Abstract
We aimed to retrospectively analyze the clinical and computed tomography (CT) characteristics of young adults with Coronavirus Disease 2019 (COVID-19) pneumonia who were critically ill and to identify the features associated with non-survival.
Thirty-eight COVID-19 patients (20–45 years old, 28 men) who had been admitted in the intensive care unit were included, including 18 non-survivors (group 1) and 20 survivors (group 2). Their clinical characteristics and initial and follow-up CT were compared between groups.
In group 1, the days from illness onset to death were 21.1 ± 10.3 days; 7 patients had underlying comorbidities. At admission, group 1 exhibited higher serum ferritin and interleukin-6 (IL-6) levels (1142.6 ± 242.4 mg/L and 33.8 ± 18.6 mmol/L) compared with group 2 (728.3 ± 150.9 mg/L and 15.2 ± 6.9 mmol/L, P < .01). Group 1 exhibited more rapidly progressive opacities and consolidation in follow-up CT (16.7 ± 3.1 scores, 15.7 ± 3.1 segments) than group 2 (11.4 ± 4.0 scores, 10.3 ± 4.6 segments, P < .01). The oxygenation index was lower (87.6 ± 19.2 vs 99.1 ± 20.4 mm Hg) and the mechanical ventilation duration was longer (14.7 ± 6.9 vs 9.7 ± 3.7 days) in group 1 compare with group 2 (P < .01).
Compared with the survivors, the non-survivors showed higher serum ferritin and IL-6 levels, more rapidly progressive opacities in CT, lower oxygenation index, and longer mechanical ventilation durations. Special attention to ferritin/IL-6 levels and oxygenation index as well as early CT application and timely reexaminations are important to identify the individuals who may be at risk of becoming critically ill.
Keywords: Coronavirus Disease 2019, computed tomography, impaired pulmonary function, short-term mortality, young adults
1. Introduction
The emerging and urgent Coronavirus Disease 2019 (COVID-19) has raised intense attention globally. The viral pneumonia was associated with a new coronavirus named SARS-CoV-2, which is highly homologous to SARS-CoV and belongs to the family Coronaviridae.[1] The disease is still spreading quickly. Until December 9, the confirmed cases have exceeded 67,530,912 worldwide.[2] Cases from >200 countries and regions have been reported.
This is the third coronavirus which causing multinational outbreaks and carrying substantial morbidity and mortality[3] in the past 2 decades. People of any age are generally susceptible. Early symptoms of COVID-19 are usually concealed and cause little vigilance. Once infected, the elderly and those with underlying diseases are more likely to become seriously ill. In a study of 138 patients with COVID-19, 36 patients were admitted to the intensive care unit (ICU). They were significantly older than those who did not require ICU admission (median of 66 years compared with 51 years) and more likely to have underlying comorbidities (72% compared with 37%). Early disease progression can be rapid, and sometimes results in severe respiratory distress syndrome, ICU admission (26%–32%), and death (4.3%–15%).[4–6] The current overall mortality rate of COVID-19 was reported lower than SARS (10%) and MERS (30%).[7,8] However, COVID-19 had ultimately proven to be more infectious as it owned a much larger infected population globally than other coronavirus did, due to rapid person to person transmission and atypical symptoms at early stage in many patients.[6,9] Most of the deceased cases were elderly patients especially those with some underlying diseases.[10] Children under the age of 12 do not usually develop viral pneumonia, although they get infected and can therefore spread the infection.[11] However, there were increasing deceased cases of young adults, to which special attention should be paid. Information is insufficient about the mechanisms behind observed episodes of sudden deterioration or the infrequent clinical demise in young subjects.
We retrospectively analyzed the clinical and computed tomography (CT) characteristics of a small cohort, whose age was 20 to 45 years old. The COVID-19 associated mortality data in young and middle-aged patients from this study will facilitate early identification of individuals who are at risk of becoming critically ill and intensive care preparedness and response, so as to reduce mortality.
2. Methods
2.1. Patients
This retrospective study was approved by our institutional review board. Informed consent was waived. From January 26, 2020 to March 20, 2020, we included 38 young adults patients, who were critically ill (patients were diagnosed as COVID-19 and classified critically ill according to the Guidance for Corona Virus Disease 2019 [6th edition] released by the National Health Commission of China)[12] and admitted to ICU. Patients were assigned to group 1 if not survived from ICU (n = 18, 13 men) and to group 2 if survived (n = 20, 15 men. Their symptoms were obviously relieved. The scale and/or density of the opacities were decreased. They were successfully removed from mechanical ventilation and transferred out of ICU). The patients had been admitted to Tongji Hospital and Wuhan Jin Yin-tan Hospital, which were the designated hospitals to treat COVID-19 patients. Their diagnosis of COVID-19 was confirmed with a positive result to real-time fluorescence polymerase chain reaction (PCR) assay for SARS-CoV-2 nucleic acid, with nasopharyngeal or oropharyngeal swab specimens. The patients were admitted consecutively. They took their first CT exam when they seek medical attention at the outpatients department. Those who were transmitted from other hospitals and the initial medical records including initial CT can’t be completely tracked were excluded.
We collected their demographic data (age, sex, chronic medical histories), symptoms and signs, lab tests on admission, and initial and follow-up CT images. Lab tests included blood routine (white blood cell [WBC] count, lymphocyte count, and platelet count), biochemical examinations (C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], D-dimer, procalcitonin, transaminase, interleukin-6 [IL-6], and ferritin, etc). Follow-up CT exams were performed during their hospitalization. Days from symptoms onset to CT exams, days from symptoms onset to ICU admission, and days from symptoms onset to death were also recorded. Parameters of pulmonary function (oxygenation index [ratio of PaO2 to FiO2, mmHg], respiratory rate, PaCO2, oxygen saturation, and duration of mechanical ventilation) were collected.
2.2. Review of CT images
All CT images were reviewed by 2 specialized radiologists (with 12 years of clinical experience) using picture archiving and communication system (PACS). For each patient, the CT images were evaluated for: presence of ground-glass opacities (GGO), consolidation, interstitial thickening or reticulation, fibrous stripes, and air bronchograms; severity of opacifications; other manifestations, including the location of the lesions, pleural effusion, and mediastinal lymph node changes (enlargement or increased number of lymph nodes). GGO was defined as increased lung attenuation with preservation of bronchial and vascular margins and consolidation was defined as opacification in which the underlying vasculature was obscured.[13,14]
Each lobe of the lungs was assessed for opacifications and the lesion size was graded as 0 (none), 1 (diameter <1 cm), 2 (diameter 1 to <3 cm), 3 (diameter 3 cm to <50% of the lobe), or 4 (50%–100% of the lobe). Scores of 5 lobes were summed (sum score, range from 0 to 20) and the numbers of segments involved were recorded to calculate the overall severity of opacifications. The numbers of segments with consolidation lesions were also recorded (range from 0 to 18).
2.3. Statistical analysis
The descriptive data were expressed as mean ± standard deviation for continuous variables and number (%) for categorical variables. The differences between non-survivors and survivors were accessed using a Mann–Whitney U test for continuous variables and a Fisher exact test for categorical variables, with a statistical significance set at P < .05. All statistical analysis procedures were conducted using SPSS 22.0 software (IBM, Armonk, NY).
3. Results
The demographic data, laboratory tests, and symptoms of the 2 groups were listed in Tables 1 and 2. The difference in mean age was not significant (37.7 ± 8.2 vs 35. 8 ± 4.1 years old; P = .38). The most common complaints were fever, cough, fatigue, and dyspnea in both groups. All the 38 patients were admitted to ICU due to respiratory failure, requiring mechanical ventilator supports. The duration from illness onset to initial CT was 5.0 ± 2.6 days in group 1 and was 4.5 ± 2.1 days in group 2 (Table 3). The duration from onset to ICU admission was 15.4 ± 8.4 days in group 1 and was 15.8 ± 9.4 days in group 2. The duration from onset to death was 21.1 ± 10.3 days (range, 7–39 days, median 21 days) in group 1. Three male patients (2 non-survivors and 1 survivor) were health-care workers. Seven patients of group 1 had underlying diseases before COVID-19 infection (diabetes [n = 2], hypertension [n = 2], cirrhosis [n = 1], breast cancer [n = 1], and leukemia [n = 1]). Patients in group 2 reported no obvious history of underlying diseases.
Table 1.
Demographics and comorbidities of all patients with severe COVID-19 infection.
| Age | Gender | Days from onset to CT | Days from onset to ICU | Days from onset to death | Comorbidity | |
| Group 1 (non-survivors) | ||||||
| 1 | 45 | M | 4 | 23 | 28 | Hypertension |
| 2 | 45 | M | 7 | 14 | 19 | Cirrhosis |
| 3 | 39 | M | 4 | 5 | 9 | None |
| 4 | 38 | M | 3 | 4 | 7 | None |
| 5 | 26 | M | 4 | 11 | 12 | None |
| 6 | 41 | M | 4 | 10 | 13 | Hypertension |
| 7 | 43 | M | 3 | 9 | 12 | None |
| 8 | 34 | M | 1 | 27 | 28 | None |
| 9 | 29 | M | 1 | 18 | 28 | None |
| 10 | 20 | M | 2 | 30 | 33 | Leukemia |
| 11 | 32 | F | 9 | 22 | 39 | None |
| 12 | 45 | F | 4 | 6 | 7 | None |
| 13 | 45 | F | 7 | 32 | 37 | Type 2 diabetes |
| 14 | 24 | F | 5 | 12 | 19 | Breast cancer |
| 15 | 43 | F | 8 | 13 | 23 | None |
| 16 | 42 | M | 9 | 17 | 28 | Type 2 diabetes |
| 17 | 44 | M | 8 | 15 | 24 | None |
| 18 | 44 | M | 7 | 10 | 12 | None |
| Group 2 (survivors) | ||||||
| 1 | 30 | M | 7 | 32 | None | |
| 2 | 32 | M | 4 | 28 | None | |
| 3 | 34 | M | 7 | 19 | None | |
| 4 | 36 | M | 4 | 9 | None | |
| 5 | 39 | M | 4 | 12 | None | |
| 6 | 40 | M | 4 | 13 | None | |
| 7 | 40 | M | 3 | 12 | None | |
| 8 | 28 | M | 1 | 28 | None | |
| 9 | 33 | F | 1 | 33 | None | |
| 10 | 41 | F | 4 | 7 | None | |
| 11 | 31 | M | 5 | 16 | None | |
| 12 | 32 | F | 6 | 15 | None | |
| 13 | 34 | M | 4 | 15 | None | |
| 14 | 39 | M | 5 | 9 | None | |
| 15 | 39 | M | 6 | 10 | None | |
| 16 | 40 | F | 5 | 8 | None | |
| 17 | 40 | M | 4 | 10 | None | |
| 18 | 32 | M | 7 | 15 | None | |
| 19 | 38 | F | 6 | 15 | None | |
| 20 | 38 | M | 3 | 7 | None | |
Table 2.
Clinical symptoms and laboratory examinations at admission of patients with severe COVID-19 infection.
| Characteristics | Non-survivals (n = 18) | Survivals (n = 20) | P value |
| Symptoms | |||
| Fever | 18 (100) | 20 (100) | NA |
| Highest temperature, °C | 39.1 ± 0.6 | 38.7 ± 0.7 | 0.13 |
| Cough | 18 (100) | 20 (100) | NA |
| Dyspnea | 18 (100) | 20 (100) | NA |
| Fatigue | 9 (50) | 9 (45) | 1.00 |
| Myalgia | 3 (16.7) | 3 (15) | 1.00 |
| Underlying comorbidities | 7 (38.9) | 0 (0) | .003 |
| Laboratory tests | |||
| WBC count, ×109/L | 9.4 ± 5.3 | 9.7 ± 3.9 | .813 |
| > 10 × 109/L | 6 | 7 | 1.00 |
| <4 × 109/L | 4 | 2 | .395 |
| Lymphocyte count, ×109/L | 0.77 ± 0.29 | 0.99 ± 0.30 | .074 |
| <1.1 × 109/L | 16 | 12 | .067 |
| Platelet count, ×109/L | 161.3 ± 82.1 | 194.0 ± 73.5 | .253 |
| CRP, mg/L | 92.3 ± 53.6 | 73.0 ± 39.5 | .365 |
| ESR, mm/H | 49.8 ± 18.0 | 38.1 ± 18.7 | .052 |
| D-dimer, μg/mL | 4.1 ± 1.2 | 3.6 ± 0.9 | .358 |
| Ferritin, mg/L | 1142.6 ± 242.4 | 728.3 ± 150.9 | .001 |
| IL-6, mmol/L | 33.8 ± 18.6 | 15.2 ± 6.9 | .003 |
| Parameters of pulmonary function | |||
| Oxygenation index (ratio of PaO2 to FiO2, mm Hg) | 87.6 ± 19.2 | 99.1 ± 20.4 | .019 |
| Respiratory rate, bpm | 36.2 ± 10.0 | 30.1 ± 6.7 | .023 |
| PaCO2 (mm Hg) | 56.3 ± 15.1 | 56.5 ± 8.0 | .955 |
| Oxygen saturation (%) | 88.0 ± 7.6 | 90.3 ± 5.4 | .326 |
| Bacterial coinfection | 3 (16.7) | 0 (0) | .097 |
| Duration of mechanical ventilation, d | 14.7 ± 6.9 | 9.7 ± 3.7 | .012 |
Table 3.
Comparison of initial and follow-up CT findings between the 2 groups.
| No. of patients or values | |||
| CT characteristics | Non-survivals (n = 18) | Survivals (n = 20) | P value |
| Sum Score of opacities and other features on initial CT | |||
| Sum score of opacifications | 8.9 ± 4.4 | 7.5 ± 3.5 | .320 |
| No. of involved segments | 7.7 ± 4.1 | 6.8 ± 3.2 | .453 |
| Consolidation | 11 (61.1) | 8 (40) | .330 |
| No. of involved segments | 3.1 ± 2.5 | 3.4 ± 2.0 | .711 |
| Interstitial thickening | 11 (61.1) | 10 (50) | .532 |
| Air bronchogram | 7 (38.9) | 4 (20) | .288 |
| Fibrous stripes | 6 (33.3) | 6 (30) | 1.000 |
| Pleural effusion | 6 (33.3) | 2 (10) | .117 |
| Lymph nodes changesa | 7 (38.9) | 4 (20) | .288 |
| Days from symptom onset to initial CT | 5.0 ± 2.6 | 4.5 ± 2.1 | .476 |
| Sum score of opacifications on follow-up CT | |||
| Sum score of opacifications | 16.7 ± 3.1 | 11.4 ± 4.0 | .009 |
| No. of involved segments | 15.7 ± 3.1 | 10.3 ± 4.6 | .007 |
| Consolidation | 13 (100) | 10 (100) | NA |
| No. of involved segments | 7.9 ± 4.9 | 5.4 ± 2.3 | .004 |
| Days from symptom onset to CT with peak Sum score | 12.8 ± 3.4 | 20.5 ± 5.3 | .001 |
At admission, all patients exhibited increased CRP (92.3 ± 53.6 mg/L for group 1, 73.0 ± 39.5 mg/L for group 2), increased ESR (49.8 ± 18.0 mm/h for group 1, 38.1 ± 17.8 mm/h for group 2). Group 1 exhibited higher levels of serum ferritin (1142.6 ± 242.4 ng/mL vs 728.3 ± 150.9 ng/mL) and IL-6 (33.8 ± 18.6 pg/mL vs 15.2 ± 6.9 pg/mL, P < .01) and a trend toward significant difference in ESR and lymphocyte count (P = .052 and .074) compared with group 2 (Fig. 1A).
Figure 1.
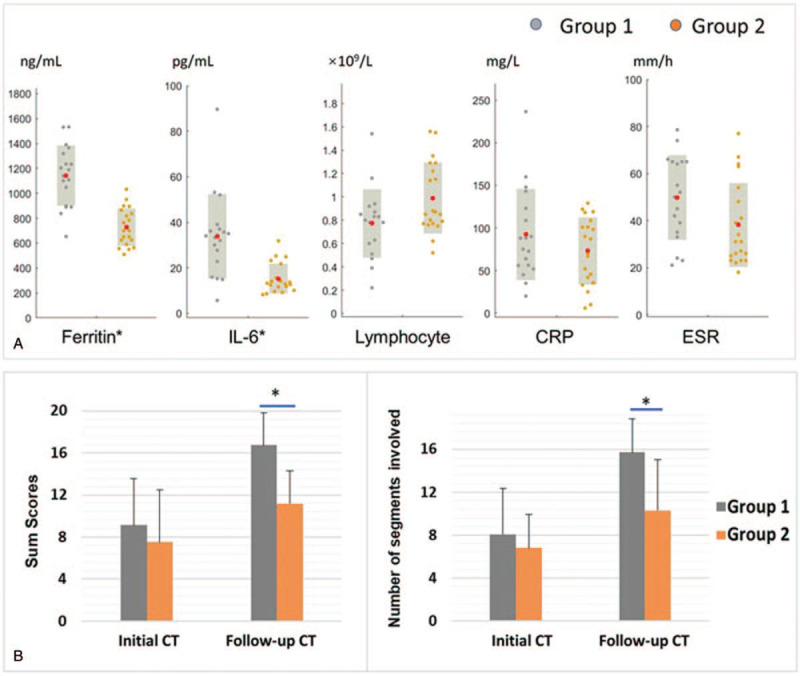
The differences in clinical and CT measurements between groups. A: The differences in levels of ferritin, IL-6, CRP, ESR, and lymphocyte count. The red solid dots denote mean values in each group. Group 1 exhibited higher levels of ferritin and IL-6 compared with that of group 2 (∗P < .01). B: The Sum score and numbers of segments involved in group 1 and group 2. There were more progressed and severer opacities and consolidations in follow-up CT in group 1 than that in group 2 (∗P < .01). CRP = C-reactive protein; CT = computed tomography; ESR = erythrocyte sedimentation rate; IL-6 = interleukin-6.
All patients underwent chest CT exams during the active phase of the disease. The details of the CT findings are summarized in Table 3. GGO was the earliest and fundamental CT finding (Fig. 2), which may appear in approximately 2 to 7 days after onset of symptoms. Combination of GGO and consolidation, interlobular septum and intralobular, interstitial thickening and fibrosis (Figs. 2 and 3), pleural and/or pericardial effusion (Fig. 4) were observed. The lower lobes were more commonly involved in both groups. In groups 1, 17 patients showed fast progressive deterioration until the lungs became completely “white lungs” (Figs. 3 and 4). One patient showed the opacities had decreased for a short period, however deteriorated quickly after that (Fig. 5). The Sum score of opacifications and the numbers of segments involved were higher in group 1 than those in group 2 in the follow-up CT exams. The number of segments with consolidations was also higher in group 1 than that in group 2 (detailed in Table 3, Fig. 1B, P < .01). The duration from onset to the day, when most obvious opacifications were observed in CT, was shorter in group 1 compare with group 2 (12.8 ± 3.4 days vs 20.5 ± 5.3 days, P < .01). For patients in group 1, most obvious opacifications were usually observed in their last CT exams.
Figure 2.

The initial and follow-up CT images of a 31 years old male patient in group 2. A: Scattered GGO were observed in the subpleural area. B: Patchy GGO and consolidation located bilaterally, indicating a progression. C: After absorption of some opacities, fibrous stripes, and interstitial thickening could be seen during the remission stage. Images in different lines represent different cross sections. CT = computed tomography; GGO = ground-glass opacities.
Figure 3.
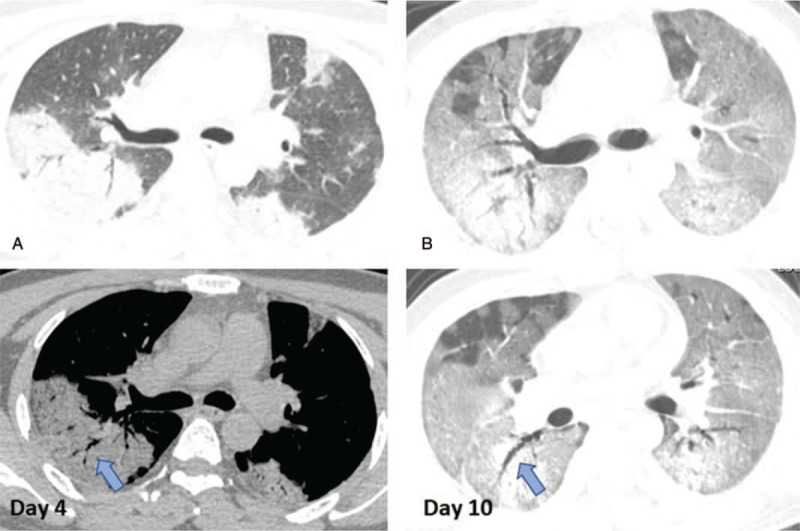
The initial and follow-up CT images of a 41 years old male patient in group 1. A: On initial CT images (day 4 after symptom onset), obvious GGO with consolidation and interstitial thickening distributed bilaterally. Air bronchograms (arrows) were seen. B: More diffuse lesions located in the lungs bilaterally, which showed as “white lungs” (day 10). CT = computed tomography; GGO = ground-glass opacities.
Figure 4.

The initial and follow-up CT images of a 43 years old male patient in group 1. Besides GGO and consolidation, pleural effusion and pericardial effusion were observed. CT = computed tomography; GGO = ground-glass opacities.
Figure 5.

The initial and follow-up CT images of a 20 years old male patient in group 1. A: On initial CT images, multiple and small patchy GGO were observed in the parabronchial area. B: After treatment, the scale and density of opacities had decreased for a period. C: The lesions deteriorated again after that, manifested as more obvious and diffuse GGO with larger scale and higher density. Images in different lines represent different cross sections. CT = computed tomography; GGO = ground-glass opacities.
All the patients presented dyspnea and other respiratory symptoms, and developed to acute respiratory distress syndrome (ARDS). The mean oxygenation index was very low in both groups and even lower in group 1 (P = .019). The mean respiratory rate was faster in group 1 than in group 2 (P = .023). The decreased oxygen saturation (%) was observed in both groups (88.0 ± 7.6 for group 1, 90.3 ± 5.4 for group 2). Of the 18 patients who died in the ICU, 3 had developed confirmed secondary infection isolated from the nasopharyngeal aspirate, as follows: pseudomonas species (n = 1), methicillin- resistant staphylococcus aureus (n = 1), and Acinetobacter baumannii and Klebsiella pneumoniae (n = 1). The mechanical ventilation duration was longer in group 1 than in group 2 (14.7 ± 6.9 vs 9.7 ± 3.7 days; P = .012).
4. Discussion
The vast majority of deaths were among the elderly patients >50 years old.[10] In this study of a younger deceased patients group, we made several observations. According to CT findings, their pulmonary opacifications developed very fast in the early stage. Meanwhile, some inflammatory chemokines and cytokines levels in serum significantly increased, especially including the ferritin and IL-6, which may be involved in the immune storm post coronavirus infection. After admitted in ICU, they required a more intensive care and a longer mechanical ventilation support duration.
The ground-glass opacities were observed to be the earliest CT finding,[15] followed by combinations with consolidation, pleural effusion, and interlobular thickening. There was a preference for lesion involvement in the lower lobes, and most lesions were peripherally located. Our results suggested that the rapidly progressed GGO and consolidations were responsible for ensuing ARDS in most patients. The alterations in Sum scores of opacities between initial and follow-up CT was greater in group 1 compared with group 2, which likely represented a rapid progression of the lesions that occupied maximum areas of the lungs. Radiological findings were consistent with the adverse clinical outcome, including the very low oxygenation index and oxygen saturation. Group 1 exhibited a higher respiratory rate and a longer mechanical ventilation duration requirement. Concomitant bacterial infections of 3 patients in group 1 could be also responsible for the progression of the pulmonary function failure.
Previous studies on severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) reported that rapid progression of the disease with high chest radiographic score was associated with high mortality.[16,17] The non-survivors in group 1 had evident pulmonary opacities, measured by the high Sum scores (16.7 ± 3.1) in follow-up CT. The deteriorations were quick, despite various medical treatments. The non-survivors also had more evident consolidations, semi-quantitatively measured by segmental involvement, than the survivors group. Subpleural focal GGO and consolidations rapidly progressed to the rest of the lungs were present in most patients in group 1 and could be an omen of adverse clinical consequences.
The characteristic clinical symptoms, contact history with an established COVID-19 patient, early GGO appearance in chest CT and lymphopenia should raise a high suspicion of COVID-19. Subpleural focal GGO have also been noted in cases of atypical pneumonia caused by chlamydia species, mycoplasma species, legionella species, H1N1 influenza, and other types of viruses.[18] In our cohort of deceased COVID-19 patients under 45 years old, consolidations were evident, along with certain occurrences of pleural effusion (6/18 cases). As seen in 2 cases, the pulmonary lesions in initial CT could also be very mild, presented as subtle GGO (Sum score range 1–3). What drew more of our attention was that the progression of GGO and consolidations of these COVID-19 patients, particularly of group 1, was very fast. The mean duration, from symptoms onset to the day when CT exhibited most obvious opacities, was only approximately 12 days in group 1.
Like MERS and SARS, there are no distinguishing clinical features of COVID-19; symptoms can overlap with other severe acute respiratory infections.[19] Lower lymphocytic count and lower platelet count are the clinical indications of fatal outcome in MERS.[20] Pronounced lymphopenia was observed both in group 1 and group 2. In addition, higher ferritin and IL-6 levels in group 1 suggested that a significant inflammatory state may associated with poorer prognosis. In patients with SARS, increased proinflammatory cytokines in serum (e.g., IL-1β, IL-6, IL-12, Interferon-gamma (IFN-γ), Interferon-inducible protein-10 (IP-10) and Monocyte chemoattractant protein-1 (MCP-1)) were associated with pulmonary inflammation and lung damage degree.[21] A MERS-CoV related study also reported the significantly increased levels of proinflammatory cytokines (IFN-γ, TNFα, IL-15, and IL-17).[22] Previous studies reported that COVID-19 patients, who were admitted to ICU, had higher levels of cytokines (e.g., Granulocyte colony stimulating factor, IP-10, MCP-1, MIP-1α, and TNF-α) than did those not requiring ICU admission, suggesting that the cytokine storm was associated with disease severity.[4]
Seven of the 18 patients in group 1 had underlying comorbidities, which would impact the choice and quality of medication, and likely aggravate the illness condition after infection. Of 3 of the health-care workers in our cohort, 2 had sacrificed their lives. Health-care workers were most likely to be infected by contacting with severely infected individuals. They had fought on the front lines against COVID-19, putting themselves at higher risk of infection than general population.
COVID-19 is a newly recognized viral infection, and the details of the pathologic process are being revealed step by step, some are still yet to be understood. Early symptoms of COVID-19 are not obvious and typical, which may cause little vigilance. The clinical spectrum of COVID-19 ranges from mild to critically ill cases, and the progressive process varies from person to person. In some cases, the process was quite rapid within days, owning to the viral load and extent and probably the individual susceptibility. The duration from onset to death ranged from 7 to 39 days, and duration from onset to ICU admission was 15.4 ± 8.4 days in group 1. The short incubation period with rapid progression to ARDS in group 1 gave a very small window of opportunity for successful managements of these high-risk patients. Hence, an early diagnosis and timely CT examinations, as well as inflammatory cytokines/chemokines tests, may help stratify patients, predict who are at risk of becoming critically ill, and facilitate effective treatment.
This study has some limitations. First, the sample size was small. Deceased cases under 45 years, with complete clinical, laboratory, and serial imaging data, were relatively rare during the study time window. Possible selection bias should be noted and further study of a larger cohort is required. Second, the semi-quantitative methods for measuring the pulmonary lesions may have certain subjectivity. Third, the histopathologic evidence of the inflammatory process for the patients who died in the course of the disease was lacked. Relevant pathological research is needed and in progress.
5. Conclusion
So far, the vaccines of COVID-19 have not been widely used. Oxygen inhalation, anti-infection therapy, and other supportive treatments to protect multiorgan functions are of great importance. In summary, the very fast-progressive opacities and consolidations according to CT findings probably indicated a poor prognosis. The higher serum inflammatory cytokines or chemokines levels (such as ferritin and IL-6) should also be noted as they may indicate an immune storm post coronavirus infection. This highlights vigilance about prompt seeking of medical care and earlier referral to intensive care unit for the high-risk young adults patients.
Acknowledgments
The authors thank all the patients and their families involved in this study, as well as all the front-line medical staffs of China for their efforts in COVID-19 prevention and control.
Author contributions
Conceptualization: Qiang Zhang, Ying Xiong.
Data curation: Qiang Zhang, Ting Wu.
Funding acquisition: Ying Xiong.
Supervision: Ying Xiong.
Writing – original draft: Qiang Zhang.
Writing – review & editing: Ying Xiong, Wenzhen Zhu.
Footnotes
Abbreviations: ARDS = acute respiratory distress syndrome, COVID-19 = Coronavirus Disease 2019, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, GGO = ground-glass opacities, IL-6 = interleukin-6, real-time PCR = real-time fluorescence polymerase chain reaction, WBC = white blood cell.
How to cite this article: Zhang Q, Xiong Y, Wu T, Zhu W. Very fast-progressive pulmonary opacities and high inflammatory factors levels are associated with decease of young Coronavirus Disease 2019 patients. Medicine. 2021;100:7(e24668).
This work was supported by the National Natural Science Foundation of China (grant number 81601480).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files];
COVID-19 = Coronavirus Disease 2019; CT = computed tomography.
Continues data are expressed as mean ± SD and categorical data are presented as n (%). The P values were calculated using Mann–Whitney U test on nonparametric data for continuous variables and Fisher exact test for categorical variables.
COVID-19 = Coronavirus Disease 2019; CRP = C-reactive protein; CT = computed tomography; ESR = erythrocyte sedimentation rate; IL-6 = interleukin-6.
CT = computed tomography.
Lymph nodes changes means mediastinal lymph nodes number >5, or short-axis diameter >1 cm.
References
- [1].International Committee on Taxonomy of Viruses. Naming the 2019 Coronavirus. Available at: https://talk.ictvonline.org/. Accessed February 11, 2020. [Google Scholar]
- [2].World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/. Accessed December 9, 2020. [Google Scholar]
- [3].Arabi YM, Murthy S, Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med 2020;46:833–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at: https://www.who.int/csr/sars/country/table2003_09_23/en/. Accessed September 26, 2003. [Google Scholar]
- [8].World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV). Available at: https://www.who.int/en/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov). Accessed March 11, 2019. [Google Scholar]
- [9].Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl 2020;26:832–4. [DOI] [PubMed] [Google Scholar]
- [12].New coronavirus pneumonia prevention and control program (6th ed.) (in Chinese); 2020. Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202003/4856d5b0458141fa9f376853224d41d7/files/4132bf035bc242478a6eaf157eb0d979.pdf. Accessed March 7, 2020. [Google Scholar]
- [13].Lei J, Li J, Li X, et al. CT Imaging of the 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 2020;295:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hansell DM, Bankier AA, MacMahon H, et al. Fleischner society: glossary of terms for thoracic imaging. Radiology 2008;246:697–722. [DOI] [PubMed] [Google Scholar]
- [15].Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020;20:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wong KT, Antonio GE, Hui DS, et al. Severe acute respiratory syndrome: radiographic appearances and pattern of progression in 138 patients. Radiology 2003;228:401–6. [DOI] [PubMed] [Google Scholar]
- [17].Das KM, Lee EY, Enani MA, et al. CT correlation with outcomes in 15 patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol 2015;204:736–42. [DOI] [PubMed] [Google Scholar]
- [18].Kim EA, Lee KS, Primack SL, et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics 2002;22:S137–49. [DOI] [PubMed] [Google Scholar]
- [19].Kooraki S, Hosseiny M, Myers L, et al. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol 2020;17:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med 2014;160:389–97. [DOI] [PubMed] [Google Scholar]
- [21].Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mahallawi WH, Khabour OF, Zhang Q, et al. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine 2018;104:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]


