Abstract
Background:
To explore the effectiveness of platelet-rich plasma (PRP) injection regarding functional recovery, pain relief, and range of motion (ROM) of shoulder compared with the corticosteroid injection in patients with rotator cuff lesions treated non-operatively.
Methods:
An electronic literature search was performed by 2 authors in the PubMed, Embase, Cochrane Library, and Web of Science databases to identify relevant randomized controlled trial (RCTs) that were published up to July 20, 2020. The quality of the included RCTs was evaluated using the approach recommended by the Cochrane Handbook for Systematic Reviews of Interventions. Standardized mean differences (SMDs) or mean differences (MDs) with 95% confidence intervals (CIs) were applied to calculate the pooled effect sizes.
Results:
Six RCTs were included in this systematic review. Meta-analysis revealed that corticosteroid injection yielded statistically significant superior functional recovery (SMD = −0.80; 95% CI, −1.42 to −0.18; P = .01) and pain relief (MD = 1.59; 95% CI, 0.30–2.89; P = .02) compared with PRP injection for rotator cuff lesions during the short-term follow-up period. However, at the medium-term and long-term follow-up, no statistically significant difference was identified between the 2 groups. Regarding the ROM of shoulder, no statistically significant difference was found between the 2 groups during the whole follow-up period.
Conclusions:
The current clinical evidence revealed short-term efficacy of corticosteroid injection and no significant medium- to long-term difference between corticosteroid and PRP injection in the treatment of rotator cuff lesions. Additional studies with longer follow-ups, larger sample sizes, and more rigorous designs are needed to draw more reliable and accurate conclusions.
Keywords: corticosteroid, injection, meta-analysis, platelet-rich plasma, rotator cuff
1. Introduction
The shoulder is considered to be one of the most complex joints in the human body due to its huge range of motion (ROM).[1] Rotator cuff lesions account for the vast majority of shoulder injuries in adult and are a common cause of chronic shoulder pain, deterioration of daily activities, and disability. The incidence of rotator cuff lesions is increasing along with an aging population.[2] Currently, several methods have been used for treating rotator cuff lesions including activity modification,[3] nonsteroidal anti-inflammatory drug,[4] physical therapy,[5] local corticosteroid injection[6] and platelet-rich plasma (PRP) injection.[7] The clinical efficacy of PRP vs corticosteroid injections has recently gained significant attention as conservative treatment options for rotator cuff lesions in the orthopedic sports medicine community.[8,9]
Corticosteroid injection is often used for tendinous lesions.[10] The efficacy of corticosteroid injection has been widely confirmed in reducing pain and improving function,[11–13] and it is considered by many practitioners as a cheap and effective therapeutic option, but the adverse reactions of glucocorticoids cause concern in clinical practice.[14] PRP is a concentrate of platelet-rich plasma protein derived from whole blood, which has a higher concentration of platelets above that of the baseline.[15,16] It contains several specific growth factors (such as vascular endothelial growth factor, epidermal growth factor, transforming growth factor-b, and insulin-like growth factor) that can stimulate healing, promote the inflammatory cascade, and accelerate tissue regeneration.[16–18] Recent study[19] reported that PRP injection play an effective and important role in the treatment of rotator cuff lesions, in cases where physiotherapy has been unsuccessful.
Several studies [8,9,20,21] have compared PRP injection with corticosteroid injection in the treatment of rotator cuff lesions; however, which method is more effective remains controversial. For example, a randomized controlled trial (RCT)[20] has indicated that PRP injection is superior to corticosteroid injection in functional recovery and pain relief. Conversely, another trial[21] has shown that corticosteroid injection is superior to PRP injection in functional recovery and pain relief. In addition, in some studies,[8,9] there were no significant differences between the PRP and corticosteroid injections in treating rotator cuff lesions. To the best of our knowledge, no systematic reviews have compared the effects of PRP injection with that corticosteroid injection in patients with rotator cuff lesions. Therefore, we conduct this systematic review and meta-analysis to further compare the clinical efficacy of PRP injection and corticosteroids injection for conservative treatment of patients with rotator cuff lesions. The primary aims of this study were to compare the efficacy of PRP injection and corticosteroids injection on functional recovery and pain relief. The secondary aim was to compare the efficacy of PRP injection and corticosteroids injection on ROM of the shoulder.
2. Methods
We conducted this systematic review and meta-analysis by following the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines.[22] The research protocol was registered in PROSPERO (registration number CRD42020202208). Ethics approval is not required as this study is a meta-analysis based on published studies.
2.1. Search strategy
A comprehensive electronic literature search was performed by 2 authors in the PubMed, Embase, Cochrane Library, and Web of Science databases to identify relevant studies that were published up to July 20, 2020. Further relevant publications were identified through the reference lists of the included studies and previous related systematic reviews. For each database, the search strategy was customized. For example, the key search terms for PubMed were a combination of medical subject heading (MeSH) terms and entry terms. Detailed information about the search terms and search results of each database is available in Supplemental Digital Content 1.
2.2. Inclusion criteria
Studies were included in this systematic review if they met the following criteria:
-
1.
Population: participants were diagnosed with rotator cuff lesions by either imaging examination or clinical evaluation. In accordance with previous systematic reviews,[23,24] the rotator cuff lesions comprised partial tendon tear, full-thickness tear, rotator cuff tendinosis, and rotator cuff impingement syndrome;
-
2.
Interventions: conservative treatment studies that had allocated a PRP injection group and a corticosteroid injection group. Trials comparing the role of PRP injection with steroid injection in orthopedic surgery were excluded;
-
3.
Outcomes: we designated functional recovery and pain relief as the primary outcomes, ROM of the shoulder as the secondary outcome; and
-
4.
Study design: to achieve high levels of evidence, we included only RCTs.
2.3. Study selection and data extraction
All of the searched records were imported into EndNote X9 to eliminate duplicate studies. The 2 authors worked independently to identify studies that met the inclusion criteria. To further evaluate the eligibility of potential studies, we obtained full-text publications and discussed any disagreements with the third author. Data were extracted from the included studies by 2 independent authors using the standardized data extraction tool. From each included study, we extracted data including the author, publication year, country, sample size, patient’ mean age, treatment details (e.g., injection dose, guidance method, and injection location), follow-up times, and outcome measurement tools. Any disagreements between the 2 independent authors were resolved by the third authors.
2.4. Risk of bias assessment
The risk of bias in the included studies was assessed using the approach recommended by the Cochrane Handbook for Systematic Reviews of Interventions.[25] The 7 recommended items included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias. All included studies were independently evaluated, and the risk of bias for each item was rated as “low risk”, “unclear” or “high risk”. Disagreements between the 2 authors were resolved by the third author.
2.5. Data analysis
Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were used when studies used different outcome scales, and mean differences (MDs) with 95% CIs were applied when studies used the same outcome scales. The level of heterogeneity was evaluated by the I-square (I2) method, and a value of I2 > 50% was regarded as significant heterogeneity.[26] The fixed-effects model was used to calculate the pooled effect size if the data were not significantly heterogeneous. Conversely, the random-effects model was used. Publication bias was evaluated by visual inspection of funnel plot.[27] RevMan 5.3 provided by the Cochrane Collaboration was used for all statistical calculations, and a P value <.05 was considered statistically significant.
Subgroup analysis was performed to explore the short-term, medium-term, and long-term effects after injection. With reference to the previous systematic reviews [28,29] and the recovery speed of rotator cuff lesions,[30,31] the post-injection follow-ups was defined as short-term (3–6 weeks), medium-term (8–12 weeks), and long-term (over 12 weeks).
3. Results
3.1. Study selection
A total of 8093 records were identified from the electronic databases in the final search, with an additional 2 records identified through other sources. After removal of duplicates and obviously irrelevant records, we retrieved 25 full-text articles to further evaluate their eligibility. In total 19 articles were excluded because they did not meet the inclusion criteria. Eventually, a total of 6 studies [8,9,20,21,32,33] met all the inclusion criteria and were included in this systematic review. The detailed screening process is illustrated in Figure 1.
Figure 1.
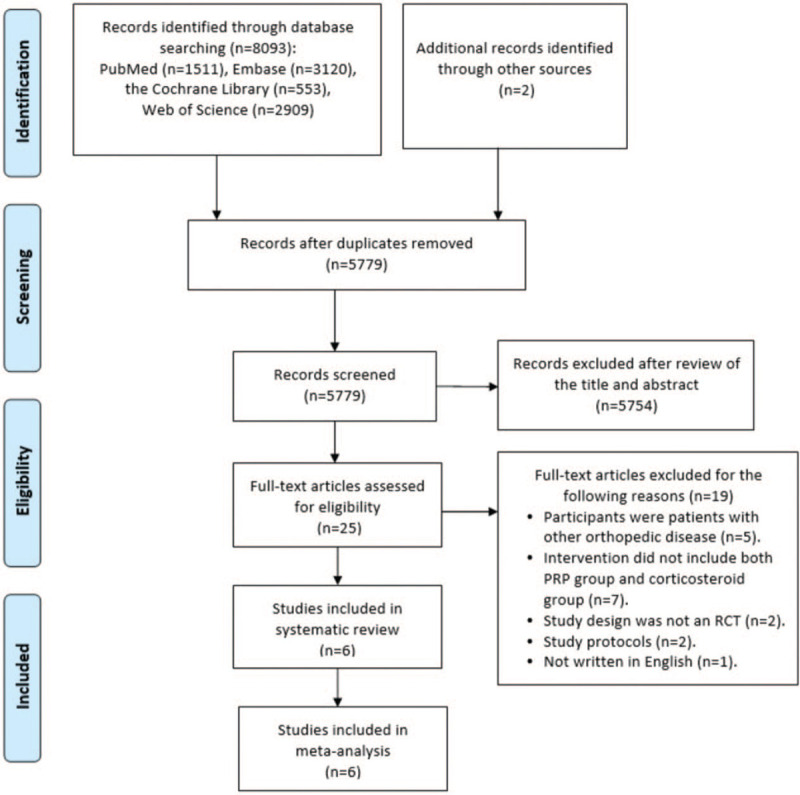
Flow diagram for search and selection of the included studies.
3.2. Characteristics of the included studies
The main characteristics of the included studies are shown in Table 1. In total 301 patients with rotator cuff lesions were enrolled in these 6 RCTs: 181 women (60.1%) and 120 men (39.9%). The mean age of the enrolled patients ranged from 41.5 ± 12.5 years to 53.2 ± 9.4 years. The duration of follow up ranged from 3 weeks to 6 months. Three of the included trials were conducted in the Turkey,[21,32,33] 2 were conducted in Egypt,[9,20] and 1 was conducted in Brazil.[8] All of the included RCTs reported clear inclusion and exclusion criteria for their patients. The diagnosis of rotator cuff lesions was based on magnetic resonance imaging in 1 study,[20] was based on clinical examination and confirmed by magnetic resonance imaging in 3 studies,[21,32,33] and was based on ultrasonography in the other 2 studies.[8,9] Among the 6 studies, 2 used ultrasound-guided injection,[9,33] 1 used blind injection,[8] and the remaining studies were not described in detail.[20,21,32] For all of the included studies, only single dose injection was administered to each patient in PRP group and corticosteroid group. Regarding the injection location, 2 studies performed the lateral subacromial injection method,[9,33] 2 with dorsolateral subacromial injection method,[20,21] 1 with posterior subacromial injection method,[8] and 1 with subacromial space injection method.[33]
Table 1.
Characteristics of the studies included in this meta-analysis.
| Participants | Rx dose /Guidance Method /Injection Location | |||||||
| Study, year, country | Study design | Number PRP/Cor | Age(years) PRP/Cor | Male% PRP/Cor | PRP | Cor | Follow up | Outcomes |
| Barreto et al., 2019, Brazil | RCT | 26/25 | 53.2 ± 9.4/53 ± 11 | 42.3%/28% | 3 ml of PRP extracted from centrifuged 15 mL of patient's peripheral blood and manual platelet separation with micropipettes/ Not guided by ultrasound subacromial/ Subacromial space through a posterior approach | Not available | 1 month, 3 months, 6 months | UCLA SRS, CMS, DASH |
| Ibrahim et al., 2019, Egypt | RCT | 15/15 | 46.8 ± 10.6/41.5 ± 12.5 | 40%/46.7% | 2 ml of PRP extracted from centrifuged 20 ml of patient's blood/ Under ultrasound guidance/ Subacromial lateral approach | 1 ml of methylprednisolone acetate and 1 ml of local anesthetic (lidocaine)/Under ultrasound guidance/Subacromial lateral approach | 2 months | VAS, SDQ, ROM, Ultrasound findings |
| Pasin et al., 2019, Turkey | RCT | 30/30 | 49.4 ± 9.1/47.7 ± 9.6 | 41.1% | 4 ml of PRP extracted from centrifuged 10 ml of patient's blood/ Not available/ The subacromial space | A single dose of 40 mg/ml triamcinolone acetonide and 3 ml lidocaine/Not available/The subacromial space | 3 weeks, 8 weeks | VAS, SDQ, Quick DASH, UCLA SRS, SF-36 scores |
| Sari et al., 2020, Turkey | RCT | 30/30 | 52.1 ± 10.8 | 35.8% | 5 ml of PRP extracted from centrifuged 10 ml of patient's blood/ Under ultrasound guidance/ The lateral subacromial injection method | 2 ml 40 mg triamcinolone acetonide (Artropan),2 ml 1% lidocaine and 1 ml saline/under ultrasound guidance/The lateral subacromial injection method | 3 weeks, 12 weeks, 24 weeks | VAS, ASES, WORC |
| Say et al., 2016, Turkey | RCT | 30/30 | 49.2 ± 7/50.2 ± 2.7 | 33.3%/40% | 2.5 ml of PRP extracted from centrifuged 30 cc of patient's peripheral blood/ Not available/ Administered via a dorsolateral approach through the interval just beneath the dorsal acromial edge | A mixture of 1 ml 40 mg methylprednisolone and 8 ml prilocaine/Not available/ Administered via a dorsolateral approach through the interval just beneath the dorsal acromial edge | 6 weeks, 6 months | The Constant score, VAS, ROM |
| Shams et al., 2016, Egypt | RCT | 20/20 | 52 ± 12/50 ± 10 | 50%/55% | 2–2.5 ml of PRP extracted from centrifuged 10 ml of patient's blood/ Not available/Posterolateral approach | Kenacort-A 40 mg, (triamcinolone acetonide, suspension)/Not available/ Posterolateral approach | 6 weeks, 12 weeks, 6 months | ASES, CMS, SST, VAS, MRI grades |
3.3. Risk of bias assessment
The risk of bias assessment for the included studies are presented in Figures 2 and 3. Most of the included RCTs reported randomization, but some trials did not describe the allocation concealment details, which could cause potential selection bias. In performance bias and detection bias, 5 studies were judged as having an unclear risk of bias because there was no description provided for the blinding method. The risk of attrition bias was categorized as high in 1 study because of the uneven number of patients lost to follow-up in each group. There was no evidence of reporting bias or other bias in any of the included studies; therefore, the risk of bias for these items was determined to be low.
Figure 2.
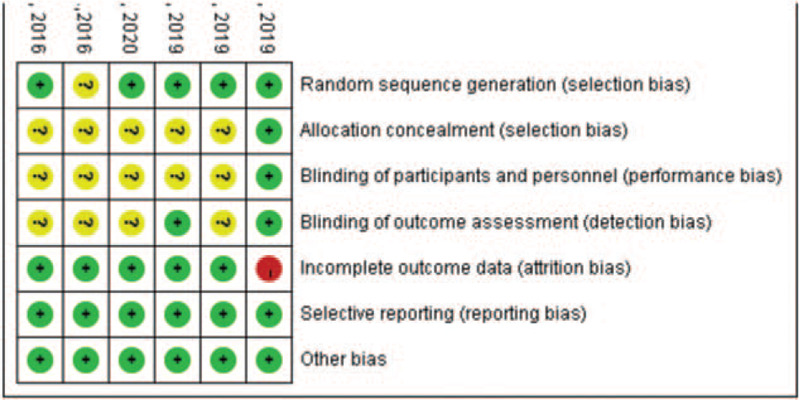
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study.
Figure 3.
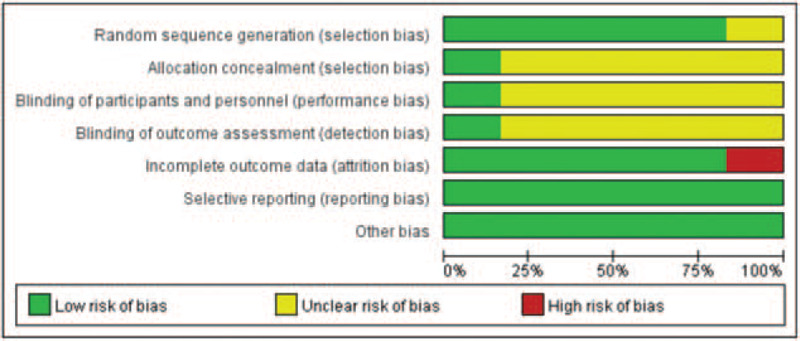
Risk of bias graph: review authors’ judgments about each risk of bias item, presented as percentage of included studies.
3.4. Results of meta-analysis
3.4.1. Primary outcome: functional recovery
All 6 studies compared the effects of PRP injection vs corticosteroid injection on functional recovery using the Constant-Murley Shoulder Outcome Score,[8,21] Shoulder Disability Questionnaire,[9] University of California Los Angeles Shoulder Rating Scale,[32] and American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form.[20,33] Because of the different assessment tools, we used the SMD to represent the pooled effect size. In the short-term subgroup, the meta-analysis showed that patients in the corticosteroid group exhibited a significant amelioration of shoulder function compared with PRP group (n = 260; SMD = −0.80; 95% CI, −1.42 to −0.18; P = .01; I2 = 82%, random-effects model; Fig. 4). In the medium-term subgroup, the results from 5 studies revealed that PRP injection improved the shoulder function with an SMD score of 0.35 compared with corticosteroid injection, but the difference was not statistically significant (n = 217; SMD = 0.35; 95% CI, −0.35−1.04; P = .33; I2 = 83%, random-effects model; Fig. 4). In the long-term subgroup, the results from 4 studies showed a similar effect (n = 182; SMD = 0.12; 95% CI, −0.73–0.96; P = .79; I2 = 87%, random-effects model; Fig. 4).
Figure 4.
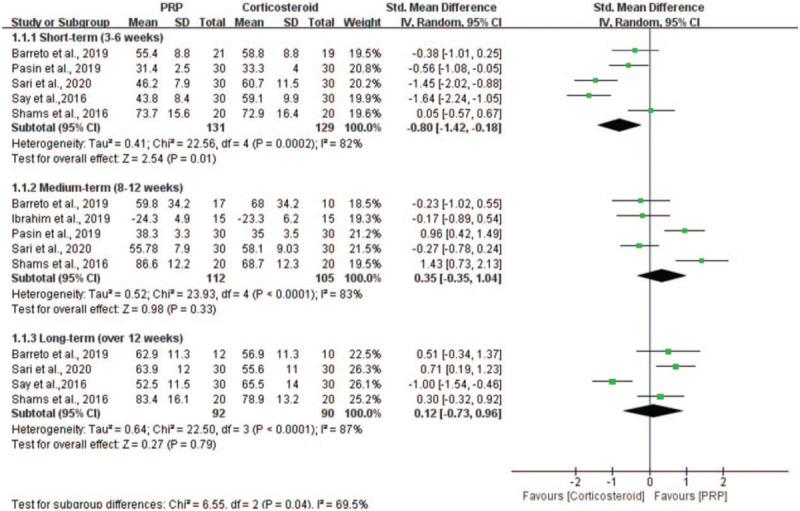
Forest plot for the function scores.
3.4.2. Primary outcome: pain relief
Four studies evaluated the effectiveness of PRP injection in comparison with corticosteroid injection on pain relief measured by the Visual Analogue Scale.[9,21,32,33] Because the measuring tool was the same, we applied the MD to represent the pooled effect size. In the short-term subgroup, the results indicated that the patients in the corticosteroid group had a significant reduction of shoulder pain compared with the patients in the PRP group (n = 180; MD = 1.59; 95% CI, 0.30−2.89; P = .02; I2 = 91%, random-effects model; Fig. 5). In the medium-term subgroup, the results revealed that PRP injection relieved the shoulder pain with a MD score of −0.17 compared with corticosteroid injection, but the difference was not statistically significant (n = 150; MD = −0.17; 95% CI, −0.97−0.63; P = .68; I2 = 76%, random-effects model; Fig. 5). In the long-term subgroup, the difference between the PRP and corticosteroid groups was also not statistically significant (n = 120; MD = 1.00; 95% CI, −3.31−5.31; P = .65; I2 = 99%, fixed-effects model; Fig. 5).
Figure 5.
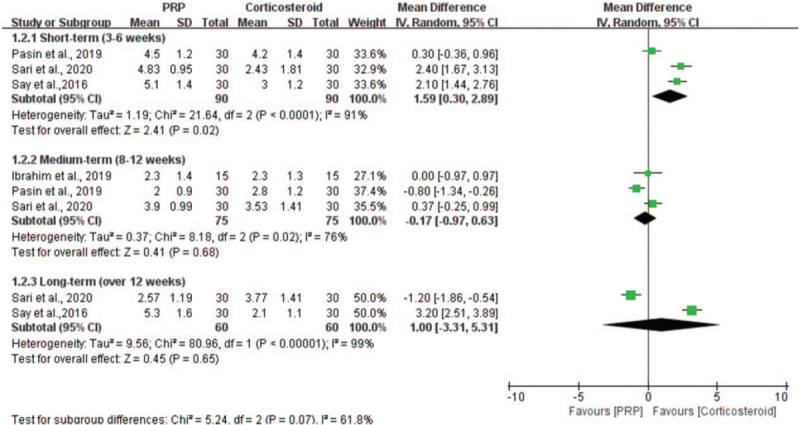
Forest plot for the pain scores.
3.4.3. Secondary outcome: ROM
Two studies assessed the efficacy of PRP injection in comparison with corticosteroid injection on ROM measured by the goniometer.[9,21] Because the measuring tool was the same, we applied the MD to represent the pooled effect size. The parameters reflecting the ROM of shoulder include flexion, abduction, internal rotation and external rotation. The results of the meta-analysis showed no significant differences between the PRP group and the corticosteroid group in the flexion (n = 90; MD = 2.39; 95% CI, −3.04−7.83; P = .86; I2 = 0%, fixed-effects model; Fig. 6), abduction (n = 90; MD = 4.71; 95% CI, −1.17−10.59; P = .12; I2 = 0%, fixed-effects model; Fig. 6), internal rotation (n = 90; MD = 0.48; 95% CI, −3.46−4.42; P = .81; I2 = 0%, fixed-effects model; Fig. 6) and external rotation (n = 90; MD = −0.39; 95% CI, −3.70−2.92; P = .82; I2 = 0%, fixed-effects model; Fig. 6) of shoulder.
Figure 6.
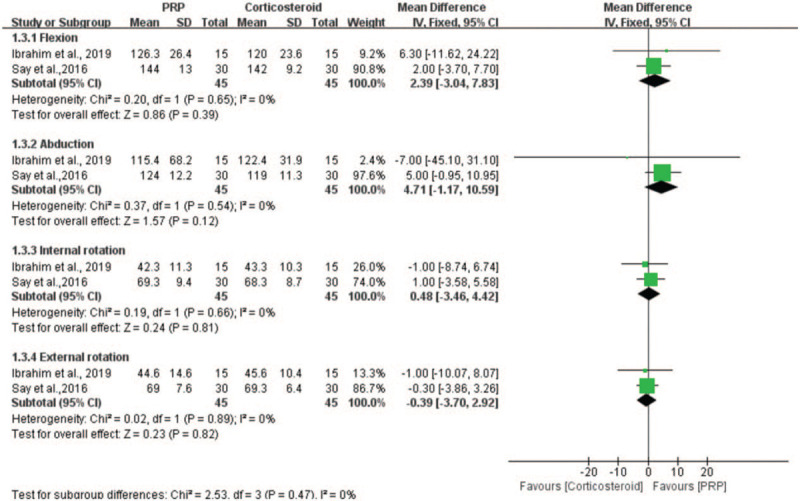
Forest plot for the ROM.
3.5. Publication bias
Visual inspection of the funnel plots did not reveal evidence of potential publication bias. The funnel plots are shown in Supplemental Digital Content 2.
4. Discussion
4.1. Summary and interpretation of main findings
Our meta-analysis revealed that corticosteroid injection yielded statistically significant superior functional recovery and pain relief compared with PRP injection for rotator cuff lesions during the short-term follow-up period (3–6 weeks). However, at the medium-term (8–12 weeks) and long-term (over 12 weeks) follow-up, no statistically significant difference was identified between the 2 groups. Regarding the ROM of shoulder, no significant difference was found between the 2 groups during the whole follow-up period (3 weeks-24 weeks).
In recent years, the efficacy of PRP injection vs corticosteroid injection in the treatment of a variety of musculoskeletal disorders including plantar fasciitis,[34] elbow epicondylitis,[35] knee osteoarthritis,[36] greater trochanteric pain syndrome,[37] and rotator cuff lesions[38] has been compared. Nevertheless, the efficacy of PRP injection vs corticosteroid injection as a conservative treatment for rotator cuff lesions remains uncertain. Corticosteroid injection was first introduced since the 1950 second[39] and has frequently been used in tendinous lesions.[10,40] A number of clinic trials have revealed the short-term effects of corticosteroid injection for the treatment of rotator cuff lesions,[41,42] however, no reliable evidence for its long-term effects. Recently published systematic reviews have shown that corticosteroid injection is superior to PRP injection in treatment of elbow epicondylitis [29,43] and hip osteoarthritis [44] in the short-term. Some new emerging RCTs have compared the efficacy of PRP vs corticosteroid injection in patients with rotator cuff lesions [9,32,33]; however, studies have shown controversial results. The current meta-analysis demonstrated that corticosteroid injection yielded statistically significant superior in short-term functional recovery and pain relief compared with PRP injection for rotator cuff lesions, and the result is in agreement with existing evidences. On the other hand, corticosteroid injection may have side effects such as permanent damage within the tendon ultrastructure, subcutaneous atrophy, relapse, effusion, systemic absorption, and subcutaneous tendon rupture.[14,45,46] Considering the short-term efficacy and potential side effects, alternative therapies were needed to improve the status of treatment.
Over the past few decades, the model of overuse injury or denaturation has been generally accepted to explain the pathophysiology of rotator cuff tendinopathy.[47] As we all know, the ability of tendons to regenerate is limited.[48] It has been hypothesized that the main cause of chronic tendinopathy is not inflammation but a lack of healing potential.[47] Therefore, new biological therapeutic agents such as PRP may be an option to treat this pathology. PRP contains growth factors, bioactive cytokines, and other chemokines, which are believed to promote tissue healing and induce tissue regeneration by improving cellular proliferation, augmenting cellular migration, accelerating angiogenesis, and increasing matrix deposition.[49,50] Several clinic trials[51,52] have shown that PRP injection had long-term effects. Cai et al [51] reported that treatment of patients with partial-thickness rotator cuff tears with PRP significantly improved rotator cuff function and pain at 12 months. And MRI results revealed that the size of rotator cuff tear in the PRP group was significantly decreased compared with normal saline group. Rha et al [52] indicated that PRP injection lead to a progressive amelioration in the disability and pain when compared to dry needling. This benefit was certainly still existing at 6 months after injection. Animal experiments suggested that the application of PRP significantly improved biomechanical and histologic properties of rotator cuff repair, resulting in increased vascular proliferation and fibroblastic response at all time points, which may be responsible for the long-term efficacy of PRP in the treatment of rotator cuff lesions.[53,54] Both corticosteroid and PRP can reduce inflammation, but by contrast, corticosteroid has no such biological regenerative property, and consequently its efficacy will be merely in decreasing inflammation and thus is short-lived. The present meta-analysis failed to demonstrate that PRP injection provided benefit in functional recovery and pain relief over corticosteroid injection at the medium-term and long-term follow-up, which was not in line with the findings of above studies. The possible reasons for these inconsistent results may be the diversity in the measurement tools, the different methods of preparation of PRP, and the differences in research design across the different studies.
ROM of shoulder is also an important indicator to assess the effect of various treatments. The parameters reflecting the ROM of shoulder include flexion, abduction, internal rotation and external rotation. In this meta-analysis, the results from 2 studies [9,21] revealed that the ROM outcome was comparable between the PRP and corticosteroid groups in the treatment of rotator cuff lesions. Ibrahim et al [9] found that the ROM of shoulder was significantly improved in both groups after injection, but the difference between the groups was not statistically significant. Say et al [21] found similar result; there was no significant difference in ROM of shoulder between the PRP and corticosteroid groups. On the other hand, Kothari et al [55] reported that PRP injection was better than corticosteroid injection in the treatment of periarthritis shoulder by improving passive and active ROM of shoulder. For the above inconsistent results, the possible reason is that the pathological mechanisms of periarthritis shoulder and rotator cuff lesions are different. Since the result of our systematic review regarding the efficacy of PRP injection on ROM of shoulder compared with the corticosteroid injection in patients with rotator cuff lesions is based on only 2 studies, the evidence is limited and further studies are needed.
4.2. Implication for clinical practice and future research
Our meta-analysis revealed short-term efficacy of corticosteroid injection and no significant medium- to long-term difference between corticosteroid and PRP injection in the treatment of rotator cuff lesions. Taking into account the short-term efficacy and potential side effects of corticosteroid injection, PRP as a new biological therapeutic agent may be preferred in clinical practice. Several issues should be considered when applying injection therapies. First, it is important to inject PRP with containing the appropriate concentrations and amounts of relevant components, such as white blood cells. Belk et al [56] found that leukocyte-poor PRP may be superior to leukocyte-rich PRP for treatment of knee osteoarthritis. Therefore, additional studies are needed to directly compare the efficacy of PRP injection with varying leucocyte content in treating rotator cuff lesions. Second, when PRP injection is used to treat rotator cuff lesions, the injection frequency, injection volume, and injection location should be strictly controlled. In a study by Vilchez-Cavazos et al,[57] multiple PRP injections seemed more effective in improving joint function than was a single PRP injection. Because the available evidence is still insufficient in this field, more research is needs to be conducted in the future to explore the optimal injection frequency, injection volume, and injection location in the treatment of rotator cuff lesions. Third, potential side effects are also a major concern after injection therapies. Local infection, skin adhesion, skin atrophy, exacerbation of pain, rashes, and fevers are commonly associated with injection therapies. Injection therapies will have less clinical value if there was a relatively high incidence of side effects. Therefore, future studies should pay attention to monitoring the complications of injection therapies, and clinicians should comprehensively evaluate the advantages and disadvantages of various injection therapies. Fourth, as far as we know, no study exists comparing the efficacy of PRP and corticosteroid on inflammatory cytokines in rotator cuff lesions, and thus this remains of interest for further study. Finally, the included studies had a relatively short duration of follow-up (up to 24 weeks). Double-blind, multicenter RCTs with longer follow-ups and larger groups of patients are needed to achieve more reliable results and to guide clinical practice.
4.3. Limitations
This systematic review has several limitations. Firstly, only 6 RCTs were included in our study and sample sizes were small. The limited number of trials and participants limits the strength of the evidence. Consequently, the results of this meta-analysis should be interpreted with caution. Secondly, for all of the included studies, PRP injection or corticosteroid injection was only administered once for each patient. Therefore, subgroup analysis based on the number of injections was not performed. Thirdly, the guidance method for injection may influence the treatment efficacy of PRP or corticosteroid. Two of the included trials in our meta-analysis used ultrasound-guided injection, 1 used blind injection, and no detailed descriptions regarding guidance method were available in the remaining trials. Thus, we could not compare the potential efficacy of different guidance methods. Fourthly, there was relatively high heterogeneity among included studies. The differences in the PRP preparation methods, injection volume, and PRP composition across each study may contribute to this heterogeneity. Lastly, all of the included studies were from developing countries, which may also introduce bias. Developed countries also need to conduct research in this field to provide sufficient evidence for clinical practice.
5. Conclusions
In patients with rotator cuff lesions, the current clinical evidence demonstrated that corticosteroid injection yielded statistically significant superior functional recovery and pain relief compared with PRP injection during the short-term follow-up period (3–6 weeks). However, at the medium-term (8–12 weeks) and long-term (over 12 weeks) follow-up, no statistically significant difference was identified between the 2 groups. Regarding the ROM of shoulder, no significant difference was found between the 2 groups during the whole follow-up period (3 weeks-24 weeks). Additional studies with longer follow-ups, larger sample sizes, and more rigorous designs are needed to draw more reliable and accurate conclusions.
Author contributions
Conceptualization: Chenglong Wang, Qingsan Zhu.
Formal analysis: Chenglong Wang, Xiangji Liu.
Methodology: Chenglong Wang, Yihang Ma.
Software: Chenglong Wang, Zhuo Zhang.
Supervision: Qingsan Zhu.
Writing – original draft: Chenglong Wang.
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, MD = mean difference, PRP = platelet-rich plasma, RCT = randomized controlled trial, ROM = range of motion, SMD = standardized mean difference.
How to cite this article: Wang C, Zhang Z, Ma Y, Liu X, Zhu Q. Platelet-rich plasma injection versus corticosteroid injection for conservative treatment of rotator cuff lesions: a systematic review and meta-analysis. Medicine. 2021;100:7(e24680).
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
The authors report no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article.
ASES = American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, CMS = Constant-Murley shoulder outcome score, Cor = corticosteroid, DASH = Disabilities of the Arm, Shoulder and Hand, MRI = Magnetic Resonance Imaging, PRP = platelet-rich plasma, RCT = randomized controlled trial, ROM = range of motion, SDQ = Shoulder Disability Questionnaire, SF-36 scores = Short Form 36 scores, SST = the Simple Shoulder Test, UCLA = University of California Los Angeles shoulder rating scale, VAS = visual analogue scale, WORC = Western Ontario Rotator Cuff Index.
References
- [1].Liang H, Zhu C, Iwata Y, et al. Feature extraction of shoulder joint's voluntary flexion-extension movement based on electroencephalography signals for power assistance. Bioengineering 2018;6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Via AG, Cupis MD, Spoliti M, et al. Clinical and biological aspects of rotator cuff tears. Muscles Ligaments Tendons J 2013;3:70–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gutiérrez-Espinoza H, Araya-Quintanilla F, Pinto-Concha S, et al. Effectiveness of supervised early exercise program in patients with arthroscopic rotator cuff repair: study protocol clinical trial. Medicine 2020;99:e18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cohen DB, Kawamura S, Ehteshami JR, et al. Indomethacin and celecoxib impair rotator cuff tendon to-bone healing. Am J Sports Med 2006;34:362–9. [DOI] [PubMed] [Google Scholar]
- [5].Dickinson RN, Ayers GD, Archer KR, et al. Physical therapy versus natural history in outcomes of rotator cuff tears: the Rotator Cuff Outcomes Workgroup (ROW) cohort study. J Shoulder Elbow Surg 2019;28:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Louwerens JKG, Sierevelt IN, Kramer ET, et al. Comparing ultrasound-guided needling combined with a subacromial corticosteroid injection versus high-energy extracorporeal shockwave therapy for calcific tendinitis of the rotator cuff: a randomized controlled trial. Arthroscopy 2020;36:1823–33. [DOI] [PubMed] [Google Scholar]
- [7].Walsh MR, Nelson BJ, Braman JP, et al. Platelet-rich plasma in fibrin matrix to augment rotator cuff repair: a prospective, single-blinded, randomized study with 2-year follow-up. J Shoulder Elbow Surg 2018;27:1553–63. [DOI] [PubMed] [Google Scholar]
- [8].Barreto RB, Azevedo AR, Gois MCd, et al. Platelet-rich plasma and corticosteroid in the treatment of rotator cuff impingement syndrome: randomized clinical trial. Trial Rev Bras Ortop 2019;54:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ibrahim DH, El-Gazzar NM, El-Saadany HM, et al. Ultrasound-guided injection of platelet rich plasma versus corticosteroid for treatment of rotator cuff tendinopathy: effect on shoulder pain, disability, range of motion and ultrasonographic findings. Egyptian Rheumatologist 2019;41:157–61. [Google Scholar]
- [10].Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. Rheumatology 2006;45:508–21. [DOI] [PubMed] [Google Scholar]
- [11].Wang W, Shi M, Zhou C, et al. Effectiveness of corticosteroid injections in adhesive capsulitis of shoulder: a meta-analysis. Medicine 2017;96:e7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Şahin Onat Ş, Biçer S, Şahin Z, et al. Effectiveness of kinesiotaping and subacromial corticosteroid injection in shoulder impingement syndrome. Am J Phys Med Rehabil 2016;95:553–60. [DOI] [PubMed] [Google Scholar]
- [13].Li S, Wang K, Sun H, et al. Clinical effects of extracorporeal shock-wave therapy and ultrasound-guided local corticosteroid injections for plantar fasciitis in adults: a meta-analysis of randomized controlled trials. Medicine 2018;97:e13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Puzzitiello RN, Patel BH, Nwachukwu BU, et al. Adverse impact of corticosteroid injection on rotator cuff tendon health and repair: a systematic review. Arthroscopy 2020;36:1468–75. [DOI] [PubMed] [Google Scholar]
- [15].Ancer-Arellano J, Villarreal-Martinez L, Vazquez-Martínez O, et al. Consistency and reproducibility in the platelet-rich plasma preparation method: mexican experience. Int J Lab Hematol 2019;41:e148–51. [DOI] [PubMed] [Google Scholar]
- [16].Amable PR, Carias RB, Teixeira MV, et al. Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 2013;4:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pauly S, Klatte-Schulz F, Stahnke K, et al. The effect of autologous platelet rich plasma on tenocytes of the human rotator cuff. BMC Musculoskelet Disord 2018;19:422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anitua E, Andia I, Ardanza B, et al. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost 2004;91:4–15. [DOI] [PubMed] [Google Scholar]
- [19].Kim SJ, Yeo SM, Noh SJ, et al. Effect of platelet-rich plasma on the degenerative rotator cuff tendinopathy according to the compositions. J Orthop Surg Res 2019;14:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shams A, El-Sayed M, Gamal O, et al. Subacromial injection of autologous platelet-rich plasma versus corticosteroid for the treatment of symptomatic partial rotator cuff tears. Eur J Orthop Surg Traumatol 2016;26:837–42. [DOI] [PubMed] [Google Scholar]
- [21].Say F, Gurler D, Bulbul M. Platelet-rich plasma versus steroid injection for subacromial impingement syndrome. J Orthop Surg 2016;24:62–6. [DOI] [PubMed] [Google Scholar]
- [22].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010;376:1751–67. [DOI] [PubMed] [Google Scholar]
- [24].Lin MT, Wei KC, Wu CH. Effectiveness of platelet-rich plasma injection in rotator cuff tendinopathy: a systematic review and meta-analysis of randomized controlled trials. Diagnostics 2020;10:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 Updated March 2011. London, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- [26].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res 1993;2:121–45. [DOI] [PubMed] [Google Scholar]
- [28].Siqi T, Xiaoshuai W, Peihui W, et al. Platelet-rich plasma vs autologous blood vs corticosteroid injections in the treatment of lateral epicondylitis: a systematic review, pairwise and network meta-analysis of randomized controlled trials. PM R 2020;12:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li A, Wang H, Yu Z, et al. Platelet-rich plasma vs corticosteroids for elbow epicondylitis: a systematic review and meta-analysis. Medicine 2019;98:e18358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Dragomir M, Jennifer K, Derek B, et al. Effect of biceps tenodesis on speed of recovery after arthroscopic rotator cuff repair. JSES Int 2020;4:341–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Amos S, Joshua D, JGregory DA, et al. Comparative time to improvement in nonoperative and operative treatment of rotator cuff tears. J Bone Joint Surg Am 2020;102:1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pasin T, Ataoğlu S, Pasin Ö, et al. Comparison of the effectiveness of platelet-rich plasma, corticosteroid, and physical therapy in subacromial impingement syndrome. Arch Rheumatol 2019;34:308–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sari A, Eroglu A. Comparison of ultrasound-guided platelet-rich plasma, prolotherapy, and corticosteroid injections in rotator cuff lesions. J Back Musculoskelet Rehabil 2020;33:387–96. [DOI] [PubMed] [Google Scholar]
- [34].Tabrizi A, Dindarian S, Mohammadi S. The effect of corticosteroid local injection versus platelet-rich plasma for the treatment of plantar fasciitis in obese patients: a single-blind. Randomized Clinical Trial J Foot Ankle Surg 2020;59:64–8. [DOI] [PubMed] [Google Scholar]
- [35].Varshney A, Maheshwari R, Juyal A, et al. Autologous platelet-rich plasma versus corticosteroid in the management of elbow epicondylitis: a randomized study. Int J Appl Basic Med Res 2017;7:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Elksniņš-Finogejevs A, Vidal L, Peredistijs A. Intra-articular platelet-rich plasma vs corticosteroids in the treatment of moderate knee osteoarthritis: a single-center prospective randomized controlled study with a 1-year follow up. J Orthop Surg Res 2020;15:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Begkas D, Chatzopoulos ST, Touzopoulos P, et al. Ultrasound-guided platelet-rich plasma application versus corticosteroid injections for the treatment of greater trochanteric pain syndrome: a prospective controlled randomized comparative clinical study. Cureus 2020;12:e6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Von Wehren L, Blanke F, Todorov A, et al. The effect of subacromial injections of autologous conditioned plasma versus cortisone for the treatment of symptomatic partial rotator cuff tears. Knee Surg Sports Traumatol Arthrosc 2016;24:3787–92. [DOI] [PubMed] [Google Scholar]
- [39].Hollander JL. Intra-articular hydrocortisone in arthritis and allied conditions; a summary of two years’ clinical experience. J Bone Joint Surg Am 1953;35:983–90. [PubMed] [Google Scholar]
- [40].Cho CH, Jin HJ, Kim DH. Comparison of clinical outcomes between idiopathic frozen shoulder and diabetic frozen shoulder after a single ultrasound-guided intra-articular corticosteroid injection. Diagnostics 2020;10:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim YS, Jin HK, Lee HJ, et al. Is it safe to inject corticosteroids into the glenohumeral joint after arthroscopic rotator cuff repair? Am J Sports Med 2019;47:1694–700. [DOI] [PubMed] [Google Scholar]
- [42].Shin SJ, Do NH, Lee J, et al. Efficacy of a subacromial corticosteroid injection for persistent pain after arthroscopic rotator cuff repair. Am J Sports Med 2016;44:2231–6. [DOI] [PubMed] [Google Scholar]
- [43].Huang K, Giddins G, Wu LD. Platelet-rich plasma versus corticosteroid injections in the management of elbow epicondylitis and plantar fasciitis: an updated systematic review and meta-analysis. Am J Sports Med 2020;48:2572–85. [DOI] [PubMed] [Google Scholar]
- [44].Zhao Z, Ma JX, Ma XL. Different intra-articular injections as therapy for hip osteoarthritis: a systematic review and network meta-analysis. Arthroscopy 2020;36:1452–64. [DOI] [PubMed] [Google Scholar]
- [45].Krogh TP, Fredberg U, Stengaard-Pedersen K, et al. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. Am J Sports Med 2013;41:625–35. [DOI] [PubMed] [Google Scholar]
- [46].Rees JD, Maffulli N, Cook J. Management of tendinopathy. Am J Sports Med 2009;37:1855–67. [DOI] [PubMed] [Google Scholar]
- [47].Lewis JS. Rotator cuff tendinopathy. Br J Sports Med 2009;43:236–41. [DOI] [PubMed] [Google Scholar]
- [48].Ho JO, Sawadkar P, Mudera V. A review on the use of cell therapy in the treatment of tendon disease and injuries. J Tissue Eng 2014;5:2041731414549678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bos-Mikich A, de Oliveira R, Frantz N. Platelet-rich plasma therapy and reproductive medicine. Assist Reprod Genet 2018;35:753–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Baksh N, Hannon CP, Murawski CD, et al. Platelet-rich plasma in tendon models: a systematic review of basic science literature. Arthroscopy 2013;29:596–607. [DOI] [PubMed] [Google Scholar]
- [51].Cai YU, Sun Z, Liao B, et al. Sodium hyaluronate and platelet-rich plasma for partial-thickness rotator cuff tears. Med Sci Sports Exerc 2019;51:227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rha DW, Park GY, Kim YK, et al. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil 2013;27:113–22. [DOI] [PubMed] [Google Scholar]
- [53].Ersen A, Demirhan M, Atalar AC, et al. Platelet-rich plasma for enhancing surgical rotator cuff repair: evaluation and comparison of two application methods in a rat model. Arch Orthop Trauma Surg 2014;134:405–11. [DOI] [PubMed] [Google Scholar]
- [54].Beck J, Evans D, Tonino PM, et al. The biomechanical and histologic effects of platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med 2012;40:2037–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kothari SY, Srikumar V, Singh N. Comparative efficacy of platelet rich plasma injection, corticosteroid injection and ultrasonic therapy in the treatment of periarthritis shoulder. J Clin Diagn Res 2017;11:RC15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Belk JW, Kraeutler MJ, Houck DA, et al. Platelet-rich plasma versus hyaluronic acid for knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Am J Sports Med 2020. 363546520909397.Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [57].Vilchez-Cavazos F, Millán-Alanís JM, Blázquez-Saldaña J, et al. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop J Sports Med 2019;7:2325967119887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


