Abstract
Purpose
The purpose of this study was to examine whether long-term ingestion of low-dose milk protein supplementation causes a greater increase in muscle mass and strength of older adults during low-to-moderate intensity exercise training intervention than isocaloric carbohydrate.
Methods
In a randomized, double-blind, and placebo-controlled design, 122 healthy older adults (60–84 year) received either an acidified milk protein drink containing 10 g of milk protein (MILK; n = 61) or an isocaloric placebo drink (PLA; n = 61) daily throughout 6 months of body weight and medicine ball exercise training. Measurements before and after the intervention included body composition, physical performance and blood biochemistry.
Results
Lean body mass significantly increased in the MILK group (+ 0.54 kg, p < 0.001), but did not change in the PLA group (− 0.10 kg, p = 0.534). The increases in the MILK group were significantly greater than in the PLA group (p = 0.004). Fat mass (− 0.77 kg) and plasma uric acid levels (− 0.3 mg/dL) significantly decreased only in the MILK group (p < 0.001), with a significant group difference (p = 0.002 and p < 0.001, respectively). Most of the physical performance tests significantly improved in both groups, but no group differences were found.
Conclusion
We conclude that low-dose milk protein supplementation (10 g of protein/day) combined with low-to-moderate intensity exercise training is associated with increased muscle mass, but not improved physical performance compared to carbohydrate combined with exercise in healthy older adults. This study was registered in the UMIN Clinical Trials Registry (UMIN000032189).
Electronic supplementary material
The online version of this article (10.1007/s00394-020-02302-4) contains supplementary material, which is available to authorized users.
Keywords: Sarcopenia, Aging, Elderly, Hypertrophy, Lean body mass, Physical performance
Introduction
Peak muscle mass occurs between the ages of 20 and 40 years, and then naturally declines as one ages, accelerating in older adults [1]. Sarcopenia, a marked decline in muscle mass in older adults, contributes to loss of independence because the loss of muscle mass reduces strength and functional capacity, both of which are needed to perform one’s activities of daily living. The loss of muscle mass is also associated with risk of diabetes [2] or heart disease [3]. Sarcopenia is a global problem for society, so it is important to develop effective strategies to maintain or increase muscle mass in older adults to combat sarcopenia.
Protein supplementation combined with resistance training may be an effective intervention to increase muscle mass regardless of age [4–7]. According to previous studies in older adults, a large dose of protein supplementation (> 20 g/day) increases muscle mass with [8–13] or without [14–16] resistance training. However, as the dose of protein increases, the size of the supplementation (foods or drinks) become bigger, and that may be a burden on older adults. Lower amounts of protein supplementation can ease the burden and can be incorporated easily into a daily routine, but the effects of low-dose protein intake (≤ 20 g/day) on muscle mass in older adults is unclear.
To detect the positive effects of low-dose protein supplementation on muscle mass in older adults, we designed the present study focusing on three points: high compliance, a longer intervention period, and high quality protein supplementation. First, we selected low-to-moderate intensity exercise training to maintain high compliance and minimize dropout. Second, we selected 6 months as the intervention period. Previous studies intervened for 10 weeks or more to detect the positive effects of a large dose of protein supplementation (> 20 g/day). In the present study, we set the protein dose to 10 g/day, less than half of the previous study design. We assumed that at least 20 weeks were required, so we set the intervention period to 6 months (26 weeks). Finally, we selected the acidified bovine milk we recently developed [17] as the protein supplementation. In a previous animal study, we showed that acidified milk had a greater effect on post-exercise muscle protein synthesis compared with skim milk, although both kinds of milk contained the same amount of bovine milk protein [17]. Bovine milk proteins are of the highest quality because they possess a complete profile of essential amino acids [18], have high amino acid absorptivity [19, 20], and stimulate muscle protein synthesis [21, 22]. The acidified milk might be more effective to increase muscle mass than normal bovine milk protein, such as skim milk, so we selected acidified milk as the intervention in the present study.
We examined whether 6 months of continuous ingestion of low-dose (10 g/day) milk protein supplementation caused a greater increase in muscle mass, strength and function in older adults compared with an isocaloric carbohydrate placebo drink during low-to-moderate intensity exercise training intervention.
Methods
Study design
The present study used a double-blind, placebo-controlled, parallel-group, randomized design. After acceptance into the study, participants were randomly allocated to either of two supplemented groups. All participants then started a 6-month exercise and supplement intervention. Before and after the intervention, body composition assessment, physical performance tests and blood sampling were performed. The trial was conducted at Obihiro University of Agriculture and Veterinary.
Medicine (Hokkaido, Japan) between February and November 2018. The data were analyzed between December 2018 and March 2019. The target number of participants was set at 128 (i.e., 64 participants per group) to detect a difference of 0.5 kg in the lean body mass change [6], assuming a standard deviation of 1.0 kg [11], to attain 80% statistical power using a two-sided α of 0.05.
Participants
Participants were recruited from senior citizens’ clubs in Obihiro, Hokkaido, Japan. All participants completed health history and physical activity questionnaires and met the following inclusion criteria: 60 years or older and physically independent. Individuals were excluded if they (1) had current or previous histories of significant liver, cardiovascular, pulmonary, renal or digestive diseases; (2) had significant orthopedic injuries; (3) had food allergies; (4) restricted protein intake due to medical reasons; (5) were involved in regular resistance training (> 2 times/week); (6) were participating in other clinical studies; (7) or were judged as inappropriate for the study by the principal investigator due to abnormal blood pressure or parameters, or other reasons.
Randomization and blinding
Stratification based on sex and age was performed after eligibility assessment for study participation. Participants were divided into four strata: (1) males less than 70 years old; (2) females less than 70 years old; (3) males 70 years or older; (4) females 70 years or older. In each strata, computer-generated random numbers were assigned to the participants who were then sorted and divided into two equal groups. The groups were randomly assigned to either the acidified milk (MILK) group or placebo (PLA) group, by an individual who was accountable for preparing the test drinks but was not involved in the plan, enrollment, evaluation, intervention, or analysis. The participants, investigators, and all staff members involved in the trial were blinded to group allocation. The randomization code was opened after the study data were checked, collated, and finalized.
Interventions
Supplementation protocol
Participants ingested a 200 mL polyethylene terephthalate bottle drink daily of either an acidified milk protein drink [17] or a placebo drink for 6 months. The acidified milk protein drink was made from milk protein concentrate, trehalose, soybean polysaccharide, pectin, fermented cellulose, citric acid, malic acid and food flavors, and contained 7.0 g of carbohydrate, 10.1 g of protein and 0.2 g of fat, providing 68 kcal of energy per bottle. The amino acid profile of the acidified milk protein drink is shown in Table 1. The placebo drink was made from maltodextrin, trehalose, soybean polysaccharide, pectin, fermented cellulose, citric acid, malic acid and food flavors, and contained 16.0 g of carbohydrate, 0.1 g of protein and 0.5 g of fat, providing 68 kcal of energy per bottle. The test drinks had an identical pH (4.2) and appearance. The participants were instructed to keep the test drinks in their refrigerator and consume one bottle per day within an hour after each exercise training session. If the participants did not perform exercise training for some reason, they were instructed to consume a test drink anytime they wanted.
Table 1.
Amino acid profile of the acidified milk protein drink
| Amino acids | g/200 mL acidified milk |
|---|---|
| Alanine | 0.37 |
| Arginine | 0.36 |
| Asparagine + Aspartic acid | 0.80 |
| Cysteine | 0.05 |
| Glutamine + Glutamic acid | 1.86 |
| Glycine | 0.20 |
| Histidine | 0.25 |
| Isoleucine | 0.50 |
| Leucine | 0.95 |
| Lysine | 0.69 |
| Methionine | 0.23 |
| Phenylalanine | 0.44 |
| Proline | 0.96 |
| Serine | 0.54 |
| Threonine | 0.44 |
| Tryptophan | 0.12 |
| Tyrosine | 0.52 |
| Valine | 0.73 |
Exercise training program
The exercise training program is shown in Table 2. All participants were instructed to perform an exercise training program daily for 6 months. The exercise training program was composed of 6 body weight exercises and 5 medicine ball exercises. The criteria for using low-to-moderate intensity exercise training [23] was that the participants were able to repeat the exercises at least 12 times (< 70% of 1-repetition maximum [24]). Part of the program was changed every 2 months to maintain participants’ motivation. Participants followed a monthly exercise training lesson throughout the 6-month intervention period (seven lessons in total).
Table 2.
Exercise training program
| 0–2 months | 2–4 months | 4–6 months | |||
|---|---|---|---|---|---|
| Bodyweight exercises | Reps | Bodyweight exercises | Reps | Bodyweight exercises | Reps |
| Standing calf raise | 20 | Single leg calf raise | 10 | Single leg calf raise | 10 |
| Squat | 20 | Side step squat | 20 | Squat | 20 |
| Abdominal crunch | 10 | Abdominal crunch | 10 | Abdominal crunch | 10 |
| Hip bridge | 10 | Single leg bridge | 10 | Standing leg curl | 16 |
| Side leg raise | 10 | Single leg raise | 10 | Single leg raise | 10 |
| Push up | 10 | Push up | 10 | Push up | 10 |
| Medicine ball exercises | Reps | Medicine ball exercises | Reps | Medicine ball exercises | Reps |
| Release and catch | 30 | Release and catch (half-rising) | 20 | Release and catch | 30 |
| Release and catch (with step) | 20 | Release and catch (with step) | 20 | Release and catch (with step) | 20 |
| Biceps curl | 20 | Biceps curl | 30 | Biceps curl | 30 |
| Upper body rotation | 10 | Upper body rotation | 10 | Upper body rotation | 10 |
| Leg flexion and extension | 10 | Leg flexion and extension | 10 | Leg flexion and extension | 10 |
For the medicine ball exercises, participants used one or two 1 kg soft medicine balls.
Release and catch: Participants stood on the floor holding a medicine ball in front of their body in one hand. They released the ball and immediately caught the ball in their opposite hand with the palm facing down. They repeated the motion a given number of times (Table 2).
Biceps curl: Participants stood on the floor holding a medicine ball in each hand with their arms hanging by their sides. They performed a biceps curl a given number of times (Table 2).
Upper body rotation: Participants stood on the floor holding a medicine ball in front of their body in each hand. Then they rotated their upper body once with outstretched arms. They repeated the motion 10 times alternating from clockwise to counterclockwise.
Leg flexion and extension: Participants sat on the floor and put a medicine ball between their feet with their knees bent and their feet flat on the floor. They extended and bent their legs without dropping the ball. They repeated the motion 10 times.
Physical activity
Participants counted and recorded the number of steps daily using a triaxial accelerometer (FB-736, TANITA corporation, Tokyo, Japan) during the intervention period.
Measurements
Body composition assessment, physical performance tests and blood sampling were performed at baseline and after 6 months of intervention by experienced staff members who were blinded to group allocation. On the day before the measurement, participants were barred from drinking alcohol and had dinner between 6 and 10 PM. After the dinner, participants were allowed to drink only water until the measurements started.
Body composition assessments
A direct segmental multifrequency (5 kHz, 50 kHz and 250 kHz) bioelectrical impedance analysis (DSM-BIA) device using an 8-point tactile electrode system (InBody 430, Biospace, Seoul, Korea) [25, 26] was used to measure body weight, lean body mass and fat mass. Participants were measured wearing the same shirt and pair of shorts, which weighed 0.6 kg, so the weight adjustment for clothing was set to 0.6 kg. Body composition assessments were performed between 8.30 and 11:30 AM in fasted states before physical performance tests.
Physical performance tests
On the measurement days, participants practiced each test twice with submaximal efforts to minimize the learning effect before making the actual test. They practiced the push-up motion twice.
Grip strength: A digital handgrip dynamometer (Grip-D, Takei Scientific Instruments Co. Ltd., Niigata, Japan) was used to measure grip strength. Subjects were allowed to adjust the grip on the apparatus, and then performed a maximum force grip with the right hand (isometric exercise) while the left arm was hanging free by the side. The test was carried out twice at an interval of 1 min and the best result was recorded as the grip strength.
Maximal walking speed: Participants walked on a flat, straight, 7-m-long walkway two times at their maximum speed. The time to walk 5-m was measured using diffuse-reflective photoelectric sensors (Yagami Inc., Aichi, Japan) which were placed at the 1-m and 6-m points. For maximum walking speed, the faster time recorded was used and converted into speed (m/s).
Knee extension and flexion strength: Knee extension and flexion strength were evaluated using an isokinetic dynamometer (Biodex System 4, Biodex Medical Systems, Inc. NY, USA) at an angular speed of 60°/s. Full knee flexion (start-position) was set to 100° and full knee extension was set to 30°. Following a familiarizing practice trial, participants extended and then flexed their right knees with full strength while crossing their arms in front of their chests. The test was repeated 3 times at an interval of 30 s and the best result was recorded as the knee extension and flexion strength.
Timed Up and Go test: The Timed Up and Go test measures speed during several functional maneuvers, which include standing up, walking, and turning and sitting down. The test is a reliable and valid test for quantifying functional mobility in older adults [27]. Participants were seated in a normal chair (41 cm high) with their back against the chair and their hands on their thighs. They were instructed to stand up, walk 3-m as quickly and safely as possible, past a cone on the floor, turn around, walk back to the chair, and sit down with back against the chair again [28]. A stop-watch was used to time one test. The test was carried out twice and the best time was recorded.
Sit-to-stand test: The sit-to-stand is often used as a measure of lower-limb strength in older adults [29]. The test measures the time taken to stand from a seated position either one, three, five or 10 times. In this study, participants were seated in a normal chair (41 cm high) and rose from the chair five times as fast as possible while crossing their arms in front of their chests. A stopwatch was used to time the test. The test was carried out twice and the best time was recorded.
Push-up: Participants fully extended their elbows and placed hands on the exercise mat directly under the shoulders, with knees together also touching the mat and trunk and thighs fully stretched. From this position, the elbows were flexed until just touching the mat with their chest and then immediately extended again. They repeated the push-up as many times as possible, and the number of repetitions was recorded.
Blood biochemistry
A blood sample was drawn from each participant in a fasted state at baseline. After 6 months of intervention, blood sampling was performed twice; the first sampling was performed in a fasted state, and the second sampling was performed 30 min after ingestion of 200 mL of the test drink (either an acidified milk or a carbohydrate drink). Immediately before ingestion of the test drink, participants performed the exercise training program (4–6 month version as described in Table 2). The second blood sampling was performed after body composition assessments and before physical performance tests. Plasma leucine was measured using liquid chromatography tandem-mass spectrometry (ACQUITY TQD, Waters Corporation, MA, USA). Other blood parameters were assayed by an independent laboratory (BML, Inc., Tokyo, Japan). Plasma albumin was measured using a bromocresol green method (Clinimate ALB, Sekisui Medical Co., Ltd., Tokyo, Japan). Plasma creatinine (Sikarikid-S CRE, Kanto Chemical Co., Inc., Tokyo Japan), uric acid (Pureauto S UA, Sekisui Medical Co., Ltd., Tokyo, Japan), low-density lipoprotein cholesterol (LDL-C) (Cholestest LDL, Sekisui Medical Co., Ltd., Tokyo, Japan), high-density lipoprotein cholesterol (HDL-C) (Cholestest N HDL, Sekisui Medical Co., Ltd., Tokyo, Japan) and triglycerides (Pureauto S TG-N, Sekisui Medical Co., Ltd., Tokyo, Japan) were measured using enzymatic methods. Plasma glucose was measured using a hexokinase assay (Pureauto S GLU, Sekisui Medical Co., Ltd., Tokyo, Japan). Plasma insulin was measured using a chemiluminescence immunoassay (Chemilumi Insulin, Siemens Healthcare Diagnostics, Inc., Tokyo, Japan). Plasma insulin-like growth factor-1 (IGF-1) was measured using an electro chemiluminescence immunoassay (Elecsys IGF-1, Roche Diagnostics K.K., Tokyo, Japan).
Dietary analysis
Before and during the 3rd and 6th month of the intervention period, participants recorded total food consumption for 3 consecutive days to determine their daily macronutrient intake exclusive of the test drinks. Food quantities were measured by using standard measuring glasses, spoons, and digital scales. All dietary data were analyzed by an independent laboratory (THF Co., Ltd., Ibaraki, Japan) using Excel Eiyo-kun, version 7.0 (Kenpakusha Co., Ltd., Tokyo, Japan).
Statistical analysis
All statistical analyses were performed by an independent organization (edihas K.K., Hokkaido, Japan) using IBM SPSS Statistics 24 (IBM Japan, Ltd., Tokyo, Japan). Analyses were done on the full analysis set (FAS) with missing values imputed by the last observation carried forward. All values are expressed as mean ± standard error of the mean (SEM). The Student t-test for independent samples was used to compare differences between groups in participant characteristics at baseline, compliance rate for exercise training and consumption of the test drinks, daily average of the number of steps, and changes of values during the intervention period. A two-factor, repeated-measures analysis of variance (ANOVA) with time as the within-participants factor and group as the between-participants factor were carried out for dietary intake, body composition, physical performance, and blood parameters. When the F-ratio was significant, the Bonferroni post hoc test was employed to identify mean differences. For all statistical analyses, significance was set at p < 0.05.
Result
Participants
The participant flow through the protocol is shown in Fig. 1. Written informed consent was obtained from 272 participants. Eighteen participants declined to participate in this study before the screening examination and 254 participants were screened. Finally, 126 participants were included in the study and randomly allocated to the MILK (n = 63) or PLA (n = 63) group. Four participants did not receive an allocated intervention due to a doctor’s orders (MILK = 2) or they changed their mind (PLA = 2); 122 participants (60–84 year, MILK = 61, PLA = 61) received the allocated intervention. Three participants discontinued the intervention due to poor physical condition (MILK = 2) or hospitalization (PLA = 1) not related to the study, so 119 participants completed the 6-month intervention. According to the FAS principle, the 122 participants (MILK = 61, PLA = 61) who began the allocated intervention were included in the analysis. The representative characteristics of the participants measured at baseline are presented in Table 3. There were no significant differences in any study outcomes between the two groups at baseline. No differences in the mean number of steps daily during the 6-month intervention period were observed (MILK: 6426 ± 299 steps/d, PLA: 6970 ± 326 steps/d, P = 0.220).
Fig. 1.
CONSORT flowchart for the human intervention study
Table 3.
Baseline participant characteristics
| MILK (n = 61) | PLA (n = 61) | p | |
|---|---|---|---|
| Female, n (%) | 45 (74) | 47 (77) | – |
| Age, year | 71.4 (0.8) | 70.4 (0.7) | 0.366 |
| Height, cm | 155.9 (1.1) | 155.7 (1.0) | 0.878 |
| Body mass, kg | 56.4 (1.3) | 55.6 (1.3) | 0.704 |
| BMI, kg/m2 | 23.1 (0.4) | 22.8 (0.4) | 0.582 |
| Body fat, % | 29.0 (1.0) | 27.7 (1.0) | 0.363 |
Data are presented as mean (SEM)
BMI body mass index
Dietary intake
Dietary intake (except for test drinks) before and during the 3rd and 6th month of the intervention period is presented in Table 4. During the intervention period, the total energy, protein and carbohydrate intake levels did not change but the fat intake significantly increased in both groups. There were no significant differences between groups in any dietary intake levels.
Table 4.
Daily dietary intake (except for test drinks) before and during the intervention period
| MILK (n = 61) | PLA (n = 61) | p (ANOVA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | 3 months | 6 months | Before | 3 months | 6 months | Time | Group | Interaction | |
| Total energy, kcal/day | 1864 (43) | 1863 (48) | 1906 (45) | 1763 (39) | 1803 (49) | 1826 (50) | 0.164 | 0.155 | 0.767 |
| Protein, g/day | 72.6 (1.9) | 73.0 (2.0) | 73.1 (2.1) | 68.9 (1.9) | 70.5 (1.9) | 70.0 (2.1) | 0.723 | 0.191 | 0.896 |
| Fat, g/day | 59.3 (2.0) | 60.1 (2.3) | 63.7 (2.5)* | 54.7 (1.6) | 57.7 (2.1) | 60.8 (2.3)* | 0.003 | 0.186 | 0.750 |
| Carbohydrate, g/day | 240.7 (5.9) | 238.9 (5.8) | 244.5 (5.8) | 238.3 (6.0) | 240.2 (6.8) | 242.0 (6.9) | 0.547 | 0.872 | 0.858 |
Data are presented as mean (SEM)
*Significantly different from before the intervention
Protocol compliance
All participants followed the first exercise training lesson. There were no significant differences between groups in lesson compliance (MILK: 93.0%, PLA: 95.3%, p = 0.295), exercise training compliance (MILK: 92.6%, PLA: 92.0%, p = 0.790), or supplementation intake compliance (MILK: 96.4%, PLA: 97.3%, p = 0.577).
Body composition
Body weight, lean body mass and fat mass before and after the intervention are presented in Table 5. Significant time × treatment interactions were found in the mean lean body mass and fat mass. Lean body mass significantly increased in the MILK group (+ 0.54 ± 0.11 kg, p < 0.001), but did not change in the PLA group (− 0.10 ± 0.19 kg, p = 0.534), following 6 months of the intervention (Fig. 2). The increases in lean body mass during the intervention period in the MILK group were significantly greater than those in the PLA group (p = 0.004). Fat mass significantly decreased in the MILK group (− 0.77 ± 0.15 kg, p < 0.001), but not in the PLA group (0.00 ± 0.20 kg, p = 0.990), following 6 months of the intervention (Fig. 2). The decreases in fat mass during the intervention period in the MILK group were significantly greater than those in the PLA group (p = 0.002). There were no significant changes or group differences in total body mass.
Table 5.
Body composition before and after the intervention (6 months)
| MILK (n = 61) | PLA (n = 61) | p (ANOVA) | |||||
|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Time | Group | Interaction | |
| Body weight, kg | 56.4 (1.3) | 56.1 (1.3) | 55.6 (1.3) | 55.5 (1.3) | 0.206 | 0.693 | 0.609 |
| Lean body mass, kg | 39.7 (0.9) | 40.3 (0.9)* | 39.9 (1.0) | 39.8 (0.9) | 0.044 | 0.924 | 0.004 |
| Fat mass, kg | 16.6 (0.8) | 15.8 (0.7)* | 15.6 (0.8) | 15.6 (0.8) | 0.002 | 0.585 | 0.002 |
Data are presented as mean (SEM)
*Significantly different from before the intervention
Fig. 2.
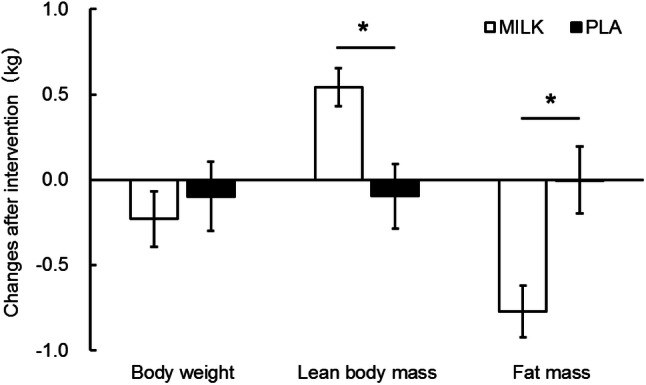
Changes in body weight, lean body mass, and fat mass after the intervention (6 months). Data are presented as mean ± SEM. *Significant differences between MILK and PLA
Physical performance tests
Physical performance test values before and after the intervention are presented in Table 6. Maximal walking speed, knee extension or flexion strength, Timed Up and Go test time, sit-to-stand test time, and push-up repetitions improved in both groups following 6 months of intervention (p < 0.001), but there were no significant differences between groups. Grip strength did not improve in either group following 6 months of the intervention.
Table 6.
Physical performance test values before and after intervention (6 months)
| MILK (n = 61) | PLA (n = 61) | p (ANOVA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Before | After | Change | Time | Group | Interaction | |
| Grip strength, kg | 27.3 (0.9) | 27.4 (0.9) | 0.0 (0.2) | 28.1 (0.9) | 27.5 (0.9) | − 0.6 (0.3) | 0.176 | 0.721 | 0.132 |
| Maximal walking speed, m/s | 1.82 (0.04) | 1.99 (0.04)* | 0.17 (0.03) | 1.90 (0.03) | 2.03 (0.03)* | 0.13 (0.02) | < 0.001 | 0.223 | 0.342 |
| Knee extension strength, Nm | 81.3 (3.0) | 89.7 (3.2)* | 8.4 (1.8) | 80.9 (2.9) | 91.1 (3.4)* | 10.2 (1.4) | < 0.001 | 0.896 | 0.427 |
| Knee flexion strength, Nm | 44.1 (2.0) | 49.0 (1.9)* | 4.8 (0.8) | 43.1 (1.8) | 48.6 (1.9)* | 5.4 (0.9) | < 0.001 | 0.790 | 0.613 |
| Timed Up and Go test, s | 5.83 (0.10) | 5.55 (0.10)* | − 0.28 (0.07) | 5.82 (0.10) | 5.59 (0.09)* | − 0.24 (0.07) | < 0.001 | 0.894 | 0.631 |
| Sit-to-stand test, s | 7.86 (0.23) | 6.93 (0.20)* | − 0.93 (0.15) | 7.69 (0.19) | 7.00 (0.18)* | − 0.69 (0.15) | < 0.001 | 0.844 | 0.262 |
| Push-up, reps | 14.7 (1.4) | 22.5 (1.5)* | 7.9 (1.4) | 14.1 (1.6) | 20.7 (1.8)* | 6.6 (1.4) | < 0.001 | 0.555 | 0.535 |
Data are presented as mean (SEM)
*Significantly different from before the intervention
Blood biochemistry
Fasting blood parameters before and after the intervention are presented in Table 7. There were significant time × treatment interactions in plasma creatinine and uric acid levels. Plasma creatinine significantly increased in the PLA group (p = 0.012) but did not change in the MILK group (p = 0.338) following 6 months of the intervention. There was a significant difference between groups in the changes in plasma creatinine levels (p = 0.014). Plasma uric acid significantly decreased in the MILK group (p < 0.001) but did not change in the PLA group (p = 0.528) following 6 months of the intervention. There was a significant difference between groups in the change in plasma uric acid levels (p < 0.001). Plasma IGF-1 in the MILK group (p = 0.043), HDL-C in the PLA group (p = 0.023), and triglyceride levels in both groups (MILK: p = 0.014, PLA: p = 0.045) significantly increased following 6 months of the intervention.
Table 7.
Fasting blood parameters before and after intervention (6 months)
| MILK (n = 61) | PLA (n = 61) | p (ANOVA) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Change | Before | After | Change | Time | Group | Interaction | |
| Albumin, g/L | 42.6 (0.3) | 42.9 (0.3) | 0.3 (0.2) | 43.0 (0.3) | 43.0 (0.3) | 0.0 (0.2) | 0.345 | 0.471 | 0.291 |
| Creatinine, mg/dL | 0.69 (0.02) | 0.69 (0.02) | − 0.01 (0.01)† | 0.70 (0.02) | 0.71 (0.02)* | 0.01 (0.01) | 0.264 | 0.423 | 0.014 |
| Uric acid, mg/dL | 5.1 (0.1) | 4.8 (0.2)* | − 0.3 (0.0)† | 4.9 (0.1) | 5.0 (0.1) | 0.0 (0.1) | 0.008 | 0.977 | < 0.001 |
| Glucose, mg/dL | 95.3 (1.6) | 94.2 (1.7) | − 1.1 (0.8) | 94.2 (1.2) | 93.5 (1.3) | − 0.7 (1.0) | 0.165 | 0.669 | 0.780 |
| Insulin, μU/mL | 5.0 (0.6) | 5.3 (0.5) | 0.3 (0.4) | 4.3 (0.5) | 4.7 (0.3) | 0.4 (0.5) | 0.291 | 0.276 | 0.898 |
| IGF-1, ng/mL | 117.0 (3.5) | 120.9 (3.7)* | 3.9 (2.1) | 109.2 (3.4) | 111.5 (3.7) | 2.3 (1.7) | 0.023 | 0.083 | 0.561 |
| LDL-C, mg/dL | 128.1 (3.6) | 124.9 (3.1) | − 3.2 (2.3) | 125.2 (4.4) | 127.1 (4.3) | 1.9 (3.8) | 0.774 | 0.945 | 0.251 |
| HDL-C, mg/dL | 70.5 (1.8) | 71.4 (1.9) | 0.9 (0.9) | 70.0 (2.0) | 72.1 (2.2)* | 2.2 (1.0) | 0.023 | 0.988 | 0.344 |
| Triglyceride, mg/dL | 83.8 (5.5) | 93.6 (6.1)* | 9.9 (3.0) | 85.3 (4.8) | 93.4 (5.9)* | 8.0 (4.8) | 0.002 | 0.932 | 0.746 |
Data are presented as mean (SEM)
*Significantly different from before the intervention
†Significantly different from PLA
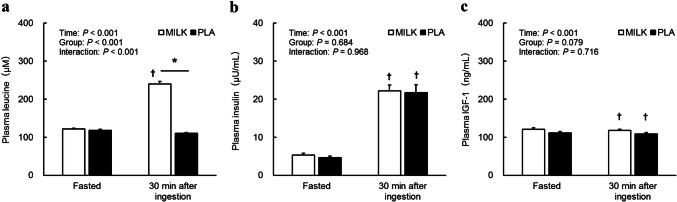
Blood parameters 30 min after a post-exercise ingestion of 200 mL of the test drink are presented in Fig. 3a–c. Plasma leucine levels in the MILK group significantly increased 30 min after exercise and ingestion compared to a fasted state (Fig. 3a). The mean value of the plasma leucine increase was 119 ± 6 μM in the MILK group. In both groups, plasma insulin significantly increased (Fig. 3b) and plasma IGF-1 significantly decreased (Fig. 3c) 30 min after exercise and ingestion compared to a fasted state, with no difference between groups.
Fig. 3.
Plasma leucine (a), insulin (b), and IGF-1 (c) 30 min after ingestion of test drink following an exercise training session. *Significant differences between MILK and PLA. †Significantly different from Fasted
Discussion
In this study, we found that post-exercise acidified milk protein supplementation throughout 6 months of low-to-moderate intensity exercise training (body weight exercises and medicine ball exercises) increased lean body mass and decreased fat mass in healthy older men and women compared with isocaloric carbohydrate supplementation. To our knowledge, this is the first study to demonstrate that the combination of low-dose milk protein supplementation (10 g of protein/day) and low-to-moderate intensity exercise training is beneficial to increase muscle mass in healthy older adults.
Many previous studies have investigated the effects of protein supplementation during exercise training on muscle mass in older adults. With adequate protein intake (> 20 g/day), the positive effects of protein supplementation on muscle mass have been reported frequently [8–13], but not always [30, 31]. At lower doses (≤ 20 g/day), significant effects of protein supplementation on muscle mass have not been found in any previous studies in exercised older adults [32–38]. In the present study, low-dose protein supplementation (10 g/day) was associated with increasing lean body mass in older adults, which is inconsistent with results in the previous studies. We propose three reasons for this. First, we speculate that exercise training intensity is associated with the effects of protein supplementation on muscle mass. It is well-known that high-intensity resistance training induces greater muscle protein synthesis [39] and muscle mass gain [40–42] compared with low-intensity resistance training. Most of the previous studies combined protein supplementation with high-intensity resistance training using weight machines or free weight training equipment (e.g., barbells) [8, 10, 12, 13, 30–32, 34–38], and those studies, except for one [13], reported a significant training effect on muscle mass without protein supplementation. In the present study, we selected low-to-moderate intensity exercise training to maintain high compliance and minimize dropout, and a significant training effect on muscle mass was not found. Two previous studies chose low (walking) [11] or moderate (body weight training and band training) [9] intensity exercise intervention, and significant protein supplementation effects on muscle mass were found although significant exercise effects were not. So it is possible that the large effects of high-intensity training obscured the effects of low-dose protein supplementation on muscle mass in the previous studies. Second, long-term, daily and high compliance protein supplementation increased total protein supplementation intake, and this may contribute to the gain of muscle mass. Although the daily supplementation dose in the present study was low, the total protein supplementation intake during 6 months was greater (approx. 1800 g) than in other low dose studies (< 1300 g) [32–35, 37, 38]. Krause [33] showed that a combination of low-dose protein intake (approx. 12 g/day) and low-to-moderate intensity exercise training, similar to conditions in our study, did not cause further muscle mass increase compared with the exercise-only group. That may be due to lower total protein intake (approx. 1000 g) during the intervention period or simply due to a shorter intervention period (12 weeks) than in the present study. Finally, the acidified milk protein drink, which was ingested in the present study, may have the potential to stimulate muscle protein synthesis more than other protein supplementations. We previously showed in an animal study that the acidified milk ingestion is associated with greater stimulation of post-exercise muscle protein synthesis compared with skim milk [17], although skim milk protein is also associated with marked changes in muscle protein synthesis [21] and muscle mass [43]. The acidified milk protein drink induced an acute increase in plasma essential amino acids, including leucine. Leucine is a potent activator of muscle protein synthesis [44, 45], and elevation of plasma leucine is associated with muscle protein synthesis stimulation: the ‘leucine trigger’ hypothesis states that there may be a threshold level of plasma leucine to trigger muscle protein synthesis [46]. Aging would increase the threshold [47, 48], so a large increase in plasma leucine levels is required to stimulate muscle protein synthesis in older adults. The acidified milk protein drink would meet the requirement in spite of the low protein dose in the present study. Actually, the mean value of plasma leucine increase (119 ± 6 μM) in the MILK group after ingestion of the acidified milk protein drink was comparable to previous studies [49, 50], which shows positive effects of protein source ingestion on muscle protein anabolic response in older adults. However, we have compared the effects of the acidified milk protein drink on muscle mass only with a carbohydrate drink, so further studies that compare acidified milk with other protein sources are necessary to confirm this finding.
Unlike the results on body composition, physical performance improved equally in both groups. In the MILK group, knee extension and flexion strength improved 10.3% and 11.1%, respectively, while lean body mass increased 1.5% during the intervention period. Not only muscle mass but also neuromuscular activity influences muscle strength [51]. Exercise is known to have beneficial effects on the neuromuscular junction through neuromuscular junction preservation, hypertrophy and sprouting [52, 53]. We propose that the effects of low-to-moderate intensity training on neuromuscular activity cause this physical performance improvement in both groups. We found that low-to-moderate intensity exercise training in the present study is a valuable method to improve physical performance in older adults, but low-dose milk protein supplementation did not result in significant benefits on physical performance.
Our intervention in the present study did not have a large effect on blood parameters, except for plasma uric acid levels. The acidified milk protein drink decreased plasma uric acid levels, and that may be a specific effect of milk protein. Several studies showed that skim milk or milk protein ingestion acutely decreased serum uric acid levels, but this did not apply to soy protein [54, 55]. A cross-sectional study also shows dairy consumption is associated with lower serum uric acid levels, but meat and seafood consumption are associated with higher serum uric acid levels [56]. Furthermore, there was a chronic urate-lowering effect of long-term milk protein ingestion in the present study. High blood uric acid levels, known as hyperuricemia, can lead to a disease called gout that causes painful joints that accumulate urate crystals. Long-term ingestion of milk protein would prevent hyperuricemia.
There are several limitations to the present study. First, the study design did not include a low-dose protein supplementation alone group, and that may limit the interpretation of the study results. It is not clear if the increase in muscle mass was due to low-dose protein supplementation alone or an interactive effect of protein supplementation and exercise although the 6-mo exercise alone did not result in an increase in muscle mass. Second, it is possible that amount of exercise performed over the intervention period in the present study might not be sufficient as opposed to what the participants reported. Krause et al. [33] showed an improvement in muscle mass over 12 weeks of a low-to-moderate intensity exercise intervention. The participants in Krause’s study performed a supervised exercise program whereas those in the present study were “instructed” to perform their exercises on their own. Insufficient amount of exercise compared to our instruction could be related to the fact that the low-to-moderate intensity exercise program used in the present study did not induce an increase in muscle mass. Finally, the participants in this study were healthy older adults whereas older adults often have co-morbidities, so the findings in this study should be interpreted carefully and applied only to healthy older adults. This healthy population may explain in part why we did not observe many changes in blood parameters.
In conclusion, the present study has demonstrated that long-term low-dose milk protein supplementation (10 g of protein/d) combined with low-to-moderate intensity exercise training is effective to increase muscle mass in healthy older adults. Furthermore, the intervention is associated with decreasing fat mass and plasma uric acid levels. However, during low-to-moderate intensity exercise training intervention, the low-dose milk protein supplementation did not have significant effects on muscle strength and function compared to isocaloric carbohydrate supplementation, so the physiological benefits of low-dose milk protein supplementation are limited. We also found that low-to-moderate intensity exercise training is a valuable method to improve physical performance in healthy older adults. Low-dose protein intake and low-to-moderate intensity exercise training are the key interventions in this study, and contribute to the low dropout rate and high compliance. We are encouraged that the nutrition and exercise strategies proposed in this study will improve the health of many older adults.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all volunteers for taking part in this study. We also thank Dr. Hideaki Hoshino and Dr. Hiroki Bochimoto for their medical judgment, the staff of edihas K.K. for measurements, data collection and statistical analysis, the staff of CHIKUDAI KIP for support of exercise training lessons, and the staff of the Food Valley Tokachi Council for recruitment of participants.
Author’s contribution
KN, CS, KM and TU designed the study. KN, YS and KM conducted the study. KN wrote the paper. All authors approved the final version of the paper.
Funding
This study was based on a collaborative research agreement between the Obihiro University of Agriculture & Veterinary Medicine and Meiji Co., Ltd. The funding for this study was provided by Meiji Co., Ltd.
Availability of data and material
This manuscript has datasets included as electronic supplementary material.
Compliance with ethical standards
Conflict of interest
KN, YS and CS are employees of Meiji Co., Ltd.
Ethics approval and consent to participate
The procedures in this study were approved by the Obihiro University of Agriculture and Veterinary Medicine Institutional Review Board of Clinical Research (Hokkaido, Japan) and the Meiji Institutional Review Board (Tokyo, Japan). It was conducted in accordance with ethical principles laid down by the Declaration of Helsinki and Ethical Guidelines for Medical and Health Research Involving Human Subjects (Ministry of Health, Labor and Welfare, Japan). Written informed consent was obtained from each participant before randomization.
References
- 1.Holloszy JO (2000) The biology of aging. Mayo Clin Proc 75 Suppl: S3–8, (discussion S8–9) [PubMed]
- 2.Jung KJ, Kimm H, Yun JE, Jee SH. Thigh circumference and diabetes: obesity as a potential effect modifier. J Epidemiol. 2013;23(5):329–336. doi: 10.2188/jea.je20120174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heitmann BL, Frederiksen P. Thigh circumference and risk of heart disease and premature death: prospective cohort study. BMJ. 2009;339:b3292. doi: 10.1136/bmj.b3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morton RW, Murphy KT, McKellar SR, Schoenfeld BJ, Henselmans M, Helms E, Aragon AA, Devries MC, Banfield L, Krieger JW, Phillips SM. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasiakos SM, McLellan TM, Lieberman HR. The effects of protein supplements on muscle mass, strength, and aerobic and anaerobic power in healthy adults: a systematic review. Sports Med. 2015;45(1):111–131. doi: 10.1007/s40279-014-0242-2. [DOI] [PubMed] [Google Scholar]
- 6.Cermak NM, Res PT, de Groot LC, Saris WH, van Loon LJ. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–1464. doi: 10.3945/ajcn.112.037556. [DOI] [PubMed] [Google Scholar]
- 7.Hou L, Lei Y, Li X, Huo C, Jia X, Yang J, Xu R, Wang X. Effect of protein supplementation combined with resistance training on muscle mass, strength and function in the elderly: a systematic review and meta-analysis. J Nutr Health Aging. 2019;23(5):451–458. doi: 10.1007/s12603-019-1181-2. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara Junior P, Ribeiro AS, Nabuco HCG, Fernandes RR, Tomeleri CM, Cunha PM, Venturini D, Barbosa DS, Schoenfeld BJ, Cyrino ES. Effects of whey protein supplementation associated with resistance training on muscular strength, hypertrophy, and muscle quality in preconditioned older women. Int J Sport Nutr Exerc Metab. 2018;28(5):528–535. doi: 10.1123/ijsnem.2017-0253. [DOI] [PubMed] [Google Scholar]
- 9.Mori H, Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: a randomized controlled trial. Geriatr Gerontol Int. 2018;18(9):1398–1404. doi: 10.1111/ggi.13499. [DOI] [PubMed] [Google Scholar]
- 10.Nabuco HCG, Tomeleri CM, Sugihara PJ, Fernandes RR, Cavalcante EF, Dos Santos L, Silva AM, Sardinha LB, Cyrino ES. Effect of whey protein supplementation combined with resistance training on cellular health in pre-conditioned older women: a randomized, double-blind, placebo-controlled trial. Arch Gerontol Geriatr. 2019;82:232–237. doi: 10.1016/j.archger.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Ten Haaf DSM, Eijsvogels TMH, Bongers C, Horstman AMH, Timmers S, de Groot L, Hopman MTE. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2019;10(2):298–310. doi: 10.1002/jcsm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candow DG, Little JP, Chilibeck PD, Abeysekara S, Zello GA, Kazachkov M, Cornish SM, Yu PH. Low-dose creatine combined with protein during resistance training in older men. Med Sci Sports Exerc. 2008;40(9):1645–1652. doi: 10.1249/MSS.0b013e318176b310. [DOI] [PubMed] [Google Scholar]
- 13.Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, van de Rest O, de Groot LC, van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–719. doi: 10.1016/j.jamda.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Kerstetter JE, Bihuniak JD, Brindisi J, Sullivan RR, Mangano KM, Larocque S, Kotler BM, Simpson CA, Cusano AM, Gaffney-Stomberg E, Kleppinger A, Reynolds J, Dziura J, Kenny AM, Insogna KL. The effect of a whey protein supplement on bone mass in older caucasian adults. J Clin Endocrinol Metab. 2015;100(6):2214–2222. doi: 10.1210/jc.2014-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norton C, Toomey C, McCormack WG, Francis P, Saunders J, Kerin E, Jakeman P. Protein supplementation at breakfast and lunch for 24 weeks beyond habitual intakes increases whole-body lean tissue mass in healthy older adults. J Nutr. 2016;146(1):65–69. doi: 10.3945/jn.115.219022. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell CJ, Milan AM, Mitchell SM, Zeng N, Ramzan F, Sharma P, Knowles SO, Roy NC, Sjodin A, Wagner KH, Cameron-Smith D. The effects of dietary protein intake on appendicular lean mass and muscle function in elderly men: a 10-wk randomized controlled trial. Am J Clin Nutr. 2017;106(6):1375–1383. doi: 10.3945/ajcn.117.160325. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama K, Kanda A, Tagawa R, Sanbongi C, Ikegami S, Itoh H (2017) Post-exercise muscle protein synthesis in rats after ingestion of acidified bovine milk compared with skim milk. Nutrients 9 (10). doi: 10.3390/nu9101071 [DOI] [PMC free article] [PubMed]
- 18.WHO Protein and amino acid requirements in human nutrition. World Health Organ Tech Rep Ser. 2007;935:1–265. [PubMed] [Google Scholar]
- 19.Rutherfurd SM, Fanning AC, Miller BJ, Moughan PJ. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J Nutr. 2015;145(2):372–379. doi: 10.3945/jn.114.195438. [DOI] [PubMed] [Google Scholar]
- 20.Sarwar G. The protein digestibility-corrected amino acid score method overestimates quality of proteins containing antinutritional factors and of poorly digestible proteins supplemented with limiting amino acids in rats. J Nutr. 1997;127(5):758–764. doi: 10.1093/jn/127.5.758. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85(4):1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 22.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc. 2006;38(4):667–674. doi: 10.1249/01.mss.0000210190.64458.25. [DOI] [PubMed] [Google Scholar]
- 23.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 24.Baechle TR, Earle RW, Wathen D. Resistance training. In: Baechle TR, Earle RW, editors. Essentials of strength training and conditioning. 3. Illinois: Human Kinetics; 2008. pp. 381–412. [Google Scholar]
- 25.Ishii S, Tanaka T, Shibasaki K, Ouchi Y, Kikutani T, Higashiguchi T, Obuchi SP, Ishikawa-Takata K, Hirano H, Kawai H, Tsuji T, Iijima K. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(1):93–101. doi: 10.1111/ggi.12197. [DOI] [PubMed] [Google Scholar]
- 26.Seino S, Shinkai S, Iijima K, Obuchi S, Fujiwara Y, Yoshida H, Kawai H, Nishi M, Murayama H, Taniguchi Y, Amano H, Takahashi R. Reference values and age differences in body composition of community-dwelling older Japanese men and women: a pooled analysis of four cohort studies. PLoS ONE. 2015;10(7):e0131975. doi: 10.1371/journal.pone.0131975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 28.Isles RC, Choy NL, Steer M, Nitz JC. Normal values of balance tests in women aged 20–80. J Am Geriatr Soc. 2004;52(8):1367–1372. doi: 10.1111/j.1532-5415.2004.52370.x. [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW. Reference values for the five-repetition sit-to-stand test: a descriptive meta-analysis of data from elders. Percept Mot Skills. 2006;103(1):215–222. doi: 10.2466/pms.103.1.215-222. [DOI] [PubMed] [Google Scholar]
- 30.Candow DG, Chilibeck PD, Facci M, Abeysekara S, Zello GA. Protein supplementation before and after resistance training in older men. Eur J Appl Physiol. 2006;97(5):548–556. doi: 10.1007/s00421-006-0223-8. [DOI] [PubMed] [Google Scholar]
- 31.Chale A, Cloutier GJ, Hau C, Phillips EM, Dallal GE, Fielding RA. Efficacy of whey protein supplementation on resistance exercise-induced changes in lean mass, muscle strength, and physical function in mobility-limited older adults. J Gerontol A Biol Sci Med Sci. 2013;68(6):682–690. doi: 10.1093/gerona/gls221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maltais ML, Ladouceur JP, Dionne IJ. The effect of resistance training and different sources of postexercise protein supplementation on muscle mass and physical capacity in sarcopenic elderly men. J Strength Cond Res. 2016;30(6):1680–1687. doi: 10.1519/jsc.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 33.Krause M, Crognale D, Cogan K, Contarelli S, Egan B, Newsholme P, De Vito G. The effects of a combined bodyweight-based and elastic bands resistance training, with or without protein supplementation, on muscle mass, signaling and heat shock response in healthy older people. Exp Gerontol. 2019;115:104–113. doi: 10.1016/j.exger.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 34.Arnarson A, Gudny Geirsdottir O, Ramel A, Briem K, Jonsson PV, Thorsdottir I. Effects of whey proteins and carbohydrates on the efficacy of resistance training in elderly people: double blind, randomised controlled trial. Eur J Clin Nutr. 2013;67(8):821–826. doi: 10.1038/ejcn.2013.40. [DOI] [PubMed] [Google Scholar]
- 35.Verdijk LB, Jonkers RA, Gleeson BG, Beelen M, Meijer K, Savelberg HH, Wodzig WK, Dendale P, van Loon LJ. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89(2):608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 36.Leenders M, Verdijk LB, Van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig WK, Senden JM, Keizer HA, Van Loon LJ. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc. 2013;45(3):542–552. doi: 10.1249/MSS.0b013e318272fcdb. [DOI] [PubMed] [Google Scholar]
- 37.Gryson C, Ratel S, Rance M, Penando S, Bonhomme C, Le Ruyet P, Duclos M, Boirie Y, Walrand S. Four-month course of soluble milk proteins interacts with exercise to improve muscle strength and delay fatigue in elderly participants. J Am Med Dir Assoc. 2014;15(12):958.e951–959. doi: 10.1016/j.jamda.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Maltais ML, Perreault K, Courchesne-Loyer A, Lagace JC, Barsalani R, Dionne IJ. Effect of resistance training and various sources of protein supplementation on body fat mass and metabolic profile in sarcopenic overweight older adult men: a pilot study. Int J Sport Nutr Exerc Metab. 2016;26(1):71–77. doi: 10.1123/ijsnem.2015-0160. [DOI] [PubMed] [Google Scholar]
- 39.Kumar V, Selby A, Rankin D, Patel R, Atherton P, Hildebrandt W, Williams J, Smith K, Seynnes O, Hiscock N, Rennie MJ. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587(1):211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holm L, Reitelseder S, Pedersen TG, Doessing S, Petersen SG, Flyvbjerg A, Andersen JL, Aagaard P. Kjaer M (2008) Changes in muscle size and MHC composition in response to resistance exercise with heavy and light loading intensity. J Appl Physiol. 1985;105(5):1454–1461. doi: 10.1152/japplphysiol.90538.2008. [DOI] [PubMed] [Google Scholar]
- 41.Mangine GT, Hoffman JR, Gonzalez AM, Townsend JR, Wells AJ, Jajtner AR, Beyer KS, Boone CH, Miramonti AA, Wang R, LaMonica MB, Fukuda DH, Ratamess NA, Stout JR (2015) The effect of training volume and intensity on improvements in muscular strength and size in resistance-trained men. Physiol Rep 3 (8). doi: 10.14814/phy2.12472 [DOI] [PMC free article] [PubMed]
- 42.Csapo R, Alegre LM. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: a meta-analysis. Scand J Med Sci Sports. 2016;26(9):995–1006. doi: 10.1111/sms.12536. [DOI] [PubMed] [Google Scholar]
- 43.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86(2):373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 44.Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr. 2005;135(6):1553s–1556s. doi: 10.1093/jn/135.6.1553S. [DOI] [PubMed] [Google Scholar]
- 45.Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS. Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr. 2005;135(3):376–382. doi: 10.1093/jn/135.3.376. [DOI] [PubMed] [Google Scholar]
- 46.Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med. 2014;44(1):S71–77. doi: 10.1007/s40279-014-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291(2):E381–387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 48.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19(3):422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Churchward-Venne TA, Burd NA, Breen L, Tarnopolsky MA, Phillips SM. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr Metab (Lond) 2012;9(1):57. doi: 10.1186/1743-7075-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holwerda AM, Paulussen KJM, Overkamp M, Goessens JPB, Kramer IF, Wodzig W, Verdijk LB, van Loon LJC. Dose-dependent increases in whole-body net protein balance and dietary protein-derived amino acid incorporation into myofibrillar protein during recovery from resistance exercise in older men. J Nutr. 2019;149(2):221–230. doi: 10.1093/jn/nxy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cormie P, McGuigan MR, Newton RU. Developing maximal neuromuscular power: Part 1–biological basis of maximal power production. Sports Med. 2011;41(1):17–38. doi: 10.2165/11537690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 52.Nishimune H, Stanford JA, Mori Y. Role of exercise in maintaining the integrity of the neuromuscular junction. Muscle Nerve. 2014;49(3):315–324. doi: 10.1002/mus.24095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krause Neto W, Ciena AP, Anaruma CA, de Souza RR, Gama EF. Effects of exercise on neuromuscular junction components across age: systematic review of animal experimental studies. BMC Res Notes. 2015;8:713. doi: 10.1186/s13104-015-1644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garrel DR, Verdy M, PetitClerc C, Martin C, Brule D, Hamet P. Milk- and soy-protein ingestion: acute effect on serum uric acid concentration. Am J Clin Nutr. 1991;53(3):665–669. doi: 10.1093/ajcn/53.3.665. [DOI] [PubMed] [Google Scholar]
- 55.Dalbeth N, Wong S, Gamble GD, Horne A, Mason B, Pool B, Fairbanks L, McQueen FM, Cornish J, Reid IR, Palmano K. Acute effect of milk on serum urate concentrations: a randomised controlled crossover trial. Ann Rheum Dis. 2010;69(9):1677–1682. doi: 10.1136/ard.2009.124230. [DOI] [PubMed] [Google Scholar]
- 56.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52(1):283–289. doi: 10.1002/art.20761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript has datasets included as electronic supplementary material.