Abstract
Experience and thoughts that are unrelated to the external surroundings are pervasive features of human cognition. Research under the rubric of mind-wandering suggests that such internal experience is context-dependent, and that the content of ongoing thought differentially influences a range of associated outcomes. However, evidence on how the extent of mind-wandering and its content translate from the laboratory to daily life settings is scarce. Furthermore, the relationship between such patterns of thought with markers of stress in daily life remains underexplored. In the current study, we examined multiple aspects of mind-wandering of ninety-three healthy participants (47 women, 25.4 ± 3.9 years) in both the laboratory and daily life and explored two questions: (a) how are mind-wandering extent and content correlated across both settings, and (b) what are their relationships with subjective stress and salivary cortisol levels in daily life? Our results suggest that the extent of off-task thinking is not correlated across contexts, while features of content—i.e., social, future-directed and negative thought content—robustly translate. We also found that daily life subjective stress was linked to more on-task, negative, and future-directed thinking, suggesting stress was linked with the need to act on personally relevant goals. Based on these results we speculate that differences in the links between stress and ongoing thought in daily life may be one reason why patterns of thinking vary from lab to everyday life. More generally, these findings underline the need to consider both context and content in investigating mind-wandering and associated features of subjective experience, and call for caution in generalizing laboratory findings to participants’ daily lives.
Introduction
Disengaging from external stimulation and letting the mind wander from the here-and-now is a common phenomenon in everyday life. In fact, humans tend to engage in thoughts that are at least partially unrelated to their current task or environment for up to half of their conscious time (Kane et al., 2007; Kane et al., 2017; Killingsworth & Gilbert, 2010; Seli et al., 2018). Accordingly, scientific interest into what is generally referred to as “mind-wandering” remains high (Smallwood & Schooler, 2015). Current research has called for a more nuanced understanding of this construct and has highlighted the heterogeneity of related experiences (e.g., Seli et al., 2018; Wang et al., 2017). In particular, the differentiation of mind-wandering qualities such as content and form (Smallwood et al., 2016), and the exploration of specific contexts in which self-generated, task-unrelated thoughts occur, are argued to be important avenues to advance our understanding of the costs and benefits of different aspects of experience (Smallwood & Andrews-Hanna, 2013; Wang et al., 2017).
Studies routinely capture individuals’ internal experiences using experience sampling techniques (Csikszentmihalyi & Larson, 1987) both in the laboratory and in daily life. In the lab, researchers often exploit the ability to constrain task context to either induce mind-wandering (typically by keeping cognitive demands low; Smallwood, Nind, & O’Connor, 2009) or to detect the consequences of off-task thought using tasks which demand continuous external attention (e.g., McVay & Kane, 2009), or both (Turnbull et al., 2019). In contrast, daily life situations present us with more complex ecological contexts which may be less readily comparable to the lab situation. In contexts requiring high cognitive (e.g., attentional) capacities, mind-wandering has been related to disruptions of performance in complex tasks such as measures of intelligence (Mrazek et al., 2012) or reading (Schooler, 2004). However, studies show that ongoing experience is generally adjusted to current demands (Kane et al., 2007; Rummel & Boywitt, 2014). When demands are low, mind-wandering can be associated with beneficial outcomes such as facilitated prospection (Baumeister & Masicampo, 2010) or attenuated low mood through ‘mental breaks’ from monotonous occupation (Ruby, Smallwood, Engen, & Singer, 2013a). Mind-wandering has further been linked to creative thinking (see Fox & Beaty, 2019 for a recent review) and may aide creative problem solving (Baird et al., 2012), although conflicting results have been found (Smeekens & Kane, 2016). The importance of specifying the task context in understanding the links between internal experience and aspects of psychological functioning is known as the context regulation hypothesis (Smallwood & Andrews-Hanna, 2013).
Studies on the content of mind-wandering indicate a prospective bias (i.e., a tendency to engage in more future-directed thoughts; Smallwood & Schooler, 2015). This prospective bias is primarily regarded as reflecting the utility of future planning (Baird, Smallwood, & Schooler, 2011). It is thought to depend on autobiographical memories (Smallwood et al., 2011) and may afford the refinement of personal goals (Medea et al., 2018). Past-oriented thoughts, on the other hand, have been shown to follow unhappy moods (Smallwood & O’Connor, 2011). While prior accounts have suggested a general link of mind-wandering to unhappiness (Killingsworth & Gilbert, 2010), later findings taking the specific thought content into account have produced a more complex picture. Thus, the affective consequences of ongoing thought depend on its specific socio-temporal content both in the laboratory (Ruby et al., 2013a) and in daily life (Welz, Reinhard, Alpers, & Kuehner, 2018), and on the level of interest in the respective mind-wandering episodes (Franklin et al., 2013). Also, individual differences in thought content differentially relate to measures of emotional well-being (Andrews-Hanna et al., 2013). Taken together, the content regulation hypothesis formalises these differential accounts and stresses that the relation of psychological well-being and self-generated thought is dependent on an individual’s capacity to regulate their thoughts’ content (Smallwood & Andrews-Hanna, 2013).
Mind-wandering is an umbrella term for multiple conceptualizations of cognitive content (Seli et al., 2018). It shares overlap with perseverative cognition (Ottaviani, Shapiro, & Couyoumdjian, 2013), which refers to two particularly prominent types of thought patterns characterized by repetitive thinking with a negative focus on events in the past or future (rumination or worry; Brosschot, Gerin, & Thayer, 2006). Perseverative cognition has been associated with psychopathology (McLaughlin & Nolen-Hoeksema, 2011) and conceptually ties stress and its physiological correlates to somatic health risks (Ottaviani et al., 2016). Recent studies into mind–body interactions within the framework of stress have identified associations of thought content and stress-induced cortisol output both in the laboratory setting (Engert, Smallwood, & Singer, 2014) and daily life (Linz, Singer, & Engert, 2018). Specifically, more negative emotional and a pattern of less future- and self-focused thoughts were linked to increased cortisol levels both at rest and after an acute laboratory stress paradigm (Engert et al., 2014). In daily life, more past-focused thoughts were associated with increased cortisol in the absence of stress. When experiencing stress, however, more negative and more future-directed thoughts predicted increased cortisol release (Linz et al., 2018). In contrast to the lab study (Engert et al., 2014), and highlighting the context specificity of where thoughts are assessed, the social thought dimension was unrelated to subjective stress or cortisol in daily life (Linz et al., 2018).
Given the ubiquity of internal experience such as mind-wandering, studies have begun to explore its relationship in different contexts. The first study to investigate mind-wandering in both laboratory and daily life within the same population found the amount of reported mind-wandering to relate between contexts, concluding mind-wandering to be a stable cognitive characteristic (McVay, Kane, & Kwapil, 2009). Moreover, more off-task thoughts in the lab predicted more worrying in daily life (McVay et al., 2009). A longitudinal study by Ottaviani and Couyoumdjian (2013) found that the frequency of laboratory mind-wandering episodes correlated with those in daily life after more than year, suggesting a stable individual disposition. A more recent study questioned these results by showing that the mind-wandering rate in the laboratory only had a marginal relationship to the same measure in daily life, and this relationship was less robust than links with contextual predictors such as the current activity or affective state (Kane et al., 2017). Regarding the content of thoughts, little is known about how well laboratory findings generalise to daily life.
Current study: aims and hypotheses
There were two aims to the current study. First, we analysed how both the content and focus of experience, i.e., specific content dimensions and the extent of off-task thinking, correlated within individuals from a controlled laboratory setting to daily life. Second, we explored moment-to-moment associations of subjective experience with both subjective and physiological stress markers, captured over 2 days of participants’ daily lives.
We aimed to compare the amount of off-task thinking in the laboratory and daily life based on the notion that the extent of mind-wandering is dependent on the demands of a given context (Kane et al., 2007), and recent evidence challenging the assumption of a consistent association between contexts (Kane et al., 2017). Emerging evidence suggests that a prospective bias is present in both the lab and daily life (e.g., Smallwood et al., 2009; Linz et al., 2018), and that it is most pronounced in situations with the lowest tasks demands. Future-directed thoughts have been shown to covary with self-focused thoughts (Ruby et al., 2013a), perhaps because of a reliance on underlying autobiographical, self-referential processes (Baird et al., 2011; Smallwood et al., 2011) which are particularly relevant to the individual (Stawarczyk, Cassol, & D’Argembeau, 2013). Accordingly, we aimed to identify whether the degree of future-directed and self-focused thought is particularly stable between contexts. Furthermore, as negative thought patterns such as rumination or worry are not only repetitive but also tend to occur habitually (Watkins, 2008), the emotional valence of thoughts may generalize well from one context to the other, particularly so when negative.
We expected subjective stress to be associated with momentary demands and thus to inversely relate to off-task thinking. Regarding thought content, we expected stress to be primarily related to negative (and inversely to positive) thoughts, as seen in Linz et al. (2018). Perseverative cognition implies a self-referential component of thought irrespective of the temporal focus (Brosschot, 2010), which may thus be associated with higher levels of subjective stress. Finally, based on our previous findings, we hypothesized a link between negative thought content and cortisol (Engert et al., 2014; Linz et al., 2018).
Methods
Participants
Ninety-three participants (47 women, age mean ± SD = 25.4 ± 3.9 years, age range 18–35 years) provided daily life experience sampling data for the present study. Repeated unavailability or insufficient compliance with the study protocol lead to the exclusion of six initially eligible participants, who had previously taken part in the laboratory testing session (reported in Engert et al., 2014). The majority of participants were students (77%), 14 (15%) were employed, eight (9%) held no job. A higher education degree was held by 27% of participants, 69% held a higher education entrance qualification (e.g., high-school diploma). Compliance with eligibility criteria was ascertained in a structured telephone interview targeting current and recent history of psychological and physiological disorders as well as medication and drug consumption. To avoid confounding effects on both subjective measures and cortisol levels, exclusion criteria were regular smoking or recreational drug use, chronic illness, current psychological disorder and medication intake affecting the HPA axis. To limit effects of sex hormones on cortisol levels (Kajantie & Phillips, 2006), female participants did not use hormonal contraceptives and were tested in the luteal phase of their cycle. The study was approved by the Research Ethics Board of Leipzig University (ethics number: 360-10-13122010). Participants gave written informed consent, could withdraw from the study at any time and received financial compensation.
Procedure
Participants completed 2 days of experience sampling in daily life. In addition, the majority of participants (N = 88) had previously taken part in a laboratory study assessing mind-wandering in relation to a psychosocial stress paradigm (reported in Engert et al., 2014), from which the baseline mind-wandering measurement was obtained for comparison with current daily life mind-wandering parameters. Daily life sampling was completed within the week following the lab testing session. Participants were advised to choose 2 regular consecutive weekdays representative of their daily life routines (Monday/Tuesday, Wednesday/Thursday or Thursday/Friday, depending on participant availability). All experience sampling data were gathered using mobile devices, which were equipped with a custom inhouse software app. At each designated sampling time point, the app prompted the respective subjective experience questionnaires and reminded participants to take a saliva sample. To ensure proficiency in handling the mobile device and self-administering saliva samples, participants received an introductory training before data collection. Saliva sampling started immediately upon free awakening (while still in bed). Further sampling times were 30 and 60 min later, as well as at 12:00, 3:00, 6:00 and 9:00 pm. Experience sampling questionnaires overlapped with the cortisol sampling schedule, except for the wakeup sample for which no experience sampling was recorded. Sampling times during the day had a margin of fluctuation (± 15 min of the fixed interval) to avoid complete predictability. The thought sampling procedure of the laboratory testing is described in detail in Engert et al. (2014). In short, participants followed a routine paradigm for sampling self-generated thoughts (e.g., Smallwood et al., 2011), which consisted of repeated thought content probes intermittent of two cognitive tasks repeatedly employed in studying mind-wandering (see Engert et al., 2014 and below for details).
Measures
Daily life subjective experience
Throughout the day, participants provided information on their momentary subjective experience including their content of thought and the extent of off-task thinking, affect and arousal as well as the occurrence of subjective stress.
Thought content and extent of mind-wandering In analogy to the laboratory testing (Engert et al., 2014), participants indicated at each sampling time point their current thought content in six separate content items (on a scale of 1–10): positive and negative (valence), future-directed and past-directed (temporal), and self-focused and other-oriented (social). In addition, they rated the extent to which their current thoughts were unrelated to their current task (off-task, on a scale from 1 to 10) as a proxy for the mind-wandering extent. Laboratory mind-wandering was sampled during two counterbalanced sessions of both the choice reaction time task (CRT) and the working memory task (WM), two tasks frequently used in previous laboratory mind-wandering studies (Engert et al., 2014; Smallwood, Ruby, & Singer, 2013; Smallwood et al., 2011).
Affect and arousal Concurrent affect and arousal were obtained using the Affect Grid (Russel, Weiss, & Mendelsohn, 1989), a single item scale assessing the dimensions pleasure-displeasure and arousal-sleepiness (both on a scale from 1 to 9) with adequate reliability, convergent and discriminant validity.
Subjective stress At each sample, participants further indicated how stressed they felt (on a visual analogue scale ranging from 1 to 10).
Salivary cortisol
Saliva was sampled into Salivette collection devices (Sarstedt, Nümbrecht, Germany). Participants were instructed to place the collection swabs in their mouths and to refrain from chewing for 2 min. They were asked to refrain from any oral intake during the 10 min before sampling, and otherwise followed their normal daily routine. Participants were instructed to store the salivettes in the freezer as soon as their daily routines permitted. Upon return to the laboratory, salivettes were stored at − 30 °C until assay (at the Department of Biological and Clinical Psychology, University of Trier, Germany). Cortisol levels (expressed in nmol/l) were determined using a time-resolved fluorescence immunoassay with intra-/inter-assay variabilities of < 10%/12%.
Statistical analysis
We applied principal component analysis with varimax rotation to decompose all daily life thought samples across participants into components reflecting patterns of covarying thought content dimensions (see Engert et al., 2014 for analogous decomposition of laboratory samples). To analyse associations of mind-wandering content between the two contexts, we used multiple correlations to relate averages of each content between daily life and laboratory. Statistical significance in this analysis was controlled for seven comparisons via Bonferroni correction and alpha threshold lowered to (0.05/7) = 0.007.
To analyse moment-to-moment associations of both subjective- and physiological measures of stress with subjective experience including thought content, we built two mixed-effects models with (1) subjective stress and (2) salivary cortisol as dependent variables of all available samples across days and subjects. Both models included a random intercept for subject. Since cortisol secretion follows a diurnal rhythm and is influenced by individual characteristics (Kudielka, Gierens, Hellhammer, Wust, & Schlotz, 2012), we included age, sex, time of sample as well as wakeup time as covariates in Model 2. In this model, we excluded all samples associated with the cortisol awakening response (wakeup, 30 min and 60 min samples) from analysis, since it has been shown to represent a distinct feature of the cortisol circadian rhythm (Wilhelm, Born, Kudielka, Schlotz, & Wüst, 2007) not readily comparable to samples later during the day (Stadler et al., 2015). Both models are specified below in Raudenbush and Bryk (2002) notation:
Model 1:
- Level 1
Ysdi = π0di + π1di (off-task) + π2di (negative) + π3di (positive) + π4di (self) + π5di (other) + π6di (future) + π7di (past) + π8di (affect) + π9di (arousal) + etdi
- Level 2
π0di = β00i + u0di
- Level 3
β00i = γ000 + r00i
Model 2:
- Level 1
Ysdi = π0di + π1di (time) + π2di (stress) + π3di (off-task) + π4di (negative) + π5di (positive) + π6di (self) + π7di (other) + π8di (future) + π9di (past) + π10di (affect) + π11di (arousal) + etdi
- Level 2
π0di = β00i + β01i (awakening time) + u0di
- Level 3
β00i = γ000 + γ001 (sex) + γ002 (age) + r00i
Taking into account the hierarchical structure of the data with multiple samples (Level 1) nested into days (Level 2) and subjects (Level 3), the respective dependent variable (subjective stress or cortisol level) was predicted by the intercept (π0di), all measures of momentary subjective experience on level 1, day-specific predictors (e.g., awakening time in model 2) on level 2 and individual predictors (e.g., sex in model 2) on level 3. Both models’ residuals displayed only very negligible deviations from normal distribution. Further checks on potential multicollinearity of the models’ predictors were negative: all variance inflation factors were < 0.15, indicating the models’ efficacy in assessing the unique influence of all predictors on the respective dependent variable. Estimates reported are restricted maximum likelihood marginal estimates using an unstructured covariance structure. Raw cortisol values were ln-transformed before analysis. All analyses were performed using R version 3.5.1 (R Core Team, 2018) and the packages lme4, car and psych. Unless stated otherwise, significance was set to a level of p (alpha) < 0.05.
Results
Descriptive statistics
Compliance with the study protocol was overall satisfactory: Regarding electronic probes of daily life thoughts, 1043 (93.4% of 1116 total) probes were completed, while 1219 (93.6% of 1302 total) cortisol samples entered analysis. Table 1 presents descriptions of subjective experience samples in daily life. We found that thoughts were more future- than past-directed [t(1042) = 21.847, p < 0.001] and more positive than negative in valence [t(1042) = 23.515, p < 0.001]. The social dimension of thought (self vs. other) was balanced [t(1042) = 0.81613, p > 0.4]. Ratings of the extent to which thoughts were off vs. on current task indicated that participants were more frequently off-task than on-task (147 out of 1043 probes [14%] were completely off-task; 104 out of 1043 probes [10%] completely on-task). Reported levels of subjective stress were on average low; specifically, in 455 out of 1043 probes (43%) participants reported the lowest possible stress rating (on a continuous scale from 1 to 10).
Table 1.
Descriptive statistics of daily experience samples
| Mean (range) | SD | n | t, p(t) | |
|---|---|---|---|---|
| Off-task | 5.47 (1–10) | 2.99 | 1043 | |
| Negative | 2.5 (1–10) | 2.17 | 1043 | 23.515, p < 0.001 |
| Positive | 5.29 (1–10) | 2.53 | 1043 | |
| Self | 4.54 (1–10) | 2.58 | 1043 | 0.81613, p = 0.414 |
| Other | 4.45 (1–10) | 2.87 | 1043 | |
| Future | 5.16 (1–10) | 2.81 | 1043 | 21.847, p < 0.001 |
| Past | 2.72 (1–10) | 2.48 | 1043 | |
| Valence (affect grid) | 6.32 (1–9) | 1.74 | 1148 | |
| Arousal (affect grid) | 4.78 (1–9) | 2.14 | 1148 | |
| Subjective stress | 2.65 (1–10) | 2.03 | 1043 |
Relation of laboratory and daily life mind-wandering: extent and content
In a first step, we applied principal component analysis (PCA) with varimax rotation to all daily life thought samples across all participants. Figure 1 shows loadings of the three components derived from the thought content samples indicating similar results as have been found in previous studies (Ruby et al., 2013a; Ruby, Smallwood, Sackur, & Singer, 2013b) and a largely overlapping set of participants as tested in the laboratory setting (Engert et al., 2014). The three components accounted for a cumulative 63% of variance and reflected (a) social–temporal thoughts directed at oneself- and the future (S–F), (b) off-task and social-temporal thoughts directed towards others and the past (O–P), and (c) thoughts both positive and negative in valence (P/N).
Fig. 1.
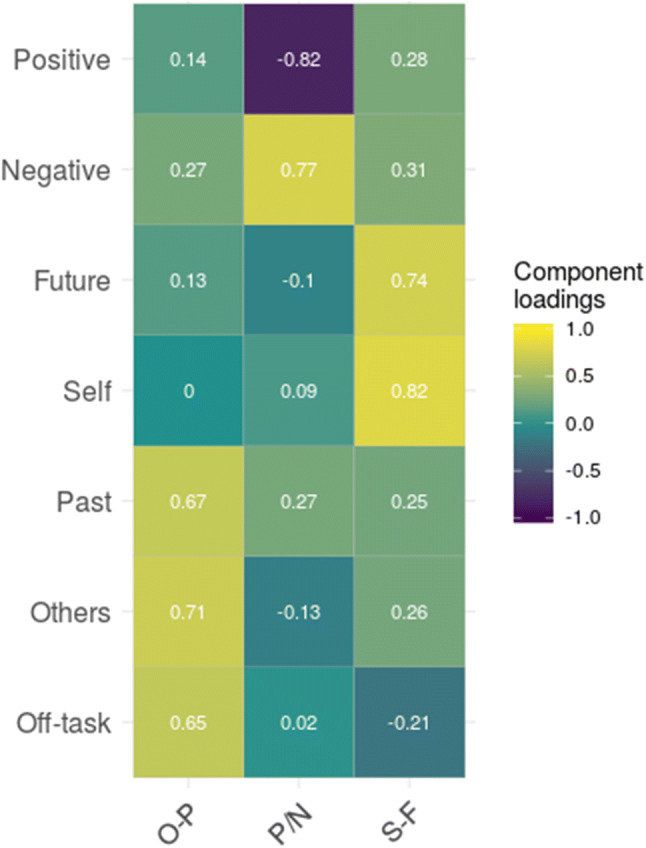
Principal components and respective rotated component loadings of daily life thought probes
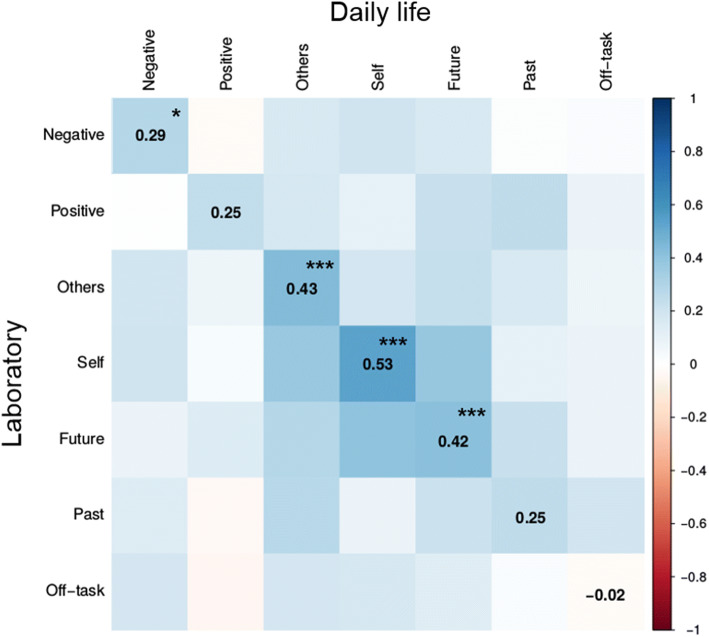
To investigate how the laboratory thought content translates to daily life, we calculated multiple within-subject correlations between the averages of specific thought content dimensions of the two contexts. Table 2 and Fig. 2 show the respective correlations. The social dimension (both self- and other-directed thoughts), future-directed thoughts, and negative thoughts showed highly significant correlations between contexts. Correlations of positive thoughts or past-directed thoughts between laboratory and daily life were not significant after Bonferroni correction for multiple comparisons. The extent to which participants’ thoughts were off-task in the laboratory session and in daily life were also not associated.
Table 2.
Correlations of respective thought content between laboratory and daily life context
| r | t | df | p | |
|---|---|---|---|---|
| Off-task | − 0.023 | − 0.214 | 86 | 0.830 |
| Negative | 0.289 | 2.808 | 86 | 0.006* |
| Positive | 0.248 | 2.381 | 86 | 0.019 |
| Self | 0.530 | 5.809 | 86 | <0.001*** |
| Other | 0.430 | 4.422 | 86 | <0.001*** |
| Future | 0.419 | 4.285 | 86 | <0.001*** |
| Past | 0.252 | 2.420 | 86 | 0.018 |
*p < 0.007; ***p < 0.00014
Fig. 2.
Correlations of mind-wandering content between daily life and laboratory context
Associations of daily life subjective experience with markers of stress
To investigate the relation of measures of subjective experience (thought content, extent of mind-wandering, affect, and arousal) with measures of stress, we ran two separate models assessing moment-to-moment associations of subjective experience with (1) subjective stress and (2) cortisol as dependent variables.
Model 1 examined the association of subjective experience with subjective stress. Estimates of Model 1 parameters are displayed in Table 3. More subjective stress was associated with more negative and less positive thoughts, more future- and less past-directed thoughts as well as more task focus (i.e., less mind-wandering) overall. In addition, subjective stress was linked to higher levels of arousal.
Table 3.
Estimates for Model 1 (predictors of subjective stress)
| Fixed effects | B (SE) | CI | p |
|---|---|---|---|
| (Intercept) | 2.65 (0.103) | 2.45 to 2.85 | < 0.001 |
| Off-task | − 0.05 (0.175) | − 0.1 to − 0.01 | 0.019 |
| Negative | 0.32 (0.035) | 0.25 to 0.39 | < 0.001 |
| Positive | − 0.12 (0.029) | − 0.18 to − 0.07 | < 0.001 |
| Self | − 0.01 (0.029) | − 0.07 to 0.05 | 0.71 |
| Other | − 0.02 (0.026) | − 0.07 to 0.03 | 0.45 |
| Past | − 0.09 (0.031) | − 0.15 to − 0.02 | 0.006 |
| Future | 0.06 (0.026) | 0.01 to 0.11 | 0.018 |
| Affect | − 0.07 (0.040) | − 0.15 to 0.01 | 0.084 |
| Arousal | 0.09 (0.034) | 0.03 to 0.16 | 0.007 |
| Random effects | Variance | SD |
|---|---|---|
| Individual (intercept) | 0.495 | 0.70 |
| Day (intercept) | 0.154 | 0.39 |
| Residual | 2.749 | 1.66 |
Model 2 examined the association of salivary cortisol levels with all subjective experience measures. Table 4 displays the estimates of Model 2 parameters including covariates sampling time, time of wakeup and sex. Sampling time was the strongest predictor of cortisol levels (β30 = 0.001, t = 3.05, p = 0.003) such that lower cortisol levels were associated with longer time between wakeup and a respective sample. Furthermore, subjective stress and earlier time of awakening were linked to higher cortisol levels. All other measures of subjective experience and sex were unassociated with concurrent cortisol levels.
Table 4.
Estimates for Model 2 (predictors of cortisol)
| Fixed effects | B (SE) | CI | p |
|---|---|---|---|
| (Intercept) | 1.73 (0.237) | 1.28 to 2.19 | < 0.001 |
| Off-task | − 7.37 (0.010) | − 0.01 to 0.03 | 0.47 |
| Negative | 6.83 (0.035) | − 0.02 to 0.04 | 0.68 |
| Positive | − 3.68 (0.013) | − 0.03 to 0.03 | 0.78 |
| Self | 9.72 (0.013) | − 0.01 to 0.03 | 0.46 |
| Other | − 1.04 (0.012) | − 0.03 to 0.01 | 0.37 |
| Past | 1.98 (0.014) | − 0.00 to 0.05 | 0.16 |
| Future | 1.11 (0.012) | − 0.01 to 0.03 | 0.36 |
| Affect | 1.69 (0.018) | − 0.01 to 0.05 | 0.34 |
| Arousal | 2.07 (0.015) | − 0.01 to 0.05 | 0.18 |
| Subjective stress | 3.37 (0.016) | − 0.01 to 0.06 | 0.03 |
| Sex | 1.07 (0.092) | − 0.07 to 0.28 | 0.25 |
| Age | − 4.57 (0.012) | − 0.02 to 0.02 | 0.99 |
| Time | − 1.24 (0.008) | − 0.14 to − 0.11 | < 0.001 |
| Awakening time | − 1.24 (0.000) | − 0.00 to − 0.00 | 0.005 |
| Random effects | Variance | SD |
|---|---|---|
| Individual (intercept) | 0.096 | 0.31 |
| Day (intercept) | 0.008 | 0.09 |
| Residual | 0.330 | 0.57 |
Discussion
Research in the mind-wandering domain has highlighted the importance of both context and content when assessing the characteristic features of ongoing thought (Smallwood & Andrews-Hanna, 2013). In particular, emerging evidence has highlighted the need to understand the extent to which laboratory findings generalize to more complex, ecologically valid contexts in daily life (Kane et al., 2017). Our study provides evidence on (1) how different aspects of ongoing thought differentially translate from the laboratory to daily life and (2) how both subjective stress as well as associated levels of the HPA axis end hormone cortisol relate to thought content and other subjective experiences such as affect and arousal in everyday life. We found that only certain aspects of ongoing thought were correlated across situations. Individual variation in social thoughts (about oneself and others), future-directed and negative thoughts displayed high stability across the lab and daily life, while past-directed and positive thoughts were less stable. Importantly, the degree of off-task thinking did not transfer from lab to daily life. Together, our findings highlight that the transferability of experiential reports from the lab to daily life is content-specific. They suggest that not only for whom and when the mind wanders (Kane et al., 2017), but also where it wanders may vary between laboratory and daily life.
We found no correlation in reports of off-task thinking between different contexts, and so our results diverge from those by McVay et al. (2009) and Ottaviani and Couyoumdjian (2013), and suggest an even less robust association than the well-powered study by Kane et al. (2017). A simple explanation for this divergence could be the way internal experience was measured. In our study, we separated multiple features of ongoing thought (task focus, temporal and social content, and affective qualities), while others have used a method in which these features were combined (i.e., concepts like perseverative cognition or everyday worries entail combinations of features of experience such as self-focus and affective content, that our study measured individually). On considering these issues, our methods of conceptualizing experience along separate dimension is possibly more conservative, because it allows us to distinguish aspects of experience that do generalize (i.e., self, future and social foci) from those that do not (being off-task). We also measured off-task thinking in a continuous manner, while others have operationalized mind-wandering dichotomously (Kane et al., 2017; McVay et al., 2009), and recent evidence suggests substantial differences in daily life off-task estimates depending on whether dichotomous or continuous rating options are employed (Seli et al., 2018).
In addition, the level of similarity between laboratory and daily life contexts likely contributes to diverging results. Ottaviani and Couyoumdjian (2013) differentiated mind-wandering from perseverative cognition and distraction, and found that the frequency of mind-wandering episodes in the laboratory predicted daily life mind-wandering a year later. Importantly, their laboratory session featured an ecological stress induction, and arguably, laboratory conditions more closely corresponding to daily life situations (where naturally occurring stressors are common) should increase correlations of mind-wandering rates.
Our study adds to an emerging picture regarding the complex links between stress and ongoing experience in daily life. We found higher subjective stress was linked to fewer off-task thoughts, a greater future focus and more negative cognition, a pattern suggesting that, in daily life, stress is often associated with the need to act. Our findings are different from those of McVay et al. (2009), who found an increased occurrence of task-unrelated thought in stressful situations and from those of Croswell and colleagues, who found unpleasant and neutral mind-wandering was associated with higher chronic stress as well as retrospective reports of daily stress (Crosswell, Coccia, & Epel, 2019). However, Kane et al. (2017) found that the mind-wandering probability was not significantly predicted by how stressful a current situation was experienced. We hypothesize that in the current study, moment-to-moment associations of stress with future-directed thoughts may reflect goal-directed planning, and potentially involve proactive efforts to alleviate ongoing or anticipated task strain. Past-directed thoughts, on the other hand, may have emerged from the ‘luxury’ of not having immediate tasks at hand (or not anticipating those). This rationale is supported by the pattern of our PCA analysis, which revealed covariation of past-focused and off-task thoughts. Negative (and, inversely, positive) thought content was the strongest predictor of subjective stress and remarkably, negativity/positivity of thought was a much stronger predictor of stress than (negative/positive) affect, which mirrors our recent daily life findings of an association of negative thoughts and cortisol levels (Linz et al., 2018). We did not find self- or other-focused thoughts to be linked to subjective stress. While a (negative) self-focus is an essential characteristic of perseverative cognition (Brosschot et al., 2006), our recent findings suggested a rather low prevalence of perseverative cognition in the daily life experience of healthy subjects (Linz et al., 2018). Conceivably, in healthy individuals, most stressful situations in daily life do not take place in isolation, but rather arise from social situations including both ourselves and others. Overall, emerging evidence illustrates links between stress and ongoing thought, and our data reinforce the need to recognize the complexity of this relationship. Future studies should profit from measuring multiple features of thought content when investigating the role of stress in daily life subjective experience.
The biomarker cortisol was not associated with any measure of subjective experience. Several reasons may explain why a link of cortisol and thought content may be less easily detectable in daily life than during a stress paradigm in the laboratory (Engert et al., 2014). Daily life stressors (and accompanying fluctuations in cortisol) are likely less pronounced than a full-blown laboratory stressor. Furthermore, while cortisol sampling in the laboratory is well controlled, samples in daily life are self-administered, and likely less reliable. Moreover, studies in ecologically valid environments are inherently noisier than controlled laboratory settings: a large proportion of variance in diurnal cortisol levels, for example, is explained by contextual factors (Kudielka et al., 2012). While we controlled for the biggest source of variance in diurnal cortisol levels, the respective time of each sample (Kudielka et al., 2012), other potential influences arising from the varying circumstances of everyday life (e.g., food or caffeine intake, physical activity, social interactions) may have obscured potential relations. In comparison to our recent finding of an interaction of stress and thought content in predicting cortisol in daily life (Linz et al., 2018), factors such as a different modelling approach, a smaller sample size and a limited age range of the investigated sample may have hindered corroborating evidence in the current study.
Several limitations of the present study need to be taken into account. First, it would have been advantageous to assess additional mind-wandering characteristics such as the form of thoughts (Smallwood et al., 2016) especially given recent evidence on the role of the default mode network in this feature of experience (Sormaz et al., 2018). On the same note, research has argued that intentional and unintentional mind-wandering are dissociable cognitive experiences (Seli, Risko, Smilek, & Schacter, 2016), which have been shown to differentially relate to the content and potential consequences of mind-wandering (Seli, Beaty, Marty-Dugas, & Smilek, 2019; Seli, Ralph, Konishi, Smilek, & Schacter, 2017). Likewise, we did not assess intrusiveness, repetitiveness, ruminative or worrysome qualities of thoughts, which would have enabled us to operationalize perseverative cognition in a more direct manner. Measuring personality traits such as trait anxiety or levels of depression may have shed light on interindividual differences mediating the relationship between thought content and stress. Because daily life episodes can be ambiguous regarding a current task or even be task-free (see Murray, Krasich, Schooler, & Seli, 2019 for an in-depth discussion), relying on a task-related operationalization of mind-wandering imposes general methodological challenges in daily life studies. Finally, cortisol measurement in ambulatory settings should be treated with some caution, unless using ways to objectively verify participants’ adherence to the sampling protocol (Stadler et al., 2015).
Before concluding, it is worth considering how our results can aid our understanding of how to link experience from the laboratory to daily life. Together, our data suggest patterns of off-task thinking are uncorrelated across situations, and in daily life are associated with lower levels of stress. Based on these data, a potential reason for why measures of on-task experience do not always generalize across contexts may be that in daily life, individuals are more likely to choose the actions they perform, and arguably are also more motivated to perform these tasks. In particular, highly constrained tasks are likely less common in daily life, but conceivably allow greater freedom to consider other topics and may have greater alignment to an individual’s goals (Murray et al., 2019). More generally, studies suggests that individuals’ off-task thoughts often have social features, and while laboratory task contexts often do not entail these social stimuli, they are frequently present in the real world. Self-generated thoughts are often assumed to reflect an individual’s current concerns (e.g., Klinger & Cox, 1987), a perspective that is supported by recent evidence implicating the dorsolateral pre-frontal cortex in the prioritisation of off-task thoughts in situations of low task demands (Turnbull, Wang, Murphy, et al., 2019). This prioritisation view of ongoing thought gains support from studies showing that the frequency of mind-wandering in the laboratory is closely linked to participants’ motivation (Seli, Cheyne, Xu, Purdon, & Smilek, 2015; Seli, Wammes, Risko, & Smilek, 2016), and that individuals can flexibly modulate their mind-wandering rates depending on upcoming demands (Seli et al., 2018; Turnbull, Wang, Schooler, et al., 2019; Turnbull, Wang, Murphy, et al., 2019). Together, these lines of evidence suggest that while thoughts with personally relevant content may be distractions in the lab (Stawarczyk, Majerus, Maj, Van der Linden, & D’Argembeau, 2011; Unsworth, McMillan, Brewer, & Spillers, 2012), they may be more closely aligned to opportunities to act in everyday life. Accordingly we suggest that if researchers want to approximate patterns of experience that correspond to those occuring in the real world, they should either assess experience outside of the lab, or measure patterns of experience in laboratory contexts with greater ecological validity and/or personal significance. We note that our data do not invalidate the exploration of patterns of ongoing experience in laboratory conditions. Instead, our study highlights the need to explicitly consider the boundary conditions of this approach when attempting to generalize from the lab to daily life.
In conclusion, we find that measures of ongoing thought differentially translate from lab to daily life. Our findings suggest that patterns of off-task thinking may not be reliably inferred from laboratory data, while specific content of thoughts, such as its social or episodic features, does transfer. Furthermore, we show a link between subjective stress and distinct thought content in daily life, with greater subjective stress linked to more on-task thoughts with a negative future focus. Taken together, our results add to a growing body of research emphasizing the heterogeneous nature of the wandering mind. We propose that findings in the mind-wandering domain should be carefully interpreted regarding their applicability to life outside the laboratory.
Acknowledgements
Open access funding provided by Max Planck Society. The data were collected in the former Department of Social Neuroscience headed by Dr. Tania Singer at the Max Planck Institute for Human Cognitive and Brain Sciences.
Author contributions
VE and JS developed the study concept. VE, JS and RL contributed to the study design. Testing, data collection and analyses were performed by RL, RP and VE. The manuscript was initially drafted by RL and VE, all authors provided critical revisions for important intellectual content. All authors approved the final version of the manuscript for submission.
Data availability
The datasets analysed during the current study are not publicly available due lacking participant consent for data-sharing with third parties (according to our current General Data Protection Regulation, GDPR), but are available from the corresponding author on reasonable request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Andrews-Hanna JR, Kaiser RH, Turner AE, Reineberg AE, Godinez D, Dimidjian S, et al. A penny for your thoughts: dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing. Frontiers in Psychology. 2013;4:900. doi: 10.3389/fpsyg.2013.00900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Mrazek MD, Kam JW, Franklin MS, Schooler JWJPS. Inspired by distraction: mind wandering facilitates creative incubation. Psychological Science. 2012;23(10):1117–1122. doi: 10.1177/0956797612446024. [DOI] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Schooler JW. Back to the future: Autobiographical planning and the functionality of mind-wandering. Consciousness and Cognition. 2011;20(4):1604–1611. doi: 10.1016/j.concog.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Masicampo EJ. Conscious thought is for facilitating social and cultural interactions: How mental simulations serve the animal-culture interface. Psychological Review. 2010;117(3):945. doi: 10.1037/a0019393. [DOI] [PubMed] [Google Scholar]
- Brosschot JF. Markers of chronic stress: prolonged physiological activation and (un)conscious perseverative cognition. Neuroscience and Biobehavioral Reviews. 2010;35(1):46–50. doi: 10.1016/j.neubiorev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Gerin W, Thayer JF. The perseverative cognition hypothesis: A review of worry, prolonged stress-related physiological activation, and health. Journal of Psychosomatic Research. 2006;60(2):113–124. doi: 10.1016/j.jpsychores.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Crosswell AD, Coccia M, Epel ES. Mind wandering and stress: When you don’t like the present moment. Emotion. 2019 doi: 10.1037/emo0000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R. Validity and reliability of the experience-sampling method. The Journal of Nervous and Mental Disease. 1987;175(9):526–536. doi: 10.1097/00005053-198709000-00004. [DOI] [PubMed] [Google Scholar]
- Engert V, Smallwood J, Singer T. Mind your thoughts: associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biological Psychology. 2014;103:283–291. doi: 10.1016/j.biopsycho.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Fox KCR, Beaty RE. Mind-wandering as creative thinking: Neural, psychological, and theoretical considerations. Current Opinion in Behavioral Sciences. 2019;27:123–130. [Google Scholar]
- Franklin M, Mrazek M, Anderson C, Smallwood J, Kingstone A, Schooler J. The silver lining of a mind in the clouds: interesting musings are associated with positive mood while mind-wandering. Frontiers in Psychology. 2013;4:583. doi: 10.3389/fpsyg.2013.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajantie E, Phillips DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31(2):151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Brown LH, McVay JC, Silvia PJ, Myin-Germeys I, Kwapil TR. For whom the mind wanders, and when: An experience-sampling study of working memory and executive control in daily life. Psychological Science. 2007;18(7):614–621. doi: 10.1111/j.1467-9280.2007.01948.x. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Gross GM, Chun CA, Smeekens BA, Meier ME, Silvia PJ, et al. For whom the mind wanders, and when, varies across laboratory and daily-life settings. Psychological Science. 2017;28(9):1271–1289. doi: 10.1177/0956797617706086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth MA, Gilbert DT. A wandering mind is an unhappy mind. Science. 2010;330(6006):932–932. doi: 10.1126/science.1192439. [DOI] [PubMed] [Google Scholar]
- Klinger E, Cox WM. Dimensions of thought flow in everyday life. Imagination, Cognition and Personality. 1987;7(2):105–128. [Google Scholar]
- Kudielka BM, Gierens A, Hellhammer DH, Wust S, Schlotz W. Salivary cortisol in ambulatory assessment–some dos, some don’ts, and some open questions. Psychosomatic Medicine. 2012;74(4):418–431. doi: 10.1097/PSY.0b013e31825434c7. [DOI] [PubMed] [Google Scholar]
- Linz R, Singer T, Engert V. Interactions of momentary thought content and subjective stress predict cortisol fluctuations in a daily life experience sampling study. Scientific Reports. 2018;8(1):15462. doi: 10.1038/s41598-018-33708-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Nolen-Hoeksema S. Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy. 2011;49(3):186–193. doi: 10.1016/j.brat.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ. Conducting the train of thought: Working memory capacity, goal neglect, and mind wandering in an executive-control task. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(1):196–204. doi: 10.1037/a0014104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay JC, Kane MJ, Kwapil TR. Tracking the train of thought from the laboratory into everyday life: An experience-sampling study of mind wandering across controlled and ecological contexts. Psychonomic Bulletin & Review. 2009;16(5):857–863. doi: 10.3758/PBR.16.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medea B, Karapanagiotidis T, Konishi M, Ottaviani C, Margulies D, Bernasconi A, et al. How do we decide what to do? Resting-state connectivity patterns and components of self-generated thought linked to the development of more concrete personal goals. Experimental Brain Research. 2018;236(9):2469–2481. doi: 10.1007/s00221-016-4729-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrazek MD, Smallwood J, Franklin MS, Chin JM, Baird B, Schooler JW. The role of mind-wandering in measurements of general aptitude. Journal of Experimental Psychology: General. 2012;141(4):788. doi: 10.1037/a0027968. [DOI] [PubMed] [Google Scholar]
- Murray, S., Krasich, K., Schooler, J., & Seli, P. (2019). What’s in a task? Complications in the study of the task-unrelated-thought (TUT) variety of mind wandering. PsyArXiv. [DOI] [PubMed]
- Ottaviani C, Couyoumdjian A. Pros and cons of a wandering mind: a prospective study. Frontiers in Psychology. 2013;4:524–524. doi: 10.3389/fpsyg.2013.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani C, Shapiro D, Couyoumdjian A. Flexibility as the key for somatic health: From mind wandering to perseverative cognition. Biological Psychology. 2013;94(1):38–43. doi: 10.1016/j.biopsycho.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Ottaviani C, Thayer JF, Verkuil B, Lonigro A, Medea B, Couyoumdjian A, et al. Physiological concomitants of perseverative cognition: A systematic review and meta-analysis. Psychological Bulletin. 2016;142(3):231. doi: 10.1037/bul0000036. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. 2. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Ruby F, Smallwood J, Engen H, Singer T. How self-generated thought shapes mood—the relation between mind-wandering and mood depends on the socio-temporal content of thoughts. PLoS One. 2013;8(10):e77554. doi: 10.1371/journal.pone.0077554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F, Smallwood J, Sackur J, Singer T. Is self-generated thought a means of social problem solving? Frontiers in Psychology. 2013;4:962. doi: 10.3389/fpsyg.2013.00962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel J, Boywitt CD. Controlling the stream of thought: Working memory capacity predicts adjustment of mind-wandering to situational demands. Psychonomic Bulletin & Review. 2014;21(5):1309–1315. doi: 10.3758/s13423-013-0580-3. [DOI] [PubMed] [Google Scholar]
- Russel JA, Weiss A, Mendelsohn GA. Affect grid: A single-item scale of pleasure and arousal. Journal of Personality and Social Psychology. 1989;57(3):493–502. [Google Scholar]
- Schooler JW. Zoning out while reading: evidence for dissociations between experience and metaconsciousness. In: Schooler JW, Reichle ED, Halpern DV, editors. Thinking and Seeing: Visual Metacognition in Adults and Children. Cambridge: MIT Press; 2004. [Google Scholar]
- Seli P, Beaty RE, Cheyne JA, Smilek D, Oakman J, Schacter DLJC, et al. How pervasive is mind wandering, really? Consciousness and Cognition. 2018;66:74–78. doi: 10.1016/j.concog.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Seli P, Beaty RE, Marty-Dugas J, Smilek D. Depression, anxiety, and stress and the distinction between intentional and unintentional mind wandering. New York: Educational Publishing Foundation; 2019. pp. 163–170. [Google Scholar]
- Seli P, Carriere JSA, Wammes JD, Risko EF, Schacter DL, Smilek D. On the clock: Evidence for the rapid and strategic modulation of mind wandering. Psychological Science. 2018;29(8):1247–1256. doi: 10.1177/0956797618761039. [DOI] [PubMed] [Google Scholar]
- Seli P, Cheyne JA, Xu M, Purdon C, Smilek D. Motivation, intentionality, and mind wandering: Implications for assessments of task-unrelated thought. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2015;41(5):1417–1425. doi: 10.1037/xlm0000116. [DOI] [PubMed] [Google Scholar]
- Seli P, Kane MJ, Smallwood J, Schacter DL, Maillet D, Schooler JW, et al. The family-resemblances framework for mind-wandering remains well clad. 2018;22(11):959–961. doi: 10.1016/j.tics.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Seli P, Ralph BCW, Konishi M, Smilek D, Schacter DL. What did you have in mind? Examining the content of intentional and unintentional types of mind wandering. Consciousness and Cognition. 2017;51:149–156. doi: 10.1016/j.concog.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Risko EF, Smilek D, Schacter DL. Mind-wandering with and without intention. Trends in Cognitive Sciences. 2016;20(8):605–617. doi: 10.1016/j.tics.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seli P, Wammes JD, Risko EF, Smilek D. On the relation between motivation and retention in educational contexts: The role of intentional and unintentional mind wandering. Psychonomic Bulletin & Review. 2016;23(4):1280–1287. doi: 10.3758/s13423-015-0979-0. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Andrews-Hanna J. Not all minds that wander are lost: the importance of a balanced perspective on the mind-wandering state. Frontiers in Psychology. 2013;4:441. doi: 10.3389/fpsyg.2013.00441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Karapanagiotidis T, Ruby F, Medea B, de Caso I, Konishi M, et al. Representing representation: integration between the temporal lobe and the posterior cingulate influences the content and form of spontaneous thought. PLoS One. 2016;11(4):e0152272. doi: 10.1371/journal.pone.0152272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Nind L, O’Connor RC. When is your head at? An exploration of the factors associated with the temporal focus of the wandering mind. Consciousness and Cognition. 2009;18(1):118–125. doi: 10.1016/j.concog.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Smallwood J, O’Connor RC. Imprisoned by the past: Unhappy moods lead to a retrospective bias to mind wandering. Cognition and Emotion. 2011;25(8):1481–1490. doi: 10.1080/02699931.2010.545263. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Ruby FJM, Singer T. Letting go of the present: Mind-wandering is associated with reduced delay discounting. Consciousness and Cognition. 2013;22(1):1–7. doi: 10.1016/j.concog.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The science of mind wandering: Empirically navigating the stream of consciousness. Annual Review of Psychology. 2015;66(1):487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW, Turk DJ, Cunningham SJ, Burns P, Macrae CN. Self-reflection and the temporal focus of the wandering mind. Consciousness and Cognition. 2011;20(4):1120–1126. doi: 10.1016/j.concog.2010.12.017. [DOI] [PubMed] [Google Scholar]
- Smeekens BA, Kane MJ. Working memory capacity, mind wandering, and creative cognition: An individual-differences investigation into the benefits of controlled versus spontaneous thought. Psychology of Aesthetics, Creativity, and the Arts. 2016;10(4):389–415. doi: 10.1037/aca0000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sormaz M, Murphy C, Wang H-T, Hymers M, Karapanagiotidis T, Poerio G, et al. Default mode network can support the level of detail in experience during active task states. Proceedings of the National Academy of Sciences. 2018;115(37):9318. doi: 10.1073/pnas.1721259115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler T, Kirschbaum C, Kudielka BM, Adam EK, Wüst S, Dockray S, et al. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2015;63:414. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Stawarczyk D, Cassol H, D’Argembeau A. Phenomenology of future-oriented mind-wandering episodes. Frontiers in Psychology. 2013;4:425. doi: 10.3389/fpsyg.2013.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maj M, Van der Linden M, D’Argembeau A. Mind-wandering: Phenomenology and function as assessed with a novel experience sampling method. Acta Psychologica. 2011;136(3):370–381. doi: 10.1016/j.actpsy.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Turnbull A, Wang HT, Murphy C, Ho NSP, Wang X, Sormaz M, et al. Left dorsolateral prefrontal cortex supports context-dependent prioritisation of off-task thought. Nature Communications. 2019;10(1):3816. doi: 10.1038/s41467-019-11764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull A, Wang H-T, Schooler JW, Jefferies E, Margulies DS, Smallwood J. The EBB and flow of attention: Between-subject variation in intrinsic connectivity and cognition associated with the dynamics of ongoing experience. NeuroImage. 2019;185:286–299. doi: 10.1016/j.neuroimage.2018.09.069. [DOI] [PubMed] [Google Scholar]
- Unsworth N, McMillan BD, Brewer GA, Spillers GJ. Everyday attention failures: An individual differences investigation. Journal of Experimental Psychology. Learning, Memory, and Cognition. 2012;38(6):1765–1772. doi: 10.1037/a0028075. [DOI] [PubMed] [Google Scholar]
- Wang H-T, Poerio G, Murphy C, Bzdok D, Jefferies E, Smallwood J. Dimensions of experience: Exploring the heterogeneity of the wandering mind. Psychological Science. 2017;29(1):56–71. doi: 10.1177/0956797617728727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins ER. Constructive and unconstructive repetitive thought. Psychological Bulletin. 2008;134(2):163–206. doi: 10.1037/0033-2909.134.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz A, Reinhard I, Alpers GW, Kuehner CJM. Happy thoughts: Mind wandering affects mood in daily life. Mindfulness. 2018;9(1):332–343. [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wüst S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32(4):358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are not publicly available due lacking participant consent for data-sharing with third parties (according to our current General Data Protection Regulation, GDPR), but are available from the corresponding author on reasonable request.