Abstract
Background:
To analyze the prevalence of latent infection of pathogens of hand, foot, and mouth disease (HFMD) in Chinese healthy population and its influencing factors, so as to provide reference for the prevention and control of HFMD.
Methods:
A systematic literature searching about the incidence of latent infection of HFMD was conducted in Chinese and English databases. The inclusion and exclusion criteria of the retrieved literature were established. The qualified literatures were screened and the data were extracted. The pooled rate and its 95% confidence interval was used to assess the latent infection rate of HFMD pathogens in healthy Chinese population, and subgroup analysis was conducted based on gender and age. All statistical analyses were performed using the STATA version 12.0 software.
Results:
A total of 31 literatures were included in this meta-analysis. The recessive infection rate of HFMD pathogens reported in the literature of Chinese healthy people ranged from 4.59% to 44.12%. The results of meta-analysis showed that the latent infection rate of human enteroviruses (HEVs) in healthy Chinese population was 17.5% (14.9–20.1%), among which, the latent infection rates of EV-A71, CV-A16, and other HEVs were 3.3% (2.2–4.4%), 1.7% (1.0–2.5%), and 15.1% (11.1–17.1%), respectively. The latent infection rates of HEVs in healthy men and women in China were 16.7% (12.9–20.4%) and 14.4% (10.8–18.0%), respectively. The latent infection rates of HEVs in the healthy population aged 0 to 5 years and over 5 years were 24.4% (20.4–28.5%) and 9.4% (6.5–12.2%), respectively. Meta regression showed that the factors affecting the latent infection rate of HEVs in Chinese healthy population included sampling period, sampling area, and study population.
Conclusion:
The latent infection rate of HEVs is high in healthy people in China, but it is mainly caused by other enteroviruses. The latent infection rate of HEVs in male was higher than that of female and was greater in people aged 0 to 5 than that of aged over 5 years. Limited by the quantity and quality of the included studies, more high-quality studies are needed for further verification in the future.
Keywords: foot and mouth disease, hand, human enterovirus, inapparent infection, meta-analysis
1. Introduction
Hand, foot, and mouth disease (HFMD) is a common acute infectious disease in children, mostly under 5 years of age, caused by several human enteroviruses (HEVs). The main clinical manifestations of HFMD are skin herpes of hand and foot and oral mucosal rash. In a few cases, there are sterile meningeal encephalitis, brainstem encephalitis, neurogenic pulmonary edema, and cardiac injury.[1] The pathogens that can cause HFMD clinically includes coxsackievirus type 2, 4, 5, 7, 9, and 10 in coxsackievirus group A, ECHO and enterovirus 71 (EV71) in group B, among which coxsackievirus group A (Cox A16) and EV71 are the most common.[2] Cox A16 was the main pathogen prevalent in the early stage of HFMD, but it did not attract wide attention due to its mild symptoms and small number of patients.[3] In recent years, in the outbreak or epidemic of Cox A16 and EV71 in mainland China and Taiwan, it has been found that coincident or alternating epidemic transmission of Cox A16 and EV71 is easy to cause severe disease or even death, and the infection rate of EV71 is increasing year by year.[4,5]
Humans are the only natural host of enterovirus, the infection sources of HFMD include patients, recessive infection.[6] HFMD virus was mainly transmitted by digestive tract (fecal–oral route) and respiratory tract (droplet, cough, sneeze). Hands, towels, toothbrushes, toys, bowls and chopsticks, milk products, and medical devices contaminated with human feces, herpes fluid, and respiratory secretions can also be spread. Among them, the contaminated hand is the key medium of transmission. Although it is not clear whether water and food are involved in transmission, studies have shown the potential for waterborne transmission of enterovirus if water is not treated effectively.[7] People are generally susceptible to enterovirus, especially infants and children.
HFMD is a public health problem that seriously affects children's health, and the number of cases is increasing year by year. At present, the related researches on HFMD mainly focus on the epidemic characteristics and pathogenesis characteristics of the diagnosed population, risk factors of severe cases, and other related aspects. However, the main transmission group of HFMD is the recessive infected person. Since most people show recessive status after human enteroviruses infection, it is difficult to be detected clinically, so it is difficult to carry out effective isolation measures, which may easily lead to further spread of the epidemic.
The investigation of the latent infection rate of enterovirus in healthy people is of great significance for the effective prevention of HFMD transmission among children, which is conducive to the understanding of the current situation of the latent infection group of HFMD. Therefore, meta-analysis was adopted in this study to comprehensively analyze the published literature on the recessive infection rate of HFMD pathogens, and to evaluate its influencing factors, so as to provide scientific basis for the prevention and control of HFMD in the future.
2. Materials and methods
2.1. Literature retrieval
The electronic databases were searched by computer for retrieval articles about the prevalence of latent infection of pathogens of hand, foot, and mouth disease in healthy people in China. The China National Knowledge Infrastructure (CNKI) and Chinese WanFang database were used for retrieval in Chinese, and PubMed was used for literature retrieval in English with the search terms of (“HFMD” OR “hand foot and mouth disease”) and (“recessive” OR “inapparent” OR “silent”). Relevant literatures published from January 1, 1996 to April 30, 2020 were searched in the above databases. Retrospective search was conducted on the references cited by relevant literature after screening to prevent the omission of literature search in the above databases.
2.1.1. Ethical approval
This study is a meta-analysis and does not involve patient and animal experiments so the ethical approval is not necessary.
2.2. Inclusion and exclusion criteria
Inclusion criteria:
-
(1)
the study type is cross-sectional study;
-
(2)
the research area is mainland China;
-
(3)
the sample size and the positive number or positive rate of hand, foot, and mouth enterovirus were available;
-
(4)
the detection method of enterovirus recessive infection was reverse transcription polymerase chain reaction (RT-PCR);
-
(5)
there are repeated published data in different literatures, and literatures with large sample size are selected.
Exclusion criteria:
-
(1)
literature on the analysis of recessive infection rate by antibody detection;
-
(2)
the latent infection rate of medical personnel and other special groups;
-
(3)
case reports, literature reviews, meeting abstracts, and other research literature;
-
(4)
repeated publication.
2.3. Literature screening and data extraction
The retrieved articles were imported into the Note Express 3.2 document management software for sorting and deduplication. The 2 researchers screened the literatures according to the inclusion and exclusion criteria. The literature finally included by the 2 researchers was compared with each other, and the inconsistencies were decided to be included or excluded through group discussion. The 2 researchers extracted information from the included literatures, including the first author, the year of publication, the study province, the positive number of enterovirus, the sample size, the type of specimen, whether the sampling period was in epidemic period, the sampling area, the study population, etc.
2.4. Quality evaluation
The methodological Quality of the included studies was assessed using 11 checklists recommended by the Agency for Healthcare Research and Quality (AHRQ).[8] If a item is answered “no” or “unclear,” the project scores “0”; if the answer is “yes,” then the item gets a score of “1.” The quality evaluation score criteria are as follows: low quality = 0 to 3, medium mass = 4 to 7, high quality = 8 to 11.
2.5. Statistical analysis
The point assessment of the recessive infection rate and the 95% confidence interval of each study were combined for the pooled rates, and the DerSimonian and Laird method (D–L method) of random effect model or the Mantel–Haenszel method (M–H method) of fixed effect model was selected according to the heterogeneity.[9] Cohran's Q test was used for qualitative evaluation of the heterogeneity. If P < .1 for Cohran's Q test, heterogeneity was indicated and random effect model was used for meta-analysis. If P ≥ .1 for Cohran's Q test, the fixed effect model was selected for meta-analysis. The heterogeneity was quantified by I2 test, and those with I2 values of 0% to 25%, 26% to 50%, and 51% to 100% were considered as low, medium, and high heterogeneity, respectively.[9]
Meta-regression analysis was used to explore the sources of heterogeneity. Potential publication bias was assessed by Begg's funnel plot and Egger's linear regression test.[10,11] In sensitivity analysis, a single study was omitted item by item to evaluate whether a certain study had a significant impact on the stability of the results. All statistical analysis was performed using STATA version 12.0 software (STATA Corporation, College Station, TX). A P value less than .05 was considered statistically significant.
3. Results
3.1. Basic information of the included literature
A total of 243 related literatures including 76 in English and 167 in Chinese were obtained through preliminary retrieval. After screening according to inclusion and exclusion criteria, we finally included 29 articles in this meta-analysis.[12–39] The literature selection process and results are shown in Figure 1. Among the 29 articles, 4 were in English and 24 were in Chinese, covering more than 20 counties and cities in 11 provinces of China, and a total of 11,921 subjects were studied. AHRQ quality score in the included literature was 6 on average, 8 on the highest score, and 5 on the lowest score. The detailed information of the included literature on the incidence of HFMD enterovirus recessive infection is shown in Table 1.
Figure 1.
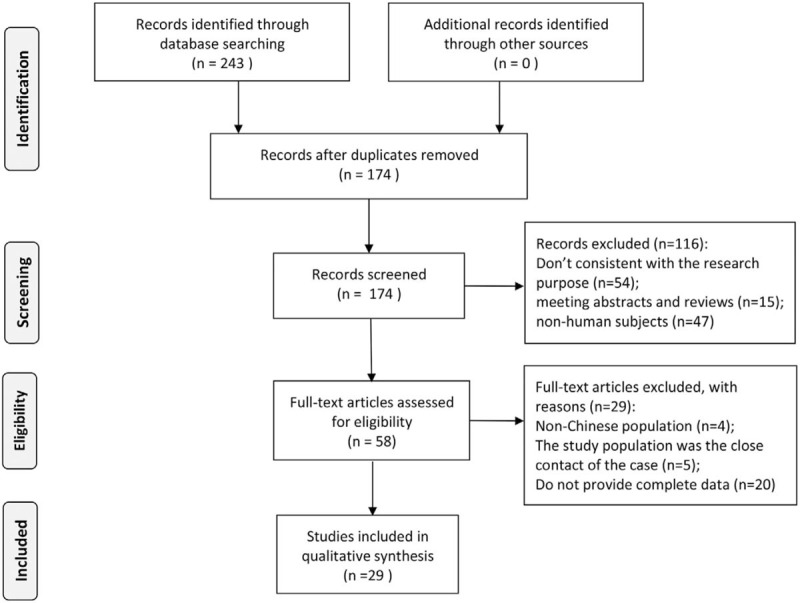
Selection of studies on the recessive infection of pathogens of HFMD in healthy people in China.
Table 1.
Basic information of the included literature on the incidence of HFMD enterovirus recessive infection.
| Refs. | First author | Publication time | Province | HEVs (+) | Sample size | Latent infection (%) | Specimen type | Sampling time | Sampling area | Population | AHRQ score |
| [12] | Yang HK | 2010 | Guangdong | 21 | 180 | 11.67 | Feces | Epidemic | Rural | Children and adults | 8 |
| [13] | Kang N | 2010 | Guangxi | 17 | 50 | 34.00 | Feces | Epidemic | Urban | Children | 6 |
| [14] | Yin FQ | 2011 | Shandong | 111 | 846 | 13.12 | Feces | Epidemic | Urban and rural | Children | 6 |
| [15] | Liu CH | 2011 | Shandong | 68 | 386 | 17.62 | Feces | Epidemic | Urban and rural | Children and adults | 5 |
| [16] | Deng HL | 2011 | Shaanxi | 13 | 207 | 6.28 | Anal swab | Epidemic | Urban and rural | Children | 7 |
| [17] | Jiang WG | 2011 | Shandong | 177 | 1235 | 14.33 | Feces | Epidemic and non-epidemic | Urban and rural | Children and adults | 5 |
| [18] | Mo LF | 2012 | Guangdong | 20 | 310 | 6.45 | Feces | Non-epidemic | Urban | Children and adults | 8 |
| [19] | Ren Y | 2012 | Guangdong | 13 | 150 | 8.67 | Feces | Epidemic and non-epidemic | Urban | Children and adults | 6 |
| [20] | Ceng HR | 2012 | Guangdong | 60 | 136 | 44.12 | Feces | Epidemic | Rural | Children | 5 |
| [21] | Liu L | 2012 | Hebei | 44 | 161 | 27.33 | Feces | Epidemic | Rural | Children | 6 |
| [22] | Wang DL | 2012 | Guangdong | 78 | 1305 | 5.98 | Anal swab | Epidemic | Urban | Children | 8 |
| [23] | He Y | 2012 | Guangdong | 18 | 392 | 4.59 | Anal swab | Epidemic and non-epidemic | Urban | Children | 7 |
| [24] | Niu WD | 2012 | Henan | 49 | 200 | 24.50 | Feces | Epidemic | Rural | Children and adults | 6 |
| [25] | Yi QH | 2013 | Jiangsu | 67 | 309 | 21.68 | Anal swab | Epidemic | Urban and rural | Children | 6 |
| [26] | Chen FY | 2013 | Hebei | 40 | 180 | 22.22 | Anal swab | Epidemic | Urban and rural | Children | 5 |
| [27] | Zhang | 2013 | Shandong | 59 | 254 | 23.23 | Feces | Epidemic | Rural | Children | 8 |
| [28] | Wu | 2013 | Guangdong | 34 | 320 | 10.63 | Feces | Epidemic and non-epidemic | Urban | Children | 7 |
| [29] | Li Y | 2013 | Henan | 71 | 200 | 35.50 | Feces | Epidemic | Rural | Children and adults | 5 |
| [30] | Cai MS | 2013 | Guangdong | 40 | 240 | 16.67 | Feces | Epidemic | Urban | Children and adults | 5 |
| [31] | Liu FR | 2014 | Guangdong | 28 | 118 | 23.73 | Anal swab | Epidemic and non-epidemic | Urban | Children | 6 |
| [32] | Sun BC | 2014 | Zhejiang | 51 | 395 | 12.91 | Anal swab | Epidemic and non-epidemic | Urban | Children | 8 |
| [33] | Hou ZY | 2015 | Henan | 26 | 106 | 24.53 | Anal swab | Epidemic and non-epidemic | Urban | Children | 5 |
| [34] | Feng X | 2015 | Jiangxi | 18 | 100 | 18.00 | Anal swab | Epidemic | Urban and rural | Children | 5 |
| [35] | Zhang L | 2015 | Shandong | 123 | 1275 | 9.65 | Feces | Epidemic and non-epidemic | Urban and rural | Children | 6 |
| [36] | Gao W | 2016 | Hebei | 11 | 130 | 8.46 | Anal swab | Epidemic and non-epidemic | Urban | Children | 6 |
| [37] | Wang HQ | 2016 | Chongqing | 211 | 1276 | 16.54 | Anal swab | Epidemic and non-epidemic | Urban and rural | Children | 8 |
| [38] | Wu | 2017 | Yunnan | 90 | 667 | 13.49 | Feces | Epidemic | Rural | Children and adults | 7 |
| [39] | Yuan W | 2018 | Sichuan | 75 | 193 | 38.86 | Feces | Epidemic | Urban and rural | Children | 5 |
| [40] | Xie Y | 2019 | Shaanxi | 123 | 600 | 20.50 | Anal swab | Epidemic and non-epidemic | Urban and rural | Children | 5 |
3.2. Meta-analysis
A total of 11,921 cases were reported in 29 literatures, among which 1756 cases were positive for enterovirus common nucleic acid. The heterogeneity test showed that there was significant heterogeneity in each analysis group, and the results of the random effect model were selected. Meta-analysis showed that the recessive infection rate of HEVs was 17.5% (14.9–20.1%) in healthy Chinese people, among which the recessive infection rate of EV-A71 was 3.3% (2.2–4.4%), the recessive infection rate of CV-A16 was 1.7% (1.0–2.5%), and the recessive infection rate of other enterovirus was 15.1% (11.1–17.1%), as shown in Figure 2.
Figure 2.
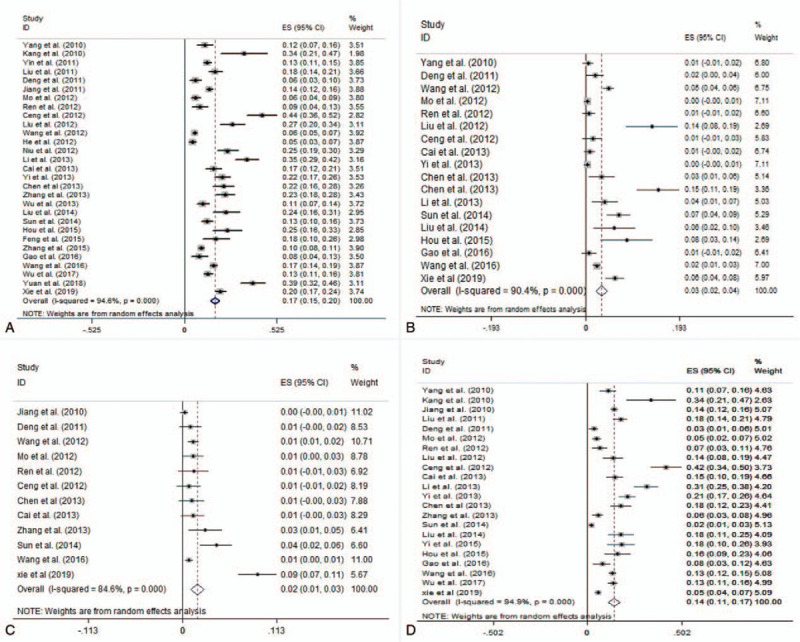
Forest plots for the pooled recessive infection of pathogens of HFMD in healthy people in China (a. HEVs, b. EV-A71, c. CV-A16, and d. other HEVs).
3.3. Subgroup analysis
We conducted a subgroup analysis based on gender and age. The results of meta-analysis showed that the rate of HEVs recessive infection was 16.7% (12.9–20.4%) in males and 14.4% (10.8–18.0%) in females. The rate of HEVs recessive infection was 24.4% (20.4–28.5%) at 0 to 5 years old, and 9.4% (6.5–12.2%) at more than 5 years old.
3.4. Meta-regression analysis
In order to further explore the source of heterogeneity, a meta-regression analysis was conducted with the Chinese population HEVs recessive infection rate as the dependent variable and the sample type, sampling period, sampling area, and study population as the covariables. The results showed that the size of the heterogeneity explained by the covariates included in the model was R2 = 34.86%, and the joint test of all covariates in the model was P = .002. The factors affecting the HEVs recessive infection rate in the Chinese population were the sampling period (t = 2.56, P = .016), the sampling area (t = 2.16, P = .039), and the study population (t = 3.03, P = .005), as shown in Table 2.
Table 2.
Meta-regression analysis results of the latent infection rate of HFMD pathogens in healthy Chinese population.
| Covariates | Coefficient | S.E. | t value | P | 95%CI |
| Sample type | |||||
| Feces | 0.056 | 0.053 | 1.08 | .291 | −0.051 to 0.164 |
| Anal swab | Reference | ||||
| Sampling period | |||||
| Epidemic | 0.094 | 0.036 | 2.56 | .016 | 0.019–0.168 |
| Non-epidemic | Reference | ||||
| Sampling area | |||||
| Rural | 0.053 | 0.024 | 2.16 | .039 | 0.003–0.104 |
| Urban | Reference | ||||
| Study population | |||||
| Children | 0.105 | 0.035 | 3.03 | .005 | 0.034–0.075 |
| Adults | Reference | ||||
| Constant | 0.167 | 0.071 | 2.37 | .025 | 0.023–0.312 |
3.5. Sensitivity analysis
Meta re-analysis was conducted by removing single studies one by one to observe the influence of a single study on the results. The sensitivity analysis results showed no significant difference in the associated recessive infection rate, indicating that the meta-analysis results were relatively stable and that a single study would not have a significant impact on the meta-analysis results, as shown in Figure 3.
Figure 3.
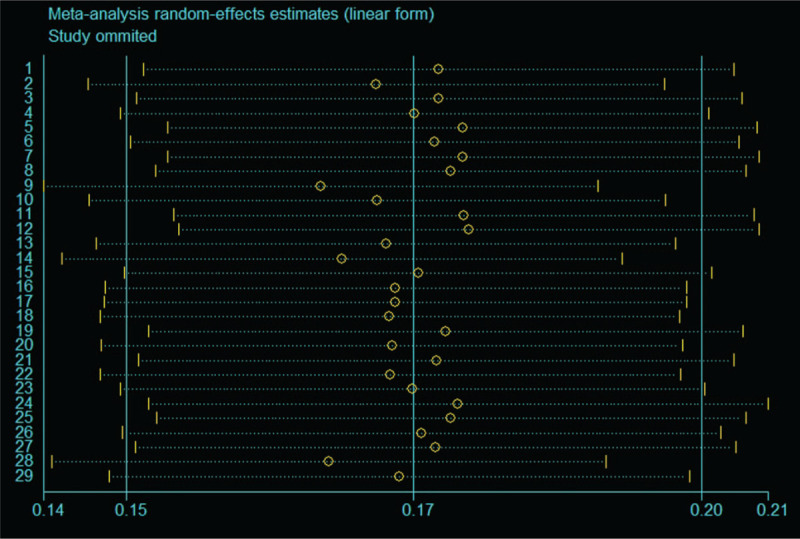
Sensitive analysis for the recessive infection of HEVs in healthy people in China by removing single studies one by one.
3.6. Publication bias analysis
The Begg's funnel plot and Egger's linear regression were used to evaluate publication bias, which showed that there was a significant publication bias, as shown in Table 3 and Figure 4.
Table 3.
Meta-analysis results of the latent infection rate of HFMD pathogens in healthy Chinese population.
| Group | No. of study | Latent infection | 95%CI | Cohran's Q test for heterogeneity (P value) | I2 (%) | Model | Begg's P value | Egger's P value |
| HEV type | ||||||||
| HEVs | 29 | 0.175 | 0.149–0.201 | <.001 | 94.6 | Random | <.001 | <.001 |
| EV71 | 18 | 0.033 | 0.022–0.044 | <.001 | 90.4 | Random | <.001 | <.001 |
| COA16 | 12 | 0.017 | 0.010–0.025 | <.001 | 85.8 | Random | .024 | .008 |
| Other HEVs | 22 | 0.151 | 0.111–0.171 | <.001 | 94.9 | Random | .010 | <.001 |
| Gender | ||||||||
| Male | 13 | 0.167 | 0.129–0.204 | <.001 | 86.5 | Random | .009 | .001 |
| Female | 13 | 0.144 | 0.108–0.180 | <.001 | 86.1 | Random | .161 | <.001 |
| Age | ||||||||
| 0–5 yr old | 27 | 0.244 | 0.204–0.285 | <.001 | 95.7 | Random | .003 | <.001 |
| >5 yr old | 17 | 0.094 | 0.065–0.122 | <.001 | 87.3 | Random | .001 | <.001 |
Figure 4.
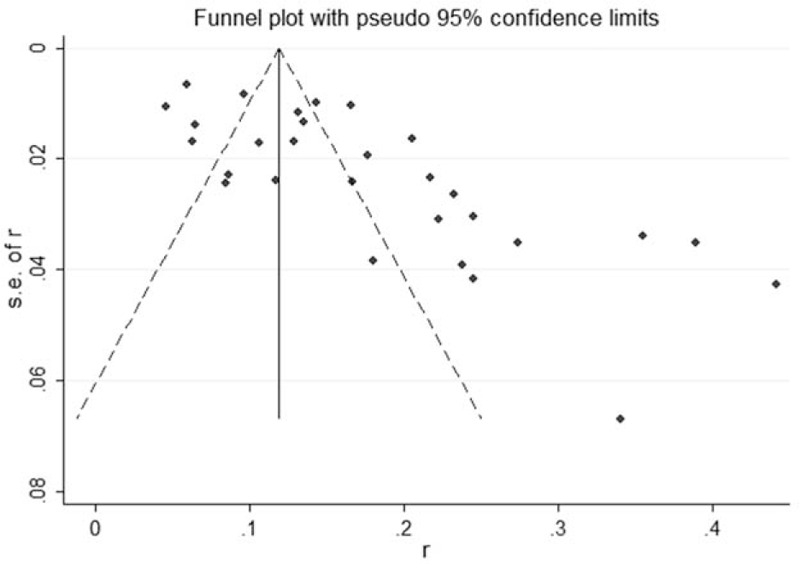
Begg's funnel plot for the pooled recessive infection of HEVs in healthy people in China.
4. Discussion
The results of the present meta-analysis showed that the recessive infection rate of HEVs was high among healthy people in China, mainly with other enteric viruses. The recessive infection rates of EV-A71 and CV-A16 were also high, at 3.3% (2.2–4.4%) and 1.7% (1.0–2.5%), respectively. Subgroup analysis showed that male HEVs recessive infection rate was slightly higher than female. The recessive infection rate of HEVs was significantly higher in the population aged 0 to 5 than in the population aged more than 5 years old. The gender and age distributions of HEVs recessive infection rate in healthy Chinese population are consistent with the distribution characteristics of HFMD cases in China.[41] It is suggested that the prevention and control measures focus on young children should be taken during the epidemic of HFMD.
Meta-regression analysis showed that the factors affecting the HEVs recessive infection rate in Chinese population included sampling period (epidemic and non-epidemic), sampling area (city and rural), and study population (children and adults), which were consistent with the epidemiological characteristics reported in China such as the epidemic period of HFMD, higher incidence in rural areas than in urban areas, and more cases concentrated in children under 5 years old. In the future, health education on HFMD prevention in nurseries and primary schools in rural areas should be strengthened, and good hygiene practices should be promoted, especially during epidemics of HFMD. Kindergartens, primary schools, and other key units should strengthen morning screening to identify cases as soon as possible. If a suspicious case is found, the one should be encouraged to return to the hospital for medical treatment and placed in home isolation. Schools should pay close attention to the physical condition of close contacts of the case, and classes may be suspended if necessary.
There was a large heterogeneity in this meta-analysis, so subgroup analysis and meta-regression analysis were used to explore the source of heterogeneity. Gender and age were selected as the grouping factors in this meta-analysis. Since most of the literature grouped these 2 factors, the analysis of these 2 subgroups could appropriately avoid the influence of other confounding factors. For other factors that may influence heterogeneity, meta-regression analysis was used to include the possible influencing factors into the covariables. However, meta regression analysis requires a certain number of covariables, usually no more than the number of included references/10, otherwise, type I error will increase significantly.[42] In this meta-regression analysis, 34.86% of the heterogeneity was explained. Other sources of heterogeneity may include geographical location, climatic factors, sample size, sampling method, etc. In order to evaluate the robustness of meta-analysis results, sensitivity analysis showed that the removal of a single reference would not have a significant impact on the combined results, indicating that the study results were relatively stable. The analysis of publication bias showed that there was significant publication bias in each comparison group. The publication bias may be caused by the unpublished literature with low recessive infection rate or the unsearched gray literatures.
The results of this meta-analysis need to be interpreted with caution and have some limitations. Firstly, the heterogeneity of the studies included in each research group is relatively large. Although subgroup analysis and meta-regression were used in this meta-analysis to explore the sources of heterogeneity, some heterogeneity could not be explained due to the lack of information in the original literature. Secondly, publication bias may lead to higher analytical results, and literature retrieval and screening may result in missing literature inspection. Finally, in the subgroup analysis, some literatures were not stratified according to the grouping factors, resulting in different numbers of literatures included in each comparison group.
In conclusion, Chinese healthy people have a high rate of HEVs recessive infection. The rate of HEVs recessive infection in males was higher than that in females. The recessive infection rate of HEVs was higher in people aged 0 to 5 than in people aged more than 5 years old. The factors influencing the HEVs recessive infection rate in Chinese population include sampling period, sampling area, and study population. Limited by the quantity and quality of the included studies, more high-quality studies are needed for further verification in the future.
Author contributions
Conceptualization: Yu-Jie Zhou, Bao-Lin Zhao.
Data curation: Yu-Jie Zhou, Xiu-De Niu, Ya-Qing Ding, Zhen Qian.
Formal analysis: Yu-Jie Zhou, Xiu-De Niu.
Methodology: Xiu-De Niu, Ya-Qing Ding, Zhen Qian.
Validation: Xiu-De Niu, Ya-Qing Ding, Zhen Qian.
Writing – original draft: Yu-Jie Zhou.
Writing – review & editing: Yu-Jie Zhou, Bao-Lin Zhao.
Footnotes
Abbreviations: Cox A16 = coxsackievirus group A, EV71 = enterovirus 71, HEVs = human enteroviruses, HFMD = hand, foot, and mouth disease, RT-PCR = reverse transcription polymerase chain reaction.
How to cite this article: Zhou YJ, Niu XD, Ding YQ, Qian Z, Zhao BL. Prevalence of recessive infection of pathogens of hand, foot, and mouth disease in healthy people in China: A meta-analysis. Medicine. 2021;100:7(e24855).
YJZ and XDN contribute equally to this work.
There was no funding support in this study.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
HEVs = human enteroviruses, HFMD = hand, foot, and mouth disease.
HFMD = hand, foot, and mouth disease.
HEVs = human enteroviruses, HFMD = hand, foot, and mouth disease.
References
- [1].Xing W, Liao Q, Viboud C, et al. Hand, foot, and mouth disease in China, 2008–12: an epidemiological study. Lancet Infect Dis 2014;14:308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Solomon T, Lewthwaite P, Perera D, et al. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect Dis 2010;10:778–90. [DOI] [PubMed] [Google Scholar]
- [3].Huang X, Wei H, Wu S, et al. Epidemiological and etiological characteristics of hand, foot, and mouth disease in Henan, China, 2008–2013. Sci Rep 2015;5:8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lai CC, Jiang DS, Wu HM, et al. A dynamic model for the outbreaks of hand, foot, and mouth disease in Taiwan. Epidemiol Infect 2016;144:1500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zeng H, Lu J, Zheng H, et al. The epidemiological study of Coxsackievirus A6 revealing hand, foot and mouth disease epidemic patterns in Guangdong, China. Sci Rep 2015;5:10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang JC, Ding R, Du YG, et al. Research on the aetiology of suspected enterovirus infected patients in Xuzhou district in 2009. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2011;25:106–8. [PubMed] [Google Scholar]
- [7].Du Z, Lawrence WR, Zhang W, et al. Bayesian spatiotemporal analysis for association of environmental factors with hand, foot, and mouth disease in Guangdong, China. Sci Rep 2018;8:15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang Y, Wang S, Zhang L, et al. Risk factors of severe hand, foot and mouth disease: a meta-analysis. Scand J Infect Dis 2014;46:515–22. [DOI] [PubMed] [Google Scholar]
- [9].Musuuza JS, Guru PK, O’Horo JC, et al. The impact of chlorhexidine bathing on hospital-acquired bloodstream infections: a systematic review and meta-analysis. BMC Infect Dis 2019;19:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Begg CB, Berlin JA. Publication bias and dissemination of clinical research. J Natl Cancer Inst 1989;81:107–15. [DOI] [PubMed] [Google Scholar]
- [11].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yang HK, Yuan DK, Huang Y, et al. Survey on epidemic situation of recessive infection of HFMD in healthy adults and children in Dongguan. Chin J Health Lab Technol 2010;20:1519–20. [Google Scholar]
- [13].Kang N, Tan Y, Bi FY, et al. In apparent infection of enteroviruses in 50 children in Nanning. Dis Surveillance 2010;25:682–3. [Google Scholar]
- [14].Yin FQ, Shi ZB. The infection of entericvirus in healthy population and the population in intimate contact with the cases of HFMD in Lanshan District. J Shandong Med Coll 2011;33:430–2. [Google Scholar]
- [15].Liu CH. Investigation and analysis on the rate of poisoning among healthy people and close contacts of HFMD in Jining city. J Prev Rehabil 2011;13:377–8. [Google Scholar]
- [16].Deng HL, Wang XY, Ma CF, et al. Pathogenic and epidemiological analysis of 1020 cases of hand foot and mouth disease and 207 healthy children. Shaanxi Med J 2011;40:1226–8. [Google Scholar]
- [17].Jiang WG, Ding SJ, Gao ZJ, et al. Investigation of the prevalence of enterovirus carriers among healthy children and their guardians during HFMD outbreaks. J Pathog Biol 2011;6:857–60. [Google Scholar]
- [18].Mo LF, Wang L, Ren Y, et al. Investigation of latent infection of hand foot and mouth disease in healthy population in Spring and Autumn in Shenzhen. J Mod Med Health 2012;28:1916–7. [Google Scholar]
- [19].Ren Y, Wang DL, Zeng YL, et al. Investigation of the HFMD carrier status of healthy adults and children in a district of Shenzhen, 2011. J Trop Med 2012;12:1025–7. [Google Scholar]
- [20].Ceng HR, Zhang YH, Sun LM, et al. Investigation on the carrying rate of enterovirus in healthy population and close contacts of HFMD cases. Chin J Health Lab Technol 2012;22:1158–60. [Google Scholar]
- [21].Liu L, Lu H, Tian HF, et al. Analysis of enterovirus-carrying situation in healthy children in Zanhuang county of Shijiazhuang. J Chin Matern Child Health 2012;27:1661–2. [Google Scholar]
- [22].Wang DL, Yao W, Feng B, et al. Investigation on in-apparent infection situation of HFMD in nursery schools in a district of Shenzhen, 2010. Chin J Health Lab Technol 2012;22: 885–857. [Google Scholar]
- [23].He Y, Zeng JR, Wei HZ, et al. Investigation and research on the carrying rate of EV71 and CoxA16 of kindergarten children. China Foreign Med Treat 2012;17:1–2. [Google Scholar]
- [24].Niu WD, Dai L, Shi J. Survey on epidemic situation of recessive infection of HFMD in healthy children and adults in Zhengzhou. Chin J Health Lab Technol 2012;22:2209–11. [Google Scholar]
- [25].Yi QH, Zhang X, Tang FY, et al. Epidemical analysis of hand-foot-mouth disease among healthy children in kindergartens of Taizhou. J Prev Med Inf 2013;29:533–5. [Google Scholar]
- [26].Chen FY. Comparison of enterovirus type between common cases of HFMD and healthy children HFMD in Xingtai City. Hebei Med J 2013;35:2190–2. [Google Scholar]
- [27].Zhang XA, Wang HY, Ding SJ, et al. Prevalence of enteroviruses in children with and without hand, foot, and mouth disease in China. BMC Infect Dis 2013;13:606.doi: 10. 1186/1471-2334-13-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wu W, Xu WB, Chen L, et al. Molecular identification and analysis of human enteroviruses isolated from healthy children in Shenzhen, China from 2010 to 2011. PLoS One 2013;8:e64889.doi: 10.1371/journal.pone.0064889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Y, Zhang XP, Bao XB. Investigation and analysis on the inapparent infection of HFMD pathogens in healthy population in Anyang in 2010 and 2011. Chin J Health Lab Technol 2013;23:2173–9. [Google Scholar]
- [30].Cai MS, Ren Y. Investigation on latent infection of Hand foot and mouth disease in healthy population in Shenzhen in 2012. Chin J Health Inspect 2013;23:2355–7. [Google Scholar]
- [31].Liu FR, Liu Q, Wang X, et al. Sub-clinical infection investigation and its risk factors analysis of hand–foot–mouth disease on healthy kindergarten children. J Trop Med 2014;14:246–8. [Google Scholar]
- [32].Sun BC, Gao J, Chen D, et al. Study on the recessive infection of HFMD virus in children. Pract Prev Med 2014;21:914–6. [Google Scholar]
- [33].Hou ZY, Niu XY. Investigation of latent infection of Hand foot and mouth disease in healthy preschool children and analysis of risk factors. J China Prescription Drug 2015;14:117–9. [Google Scholar]
- [34].Feng X, You S, Qiu Q, et al. Analysis on epidemic situation of recessive infection of HFMD in healthy population in Pingxiang. Mod Prev Med 2015;42:4178–80. [Google Scholar]
- [35].Zhang L. Analysis of detection results of Hand foot and mouth disease in Linyi city and research on prevention and control counter measures. Shandong Univ 2015. [Google Scholar]
- [36].Gao W, Yang M, Xiang D, et al. Study on the status of the recessive infection of hand-foot-mouth disease virus in healthy children and the family aggregation of parent and children. J Med Pest Control 2016;32:754–9. [Google Scholar]
- [37].Wang HQ, Wu B, Lang ZK, et al. Investigation on the carrying status of hand-foot-mouth disease pathogens among children in kindergartens in Wanzhou district of Chongqing. Chin J Health Lab Technol 2016;26:582–4. [Google Scholar]
- [38].Wu Q, Fu X, Jiang L, et al. Prevalence of enteroviruses in healthy populations and excretion of pathogens in patients with hand, foot, and mouth disease in a highly endemic area of southwest China. PLoS One 2017;12:e0181234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yuan W, Cheng XW, Liu YQ, et al. Subclinical infection of hand-foot-mouth disease in healthy kindergarten children and influencing factors. J Prev Med Inf 2018;34:1063–5. [Google Scholar]
- [40].Xie Y, Xia L, Yuan J. Investigation on inapparent infection status of hand–foot–mouth disease in Xi’an City of Shaanxi province. J Clin Med Pract 2019;23:45–7. [Google Scholar]
- [41].Koh WM, Bogich T, Siegel K, et al. The epidemiology of hand, foot and mouth disease in Asia: a systematic review and analysis. Pediatr Infect Dis J 2016;35:e285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–73. [DOI] [PubMed] [Google Scholar]


