, Hiroshi Iwamoto, Takuma Sadamori, Nobuaki Shime.
Project administration: Yasushi Horimasu, Shinichiro Ohshimo, Hiroshi Iwamoto, Noboru Hattori.
Resources: Shinichiro Ohshimo, Kakuhiro Yamaguchi, Shinjiro Sakamoto, Kazunori Fujitaka.
Software: Takuma Sadamori.
Supervision: Shinichiro Ohshimo, Hiroshi Iwamoto, Hironobu Hamada, Nobuaki Shime, Noboru Hattori.
Validation: Shinichiro Ohshimo, Kakuhiro Yamaguchi, Shinjiro Sakamoto, Takeshi Masuda, Taku Nakashima, Shintaro Miyamoto, Hiroshi Iwamoto, Kazunori Fujitaka, Hironobu Hamada, Takuma Sadamori, Noboru Hattori.
Writing – original draft: Yasushi Horimasu.
Writing – review and editing: Yasushi Horimasu, Shinichiro Ohshimo, Kakuhiro Yamaguchi, Shinjiro Sakamoto, Takeshi Masuda, Taku Nakashima, Shintaro Miyamoto, Hiroshi Iwamoto, Kazunori Fujitaka, Hironobu Hamada, Takuma Sadamori, Nobuaki Shime, Noboru Hattori.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CCQV = coarse crackles quantitative value, FCQV = fine crackles quantitative value, FVC = forced vital capacity, HRCT = high-resolution computed tomography, IIP = idiopathic interstitial pneumonia, ILD = interstitial lung disease, PFT = pulmonary function test, RHQV = rhonchi quantitative value, ROC = receiver operating characteristic, WHQV = wheezes quantitative value.
How to cite this article: Horimasu Y, Ohshimo S, Yamaguchi K, Sakamoto S, Masuda T, Nakashima T, Miyamoto S, Iwamoto H, Fujitaka K, Hamada H, Sadamori T, Shime N, Hattori N. A machine-learning based approach to quantify fine crackles in the diagnosis of interstitial pneumonia: a proof-of-concept study. Medicine. 2021;100:7(e24738).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
BMI = body mass index, CVD-ILD = collagen vascular disease related interstitial lung disease, DLco = diffusion capacity for carbon monoxide, FVC = forced vital capacity, HP = hypersensitivity pneumonia, HRCT = high-resolution computed tomography, IIPs = idiopathic interstitial pneumonia, ILD = interstitial lung disease, NA = not available.
Thirty-three participants had ILD in HRCT bilaterally and one participant had ILD in HRCT unilaterally only in left lung field.
BMI = body mass index, FCQV = fine crackles quantitative value.
HRCT = high-resolution computed tomography, ILD = interstitial lung disease.
FCQV = fine crackles quantitative value, NLR = negative likelihood ratio, PLR = positive likelihood ratio.
References
- [1].Bohadana A, Izbicki G, Kraman S. Fundamentals of lung auscultation. N Engl J Med 2014;370:744–51. [DOI] [PubMed] [Google Scholar]
- [2].Epler GR, Carrington CB, Gaensler EA. Crackles (rales) in the interstitial pulmonary diseases. Chest 2014;73:333–9. [DOI] [PubMed] [Google Scholar]
- [3].Sellarés J, Hernández-González F, Lucena C, et al. Auscultation of velcro crackles is associated with usual interstitial pneumonia. Medicine 2016;95:e2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sgalla G, Walsh S, Sverzellati N, et al. Velcro-type” crackles predict specific radiologic features of fibrotic interstitial lung disease. BMC Pulm Med 2018;18:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pramono RXA, Bowyer S, Rodriguez-Villegas E. Automatic adventitious respiratory sound analysis: a systematic review. PLoS One 2017;12:e0177926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Grzywalski T, Piecuch M, Szajek M, et al. Practical implementation of artificial intelligence algorithms in pulmonary auscultation examination. Eur J Pediatr 2019;178:883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ohshimo S, Sadamori T, Tanigawa K. Innovation in analysis of respiratory sounds. Ann Intern Med 2016;164:638–9. [DOI] [PubMed] [Google Scholar]
- [8].Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet 2012;380:689–98. [DOI] [PubMed] [Google Scholar]
- [10].Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976–87. [DOI] [PubMed] [Google Scholar]
- [11].Vasakova M, Morell F, Walsh S, et al. Hypersensitivity pneumonitis: perspectives in diagnosis and management. Am J Respir Crit Care Med 2017;196:680–9. [DOI] [PubMed] [Google Scholar]
- [12].American Thoracic Society. Standardization of Spirometry, 1994 Up-date. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- [13].Pasterkamp H, Kraman SS, Wodicka GR. Respiratory Sounds - Advances beyond the stethoscope. Am J Respir Crit Care Med 1997;156:974–87. [DOI] [PubMed] [Google Scholar]
- [14].Fukumitsu T, Obase Y, Ishimatsu Y, et al. The acoustic characteristics of fine crackles predict honeycombing on high-resolution computed tomography. BMC Pulm Med 2019;19:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vyshedskiy A, Alhashem R, Paciej R, et al. Mechanism of inspiratory and expiratory crackles. Chest 2009;135:156–64. [DOI] [PubMed] [Google Scholar]
- [16].Melbye H, Garcia-Marcos L, Brand P, et al. Wheezes, crackles and rhonchi: simplifying description of lung sounds increases the agreement on their classification: a study of 12 physicians’ classification of lung sounds from video recordings. BMJ Open Respir Res 2016;3:e000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Munakata M, Ukita H, Doi I, et al. Spectral and waveform characteristics of fine and coarse crackles. Thorax 1991;46:651–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ono H, Taniguchi Y, Shinoda K, et al. Evaluation of the usefulness of spectral analysis of inspiratory lung sounds recorded with phonopneumography in patients with interstitial pneumonia. J Nippon Med Sch 2009;76:67–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


