Abstract
Gout is a common type of acute arthritis that results from elevated serum uric acid (SUA) levels. Recent genome-wide association studies (GWASs) have revealed several novel single nucleotide polymorphism (SNPs) associated with SUA levels. Of these, rs10821905 of A1CF and rs1178977 of BAZ1B showed the greatest and the second greatest significant effect size for increasing SUA level in the Japanese population, but their association with gout is not clear. We examined their association with gout using 1411 clinically-defined Japanese gout patients and 1285 controls, and meta-analyzed our previous gout GWAS data to investigate any association with gout. Replication studies revealed both SNPs to be significantly associated with gout (P = 0.0366, odds ratio [OR] with 95% confidence interval [CI]: 1.30 [1.02–1.68] for rs10821905 of A1CF, P = 6.49 × 10–3, OR with 95% CI: 1.29 [1.07–1.55] for rs1178977 of BAZ1B). Meta-analysis also revealed a significant association with gout in both SNPs (Pmeta = 3.16 × 10–4, OR with 95% CI: 1.39 [1.17–1.66] for rs10821905 of A1CF, Pmeta = 7.28 × 10–5, OR with 95% CI 1.32 [1.15–1.51] for rs1178977 of BAZ1B). This study shows the first known association between SNPs of A1CF, BAZ1B and clinically-defined gout cases in Japanese. Our results also suggest a shared physiological/pathophysiological background between several populations, including Japanese, for both SUA increase and gout susceptibility. Our findings will not only assist the elucidation of the pathophysiology of gout and hyperuricemia, but also suggest new molecular targets.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13577-021-00485-4.
Keywords: Gout/hyperuricemia, BAZ1B/WSTF, MLXIPL/ChREBP, Apolipoprotein B (ApoB), ABCG2/BCRP
Introduction
Gout is a common disease which displays severe non-infectious acute arthritis and results from elevated serum uric acid (SUA) level, or hyperuricemia [1]. Recent genetic studies including genome-wide association studies (GWASs) have revealed several genes to be associated with SUA levels [2–7] as well as clinically-defined gout [8–14]. Of these, Köttgen et al. [7] reported 18 novel loci associated with SUA in European, Indian, African-American and Japanese populations. Two of them, rs10821905 of A1CF (chromosome 10q11.23) and rs1178977 of BAZ1B (chromosome 7q.11.23), showed the statistically significantly greatest and the second greatest effect size for increasing SUA level in a Japanese population [7]. While they showed a nominally significant association with gout in individuals of European ancestry, their association was not clarified in Japanese gout cases.
With clinically defined Japanese gout cases, we have identified several genes through a candidate gene approach [15–19] which are associated with gout. Many urate transporter genes, such as SLC22A12/URAT1 and SLC2A9/GLUT9, whose dysfunctional variants cause renal hypouricemia [20–22] are also reported to have an association with gout and hyperuricemia.
This prompted us to examine the association between common variants of A1CF, BAZ1B and gout using clinically-defined Japanese gout patients through a candidate gene approach, and to meta-analyze it with our previous gout GWAS data [10].
Methods
Patients and controls
1411 male Japanese patients in total were recruited from the outpatients of the gout clinics of Ryougoku East Gate Clinic (Tokyo, Japan), Nagase Clinic (Tokyo, Japan), Wakasa Clinic (Saitama, Japan) and Tokorozawa Central Hospital (Saitama, Japan). All the subjects had been diagnosed with primary gout according to the criteria established by the American College of Rheumatology [23]. As the control group, 1,285 Japanese males without a history of gout or hyperuricemia (SUA levels > 7.0 mg/dL) were selected from participants in the Nagoya and Shizuoka area in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) [24, 25]. The mean age and standard deviation of cases and controls were, respectively, 48.2 ± 11.7 and 53.4 ± 9.9 years, and their mean body-mass indexes were 25.2 ± 3.7 and 22.9 ± 2.9 kg/m2, respectively.
Genetic and statistical analyses
Genomic DNA was extracted from whole peripheral blood [26]. Genotyping of A1CF polymorphism (rs10821905) and BAZ1B polymorphism (rs1178977) was performed using a TaqMan assay (Applied Biosystems) employing a Lightcycler 480 (Roche Diagnostics) as reported in our previous study [27]. GWAS genotyping data were obtained from our previous study [10] which was performed using the Illumina HumanOmniExpress-12 v1.0 (Illumina) platform employing 945 clinically-ascertained cases and 1213 Japanese male controls.
For the calculations in the statistical analyses, we used R (version 4.0.1) [28] including a meta package [29] in a fixed effect model. The chi-squared test was used for the association and Hardy–Weinberg equilibrium analyses. A P value of < 0.05 was regarded as statistically significant.
Results
Table 1 shows the genotyping results for rs10821905 of A1CF and rs1178977 of BAZ1B in 1,411 gout cases and 1,285 controls. The genotyping call rate for these SNPs exceeded 98%. These SNPs in the control group were in Hardy–Weinberg equilibrium (P > 0.05), which suggested no mistyping. Table 1 shows that both SNPs showed a significant association with gout.
Table 1.
Association analysis between A1CF and BAZ1B and gout
| Gene | SNP | Genotypea | Allele frequency mode | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | P valueb | OR (95% CI) | ||||||||
| 1/1 | 1/2 | 2/2 | RAF | 1/1 | 1/2 | 2/2 | RAF | ||||
| A1CF | rs10821905 | 1252 | 150 | 3 | 0.0555 | 1168 | 106 | 2 | 0.0431 | 0.0366 | 1.30 (1.02–1.68) |
| BAZ1B | rs1178977 | 6 | 223 | 1174 | 0.916 | 14 | 240 | 1016 | 0.895 | 6.49 × 10–3 | 1.29 (1.07–1.55) |
SNP single nucleotide polymorphism, RAF risk allele frequency, OR odds ratio, CI confidence interval
aThe non-risk allele referred to as allele 1 and the risk allele as 2. Allele 1 is G and allele 2 is A in both rs10821905 and rs1178977
bP values were obtained by chi-squared tests
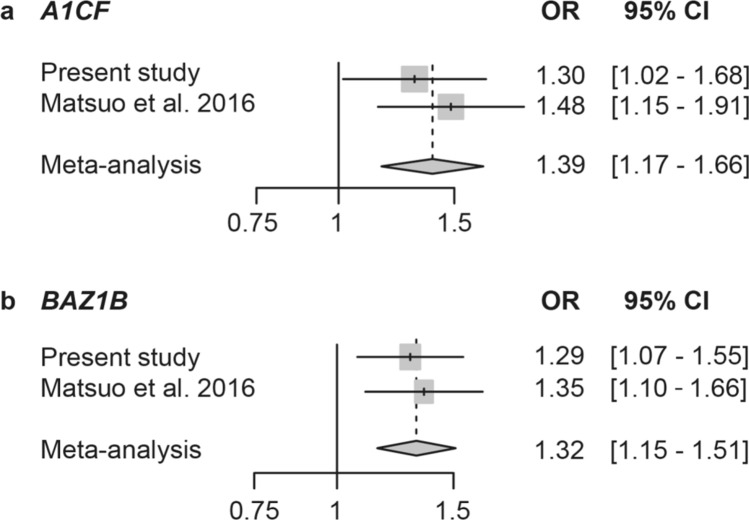
As shown in Fig. 1, the meta-analysis between the present study and previous gout GWAS [10] also showed both SNPs to have significant associations with gout (Pmeta = 3.16 × 10–4, odds ratio [OR] with 95% confidential interval [CI]: 1.39 [1.16–1.66] for rs10821905 of A1CF, Pmeta = 7.28 × 10–5, OR with 95% CI 1.32 [1.15–1.51] for rs1178977 of BAZ1B).
Fig. 1.
Meta-analysis of a rs10821905 of A1CF and b rs1178977 of BAZ1B for gout in the Japanese male population. The meta-analysis was conducted using the present study and our previous gout GWAS of Japanese male populations (Matsuo et al. [10]). Both SNPs showed a statistically significant association with gout (Pmeta = 3.16 × 10–4 for rs10821905 of A1CF, Pmeta = 7.28 × 10–5 for rs1178977 of BAZ1B). OR odds ratio, CI confidence interval
Discussion
The present study showed, for the first time, an association between rs10821905 of A1CF as well as rs1178977 of BAZ1B and gout in a Japanese population.
Since it has been established that hyperuricemia is associated with dyslipidemia [30] such as hypertriglyceridemia [31], variants of A1CF may affect urate metabolism via the following ApoB-related mechanisms. A1CF (APOBEC1 complementation factor) encodes a complementation factor which forms a multi-component enzyme complex with APOBEC1 (apolipoprotein B mRNA editing enzyme catalytic subunit 1) to deaminate mammalian apolipoprotein B (ApoB) mRNA [32]. In other words, mRNA coding ApoB-100 is converted to mRNA coding ApoB-48 by this enzyme complex containing A1CF. Variants of A1CF may, therefore, affect urate metabolism through ApoB production and/or ApoB-related insulin resistance. Moreover, A1CF is reported to be expressed in the liver, kidney and intestine [33, 34], from where urate is also mainly produced and excreted in humans. Indeed, in addition to the present study, Dong et al. and Rasheed et al. have reported an association between gout and the A1CF variant in Han Chinese and in New Zealand European and Polynesian populations [35, 36]. Nevertheless, further studies need to be conducted to elucidate the precise pathophysiological background, when taking into account the fact that rs10821905 is located at about 2 kbp upstream of the A1CF gene. In other words, while the present study showed significance at rs10821905 of A1CF, it is possible that this is a mere marker and that the true risk gene is present close by.
This is the first report to identify an association between clinically-defined gout and BAZ1B. BAZ1B is possibly involved in urate metabolism due to transcriptional changes. BAZ1B (bromodomain adjacent to zinc finger domain 1B), also known as WSTF (Williams syndrome transcription factor), encodes a member of the bromodomain protein family. BAZ1B shows ubiquitous expression in humans and is mainly involved in the chromatin-dependent regulation of transcription, including chromatin assembly, RNA polymerase I and III gene regulation, vitamin D metabolism, and DNA repair [37]. Since Köttgen et al. [7] reported rs1178977 of BAZ1B to be in linkage disequilibrium (LD) with several SNPs of MLXIPL, it is probable that BAZ1B is a mere surrogate marker of MLXIPL, since MLXIPL/ChREBP is involved in the glucose-6-phosphate production, an upstream pathway of de novo urate production [38, 39]. However, because urate is an end metabolite of purine bodies including ATP and some nucleosides, it is also possible that changes in regulation of transcription by BAZ1B variants are associated with urate metabolism.
While deletion, including BAZ1B, is known to cause Williams syndrome [40], the mechanism between SUA or gout and BAZ1B variants remains to be elucidated. It is compatible in that the same SNP (rs1178977) is reported to have an association with triglyceride levels from a previous GWAS [41], when taking into account the association between urate and triglycerides [31].
We previously reported that ABCG2 (ATP-binding cassette subfamily G member 2) is a renal and intestinal urate exporter and that its dysfunctional variants have a significant and strong effect on susceptibility to gout/hyperuricemia [8, 42, 43]. We, therefore, recalculated the results shown in Table 1 with and without these variants. As shown in Supplementary Table S1, the A1CF variant still showed a significant association with gout in the presence of the dysfunctional variants of ABCG2 but was no longer significant without those variants, while the BAZ1B variant remained significant both with and without ABCG2 dysfunction. A1CF might, therefore, have synergistic effects on susceptibility to gout with dysfunctional variants of ABCG2, for which further analyses are necessary.
As mentioned in the Introduction, both A1CF and BAZ1B were first detected in the GWAS of SUA from a European population with genome-wide significance [P = 7.40 × 10–17, beta = 0.057, and P = 1.20 × 10–12, beta = 0.0247 (unit: mg/dl)]. These SNPs also showed nominal significance with gout [P = 0.026, OR = 1.09, and P = 6.70 × 10–4, OR = 1.14, respectively] [7]. Our previous GWAS of SUA from a Japanese population also showed [44] a nominally significant association (P = 1.79 × 10–3, beta = 0.029, and P = 2.35 × 10–7, beta = 0.033). The present result and the previous reports suggest there to be a shared physiological or pathophysiological background between Japanese and European populations for both SUA increase and gout susceptibility.
Our findings should not only assist the elucidation of the pathophysiology of gout and hyperuricemia, but also suggests new molecular targets.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the participants for their generous contributions to this study. We are also grateful to members of the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) for their support. We are indebted to K. Morichika, M. Miyazawa, K. Gotanda, and M. Seki for genetic analyses, and to N. Hamajima, and K. Wakai for sample collection. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, including MEXT Kakenhi Priority Areas Number 17015018, and JSPS Kakenhi Grants Nos. 17H04128, 19K22786, 20H00566, 20K23152, 16H06277 (CoBiA), and Innovative Area No. 221S0001, as well as the Gout and Uric Acid Foundation of Japan and the Kawano Masanori Memorial Foundation for the Promotion of Pediatrics.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study was approved by the National Defense Medical College’s institutional ethical committee (No. 2914) and that of Nagoya University (No. 2011-1248-7). All procedures were performed in accordance with the Declaration of Helsinki and its later amendments.
Informed consent
Written informed consent was obtained from each subject participating in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Makoto Kawaguchi, Akiyoshi Nakayama contributed equally to this work.
References
- 1.Dalbeth N, Choi HK, Joosten LAB, Khanna PP, Matsuo H, Perez-Ruiz F, Stamp LK. Gout. Nat Rev Dis Primers. 2019;5(1):69. doi: 10.1038/s41572-019-0115-y. [DOI] [PubMed] [Google Scholar]
- 2.Dehghan A, Köttgen A, Yang Q, Hwang SJ, Kao WL, Rivadeneira F, Boerwinkle E, Levy D, Hofman A, Astor BC, Benjamin EJ, van Duijn CM, Witteman JC, Coresh J, Fox CS. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372(9654):1953–1961. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, Consortium E, Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, Consortium E, Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Volker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H, Consortium P, Doering A, Wichmann HE, Study K, Spector TD, Peltonen L, Volzke H, Nagaraja R, Vollenweider P, Caulfield M, Wtccc, Illig T, Gieger C Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5(6):e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamatani Y, Matsuda K, Okada Y, Kubo M, Hosono N, Daigo Y, Nakamura Y, Kamatani N. Genome-wide association study of hematological and biochemical traits in a Japanese population. Nat Genet. 2010;42(3):210–215. doi: 10.1038/ng.531. [DOI] [PubMed] [Google Scholar]
- 5.Tin A, Woodward OM, Kao WH, Liu CT, Lu X, Nalls MA, Shriner D, Semmo M, Akylbekova EL, Wyatt SB, Hwang SJ, Yang Q, Zonderman AB, Adeyemo AA, Palmer C, Meng Y, Reilly M, Shlipak MG, Siscovick D, Evans MK, Rotimi CN, Flessner MF, Kottgen M, Cupples LA, Fox CS, Köttgen A, Care CC. Genome-wide association study for serum urate concentrations and gout among African Americans identifies genomic risk loci and a novel URAT1 loss-of-function allele. Hum Mol Genet. 2011;20(20):4056–4068. doi: 10.1093/hmg/ddr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sulem P, Gudbjartsson DF, Walters GB, Helgadottir HT, Helgason A, Gudjonsson SA, Zanon C, Besenbacher S, Bjornsdottir G, Magnusson OT, Magnusson G, Hjartarson E, Saemundsdottir J, Gylfason A, Jonasdottir A, Holm H, Karason A, Rafnar T, Stefansson H, Andreassen OA, Pedersen JH, Pack AI, de Visser MC, Kiemeney LA, Geirsson AJ, Eyjolfsson GI, Olafsson I, Kong A, Masson G, Jonsson H, Thorsteinsdottir U, Jonsdottir I, Stefansson K. Identification of low-frequency variants associated with gout and serum uric acid levels. Nat Genet. 2011;43(11):1127–1130. doi: 10.1038/ng.972. [DOI] [PubMed] [Google Scholar]
- 7.Köttgen A, Albrecht E, Teumer A, Vitart V, Krumsiek J, Hundertmark C, Pistis G, Ruggiero D, O'Seaghdha CM, Haller T, Yang Q, Tanaka T, Johnson AD, Kutalik Z, Smith AV, Shi J, Struchalin M, Middelberg RP, Brown MJ, Gaffo AL, Pirastu N, Li G, Hayward C, Zemunik T, Huffman J, Yengo L, Zhao JH, Demirkan A, Feitosa MF, Liu X, Malerba G, Lopez LM, van der Harst P, Li X, Kleber ME, Hicks AA, Nolte IM, Johansson A, Murgia F, Wild SH, Bakker SJ, Peden JF, Dehghan A, Steri M, Tenesa A, Lagou V, Salo P, Mangino M, Rose LM, Lehtimaki T, Woodward OM, Okada Y, Tin A, Muller C, Oldmeadow C, Putku M, Czamara D, Kraft P, Frogheri L, Thun GA, Grotevendt A, Gislason GK, Harris TB, Launer LJ, McArdle P, Shuldiner AR, Boerwinkle E, Coresh J, Schmidt H, Schallert M, Martin NG, Montgomery GW, Kubo M, Nakamura Y, Tanaka T, Munroe PB, Samani NJ, Jacobs DR, Jr., Liu K, D'Adamo P, Ulivi S, Rotter JI, Psaty BM, Vollenweider P, Waeber G, Campbell S, Devuyst O, Navarro P, Kolcic I, Hastie N, Balkau B, Froguel P, Esko T, Salumets A, Khaw KT, Langenberg C, Wareham NJ, Isaacs A, Kraja A, Zhang Q, Wild PS, Scott RJ, Holliday EG, Org E, Viigimaa M, Bandinelli S, Metter JE, Lupo A, Trabetti E, Sorice R, Döring A, Lattka E, Strauch K, Theis F, Waldenberger M, Wichmann HE, Davies G, Gow AJ, Bruinenberg M, LifeLines Cohort S, Stolk RP, Kooner JS, Zhang W, Winkelmann BR, Boehm BO, Lucae S, Penninx BW, Smit JH, Curhan G, Mudgal P, Plenge RM, Portas L, Persico I, Kirin M, Wilson JF, Mateo Leach I, van Gilst WH, Goel A, Ongen H, Hofman A, Rivadeneira F, Uitterlinden AG, Imboden M, von Eckardstein A, Cucca F, Nagaraja R, Piras MG, Nauck M, Schurmann C, Budde K, Ernst F, Farrington SM, Theodoratou E, Prokopenko I, Stumvoll M, Jula A, Perola M, Salomaa V, Shin SY, Spector TD, Sala C, Ridker PM, Kahonen M, Viikari J, Hengstenberg C, Nelson CP, Consortium CA, Consortium D, Consortium I, Consortium M, Meschia JF, Nalls MA, Sharma P, Singleton AB, Kamatani N, Zeller T, Burnier M, Attia J, Laan M, Klopp N, Hillege HL, Kloiber S, Choi H, Pirastu M, Tore S, Probst-Hensch NM, Volzke H, Gudnason V, Parsa A, Schmidt R, Whitfield JB, Fornage M, Gasparini P, Siscovick DS, Polasek O, Campbell H, Rudan I, Bouatia-Naji N, Metspalu A, Loos RJ, van Duijn CM, Borecki IB, Ferrucci L, Gambaro G, Deary IJ, Wolffenbuttel BH, Chambers JC, Marz W, Pramstaller PP, Snieder H, Gyllensten U, Wright AF, Navis G, Watkins H, Witteman JC, Sanna S, Schipf S, Dunlop MG, Tonjes A, Ripatti S, Soranzo N, Toniolo D, Chasman DI, Raitakari O, Kao WH, Ciullo M, Fox CS, Caulfield M, Bochud M, Gieger C Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–154. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuo H, Takada T, Ichida K, Nakamura T, Nakayama A, Ikebuchi Y, Ito K, Kusanagi Y, Chiba T, Tadokoro S, Takada Y, Oikawa Y, Inoue H, Suzuki K, Okada R, Nishiyama J, Domoto H, Watanabe S, Fujita M, Morimoto Y, Naito M, Nishio K, Hishida A, Wakai K, Asai Y, Niwa K, Kamakura K, Nonoyama S, Sakurai Y, Hosoya T, Kanai Y, Suzuki H, Hamajima N, Shinomiya N. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1(5):5ra11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 9.Woodward OM, Köttgen A, Coresh J, Boerwinkle E, Guggino WB, Köttgen M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106(25):10338–10342. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuo H, Yamamoto K, Nakaoka H, Nakayama A, Sakiyama M, Chiba T, Takahashi A, Nakamura T, Nakashima H, Takada Y, Danjoh I, Shimizu S, Abe J, Kawamura Y, Terashige S, Ogata H, Tatsukawa S, Yin G, Okada R, Morita E, Naito M, Tokumasu A, Onoue H, Iwaya K, Ito T, Takada T, Inoue K, Kato Y, Nakamura Y, Sakurai Y, Suzuki H, Kanai Y, Hosoya T, Hamajima N, Inoue I, Kubo M, Ichida K, Ooyama H, Shimizu T, Shinomiya N. Genome-wide association study of clinically defined gout identifies multiple risk loci and its association with clinical subtypes. Ann Rheum Dis. 2016;75(4):652–659. doi: 10.1136/annrheumdis-2014-206191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Li Z, Liu S, Wang C, Han L, Cui L, Zhou J, Zou H, Liu Z, Chen J, Cheng X, Zhou Z, Ding C, Wang M, Chen T, Cui Y, He H, Zhang K, Yin C, Wang Y, Xing S, Li B, Ji J, Jia Z, Ma L, Niu J, Xin Y, Liu T, Chu N, Yu Q, Ren W, Wang X, Zhang A, Sun Y, Wang H, Lu J, Li Y, Qing Y, Chen G, Wang Y, Zhou L, Niu H, Liang J, Dong Q, Li X, Mi QS, Shi Y. Genome-wide association analysis identifies three new risk loci for gout arthritis in Han Chinese. Nat Commun. 2015;6:7041. doi: 10.1038/ncomms8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama A, Nakaoka H, Yamamoto K, Sakiyama M, Shaukat A, Toyoda Y, Okada Y, Kamatani Y, Nakamura T, Takada T, Inoue K, Yasujima T, Yuasa H, Shirahama Y, Nakashima H, Shimizu S, Higashino T, Kawamura Y, Ogata H, Kawaguchi M, Ohkawa Y, Danjoh I, Tokumasu A, Ooyama K, Ito T, Kondo T, Wakai K, Stiburkova B, Pavelka K, Stamp LK, Dalbeth N, Eurogout C, Sakurai Y, Suzuki H, Hosoyamada M, Fujimori S, Yokoo T, Hosoya T, Inoue I, Takahashi A, Kubo M, Ooyama H, Shimizu T, Ichida K, Shinomiya N, Merriman TR, Matsuo H, Eurogout Consortium GWAS of clinically defined gout and subtypes identifies multiple susceptibility loci that include urate transporter genes. Ann Rheum Dis. 2017;76(5):869–877. doi: 10.1136/annrheumdis-2016-209632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawamura Y, Nakaoka H, Nakayama A, Okada Y, Yamamoto K, Higashino T, Sakiyama M, Shimizu T, Ooyama H, Ooyama K, Nagase M, Hidaka Y, Shirahama Y, Hosomichi K, Nishida Y, Shimoshikiryo I, Hishida A, Katsuura-Kamano S, Shimizu S, Kawaguchi M, Uemura H, Ibusuki R, Hara M, Naito M, Takao M, Nakajima M, Iwasawa S, Nakashima H, Ohnaka K, Nakamura T, Stiburkova B, Merriman TR, Nakatochi M, Ichihara S, Yokota M, Takada T, Saitoh T, Kamatani Y, Takahashi A, Arisawa K, Takezaki T, Tanaka K, Wakai K, Kubo M, Hosoya T, Ichida K, Inoue I, Shinomiya N, Matsuo H. Genome-wide association study revealed novel loci which aggravate asymptomatic hyperuricaemia into gout. Ann Rheum Dis. 2019;78(10):1430–1437. doi: 10.1136/annrheumdis-2019-215521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakayama A, Nakatochi M, Kawamura Y, Yamamoto K, Nakaoka H, Shimizu S, Higashino T, Koyama T, Hishida A, Kuriki K, Watanabe M, Shimizu T, Ooyama K, Ooyama H, Nagase M, Hidaka Y, Matsui D, Tamura T, Nishiyama T, Shimanoe C, Katsuura-Kamano S, Takashima N, Shirai Y, Kawaguchi M, Takao M, Sugiyama R, Takada Y, Nakamura T, Nakashima H, Tsunoda M, Danjoh I, Hozawa A, Hosomichi K, Toyoda Y, Kubota Y, Takada T, Suzuki H, Stiburkova B, Major TJ, Merriman TR, Kuriyama N, Mikami H, Takezaki T, Matsuo K, Suzuki S, Hosoya T, Kamatani Y, Kubo M, Ichida K, Wakai K, Inoue I, Okada Y, Shinomiya N, Matsuo H, Japan Gout Genomics Consortium Subtype-specific gout susceptibility loci and enrichment of selection pressure on ABCG2 and ALDH2 identified by subtype genome-wide meta-analyses of clinically defined gout patients. Ann Rheum Dis. 2020;79(5):657–665. doi: 10.1136/annrheumdis-2019-216644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashino T, Matsuo H, Sakiyama M, Nakayama A, Nakamura T, Takada T, Ogata H, Kawamura Y, Kawaguchi M, Naito M, Kawai S, Takada Y, Ooyama H, Suzuki H, Shinomiya N. Common variant of PDZ domain containing 1 (PDZK1) gene is associated with gout susceptibility: a replication study and meta-analysis in Japanese population. Drug Metab Pharmacokinet. 2016;31(6):464–466. doi: 10.1016/j.dmpk.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Akashi A, Nakayama A, Kamatani Y, Higashino T, Shimizu S, Kawamura Y, Imoto M, Naito M, Hishida A, Kawaguchi M, Takao M, Matsuo M, Takada T, Ichida K, Ooyama H, Shinomiya N, Matsuo H. A common variant of LDL receptor related protein 2 (LRP2) gene is associated with gout susceptibility: a meta-analysis in a Japanese population. Hum Cell. 2020;33(2):303–307. doi: 10.1007/s13577-019-00318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higashino T, Matsuo H, Okada Y, Nakashima H, Shimizu S, Sakiyama M, Tadokoro S, Nakayama A, Kawaguchi M, Komatsu M, Hishida A, Nakatochi M, Ooyama H, Imaki J, Shinomiya N. A common variant of MAF/c-MAF, transcriptional factor gene in the kidney, is associated with gout susceptibility. Hum Cell. 2018;31(1):10–13. doi: 10.1007/s13577-017-0186-6. [DOI] [PubMed] [Google Scholar]
- 18.Sakiyama M, Matsuo H, Shimizu S, Chiba T, Nakayama A, Takada Y, Nakamura T, Takada T, Morita E, Naito M, Wakai K, Inoue H, Tatsukawa S, Sato J, Shimono K, Makino T, Satoh T, Suzuki H, Kanai Y, Hamajima N, Sakurai Y, Ichida K, Shimizu T, Shinomiya N. Common variant of leucine-rich repeat-containing 16A (LRRC16A) gene is associated with gout susceptibility. Hum Cell. 2014;27(1):1–4. doi: 10.1007/s13577-013-0081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakayama A, Matsuo H, Shimizu T, Ogata H, Takada Y, Nakashima H, Nakamura T, Shimizu S, Chiba T, Sakiyama M, Ushiyama C, Takada T, Inoue K, Kawai S, Hishida A, Wakai K, Hamajima N, Ichida K, Sakurai Y, Kato Y, Shimizu T, Shinomiya N. Common missense variant of monocarboxylate transporter 9 (MCT9/SLC16A9) gene is associated with renal overload gout, but not with all gout susceptibility. Hum Cell. 2013;26(4):133–136. doi: 10.1007/s13577-013-0073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417(6887):447–452. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 21.Matsuo H, Chiba T, Nagamori S, Nakayama A, Domoto H, Phetdee K, Wiriyasermkul P, Kikuchi Y, Oda T, Nishiyama J, Nakamura T, Morimoto Y, Kamakura K, Sakurai Y, Nonoyama S, Kanai Y, Shinomiya N. Mutations in glucose transporter 9 gene SLC2A9 cause renal hypouricemia. Am J Hum Genet. 2008;83(6):744–751. doi: 10.1016/j.ajhg.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama A, Matsuo H, Ohtahara A, Ogino K, Hakoda M, Hamada T, Hosoyamada M, Yamaguchi S, Hisatome I, Ichida K, Shinomiya N. Clinical practice guideline for renal hypouricemia (1st edition) Hum Cell. 2019;32(2):83–87. doi: 10.1007/s13577-019-00239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- 24.Hamajima N, J-MICC Study Group The Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) to detect gene-environment interactions for cancer. Asian Pac J Cancer Prev. 2007;8(2):317–323. [PubMed] [Google Scholar]
- 25.Asai Y, Naito M, Suzuki M, Tomoda A, Kuwabara M, Fukada Y, Okamoto A, Oishi S, Ikeda K, Nakamura T, Misu Y, Katase S, Tokumasu S, Nishio K, Ishida Y, Hishida A, Morita E, Kawai S, Okada R, Wakai K, Tamakoshi A, Hamajima N. Baseline data of Shizuoka area in the Japan Multi-Institutional Collaborative Cohort Study (J-MICC Study) Nagoya J Med Sci. 2009;71(3–4):137–144. [PMC free article] [PubMed] [Google Scholar]
- 26.Chiba T, Matsuo H, Sakiyama M, Nakayama A, Shimizu S, Wakai K, Suma S, Nakashima H, Sakurai Y, Shimizu T, Ichida K, Shinomiya N. Common variant of ALPK1 is not associated with gout: a replication study. Hum Cell. 2015;28(1):1–4. doi: 10.1007/s13577-014-0103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakayama A, Matsuo H, Shimizu T, Takada Y, Nakamura T, Shimizu S, Chiba T, Sakiyama M, Naito M, Morita E, Ichida K, Shinomiya N. Common variants of a urate-associated gene LRP2 are not associated with gout susceptibility. Rheumatol Int. 2014;34(4):473–476. doi: 10.1007/s00296-013-2924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Development Core Team. R: a language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna. 2006.
- 29.Schwarzer G. meta: Meta-analysis with R. 2014.
- 30.Schmidt MI, Watson RL, Duncan BB, Metcalf P, Brancati FL, Sharrett AR, Davis CE, Heiss G. Clustering of dyslipidemia, hyperuricemia, diabetes, and hypertension and its association with fasting insulin and central and overall obesity in a general population. Atherosclerosis risk in communities study investigators. Metabolism. 1996;45(6):699–706. doi: 10.1016/s0026-0495(96)90134-1. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi S, Yamamoto T, Moriwaki Y, Tsutsumi Z, Higashino K. Increased concentrations of serum Lp(a) lipoprotein in patients with primary gout. Ann Rheum Dis. 1995;54(2):90–93. doi: 10.1136/ard.54.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith HC, Bennett RP, Kizilyer A, McDougall WM, Prohaska KM. Functions and regulation of the APOBEC family of proteins. Semin Cell Dev Biol. 2012;23(3):258–268. doi: 10.1016/j.semcdb.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greeve J, Altkemper I, Dieterich JH, Greten H, Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993;34(8):1367–1383. [PubMed] [Google Scholar]
- 34.Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, Olsson I, Edlund K, Lundberg E, Navani S, Szigyarto CA, Odeberg J, Djureinovic D, Takanen JO, Hober S, Alm T, Edqvist PH, Berling H, Tegel H, Mulder J, Rockberg J, Nilsson P, Schwenk JM, Hamsten M, von Feilitzen K, Forsberg M, Persson L, Johansson F, Zwahlen M, von Heijne G, Nielsen J, Ponten F. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 35.Dong Z, Zhou J, Jiang S, Li Y, Zhao D, Yang C, Ma Y, Wang Y, He H, Ji H, Yang Y, Wang X, Xu X, Pang Y, Zou H, Jin L, Wang J. Effects of multiple genetic loci on the pathogenesis from serum urate to gout. Sci Rep. 2017;7:43614. doi: 10.1038/srep43614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rasheed H, Stamp LK, Dalbeth N, Merriman TR. Interaction of the GCKR and A1CF loci with alcohol consumption to influence the risk of gout. Arthritis Res Ther. 2017;19(1):161. doi: 10.1186/s13075-017-1369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnett C, Krebs JE. WSTF does it all: a multifunctional protein in transcription, repair, and replication. Biochem Cell Biol. 2011;89(1):12–23. doi: 10.1139/O10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boocock J, Leask M, Okada Y, Asian Genetic Epidemiology Network Consortium. Matsuo H, Kawamura Y, Shi Y, Li C, Mount DB, Mandal AK, Wang W, Cadzow M, Gosling AL, Major TJ, Horsfield JA, Choi HK, Fadason T, O'Sullivan J, Stahl EA, Merriman TR. Genomic dissection of 43 serum urate-associated loci provides multiple insights into molecular mechanisms of urate control. Hum Mol Genet. 2020;29(6):923–943. doi: 10.1093/hmg/ddaa013. [DOI] [PubMed] [Google Scholar]
- 39.Song Z, Yang H, Zhou L, Yang F. Glucose-sensing transcription factor MondoA/ChREBP as targets for type 2 diabetes: opportunities and challenges. Int J Mol Sci. 2019;20(20):5132. doi: 10.3390/ijms20205132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, Stock AD, Leppert M, Keating MT. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet. 1993;5(1):11–16. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- 41.Spracklen CN, Chen P, Kim YJ, Wang X, Cai H, Li S, Long J, Wu Y, Wang YX, Takeuchi F, Wu JY, Jung KJ, Hu C, Akiyama K, Zhang Y, Moon S, Johnson TA, Li H, Dorajoo R, He M, Cannon ME, Roman TS, Salfati E, Lin KH, Guo X, Sheu WHH, Absher D, Adair LS, Assimes TL, Aung T, Cai Q, Chang LC, Chen CH, Chien LH, Chuang LM, Chuang SC, Du S, Fan Q, Fann CSJ, Feranil AB, Friedlander Y, Gordon-Larsen P, Gu D, Gui L, Guo Z, Heng CK, Hixson J, Hou X, Hsiung CA, Hu Y, Hwang MY, Hwu CM, Isono M, Juang JJ, Khor CC, Kim YK, Koh WP, Kubo M, Lee IT, Lee SJ, Lee WJ, Liang KW, Lim B, Lim SH, Liu J, Nabika T, Pan WH, Peng H, Quertermous T, Sabanayagam C, Sandow K, Shi J, Sun L, Tan PC, Tan SP, Taylor KD, Teo YY, Toh SA, Tsunoda T, van Dam RM, Wang A, Wang F, Wang J, Wei WB, Xiang YB, Yao J, Yuan JM, Zhang R, Zhao W, Chen YI, Rich SS, Rotter JI, Wang TD, Wu T, Lin X, Han BG, Tanaka T, Cho YS, Katsuya T, Jia W, Jee SH, Chen YT, Kato N, Jonas JB, Cheng CY, Shu XO, He J, Zheng W, Wong TY, Huang W, Kim BJ, Tai ES, Mohlke KL, Sim X. Association analyses of East Asian individuals and trans-ancestry analyses with European individuals reveal new loci associated with cholesterol and triglyceride levels. Hum Mol Genet. 2017;26(9):1770–1784. doi: 10.1093/hmg/ddx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ichida K, Matsuo H, Takada T, Nakayama A, Murakami K, Shimizu T, Yamanashi Y, Kasuga H, Nakashima H, Nakamura T, Takada Y, Kawamura Y, Inoue H, Okada C, Utsumi Y, Ikebuchi Y, Ito K, Nakamura M, Shinohara Y, Hosoyamada M, Sakurai Y, Shinomiya N, Hosoya T, Suzuki H. Decreased extra-renal urate excretion is a common cause of hyperuricemia. Nat Commun. 2012;3:764. doi: 10.1038/ncomms1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakayama A, Matsuo H, Nakaoka H, Nakamura T, Nakashima H, Takada Y, Oikawa Y, Takada T, Sakiyama M, Shimizu S, Kawamura Y, Chiba T, Abe J, Wakai K, Kawai S, Okada R, Tamura T, Shichijo Y, Akashi A, Suzuki H, Hosoya T, Sakurai Y, Ichida K, Shinomiya N. Common dysfunctional variants of ABCG2 have stronger impact on hyperuricemia progression than typical environmental risk factors. Sci Rep. 2014;4:5227. doi: 10.1038/srep05227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakatochi M, Kanai M, Nakayama A, Hishida A, Kawamura Y, Ichihara S, Akiyama M, Ikezaki H, Furusyo N, Shimizu S, Yamamoto K, Hirata M, Okada R, Kawai S, Kawaguchi M, Nishida Y, Shimanoe C, Ibusuki R, Takezaki T, Nakajima M, Takao M, Ozaki E, Matsui D, Nishiyama T, Suzuki S, Takashima N, Kita Y, Endoh K, Kuriki K, Uemura H, Arisawa K, Oze I, Matsuo K, Nakamura Y, Mikami H, Tamura T, Nakashima H, Nakamura T, Kato N, Matsuda K, Murakami Y, Matsubara T, Naito M, Kubo M, Kamatani Y, Shinomiya N, Yokota M, Wakai K, Okada Y, Matsuo H. Genome-wide meta-analysis identifies multiple novel loci associated with serum uric acid levels in Japanese individuals. Commun Biol. 2019;2(1):115. doi: 10.1038/s42003-019-0339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.