Abstract
Objective:
This study aimed to evaluate and compare the long-term therapeutic efficacy of radiofrequency ablation (RFA) versus that of surgical resection in small hepatocellular carcinoma (HCC).
Methods:
Relevant articles in English from PubMed, EMBASE, and the Cochrane Library were retrieved. Pooled hazard ratios (HRs) were calculated to assess the prognostic value of RFA compared with that of surgical resection.
Results:
A total of 19 studies involving 15,071 patients were included. The combined HRs (95% confidence interval [CI]) of RFA for recurrence/relapse-free survival (RFS) and overall survival (OS) were 1.55 (95% CI = 1.29-1.86, I2 = 72.5%) and 1.61 (95% CI = 1.29-2.01, I2 = 60.4%), respectively, compared with surgical resection. In subgroup analyses according to study design, both RFS and OS of the prospective subgroups showed statistical significance, and no statistical heterogeneity existed between studies.
Conclusion:
Our clinical data suggest that surgical resection offers better long-term oncologic outcomes than RFA.
Keywords: hepatocellular carcinoma, long-term efficacy, meta-analysis, radiofrequency ablation, surgical resection
1. Introduction
Liver cancer is the third leading cause of mortality worldwide and includes hepatocellular carcinoma (HCC) and other primary liver cancers (for example, intrahepatic cholangiocarcinoma).[1] Treatment options for HCC currently include liver transplantation, surgical resection, radiofrequency ablation (RFA), transarterial chemoembolization, and drug therapy.[2] Based on the current guidelines of the European Association for the Study of the Liver and American Association for the Study of Liver Diseases, surgical resection and RFA, along with liver transplantation, are the recommended treatment modalities for very small HCC, based on the presence or absence of portal hypertension and associated diseases.[3,4]
While liver transplantation is regarded as an ideal choice for patients with small HCC, this option is significantly limited due to organ shortages. According to the Barcelona Clinic Liver Cancer (BCLC) system, early HCC (BCLC 0 or A) should be treated with surgical resection or RFA.[3] Although surgical resection causes greater damage to the non-tumor liver parenchyma, it is generally preferred for small HCCs because surgical resection offers the therapeutic possibility of complete eradication of satellite tumor lesions and microscopic tumor emboli in the adjacent vasculature.[5,6] RFA, which involves heating the tumor and surrounding liver tissue, is generally accepted as a priority treatment in patients with impaired liver functional reserve because of its excellent efficacy and safety as well as better tolerability.[7–9]
To date, several studies have compared the curative effects of RFA and surgical resection for HCC. The optimal management choice considering long-term overall and recurrence/relapse-free survival for patients with small HCC is a matter of debate. Several researchers have reported higher overall or disease-free survival in patients who underwent surgical resection compared to that in patients who underwent RFA.[10–12] In contrast, no such correlation was observed by Lee et al (2017).[13] To clarify this issue, a meta-analysis was designed to compare the long-term oncologic outcomes of RFA and surgical resection in patients with small HCC.
2. Materials and methods
2.1. Registration
This systematic review including a meta-analysis was prospectively registered with the PROSPERO International Prospective Register of Systematic Reviews (PROSPERO identifier CRD42020152746).[14] All analyses were based on previously published studies; thus, no ethical approval and patient consent are required.
2.2. Inclusion criteria and literature source retrieval strategy
A systematic search of PubMed, Embase, and the Cochrane Library (2006–2017) was performed. The following search items were listed: (“hepatocellular carcinoma” [All Fields]) AND (“percutaneous local ablative therapy” [All Fields] OR “radiofrequency ablation” [All Fields]) AND (“hepatectomy” [All Fields] OR “resection” [All Fields]). To be considered eligible for this meta-analysis, the enrolled studies were required to include comparative data on clinical efficacy (overall survival [OS] and adverse events of HCC [RFS]). The inclusion criteria were as follows:
-
(1)
trial design: studies with comparative data on clinical effectiveness and adverse events of RFA and surgical resection for HCC;
-
(2)
clearly documented indications for use of RFA and surgical resection;
-
(3)
treatment design: RFA vs surgical resection or surgical resection vs. RFA; and
-
(4)
characteristics of patients.
Studies were required to include relatively integrated information (demography and basic characteristics) on the enrolled patients, such as average age, percentage of males, Child-Pugh class, and tumor size. Abstracts, letters, editorials and expert opinions, reviews without original data, case reports, and studies lacking control groups were excluded in addition to studies that focused on unresectable HCC or HCC recurrence after hepatectomy, had no clearly reported outcomes of interest, evaluated patients with cholangiocellular carcinomas or liver metastases, or employed a combination of surgical resection and ablation in patients with HCC.
2.3. Data extraction
Data extraction was independently conducted by two reviewers using standardized methods. Any disagreements were settled by discussion of the relevant study data and adjudicated by an experienced reviewer (Table 1). From each study, the following data were extracted: publication details (name of the first author and year of publication) and study characteristics (study design, age, gender, study design, tumor size, Child–Pugh class, endpoints).
Table 1.
Characteristics of the included studies.
| Study | Year | Country | study design | Study period | Follow-up duration | Median age (range), years | No. of patients | Child-Pugh Class (A/B/C) | End points | Sex (M/F) | Tumor Size (cm) (Range) |
| Min-Shan Chen et al. (2006) | 2006 | China | Pro | 1999.11–2004.6 | 5 yr | RFA Median (range): 51.9 (11.2) | RFA 71, | NA | DFS OS | RFA56/15 | HCC ≤5 cm |
| SR Median (range): | SR 90 | SR 75/15 | |||||||||
| 49.4 (10.9) | |||||||||||
| Abu et al. (2008) | 2008 | Italy | Retro | 1999-2003 | 5 yr | RFA Median 67 | RFA 34, | Child-Pugh A/B | DFS OS | RFA27/7 | HCC ≤5 cm |
| SR Median 65 | SR 34 | SR 26/8 | |||||||||
| Ji-Wei Huang et al. (2010) | 2010 | China | Pro | 2003.3–2005.1 | 5 yr | RFA Mean (SD): | RFA 115, | Class A/B | RFS OS | RFA79/36 | HCC conforming to |
| 56.57 (14.30) | SR 115 | SR 85/30 | Milan criteria | ||||||||
| SR Mean (SD): | ≤5cm | ||||||||||
| 55.91 (12.68) | |||||||||||
| Hiroki et al. (2011) | 2011 | Japan | Retro | 2004-2010 | RFA 68.4 ± 8.7 | RFA 164 | Class A/B | RFS OS | RFA95/67 | RFA 2.68 ± 0.49 | |
| SR 67.4 ± 9.7 | SR 69 | SR 50/19 | SR 2.88 ± 1.06 | ||||||||
| Hung-Hsu Hung et al.(2011) | 2011 | Taipei | Retro | 2002-2007 | 60 mo | RFA 67.42 ± 11.45 | RFA 190 | Child-Pugh A/B | OS | RFA 121/69 | RFA 2.37 ± 0.92 |
| SR 60.07 ± 12.56 | SR 229 | SR 184/45 | SR 2.88 ± 1.06 | ||||||||
| Kai Feng et al. (2012) | 2012 | China | Pro | 2005.1–2008.3 | 3 yr | RFA Median (range): | RFA 84, | Class A/B | RFS OS | RFA79/5 | Small HCC |
| 51 (24–83) | SR 84 | SR 75/9 | |||||||||
| SR Median (range): | |||||||||||
| 47 (18–76) | |||||||||||
| Kiong-Ming Wong et al.(2012) | 2012 | China | Retro | 2004-2009 | Median 36mo, | RFA 63.5 ± 13 | RFA 36 | Child-Pugh class A | RFS | RFA 18/18 | RFA 1.9 ± 0.6 |
| range 6–60 mo | SR55.1 ± 12 | SR 46 | SR 30/16 | SR 2.1 ± 0.6 | |||||||
| Jing-Houng Wang et al. (2012) | 2012 | China | Retro | RFA2.5 years (1.3–4.0) | RFA2.5 yr (1.3–4.0) | NA | RFA91 | NA | OS | RFA60/31 | ≤2cm |
| SR 2.3years (1.5–3.7) | SR 2.3 yr (1.5–3.7) | SR 52 | DFS | SR 38/24 | |||||||
| Kiyoshi Hasegawa et al.(2013) | 2013 | Japan | Retro | 2000-2005 | 2.16 yr | RFA 69 (52, 80) | RFA 5548 | Child-Pugh A/B | OS | RFA 3569/1979 | RFA 20 (10, 30) |
| SR 66 (48, 77) | SR 5361 | SR 3967/1394 | SR 23 (12, 30) | ||||||||
| Kastunori Imai et al. (2013) | 2013 | Japan | Retro | 2000.1–2011.4 | RFA 38.3 mo | RFA 66.8 +-8.3 | RFA 51 | Child-Pugh class A | OS DFS | RFA25/26 | ≤2cm |
| SR 49.0 mo | SR 68 61.5 +-10.1 | SR 38 | or B | SR 25/13 | |||||||
| Maurizio Pompili et al. (2013) | 2013 | Italy | Retro | 1999-2010 | RFA 41 (33–126) mo | RFA 68 (36–88) | RFA109 | Child-Pugh class A | OS DFS | RFA175/123 | RFA 2.3 (1.0–3.0) |
| SR 38 (6–132) mo | SR 67 (35–85) | SR 99 | SR 200/46 | SR 2.5 (0.8–3.0) | |||||||
| Zhi-Peng Zhou et al. (2013) | 2014 | China | Retro | 2003.7–2008.8 | 5 yr | RFA 46.7 ± 9.8 | RFA31 | Child-Pugh class A | TFS | RFA20/11 | <2cm |
| SR 42.2 ± 7.6 | SR 21 | or B | SR 15/6 | ||||||||
| Hyo-Joon Yang et al. (2014) | 2014 | Korea | Retro | 2005.1–2006.12 | 8 yr | RFA 57.2+- 9.2, | RFA 79, | Child-Pugh class A/B | OS | RFA59/20 | ≤3cm |
| SR 55.7 +- 10.6 | SR 52 | SR 38/14 | |||||||||
| G.-A. Kim et al. (2015) | 2015 | Korea | Retro | 2000-2009 | RFA 61 (i.q.r. 40–82) | RFA 57·3 (10·3) | RFA 331, | RFS | RFA 260/71 | ≤3cm | |
| SR 66 (52–88) | SR 54·4 (8·5) | SR 273 | SR 205/68 | ||||||||
| Arnaud Hocquelet et al. (2015) | 2015 | France | Retro | 2004.1–2013.12 | January 2004 to 30 March 2014 | RFA 65 (56–74) | RFA 178, | Child-Pugh class A | OS | RFA148/30 | RFA 22 (20–28) |
| SR68 (61–74) | SR 103 | or B | SR 82/21 | SR 35 (25–40) | |||||||
| Po-Hong Liu et al. (2016) | 2016 | China | Retro | 2002-2013 | RFA 44 mo | RFA 64 | RFA128 | NA | OS DFS | RFA84/66 | RFA 2.0 (mean) |
| SR 43 mo | SR 60 | SR 109 | SR 78/72 | SR 2.6 (mean) | |||||||
| Tian-Pei Guan et al.(2016) | 2016 | China | Retro | 2006.1–2010.5 | 5 yr | RFA 54.02 ± 7.66 | RFA 102 | Child-Pugh A/B | RFS OS | RFA 90/12 | Single HCC≤5 |
| SR 52.48 ± 8.36 | SR 92 | SR 75/17 | no more than 3 lesions with the largest nodule 3 cm in diameter | ||||||||
| Kenichi Takayasu et al.(2017) | 2017 | Japan | Pro | 2000.1–2007.12 | 8 yr | 67.0 ± 8.47 | RFA 491, | Child-Pugh A/B | RFS OS | RFA297/194 | 1.47 (0.36) |
| SR 176 | SR104/72 | ||||||||||
| Seung-Ho Lee et al.(2017) | 2017 | Korea | Retro | 2008-2010 | 45 mo (range, 1–73 mo) | RFA 62 (38–88) | RFA 36 | Barcelona Clinic Liver Cancer (BCLC) stage A | PFS OS | RFA 29 /7 | RFA 3.8 (3.1–5.0) |
| SR 56 (33–76) | SR 151 | SR 111/39 | SR 4.0 (3.1–5.0) |
2.4. Statistical analysis
In this meta-analysis, recurrence/relapse-free survival (RFS), disease-free survival (DFS), progression-free survival (PFS), and tumor-free survival (TFS) in the included studies were combined and redefined as RFS. DFS was defined as the period after successful treatment in which there were no symptoms or effects of the disease. For PFS analysis, follow-up durations were estimated from the date of initial treatment to the date of disease progression.[13] TFS was defined as the time from the start of treatment to the appearance of recurrence or metastasis.[29] In general, all these terms are not synonymous but can at times represent the same outcomes. These outcomes can be reflected as local or systemic recurrences. Overall survival (OS) was defined as the time from therapy initiation until death regardless of the cause.[15,16] In terms of the effect size of each study, hazard ratio (HR) and 95% confidence interval (CI), taking into account the number and time of events, were calculated. HR referred to the sum of the differences between Kaplan-Meier survival curves and represented comparative data between two groups during a certain follow-up period. Data regarding multivariate HR and 95% CI were directly extracted from the studies. In cases where multivariate HR data were not available, univariate HR data were extracted. If both multivariate and univariate HRs were unavailable, the methodology recommended by Tierney et al[17] was applied to reconstruct HR estimates and variations based on survival data read from Kaplan-Meier survival curves using the Engauge Digitizer (version 9.4). HR> 1 implies poorer survival in patients who underwent RFA than in patients who underwent surgical resection, whereas HR< 1 implies a survival benefit in patients who underwent RFA. RevMan version 5.3 (RevMan version 5.3; The Nordic Cochrane Center, The Cochrane Collaboration) and STATA version 12.0 (STATA Corp., College Station, TX) were employed for statistical analysis. Statistical heterogeneity was measured using the chi-squared Q test and I2 statistic. Heterogeneity was considered to be > 50% 2. A fixed-effects model was used for meta-analysis when heterogeneity was not significant and a random-effects model was applied in case of significant heterogeneity. Begg and Egger tests were used to evaluate bias using STATA version 12.0. P values < .05 were considered statistically significant.
3. Results
3.1. Search results
The search process is presented in Figure 1. Searches were conducted on three databases, initially including 137, 1453, and 0 articles (1,590 in total) from Embase, PubMed, and Cochrane Library, respectively. After excluding duplications and meeting summaries, 142 qualified full-text articles were left, 85 articles that did not meet the study design were removed, 6 studies did not describe liver cancer, 3 study introduced a case report, and there was no reliable data in 10 studies. Finally, 19 studies including 15,071 patients that met the conditions of the study, published between 2006 and 2017, were included in the meta-analysis[10–13,18–30] (Fig. 1). All 19 studies reported at least one item of the applicable results.
Figure 1.
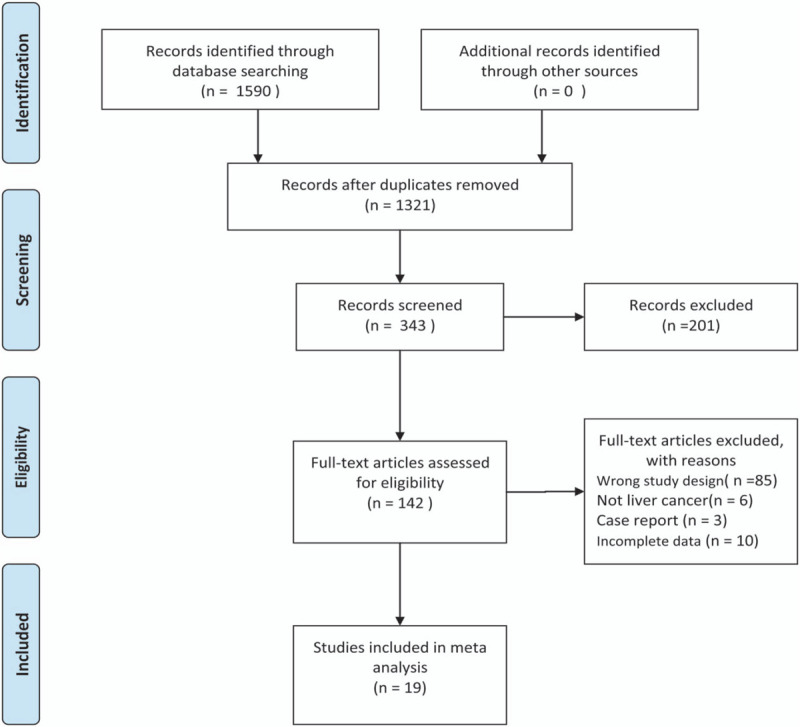
Flow diagram of study selection.
3.2. Study characteristics
Almost all the studies were conducted in Asia (9 in China, 4 in Japan, 3 in Korea,1 one in France, and 2 in Italy). Three of the included studies were prospective studies and 16 were retrospective, and one study was prospective. In total, 7 studies analyzed RFS (including 1,945 patients), 6 analyzed DFS (including 838 patients), 1 analyzed PFS (including 187 patients), 1 analyzed TFS (including 52 patients), and 14 analyzed OS (including 14,024 patients). The follow-up duration of 7 studies was ≥5 years. Tumor sizes in 10 studies were < 3 cm, with 9 studies involving tumor sizes of 0 to 5 cm. There is a huge difference in the sample size of one study compared to the others. In fact, the study by Hasegawa et al (2013)[31] included 74% of all patients evaluated overall in the 19 studies. Details of all studies were recorded, including the study period, follow-up duration, age, and number of patients (Table 1).
3.3. Literature quality evaluation
The quality of 19 studies was assessed according to the CRITICAL APPRAISAL OF PROGNOSTIC STUDIES
(https://www.cebm.net/wp-content/uploads/2018/11/Prognosis.pdf; Fig. 2). Generally, the included studies were of high quality. Four of the studies were assessed as high risk and 3 as unclear risk of bias in the domain of defined representative sample measurements, since few were non-blinded or non-randomized. In the domain of prognostic factor, that is, follow-up time measurements, there was a high risk of bias in 2 studies and an unclear risk of bias in 3 studies owing to missing follow-up data or short follow-up time. The majority of studies were well described and monitored for adverse events based on objective criteria. In the domain of prognostic factor follow-up time measurements, there was a high risk of bias in 2 studies and an unclear risk of bias in 5 studies when monitoring for adverse events based on objective criteria.
Figure 2.
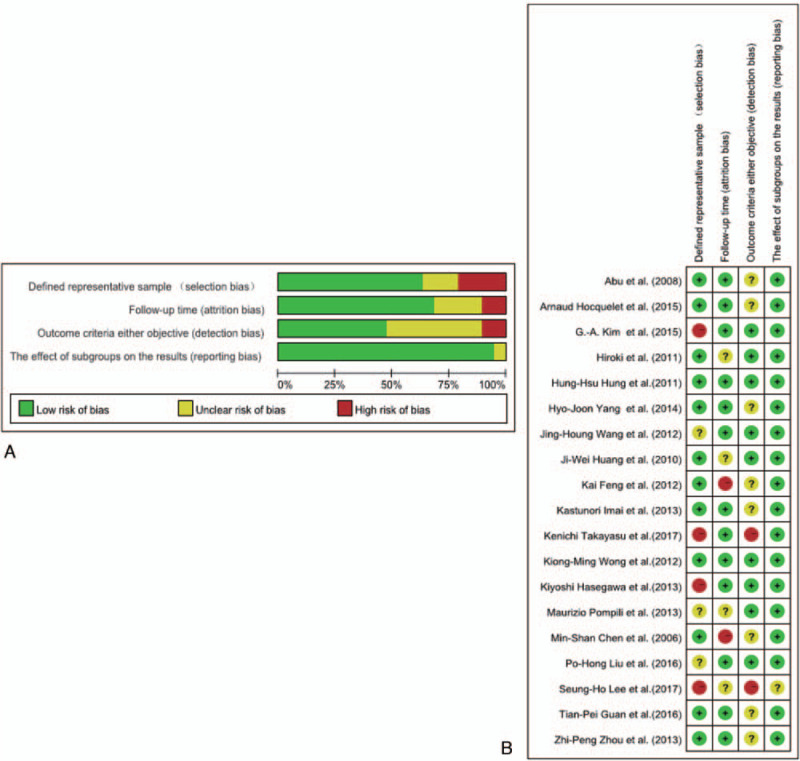
A Risk of bias graph: review author judgments on each risk of bias item presented as percentages across all included studies. B Risk of bias summary: review author judgements on individual risk of bias items for each included study.
3.4. Primary outcome: RFS
In total, 15 studies (3,323 patients) analyzed RFS as the primary endpoint. After combining HR, poorer RFS was predicted with RFA than with surgical resection. The random-effects model (HR = 1.55, 95% CI = 1.29–1.86, I2 = 72.5%; Fig. 3) showed statistical significance and heterogeneity existed between studies. Potential publication biases were assessed using Funnel plots and 2 statistical (Begg and Egger) tests. Data from Funnel plots (Fig. 4) and Begg (P = .373) and Egger (P = .342) tests (Supplemental Figure 1) indicated no significant publication biases. A sensitivity analysis was conducted to further estimate the impact on combined HRs (Fig. 5). Systematic exclusion of each study did not result in significant changes, suggesting that the results were stable.
Figure 3.
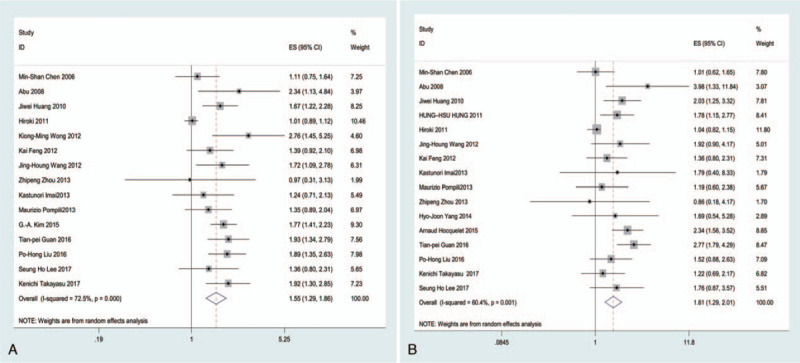
Forest plots of HR for RFS and OS in relation to the patients treated with RFA and surgical resection (A, RFS; B, OS). Chi-squared test is a measurement of heterogeneity. P < .05 indicates significant heterogeneity (Squares = individual study point estimates. Horizontal lines = 95% CI. Rhombus = summarized estimate and its 95% CI. Fixed: fixed-effects model.). ES indicates hazard ratio.
Figure 4.
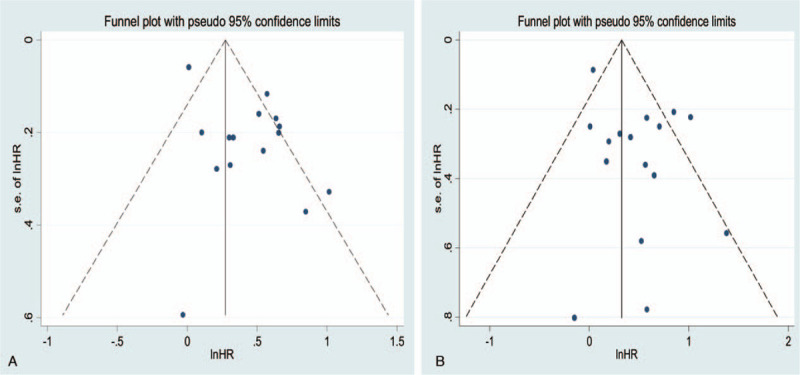
Funnel plots for RFS and OS in relation to the patients treated with RFA and surgical resection (A, RFS; B, OS). The pseudo 95% confidence interval (CI) was computed as part of the analysis to produce the Funnel plots and corresponded to the expected 95% CI for a given standard error (SE). HR indicates hazard ratio.
Figure 5.
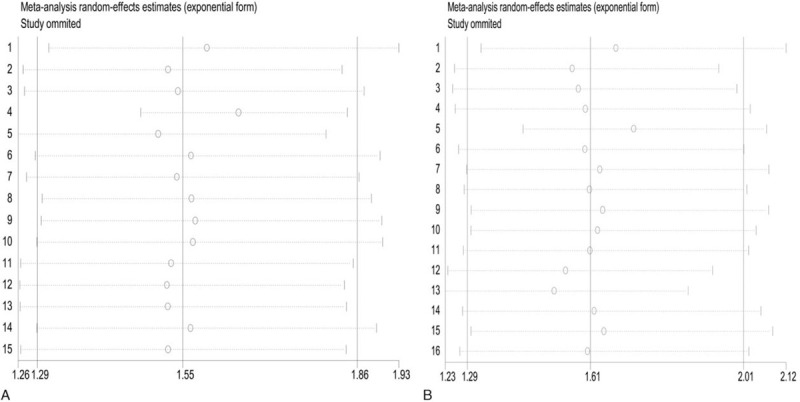
Sensitivity analysis of RFS and OS in relation to the patients treated with RFA and surgical resection.
Additional subgroup analyses were performed according to region, study design, tumor size, and endpoint (Table 2). Among the studies including RFS as an endpoint, 13 conducted in Asia had an HR of 1.54 (95% CI = 1.27–1.88, I2 = 75.3%), and 2 conducted in Europe showed significant correlations (HR = 1.08, 95% CI = 1.08–2.11, I2 = 39.2%). In terms of study design, the HR of 4 prospective studies was 1.52 (95% CI = 1.26–1.82, I2 = 31.6%) and that of 11 retrospective studies was 1.58 (95% CI = 1.25–2.00, I2 = 77.2%). According to the included tumor size, groups were divided into ≤3 and ≤5 cm subgroups. Meta-analyses illustrated that subgroups with tumor sizes of 3 cm had an HR of 1.56 (95% CI = 1.20–2.03, I2 = 77.2%) and those with tumor sizes ≤ 5 cm had an HR of 1.55 (95% CI = 1.27–1.79, I2 = 17.1%). Based on the endpoint, eligible studies were divided into RFS, DFS, PFS, and TFS groups. Subgroup analyses showed that the combined HRs of RFS and DFS were 1.64 (95% CI = 1.23–2.19, I2 = 85.4%) and 1.51 (95% CI = 1.27–1.81, I2 = 24.0%). No significant results were obtained for the PFS (HR = 1.36, 95% CI = 0.30–2.31) and TFS (HR = 0.97, 95% CI = 0.30–3.11) groups.
Table 2.
Subgroup of EFS and OS in relation to the patients treated with RFA and surgical resection.
| Endpoint | Factor | No. of studies | No. of patients | Heterogeneity test (I2) | Effect model | HR | 95%CI of HR |
| RFS | region | ||||||
| Asia | 13 | 3047 | 75.3 | radom | 1.54 | 1.27,1.88 | |
| Europe | 2 | 276 | 39.2 | fixed | 1.54 | 1.08,2.11 | |
| tumor size | |||||||
| ≤3cm | 9 | 2246 | 80.1 | radom | 1.56 | 1.20,2.03 | |
| ≤5cm | 6 | 1077 | 18.5 | fixed | 1.55 | 1.27,1.79 | |
| study design | |||||||
| Retro | 11 | 3097 | 77.2 | radom | 1.58 | 1.25,2.00 | |
| Pro | 4 | 1226 | 31.6 | fixed | 1.52 | 1.26,1.82 | |
| endpoint | |||||||
| RFS | 7 | 2178 | 85.4 | radom | 1.64 | 1.23,2.19 | |
| DFS | 6 | 906 | 24.0 | fixed | 1.51 | 1.27,1.81 | |
| PFS | 1 | 187 | - | - | 1.36 | 0.80,2.31 | |
| TFS | 1 | 52 | - | - | 0.97 | 0.30,3.11 | |
| OS | region | ||||||
| Asia | 13 | 13768 | 12.6 | fixed | 1.66 | 1.40,1.97 | |
| Europe | 3 | 557 | 83.0 | radom | 1.67 | 0.94,2.95 | |
| tumor size | |||||||
| ≤3cm | 8 | 12383 | 0.0 | fixed | 1.13 | 0.97,1.30 | |
| ≤5cm | 8 | 2002 | 49.1 | fixed | 1.89 | 1.58,2.26 | |
| study design | |||||||
| Retro | 12 | 13099 | 67.3 | radom | 1.74 | 1.30,2.33 | |
| Pro | 4 | 1226 | 27.5 | fixed | 1.37 | 1.06,1.77 | |
3.5. Primary outcome: OS
In total, 16 studies (17,025 patients) analyzed OS as the primary endpoint. After combining HR, poorer OS was predicted for RFA than for surgical resection. The random-effects model (HR = 1.61, 95% CI = 1.29–2.01, I2 = 60.4%; Fig. 3) showed statistical significance and heterogeneity existed between studies. Potential publication biases were statistically assessed using Funnel plots, Begg test and Egger test. Data from the Funnel plots (Fig. 4), Begg test (P = .62), and Egger (P = .051) test (Supplemental Figure 1) indicated no significant publication biases. A sensitivity analysis was conducted to further estimate the impact on combined HRs (Fig. 5). Systematic exclusion of each study did not result in significant changes, suggesting that the results were stable.
Additional subgroup analyses were performed according to region, study design, and tumor size (Table 2). Among the studies including OS as an endpoint, 13 conducted in Asia had an HR of 1.66 (95% CI = 1.40–1.97, I2 = 12.6%) and 3 in Europe had an HR of 1.67 (95% CI = 0.94–2.95, I2 = 83.0%). In terms of study design, the HR of 4 prospective studies was 1.37 (95% CI = 1.06–1.77, I2 = 27.5%) and that of 12 retrospective studies was 1.74 (95% CI = 1.30–2.33, I2 = 67.3%). Regarding the included tumor size, groups were subdivided into ≤3 cm and ≤5 cm subgroups. Meta-analyses illustrated that the subgroup with tumor sizes ≤3 cm had an HR of 1.13 (95% CI = 0.97–1.30, I2 = 0.0%), while those with tumors ≤5 cm had an HR of 1.89 (95% CI = 1.58–2.26, I2 = 49.1%).
4. Discussion
In clinical practice, to optimize treatment options for patients with small HCC, tumor characteristics, liver function reserve, and patient demographics are the key factors considered by clinicians or surgeons.[18,31,32] The clinical practice guidelines of the European Organization for Research and Treatment of Cancer recommend surgical resection as the first-line treatment for small HCC, with RFA considered the standard of care for patients who are not suitable for surgery.[3,4] However, this may sometimes be an extremely difficult decision. In recent years, RFA has been established as a valid alternative treatment for small HCC with comparable results to surgical resection in selected patients.[33] The potential merits of higher repeatability, greater tolerability, lower complication rates, and lower costs make RFA an attractive option.[30] Patients with comorbidities or poor liver functions are more likely to choose RFA because their comorbidities contraindicate SR. RFA is less invasive compared with SR, has a lower incidence, lower risk of complications, and is more likely to regenerate when relapsed.[34,35]
Focusing on long-term survival oncologic outcomes, meta-analysis results comprehensively and systematically showed that RFA is associated with poorer RFS (HR = 1.55, 95% CI = 1.29–1.86, I2 = 72.5%) and OS (HR = 1.61, 95% CI = 1.29–2.01, I2 = 60.4%) compared with SR. The evidence supporting this association was consistent in most subgroup analyses (region, study design, tumor size, and endpoints). In subgroup analysis performed according to region, RFS 1.54 (95% CI = 1.08–2.11, I2 = 39.2%) of the Europe and OS 1.66 (95% CI = 1.40–1.97, I2 = 12.6%) of the Asian group showed statistical significance, and no statistical heterogeneity existed between studies. 13 studies analyzed the RFS of the Asian group, which revealed statistical significance, but statistical heterogeneity existed between studies. Three studies analyzed the OS of the European group, which showed no statistical significance between studies. The majority of studies were conducted in China, while only one and two studies on European subjects analyzed RFS and OS, respectively. This phenomenon is primarily affected by insufficient statistical power or is attributed to the higher prevalence of hepatitis B virus infection and HCC in China.[36] Further research on different regions is required to establish the prognostic value of RFA or surgical resection in patients with small HCC. All included patients met the Milan criteria (i.e., solitary HCC nodules ≤5 cm or up to 3 nodules, each < 3 cm). In subgroup analyses according to tumor size, both OS of 0 to 3 cm and 0 to 5 cm subgroups showed statistical significance, and no statistical heterogeneity existed between studies. A simulation study demonstrated a 3% increase in local recurrence rate after RFA, equivalent to a 1% increase in perioperative mortality.[37] Thus, comparison of the treatment effects on the survival of patients with HCC should be performed separately for smaller (<3 cm) or intermediate (3 to 5 cm) nodules. In our meta-analysis, only Lee et al. (2017)[13] performed an analysis with RFS to compare the treatment effects on 3–5-cm nodules; however, HR showed no statistical significance (HR = 1.36, 95% CI = 0.8–2.312). We were unable to determine RFS and OS to compare the treatment effects on tumors 3 to 5 cm in size; therefore, a subgroup meta-analysis was not conducted. The difficulties in data collection limit further subgroup analysis with respect to the comparison of treatment effects on tumor sizes of 3 to 5 cm. Further randomized studies are warranted to explore the significance of 3 to 5-cm tumors on treatment-related survival of patients with HCC.
The choice of initial treatment for small HCC is largely dependent on the best interest of the individual patient and level of expertise in different institutions. Treatment algorithms to select patients who could benefit from SR or RFA are yet to be established. Randomized controlled trials provide high-level evidence by evaluating the clinical endpoints and using the most efficient and reliable method.[38] In subgroup analyses performed according to study design, both RFS (HR = 1.52, 95% CI = 1.26–1.82, I2 = 31.6%) and OS (HR = 1.37, 95% CI = 1.06–1.77, I2 = 27.5%) of the RCTs showed statistical significance, and no statistical heterogeneity existed between studies. In contrast, retrospective studies provide relatively low-level clinical evidence due to a potential selection bias. Both RFS (HR = 1.58, 95% CI = 1.25–2.00, I2 = 77.2%) and OS (HR = 1.74, 95% CI = 1.30–2.33, I2 = 67.3%) of retrospective subgroups showed statistical significance, though statistical heterogeneity existed between studies. Thus, data from both prospective and retrospective subgroups support our results.
In subgroup analyses performed according to endpoint, both RFS and DFS subgroups showed statistical significance between studies. Only 1 study analyzed PFS and one analyzed TFS, neither of which showed statistical significance. Further research on PFS or TFS should be conducted to establish the prognostic value of RFA and surgical resection in patients with small HCC.
Several previous meta-analyses[39–43] have compared the efficacy of RFA and surgical resection for improving the survival of patients with HCC through extraction of relative risk or values. To date, the results have been inconsistent. OR and relative risk are appropriate measures of dichotomous outcomes, since they simply involve the assessment of the number of events without consideration of the time when events develop. In the current study, HRs were extracted to compare the OS and RFS after surgical resection and RFA for HCC. The cumulative OS or RFS rates are time-to-event outcomes. Additionally, HRs are the most appropriate parameters to measure time-dependent outcomes.[17]
With the increasing application of laparoscopic and intraoperative ultrasound techniques, laparoscopic RFA (LRFA) presents a novel approach to local ablation. LRFA has other advantages, such as real-time security monitoring of the ablation process and accurate detection of tiny lesions, compared with percutaneous RFA. Gao et al (2016)[44] reported that LRFA led to fewer complications and lower hospitalization mortality compared with percutaneous RFA. However, limited information is available on its long-term efficacy. Microwave ablation (MWA) is also a common treatment for HCC. Several studies have found that MWA produced significantly larger zones than RFA.[45–47] Therefore, the apparent superiority of MWA over RFA has made it an alternative method to RFA, and it may be a more powerful technique than RFA. A meta-analysis showed that the efficacy of MWA in the treatment of HCC was similar to that of RFA.[48] There was no significant difference between MWA and RFA in terms of prognosis and incidence of major complications. The long-term efficacy of LRFA and MWA in the treatment of small HCC was not clear. In addition, the efficacy of LRFA and MWA in the treatment of small HCC compared with that of surgical resection or RFA was not analyzed in this study. Multicenter, randomized, controlled studies are needed to verify the efficacy of LRFA and MWA against other therapies for small HCC.
The quality of the included studies is a limitation of our study. In addition, only published English language articles were included, and the exclusion of non-English articles may introduce language biases and erroneous conclusions. Further, the studies included in this meta-analysis (19 in total) mainly included Asian authors but also included two European works (one Italian and one French). Studies in this meta-analysis were mainly conducted in China, where the incidence of HCC is higher, which may be another potential cause of bias. Moreover, only published studies were included from electronic database searches; therefore, the possibility of publication biases cannot be excluded. However, it must be noted that evaluation of publication biases supported the reliability of our analysis. Furthermore, the Engauge Digitizer was used to extract HR data from survival curves indirectly, leading to potential imprecisions. Finally, selection biases could not be completely avoided. Selection of initial treatment was largely dependent on the best interest of the individual patient and level of expertise in different institutions. Treatment algorithms to identify patients who would benefit from surgical resection or RFA are yet to be established. Finally, the follow-up time of some studies was insufficient. With longer follow-up durations, disease relapse rates would likely be higher and prognostic performance would be different. Further multi-center and randomized controlled studies are necessary to confirm our findings.
5. Conclusion
In conclusion, surgical resection was associated with higher overall and disease-free survival than RFA. Thus, surgical resection remains the preferred treatment of choice for small HCC in cases where liver transplantation is not readily available.
Author contributions
Conceptualization: Dongchun Xuan, Toufeng Jin.
Data curation: Weibo Wen, Dongyuan Xu.
Investigation: Weibo Wen, Dongyuan Xu.
Methodology: Dongchun Xuan, Dongyuan Xu.
Writing – original draft: Dongchun Xuan, Weibo Wen, Dongyuan Xu.
Writing – review & editing: Dongchun Xuan, Weibo Wen, Dongyuan Xu, Toufeng Jin.
Supplementary Material
Footnotes
Abbreviations: HCC = hepatocellular carcinoma, HR = hazard ratio, OS = overall survival, RFA = radio frequency ablation, RFS = recurrence/relapse-free survival.
How to cite this article: Xuan D, Wen W, Xu D, Jin T. Survival comparison between radiofrequency ablation and surgical resection for patients with small hepatocellular carcinoma: a systematic review and meta-analysis. Medicine. 2021;100:7(e24585).
This research was supported by the National Natural Science Foundation of China (31760330) and the Project of Education Department of the Jilin province of China (JJKH20180910KJ).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
DFS = disease-free survival, NA = not available, OS = overall survival, PFS = progression-free survival, Ptro = prospective, RCT (randomized controlled trial), RFA = radiofrequency ablation surgical resection, RFS = recurrence/relapse free survival, Rtro = retrospective, SR = surgical resection, TFS = tumor-free survival.
CI = confidence interval, DFS = disease-free survival, EFS = event-free survival, HR = hazard ratios, OS = overall survival, PFS = progression-free survival, RCT = randomized controlled trial, Retro = Retrospective, RFS = recurrence/relapse free survival, TFS = tumor-free survival.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- [2].Sangiovanni A, Colombo M. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver International. 36:124–129. [DOI] [PubMed] [Google Scholar]
- [3].Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908–43. [DOI] [PubMed] [Google Scholar]
- [5].Poon RT, Ng IO, Fan ST, et al. Clinicopathologic features of longterm survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol 2001;19:3037–44. [DOI] [PubMed] [Google Scholar]
- [6].Grieco A, Pompili M, Caminiti G, et al. Prognostic factors for survival in patients with early-intermediate hepatocellular carcinoma undergoing non-surgical therapy: comparison of Okuda, CLIP, and BCLC staging systems in a single Italian centre. Gut 2005;54:411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lau WY, Lau SHY. The current role of radiofrequency ablation in the treatment of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2017;16:122–6. [DOI] [PubMed] [Google Scholar]
- [9].Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 2011;98:1210–24. [DOI] [PubMed] [Google Scholar]
- [10].Takayasu K, Arii S, Sakamoto M, et al. Impact of resection and ablation for single hypovascular hepatocellular carcinoma ≤2 cm analysed with propensity score weighting. Liver Int 2008;38:484–93. [DOI] [PubMed] [Google Scholar]
- [11].Kim GA, Shim JH, Kim MJ, et al. Radiofrequency ablation as an alternative to hepatic resection for single small hepatocellular carcinomas. Br J Surg 2016;103:126–35. [DOI] [PubMed] [Google Scholar]
- [12].Hocquelet A, Balageas P, Laurent C, et al. Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia 2015;31:749–57. [DOI] [PubMed] [Google Scholar]
- [13].Lee SH, Jin YJ, Lee JW. Survival benefit of radiofrequency ablation for solitary (3–5 cm) hepatocellular carcinoma: an analysis for nationwide cancer registry. Medicine 2017;96:e8486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sarker A, Im HJ, Cheon GJ, et al. Prognostic implications of the SUVmax of primary tumors and metastatic lymph node measured by 18F-FDG PET in patients with uterine cervical cancer: a meta-analysis. Clin Nucl Med 2015;41:34. [DOI] [PubMed] [Google Scholar]
- [16].Im HJ, Pak K, Cheon GJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging 2015;42:241–51. [DOI] [PubMed] [Google Scholar]
- [17].Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hung HH, Chiou YY, Hsia CY, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol 2011;9:79–86. [DOI] [PubMed] [Google Scholar]
- [19].Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology 2014;271:909–18. [DOI] [PubMed] [Google Scholar]
- [20].Wang JH, Wang CC, Hung CH, et al. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 2012;56:412–8. [DOI] [PubMed] [Google Scholar]
- [21].Huang J, Zeng Y, Wu H, Chen Z, Lu Q. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903–12. [DOI] [PubMed] [Google Scholar]
- [22].Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794–802. [DOI] [PubMed] [Google Scholar]
- [23].Wong KM, Yeh ML, Chuang SC, et al. Survival comparison between surgical resection and percutaneous radiofrequency ablation for patients in Barcelona Clinic Liver Cancer early stage hepatocellular carcinoma. Indian J Gastroenterol 2013;32:253–7. [DOI] [PubMed] [Google Scholar]
- [24].Imai K, Beppu T, Chikamoto A, et al. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3 cm or less. Hepatol Res 2013;43:853–64. [DOI] [PubMed] [Google Scholar]
- [25].Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 2013;58:724–9. [DOI] [PubMed] [Google Scholar]
- [26].Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 2006;243:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu PH, Hsu CY, Hsia CY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤2 cm in a propensity score model. Ann Surg 2016;263:538–45. [DOI] [PubMed] [Google Scholar]
- [28].International BM. Retracted: a comparison between three-dimensional visualization guided hepatectomy and ultrasonography guided radiofrequency ablation in the treatment of small hepatocellular carcinoma within the milan criteria. Biomed Res Int 2017;2017:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou Z, Lei J, Li B, et al. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule < 2 cm): a single-center study. Eur J Gastroenterol Hepatol 2014;26:339–44. [DOI] [PubMed] [Google Scholar]
- [30].Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol 2013;59:89–97. [DOI] [PubMed] [Google Scholar]
- [31].Hasegawa K, Makuuchi M, Takayama T, et al. Surgical resection vs. percutaneous ablation for hepatocellular carcinoma: a preliminary report of the Japanese nationwide survey. J Hepatol 2008;49:589–94. [DOI] [PubMed] [Google Scholar]
- [32].Arii S, Yamaoka Y, Futagawa S, et al. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: A retrospective and nationwide survey in Japan. Hepatology 2000;32: [DOI] [PubMed] [Google Scholar]
- [33].Wang Y, Qianqian L, Youping L, et al. Radiofrequency Ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomized and nonrandomized controlled trials. Plos One 2014;9:e84484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Santambrogio R, Ceretti AP, Barabino M, et al. Surgical resection versus laparoscopic radiofrequency ablation for treatment of hepatocellular carcinoma. Digestive & Liver Disease 2008;40: [Google Scholar]
- [35].Livraghi T, Meloni F, Stasi MD, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 2010;47:82–9. [DOI] [PubMed] [Google Scholar]
- [36].Liu J, Fan D. Hepatitis B in China. Lancet 2007;369:1582–3. [DOI] [PubMed] [Google Scholar]
- [37].Yun KC, Kim JK, Wan TK, et al. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 2010;51:1284–90. [DOI] [PubMed] [Google Scholar]
- [38].Croswell JM, Kramer BS. Clinical trial design and evidence-based outcomes in the study of liver diseases. Hepatology 2009;50:817–26. [DOI] [PubMed] [Google Scholar]
- [39].Li L, Zhang J, Liu X, et al. Clinical outcomes of radiofrequency ablation and surgical resection for small hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol 2012;27:51–8. [DOI] [PubMed] [Google Scholar]
- [40].Liu Z, Zhou Y, Zhang P, et al. Meta-analysis of the therapeutic effect of hepatectomy versus radiofrequency ablation for the treatment of hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech 2010;20:130–40. [DOI] [PubMed] [Google Scholar]
- [41].Liu JG, Wang YZ. Radiofrequency ablation in the treatment of small hepatocellular carcinoma: a meta analysis. World J Gastroenterol 2010;16:3450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Xu G, Qi FZ, Zhang JH, et al. Meta-analysis of surgical resection and radiofrequency ablation for early hepatocellular carcinoma. World J Surg Oncol 2012;10:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. Bmc Gastroenterol 2010;10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gao J, Zhou Y, Zhang Q, et al. Early laparoscopic radiofrequency ablation for spontaneous rupture of hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A 2016;26:560–6. [DOI] [PubMed] [Google Scholar]
- [45].Qian GJ, Wang N, Shen Q, et al. Efficacy of microwave versus radiofrequency ablation for treatment of small hepatocellular carcinoma: experimental and clinical studies. Eur Radiol 2012;22:1983–90. [DOI] [PubMed] [Google Scholar]
- [46].Wright AS, Sampson LA, Warner TF, et al. Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 2005;236:132–9. [DOI] [PubMed] [Google Scholar]
- [47].Andreano A, Huang Y, Meloni MF, et al. Microwaves create larger ablations than radiofrequency when controlled for power in ex vivo tissue. Med Phys 2010;37:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wencheng, Tan, Qiwen, et al. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 2019;36:264–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


