Abstract
COVID-19 has placed a significant strain upon healthcare resources at a global level and refractory hypoxemia is the leading cause of death among COVID-19 patients. The management of limited resources such as mechanical ventilators has remained a contentious issue both at an individual and institutional level since the beginning of the pandemic. As a result, the COVID-19 pandemic has presented challenges to critical care practitioners to find innovative ways to provide supplemental oxygen therapy to their patients. We present a single-center experience: a case series of five COVID-19 infected patients managed with a novel approach to provide supplemental oxygen and positive end-expiration pressure (PEEP) via the helmet. Three of the five patients responded to therapy, did not require intubation, and survived to discharge. The other two patients continued to deteriorate clinically, required endotracheal intubation, and subsequently expired during their hospitalization. We extrapolated our accumulated experience with non-invasive oxygen support by helmet in COVID-19 patients to a non-COVID-19 postoperative patient who underwent sinus surgery and developed hypoxemic respiratory failure also resulting in avoidance of endotracheal intubation. We conclude that oxygen therapy via a helmet is a safe, cost-effective technique to prevent intubation in carefully selected patients with infectious and non-infectious causes of hypoxic respiratory failure. Our positive experience with the system warrants further large-scale study and possible technique refinement.
Keywords: ARDS, Coronavirus, Severe acute respiratory syndrome, Critical care, Hypoxia
1. Introduction
The severe acute respiratory syndrome coronavirus (SARS-CoV-2), commonly referred to as COVID-19, was first reported in Wuhan, China in late 2019 [1], and has since caused a global pandemic [2,3]. The virus primarily affects the lungs and, in serious cases, can lead to acute severe respiratory failure requiring admission to the intensive care unit (ICU) and endotracheal intubation. Mortality predominantly occurs as a result of refractory hypoxemia and the associated sequelae including multi-organ dysfunction and failure [5]. Globally, the volume of patients and the severity of illness overwhelmed the medical resources of hospitals [2] and, in the United States, the Society of Critical Care Medicine projected that our resources were at risk of being insufficient to meet the anticipated needs [6].
Resource management for items such as ICU beds, ventilators, and key health care personnel has been debated through out the ongoing pandemic [5,9,10]. In order to address the appropriate utilization of sparse resources and the possibility to reduce morbidity and increased mortality related to endotracheal intubation, we began to explore novel methods for providing respiratory support to critically ill COVID-19 patients.
European practitioners of hyperbaric oxygen therapy have reported their experience in treating hypoxic patients with a soft, clear, vinyl helmet system that could be used to provide oxygen therapy with high FiO2 and a modest amount of positive end-expiratory pressure (PEEP) outside of a hyperbaric environment [15]. Traditionally, these devices are used to provide intermittent periods of oxygen therapy (FiO2 1.0) while exposed to a hyperbaric environment in a hyperbaric chamber. This technique had been employed in Italy with negligible leakage of exhaled air around viral filters and associated aerosolized droplets [16] thereby decreasing the risk of exposure to aerosolized droplets of infectious material amongst healthcare personnel. These helmets can also create a high FiO2 environment outside of a hyperbaric chamber and thus, we designed a treatment algorithm whereby we would use these helmets in our ICU to potentially prevent the need for intubation and thereby maintain an adequate supply of available ventilators and supplies. This case series describes our experience with using the oxygen helmet in a subset of critically ill COVID-19 patients.
2. Methods
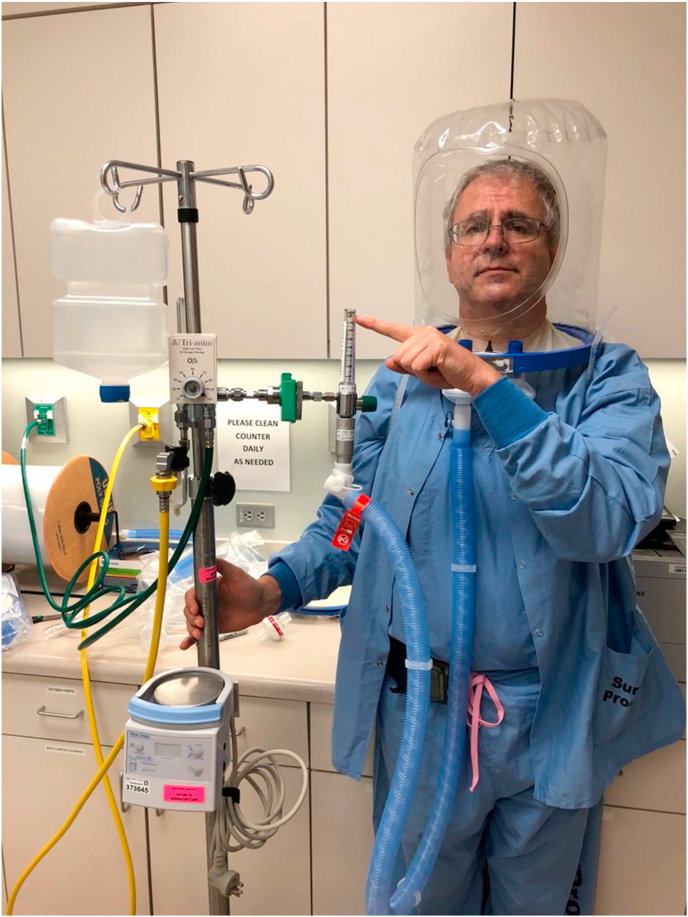
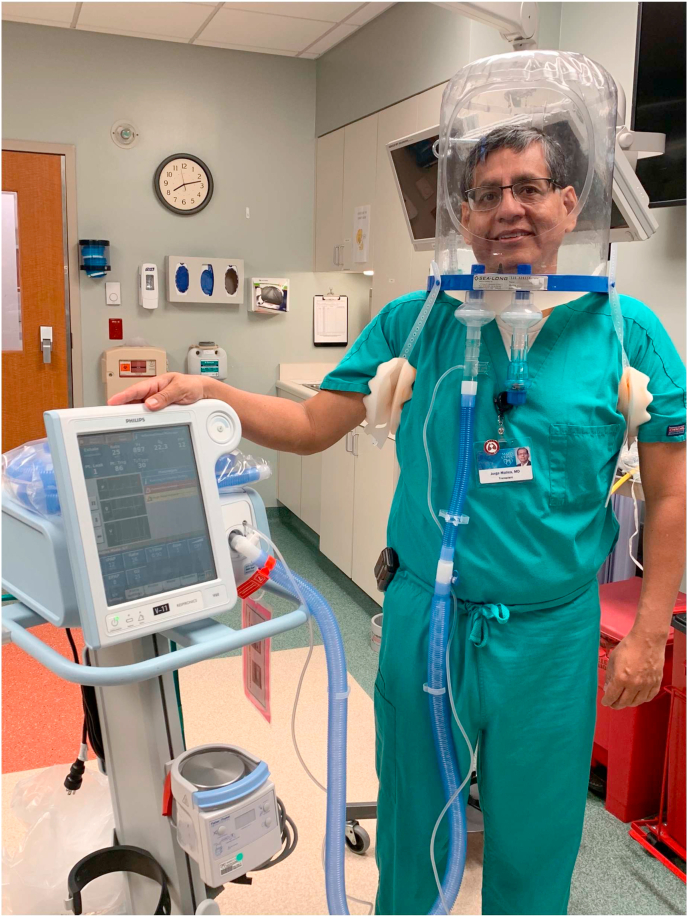
The three models of the helmet (PN 580, PN5200, and PN5202; Sea-Long Medical Systems LLCX, Texas) have a clear plastic hood that covers the patient's head. The helmet is connected to a plastic ring that is supported by a soft, rubber collar that is tailored to each patient by measuring the neck circumference. The rubber collar forms a tight seal against the skin of the patient and permits a modest amount of PEEP within the helmet. The helmet has two connection ports to which expiratory and inspiratory tubing are attached. The inspiratory tubing can be connected to high-flow nasal cannula or NIPPV device, while a viral filter is connected to the expiratory tubing. The helmet is secured to the patient by armpit braces with padding to prevent skin breakdown See Fig. 1.
Fig. 1.
High-flow (a) and NIPPV (b) via helmet demonstrated in Mayo Clinic physicians.
At our institution, HFNC was provided in conjunction with the helmet. Additionally, a PEEP valve and a viral filter were added to the circuit. All patients received high-flow nasal cannula with PEEP ranging from 5 to 10 cmH2O, the minimum flow delivered was 50 L of oxygen, and FiO2 was titrated according to the patient's arterial blood gas. A training session was provided to all clinicians and respiratory therapists using this modality in COVID-19 patients.
A systematic approach was used for the management of refractory hypoxemia in COVID-19 patients at our institution. Accordingly, patients with increasing oxygen requirements on the nasal cannula or oxygen mask were placed on a reservoir nasal cannula or non-rebreather mask and encouraged to self-prone as much as tolerated. If they continue to experience desaturations and/or further increases in their work of breathing (tachypnea, retractions, shallow respirations, or subjective fatigue), they were designated to a trial of high-flow oxygen therapy via the helmet. Initial FiO2 and flow rate settings were selected and titrated based on patient need and response to therapy. If the patient was not already on FiO2 1.0, the FiO2 was titrated by at least 0.1 at the initiation of helmet therapy. Respiratory rate, work of breathing, and arterial blood gases were frequently assessed for patients using high-flow via helmet. Patients who did not tolerate the helmet as defined by continued increased work of breathing, continued desaturation (SpO2 <88%) or continued worsening PaO2 (<60) or those who had further clinical decompensation while on high-flow via helmet were intubated endotracheally. There were no objective cutoffs defining non-tolerance to helmet therapy as this was a clinical decision based on the above-mentioned criteria. During this time period, the ICU staffing model included at least one provider, senior nurse, and respiratory therapist who had been trained to monitor patients receiving oxygen therapy via the helmet technique.
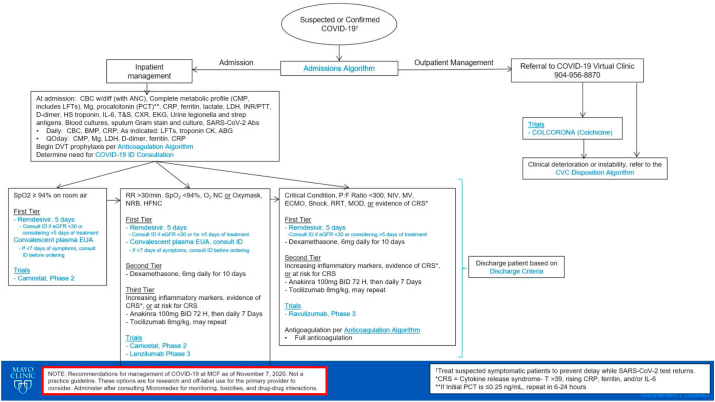
Additional COVID-19 therapies were given to patients according to our treatment algorithm (Fig. 2). Patients were assigned to receive different therapies such as dexamethasone, remdesivir, tocilizumab, lenzilumab, or convalescent plasma according to their oxygen requirements and inflammatory markers. The guidelines with respect to pharmacological treatment and standard of care for patients with COVID-19 have represented a dynamic state; the different treatment regimens provide were consistent with the standard of care at the time of the patient's hospitalization. All patients received either prophylactic or therapeutic anticoagulation according to our anticoagulation algorithm. All patients signed a research release at the time of their admission and this retrospective analysis was deemed exempt from IRB review.
Fig. 2.
Mayo Clinic Florida inpatient treatment algorithm for COVID-19 infection.
3. Case descriptions
3.1. Case #1
A 70-year-old male with a past medical history of morbid obesity (BMI = 42.0 kg/m2), hypertension, diabetes mellitus type 2, and depression presented to the emergency department with acute respiratory failure in the setting of COVID-19 pneumonia. The patient presented with oxygen saturations <70% (measured by pulse oximetry) with adequate waveforms. His respiratory rate was >40 breaths per minute and was placed on NIPPV with initial settings of inspiratory positive airway pressure (IPAP) and expiratory positive airway pressure (EPAP) of 10/5 cmH2O with FiO2 1.0. He was admitted directly to the ICU but did not tolerate NIPPV support due to agitation. The patient was provided with a trial of therapy with HFNC via helmet along with nitric oxide (40 ppm), but because of persistent hypoxemia and worsening encephalopathy, he was endotracheally intubated for mechanical ventilatory support within 8hrs of admission. His COVID-19 treatment regimen included remdesivir, tocilizumab, and dexamethasone. His 22-day ICU course was complicated by renal failure requiring dialysis, bacteremia, thrombocytopenia, and intracerebral hemorrhages. Life support measures were withdrawn after goals of care discussion as per the patient and family wishes.
3.2. Case #2
A 74-year-old male with a past medical history of obstructive sleep apnea (OSA) and obesity presented with acute hypoxic respiratory failure in the setting of COVID-19 pneumonia. On admission day 3, the patient had increasing oxygen requirements with HFNC (flow 50L, FiO2 1.0) with additional oxygen supplementation via a non-rebreather mask (flow 15L). Having failed to tolerate a brief trial (<15min) of bi-level NIPPV (10/5 cmH2O, FiO2 1.0), he was then transitioned to HFNC (Flow 45L/min, FiO2 1.0) via helmet in combination with inhaled nitric oxide (40 ppm). He received convalescent plasma, remdesivir, dexamethasone, and lenzilumab as part of his COVID-19 treatment. On admission day 6, the patient was transitioned back to the floor and was progressively weaned off helmet ventilation to the nasal cannula. He was subsequently discharged home.
3.3. Case #3
A 68-year-old male with a past medical history of hypertension, gastroesophageal reflux disease, and hyperlipidemia presented with a 4-day history of cough, fever, chest tightness, and weakness after returning from a trip from New Zealand. On admission day 3, his oxygen requirements increased as his dyspnea worsened and work of breathing intensified. He engaged in a self-proning posture with supplemental oxygen provided via a non-rebreather mask (flow 15L/min), and experienced an improvement in his hypoxia (oxygen saturations increased from 92% to 98%) and associated dyspnea. He was then transferred to the ICU for closer monitoring and placed on non-invasive helmet ventilation with HFNC (Flow 80L/min, FiO2 0.8) and inhaled nitric oxide (20 ppm). He received hydroxychloroquine, lenzilumab, and tocilizumab as part of his COVID-19 treatment. After 8 days on noninvasive ventilation via helmet, his condition continued to improve and he was transitioned to an oxygen mask and eventually nasal cannula. He was then discharged home with supplemental oxygen provided via nasal cannula (NC) (2L/min).
3.4. Case #4
A 73-year-old female with a history of hypothyroidism, gastroesophageal reflux disease, hyperlipidemia, and chronic kidney disease presented to the emergency department with nausea, fatigue, and non-productive cough in the setting of COVID-19 pneumonia. Her hospital course was complicated by hypoxemic respiratory failure with a progressively increasing need for O2 via nasal cannula to non-rebreather mask at 15L/min. Her hypoxia continued to worsen and she required HFNC (Flow 80L/min, FiO2 0.8) via helmet therapy. She received lopinavir/ritonavir, remdesivir, ribavirin, tocilizumab, and hydroxychloroquine as part of her COVID-19 treatment. After three days of helmet therapy with HFNC, she was transferred to the floor on 6L NC and was eventually discharged home on 2L NC.
3.5. Case # 5
A 76-year-old male with a past medical history of chronic obstructive pulmonary disease (COPD) and small-cell lung cancer status post-chemotherapy and radiation (>5yrs prior to presentation, in remission) presented with acute hypoxic respiratory failure due to COVID-19 pneumonia. He was initially admitted to the general medical wards; but, due to escalating oxygen requirements, he was transferred to the ICU on the third day of hospitalization. There he was placed on HFNC (Flow 70L/min, FiO2 1.0) via the helmet along with inhaled nitric oxide (40 ppm). He received remdesivir, tocilizumab, and dexamethasone as part of his COVID-19 treatment. Unfortunately, he continued to deteriorate, and due to increasing hypoxia and work of breathing, he required endotracheal intubation on day 8 of hospitalization. His clinical condition deteriorated further and, after goals of care discussion with family, the patient was transitioned to hospice care and expired.
3.6. Scope for the future - helmet therapy for non-covid-19 patients
A 66-year-old male patient with a past medical history of congestive heart failure, associated with a reduced ejection fraction, underwent maxillary sinus repair surgery. His COVID-19 negative status was confirmed pre-operatively. He was extubated postoperatively but developed hypoxia due to flash pulmonary edema while recovering in PACU on a non-rebreather. His surgical procedure presented a contra-indication for NIPPV therapy and the ICU service was consulted for the need for possible reintubation. The patient was instead managed with HFNC (Flow 70L/min, FiO2 1.0) via helmet and intravenous furosemide. He had symptomatic improvements within 24 hours and was subsequently transferred to the general ward. The remainder of his hospitalization was uneventful from a respiratory perspective and he was eventually discharged home.
4. Discussion
We used noninvasive oxygenation via the helmet in carefully selected patients to decrease dispersion of the COVID-19 virus and to reduce the need for endotracheal intubation and mechanical ventilation. We went a step further and use this modality in a COVID-negative patient with hypoxic respiratory failure as an innovative way to explore the potential of this therapy.
Our report demonstrates favorable outcomes in a majority of the patients. Those of our patients who survived their acute hypoxic illness demonstrated increased tissue oxygenation after the application of the oxygen helmet. The patients who died during hospitalization demonstrated many risk factors known for increased mortality associated with COVID-19 and include male sex, obesity, pulmonary disease including COPD and lung cancer, diabetes, hypertension, and thrombocytopenia [[24], [25], [26]]. Table 1 summarizes the main characteristics and the outcomes for the patients included in this case series.
Table 1.
Patient characteristics and outcomes.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age | 70 | 74 | 68 | 73 | 76 |
| Sex | M | M | M | F | M |
| Duration of hospitalization before helmet treatment | 2 days | 1 day | 3 days | 7 days | 3 days |
| Time elapsed between symptom onset and helmet therapy | 9 days | 6 days | 8 days | 14 days | 13 days |
| P/F Ratio (Prior to initiation of oxygen helmet) | <200 | <200 | <300 | <300 | <100 |
| CRP | 235.3 | 168.5 | 79 | 229.7 | 82.8 |
| IL-6 | >400 | 314 | 30.9 | 16.2 | 16.1 |
| d-dimer | >42000 | 678 | 751 | 1516 | 1058 |
| SOFA score (Day of admission) | 5 | 3 | 3 | 2 | 3 |
| Helmet settings | |||||
| Flow rate (L/min) | 60 | 30 | 50 | 80 | 50 |
| FiO2 | 1.0 | 0.8 | 0.8 | 0.8 | 1.0 |
| PEEP (cmH2O) | 8 | 7 | 5 | 5 | 7 |
| Average respiratory rate | 24 | 32 | 30 | 22 | 24 |
| Duration of helmet therapy | 1 day | 1 day | 7 days | 5 days | 4 days |
| Duration of NO | 16 days | 6 days | 1 day | No | 12 days |
| Intubation (yes/no) | Yes | No | No | No | Yes |
| Duration of hospital stay | 22 days | 9 days | 13 days | 17 days | 14 days |
| Outcome | Deceased | Alive | Alive | Alive | Deceased |
The patient population who would benefit from this treatment modality should be carefully selected. The most important selection criterion to be considered is the respiratory function of the patient. The helmet is dependent on the patient's ability to generate tidal volume and minute ventilation sufficient to meet their needs. Patients who are unable to tolerate the mask associated with NIPPV due to facial anatomy, facial hair, skin breakdown from prolonged use of mask, or risk of aspiration, can also benefit from this practice. The factors which helped us in selecting the patients include claustrophobia, mask intolerance, degree of CO2 retention, degree of hypoxia, risk of vomiting/aspiration, and patient/family preference.
The usage of oxygen hood in the current COVID-19 pandemic showcased several advantages. The cost of each hood is measured in hundreds of dollars as opposed to the thousands of dollars range when compared with mechanical ventilation. Furthermore, this therapy decreases overall hospital costs associated with the prolonged ICU admission mechanical ventilation entails [17]. While the rubberneck gaskets are used for single patients due to the customized sizing procedure required, the hoods can be sterilized and reused on multiple patients. The hoods are also compatible with adjunct therapy such as nitric oxide (NO) therapy, an approach that has been used with success in COVID-19 patients [[19], [20], [21]]. Oxygen supplementation with the oxygen helmet also provides the patient with the ability to drink fluids through a straw, wear glasses under the helmet, and engage in verbal communication with greater ease. More importantly, the neck seal and the viral filter reduces the risk of aerosol generation and nosocomial infection for healthcare workers and other patients. Thus, not only helmet oxygenation a cost-effective alternative, but it also has the potential to prevent intubation and preserve ventilators in the current pandemic. Table 2 provides a visual reference for relative comparison of multiple modalities of providing supplemental oxygen.
Table 2.
Relative Comparison of multiple modalities of providing supplemental oxygen.
| Oxygen helmet | HFNC | BiPAP | MEchanical Ventilation | |
|---|---|---|---|---|
| COST | $$ | $$ | $$$ | $$$ |
| RISK OF AEROSOLIZATION | Low | High | High | Low |
| INDICATIONS FOR USE: | ||||
| -HYPOXIC FAILURE | + | + | + | + |
| -HYPERCARBIC FAILURE | – | – | + | + |
| PATIENT COMFORT | Medium | Medium | Low | Low |
| USE OF INHALED NO ALONG WITH DEVICE | + | + | – | + |
There is a potential to expand the application of this method of oxygen therapy to other patients for whom traditional NIPPV or intubation is either contraindicated or sought to be avoided. We demonstrated this by preventing intubation in a COVID-negative patient, who underwent maxillary sinus surgery when NIPPV was contraindicated. Preventing intubation has many benefits including preventing ventilator-induced lung injury (VILI), ICU associated delirium, infection, and longer ICU stay & hospitalization. This practice can possibly be applied in surgical patients such as gastric bypass patients with fresh anastomoses, immunocompromised patients at risk of developing ventilator-associated pneumonia, or hypoxic patients with advanced directives precluding intubation (i.e. Do Not Intubate (DNI) orders). Thus, more trials of this innovative type of ventilation with either high-flow or NIPPV in different patient populations are necessary to fully demonstrate its potential.
While there are numerous benefits to the helmet, there are limitations that need to be considered. This includes the inability to titrate PEEP to higher levels as well as the inability to adjust the tidal volume. Furthermore, there is a risk of nitrogen dioxide (NO2) development—a pulmonary irritant with lethal potential [22]. This highlights the need for continuous patient monitoring with a trained team of critical care providers. Though the helmet was used in Europe previously [16], this practice is not widely recognized in North America and thus, its availability is low. Patient mobility was not assessed in our case series due to clinical condition. The patients with COVID-19 were prone to significant desaturation episodes with even minimal movement at the nadir of their course and this correlated with the timing of treatment with the oxygen helmet. Any periods of patient mobility that did occur were limited to repositioning in bed or moving to the bedside chair. Furthermore, while some PEEP is provided, our case series documents the use of helmet therapy to provide increased oxygen support rather than ventilatory support and it is not clear to what degree the helmet therapy may assist in a patient with profound hypercapnia.
The regimen of pharmacotherapy provided to the patients in our series may represent a confounding variable. The standards of care during the pandemic, particularly during the early phases, represented a dynamic set of guidelines supported by varying levels of evidence. The differing treatments provided to our patients reflect this state of flux but were appropriate with respect to the guidelines at the time of the individual patient's hospitalization. We recognize that certain medications and treatment regimens have subsequently fallen out of favor as the medical community's COVID-19 knowledge-base has grown.
In conclusion, further studies are required to investigate the impact of these limitations. Although a previous randomized control trial with ARDS patients demonstrated its utility in reducing intubation rates and consequently reducing the 90-day mortality, new trials are necessary to replicate our outcomes on a larger scale [11].
5. Conclusion
Supplemental oxygen therapy via the hyperbaric helmet is an innovative modality with the potential for widespread utilization in critical care practice. This cost-effective approach is well-tolerated in appropriately selected patients with escalating supplemental oxygen requirements, highlighting the benefits provided by the helmet over other modalities. However, oxygen therapy with the helmet does warrant further study to confirm its efficacy on a larger scale.
Declaration of competing interest
This manuscript has not been presented in whole or in part. No funding was provided and the authors do not have any conflicts of interest to declare.
Acknowledgements
The authors wish to acknowledge the hard work of every team member in our critical care units including the physicians, advance practice providers, nurses, respiratory therapists, patient care assistants, physical therapists, occupational therapists, without whom we could not provide the highest-quality and innovative patient care.
References
- 1.Guan W.-j. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spinelli A., Pellino G. COVID-19 pandemic: perspectives on an unfolding crisis. Br. J. Surg. 2020;107(7):785–787. doi: 10.1002/bjs.11627. Epub 2020 Mar 23. PMID: 32191340; PMCID: PMC7228411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holshue M.L. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phua J. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respiratory Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halpern N.A., Tan K. Society of Critical Care Medicine; 2020. United States Resource Availability for COVID-19; p. 3. [Google Scholar]
- 9.Tuite A.R., Fisman D.N., Greer A.L. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. CMAJ (Can. Med. Assoc. J.) 2020;192(19):E497–E505. doi: 10.1503/cmaj.200476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White D.B., Lo B. A framework for rationing ventilators and critical care beds during the COVID-19 pandemic. Jama. 2020;323(18):1773–1774. doi: 10.1001/jama.2020.5046. [DOI] [PubMed] [Google Scholar]
- 11.Patel B.K. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome: a randomized clinical trial. J. Am. Med. Assoc. 2016;315(22):2435–2441. doi: 10.1001/jama.2016.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koegel E., Sheffield P.J. Google Patents; 1986. Light-weight Oxygen Delivery Hood Assembly for Hyperbaric Chamber. [Google Scholar]
- 16.Hui D.S. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147(5):1336–1343. doi: 10.1378/chest.14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyeremanteng K. Cost analysis of noninvasive helmet ventilation compared with use of noninvasive face mask in ARDS. Can. Respir. J. J. Can. Thorac. Soc. 2018;2018:6518572. doi: 10.1155/2018/6518572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martel J. 2020. Could Nasal Nitric Oxide Help to Mitigate the Severity of COVID-19? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari M. Inhaled nitric oxide in mechanically ventilated patients with COVID-19. J. Crit. Care. 2020;60:159–160. doi: 10.1016/j.jcrc.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh R. Inhaled nitric oxide treatment in spontaneously breathing COVID-19 patients. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620933510. 1753466620933510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petit P.C. The pathophysiology of nitrogen dioxide during inhaled nitric oxide therapy. Am. Soc. Artif. Intern. Organs J. 2017;63(1) doi: 10.1097/MAT.0000000000000425. [DOI] [PubMed] [Google Scholar]
- 24.Figliozzi S. Predictors of adverse prognosis in COVID‐19: a systematic review and meta‐analysis. Eur. J. Clin. Invest. 2020;50(10) doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 25.Robilotti E.V. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robilotti E.V. Determinants of COVID-19 disease severity in patients with cancer. Nat. Med. 2020;26(8):1218–1223. doi: 10.1038/s41591-020-0979-0. [DOI] [PMC free article] [PubMed] [Google Scholar]