Summary
The precise spatiotemporal characteristics of subcellular calcium (Ca2+) transients are critical for the physiological processes. Here we report a green Ca2+ sensor called “G-CatchER+” using a protein design to report rapid local ER Ca2+ dynamics with significantly improved folding properties. G-CatchER+ exhibits a superior Ca2+ on rate to G-CEPIA1er and has a Ca2+-induced fluorescence lifetimes increase. G-CatchER+ also reports agonist/antagonist triggered Ca2+ dynamics in several cell types including primary neurons that are orchestrated by IP3Rs, RyRs, and SERCAs with an ability to differentiate expression. Upon localization to the lumen of the RyR channel (G-CatchER+-JP45), we report a rapid local Ca2+ release that is likely due to calsequestrin. Transgenic expression of G-CatchER+ in Drosophila muscle demonstrates its utility as an in vivo reporter of stimulus-evoked SR local Ca2+ dynamics. G-CatchER+ will be an invaluable tool to examine local ER/SR Ca2+ dynamics and facilitate drug development associated with ER dysfunction.
Subject areas: biochemistry, cell biology, biophysics
Graphical Abstract
Highlights
-
•
G-CatchER+ exhibits superior kinetics with 1:1 stoichiometry than G-CEPIA1er
-
•
G-CatchER+ captures spatially confined ER Ca2+ dynamics in hippocampal neurons
-
•
G-CatchER+-JP45 reports rapid Ca2+ signals adjacent to the junctional SR membrane
-
•
G-CatchER+ reports stimulus-evoked SR local Ca2+ dynamics in Drosophila muscle
Biochemistry; cell biology; biophysics
Introduction
Calcium (Ca2+) is a major modulator of a multitude of cellular, biological, and pathological processes in organisms (Clapham, 2007) that is achieved using a Ca2+ signaling toolkit consisting of Ca2+ pumps, Ca2+ channels, receptors, and Ca2+ binding proteins (CaBPs) such as calsequestrin, calreticulin, parvalbumin, calmodulin etc. (Figure 1C). As the primary intracellular Ca2+ store, the endoplasmic reticulum (ER) or the sarcoplasmic reticulum (SR) found in muscle cells is central to Ca2+ signaling by modulating Ca2+ transients with differential timescales that govern key biological functions (Berridge et al., 2003). The task of converting extracellular stimuli into a coded intracellular Ca2+ signal, wave, or oscillation lies within the ER/SR in combination with a non-uniform distribution of CaBPs. These modulators of the Ca2+ signal create spatially diverse nanocompartments with differential Ca2+ concentrations within the ER/SR lumen via their spatial distribution and response dynamics (Papp et al., 2003). The morphology of the ER, especially in neural cells, is highly heterogeneous with strong plasticity tailored to various chemical and dynamic tasks in different areas of the cell, as well as between different cell types (Bourne and Harris, 2012). On the other hand, in skeletal muscle the RyR1 of the SR has a specialized arrangement in order to ensure a quick delivery of the Ca2+ needed for muscle contraction in response to membrane depolarization (Petersen et al., 2001; Berridge, 2002). The hypothesis that differential subcellular dynamics play an essential role in the regulation of rapid biological and pathological processes has not been fully addressed due to lack of fast Ca2+ sensors.
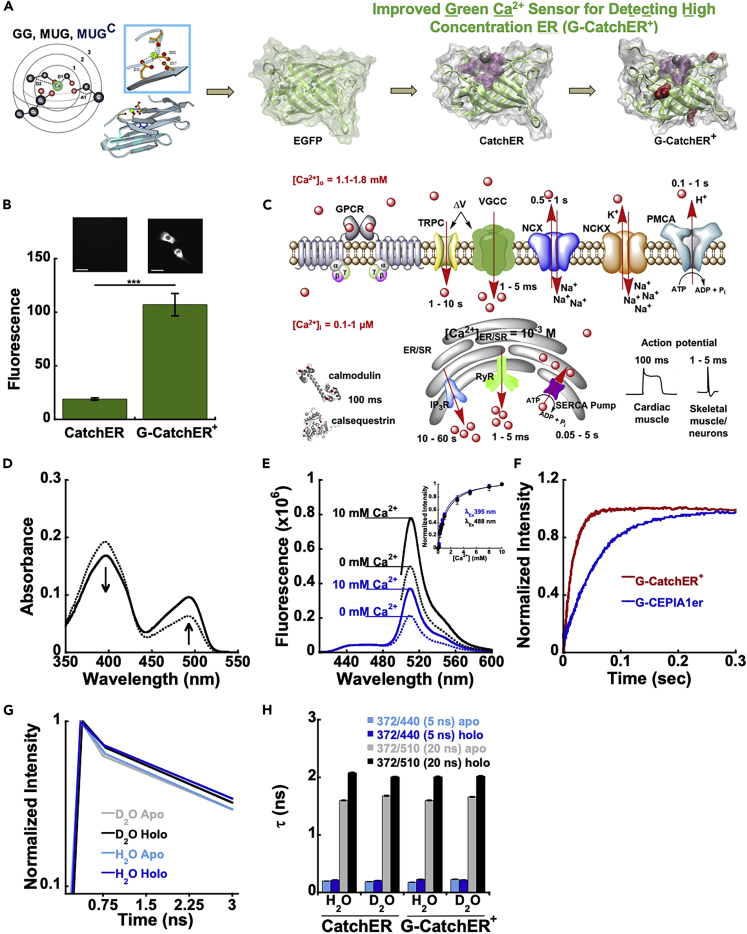
Figure 1.
Design and in vitro properties of G-CatchER+
(A) Design of Ca2+ binding sites using MUG (MUltiple Geometries) algorithm. EGFP was modified with a direct Ca2+ binding site. Both CatchER and G-CatchER+ were designed using these techniques (binding site residues are highlighted in pink, residues mutated from CatchER to G-CatchER+ are highlighted in red, and Ca2+ is represented with the silver ball).
(B) Mutations S175G, S30R, and Y39N were added to CatchER to improve its brightness and thermostability at 37°C. G-CatchER+ contains all three mutations. n = 10 for CatchER and G-CatchER+. Statistical significance was determined using unpaired student t test. p < 0.0001.
(C) Components of the Ca2+ signaling toolkit help shape the spatial-temporal signal. Calcium signaling is mediated by various pumps, channels, receptors, and CaBPs to control intracellular Ca2+ release from calcium storage organelles such as the ER/SR. The rapid action of Ca2+ release receptors and pumps lining the ER/SR membrane along with the fast conformational changes of some CaBPs rapidly convert the signal for action potential generation in muscle contraction and neuron activation.
(D) Absorbance spectra of 10 μM G-CatchER+ sample before titration with 10 μM EGTA (dashed line) and after titrating up to 10 mM Ca2+ (solid line). Arrows indicate the increase and decrease in the 488 nm and 395 nm excitation peaks with the addition of Ca2+.
(E) Fluorescence increase of G-CatchER+ in response to addition of Ca2+ excited at 395 nm (blue) and 488 nm (black), respectively, with emission monitored at 510 nm. Slit widths for excitation and emission were 0.25 mm. Inset, Binding curves were fit with a 1:1 binding equation to obtain the Kd.
(F) Comparison of Ca2+-associated kinetics of G-CatchER+ and G-CEPIA1er.
(G) Fluorescence decay curves of G-CatchER+ in both D2O and H2O when excited at 372 nm, with and without Ca2+.(H) Average lifetimes of G-CatchER+ in both D2O and H2O when excited at 372 nm, with and without Ca2+. Scale bars, 20 μm.
See also Figures S1 and S2 and Tables S1–S3.
Early efforts investigating ER Ca2+ release involved Ca2+ dyes to monitor Ca2+ transients indirectly with antipyrylazo III (Schneider et al., 1987; Melzer et al., 1987) and directly with fluo-5N (Kabbara and Allen, 2001). Genetically encoded Ca2+ indicators (GECIs) based on calmodulin (CaM) (Miyawaki et al., 1997) such as G-CEPIA1er (Suzuki et al., 2014a) and FRET pairs have been very informative but have limitations that include non-specific distribution within intracellular organelles, narrow Ca2+ affinities that are close to the intracellular environment, non-linearity due to multiple Ca2+ binding sites, and slow Ca2+ kinetics (Sztretye et al., 2011a; Landolfi et al., 1998; Jimenez-Moreno et al., 2010). Thus, there is a pressing need to design Ca2+ sensors having the capacity to quantify these differential subcellular Ca2+ dynamics in both biological and pathological states (Tang et al., 2015, 2020). To fill in this gap, we initially developed a genetically encoded green Ca2+ sensor, CatchER, by creating a single Ca2+ binding site directly onto the enhanced green florescent protein (EGFP) scaffold (Tang et al., 2011).
Here we report the development of an improved version of CatchER, G-CatchER+, with optimized folding at physiological temperature. G-CatchER+ specifically reports rapid global ER/SR Ca2+ dynamics in various cell types including neuron and muscle cells. G-CatchER+ exhibits a significantly faster Ca2+ on rate than G-CEPIA1er with enhanced Ca2+-dependent fluorescence increases as a result of increased fluorescence lifetime upon Ca2+ binding. In addition, G-CatchER+ has the capacity to monitor rapid local ER/SR Ca2+ changes in multiple cell types and in a subcellular environment in both neuron and excitation-contraction (EC) coupling processes.
Results
Generation and optimization of G-CatchER+
G-CatchER+ was designed by creating a single Ca2+ binding site with negatively charged residues directly onto EGFP like CatchER (Figure 1A)(Tang et al., 2011). To overcome folding limitations and to increase dynamic range of CatchER for mammalian cell applications, we introduced mutations S30R, Y39N, and S175G individually and in combination to create G-CatchER+ (Pedelacq et al., 2006; Siemering et al., 1996). G-CatchER+ exhibits significantly improved folding stability and subsequent fluorescence. These engineered proteins were expressed in Escherichia coli and purified using established methods (Figure S1). G-CatchER+ is among several variants that exhibited an improvement in their inherent optical properties compared with CatchER when investigated using spectroscopic methods (Table S2 and Figure S1). G-CatchER+ is also among several variants that exhibits less pH sensitivity with Ca2+ as determined by pKa values of 7.37 ± 0.01 and 6.80 ± 0.01 with and without Ca2+, respectively (Table S3 and Figure S2). C2C12 myoblast cells expressing G-CatchER+ experienced a 5-fold increase in intensity (107.2 ± 3.3) compared with CatchER (19.1 ± 0.3) at 37°C using epifluorescence microscopy (Figure 1B). Each individual mutation had minimal effects on the intensity at 37°C compared with the combination of all three (Figure S1).
Metal binding affinity of G-CatchER+in vitro and in cells
The Ca2+ binding affinity of G-CatchER+ was determined using fluorescence spectroscopy and epifluorescence microscopy. Saturation with Ca2+ resulted in a concurrent absorbance increase at 488 nm and decrease at 395 nm for G-CatchER+ (Figure 1D) and variants (Figure S1). G-CatchER+ had gradual incremental increases (~ 60%) in fluorescence intensity with a maximum peak at 510 nm upon addition of Ca2+ when excited at both 395 nm and 488 nm. Such fluorescence excitation changes at 395 nm and 488 nm were well fitted to a 1:1 binding equation with a determined Kd value for Ca2+ binding of 1.2 ± 0.2 mM (Figure 1E and Table S1). The average Ca2+ Kd in the presence of 150 mM KCl and in situ in Cos-7 and HEK293 cells in the presence of physiological concentrations of Mg2+, Na+, K+, and small molecules in the ER/SR were decreased, suggesting the role of an electrostatic interaction for Ca2+ binding (Figure S2).
Measuring the rapid kinetic properties of G-CatchER+
We next used stopped-flow spectrofluorometry to determine the time courses for Ca2+ association to and dissociation from G-CatchER+. Irrespective of the Ca2+ concentration between 0.1 mM and 5.0 mM, the fluorescence of G-CatchER+ increased with a kobs value of 62 s−1 (Figure S2), consistent with a conformational change of a Ca2+-G-CatchER+ complex being rate limiting for the overall process. When Ca2+-loaded G-CatchER+ was mixed with 2.0 mM EGTA, the fluorescence decreased with a kobs value of 63 s−1, indicating rapid release of Ca2+ from the Ca2+-G-CatchER+ complex (Figure S2). For comparison, when G-CEPIA1er was used instead of G-CatchER+, the kobs value for Ca2+ release from the complex was similar (kobs = 81 s−1 versus 63 s−1), but the kobs value for fluorescence increase associated with Ca2+ binding was four times slower (kobs = 15 s−1 versus 62 s−1) (Figures 1F and S2).
Calcium binding increases G-CatchER+’s lifetime leading to enhanced fluorescence
We next applied time-resolved optical methods and hydrogen/deuterium isotope exchange to understand the origin of Ca2+-induced optical property changes of G-CatchER+. Fluorescence decay curves of R∗OH and R∗O− were measured at pH 7.4 (Figure S2). The decay of R∗OH and the directly excited R∗O− form (at 467 nm) could be fitted to a double-exponential that gave rise to average lifetimes of 0.19 ns and 2.61 ns, respectively (Figures 1G and 1H). Upon Ca2+ binding, there was a 30% mean fluorescence lifetime increase of the indirectly excited anionic chromophore. For the indirectly excited (at 372 nm) R∗O− form, a fast quenching component within the first 2 ns was detected, which was followed by a long asymptotic decay closely approaching the lifetime of the directly excited R∗O−. Further lifetime studies in D2O confirmed that such a lifetime increase of G-CatchER+ is a result of delayed proton geminate recombination and is caused by a combination of acid-base equilibrium and arrangement differences of the proton network with the chromophore between Ca2+ free and the Ca2+ binding form (Zhuo et al., 2015). Ca2+ also exhibited a strong inhibition of the excited state proton transfer nonadiabatic geminate recombination in protic (versus deuteric) medium (Figure S2). Such a Ca2+-dependent lifetime change explains the Ca2+-dependent fluorescence increase and indicates a potential future application of G-CatchER+ for lifetime imaging.
Quantitative measurements of drug-induced ER/SR calcium changes in multiple cell types
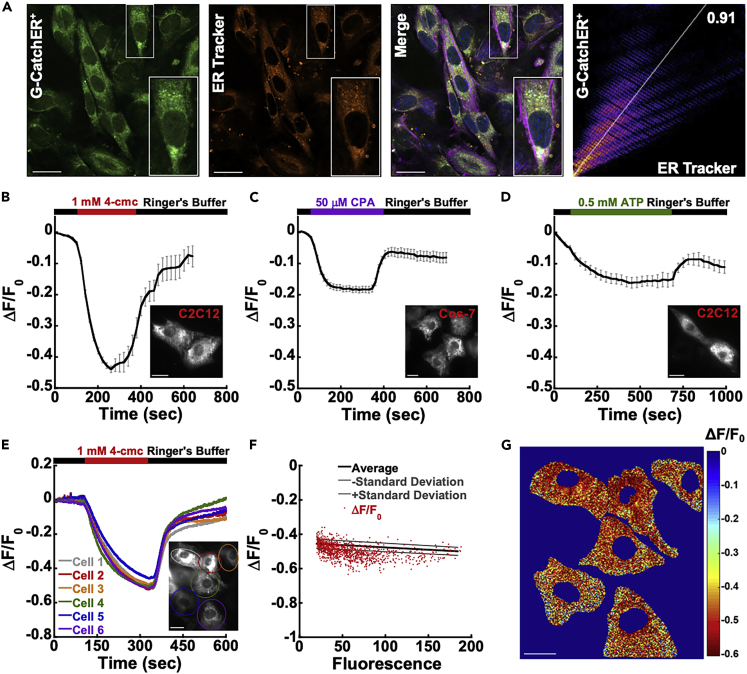
G-CatchER+ was highly enriched in the ER/SR of C2C12 myoblasts (Figure 2A) as demonstrated by labeling of C2C12 cells expressing G-CatchER+ with the ER-specific marker, ER-Tracker red, yielding a Pearson's coefficient of 0.91 (Figure 2A). G-CatchER+ could also quantitatively monitor drug-induced [Ca2+]ER changes in multiple cell types using highly inclined thin illumination (HILO) (Tokunaga et al., 2008) (Figures 2B–2D) and epifluorescence microscopy (Figure S3). Although CatchER+ is likely to have a differential expression in different cells, fluorescence response (changes) in C2C12 cells upon the addition of the RyR agonist in different cells can be accurately determined with normalization (Figure 2E). After normalizing the fluorescence change by dividing the baseline fluorescence before treatment, all cells (n = 32) have a similar degree of release responses (−0.46 ± 0.01) (Figures 2E–2G and Table 1).
Figure 2.
G-CatchER+ ER localization and HILO imaging leads to quantitative measurement of calcium responses
(A) G-CatchER+ expression at 488 nm (green), ER-Tracker red staining at 555 nm (orange), DAPI staining for the nucleus at 405 nm (blue), actin staining with phalloidin at 633 nm for structure (magenta). Insets are zoomed in regions of one cell from the outlined white box. G-CatchER+ expression has a Pearson's coefficient of 0.91 when compared with ER-Tracker red.
(B) 1 mM of 4-cmc was added to initiate a release of Ca2+ from the ER in C2C12 cells. n = 21 cells. F0 is the fluorescence intensity at the initial time point of measurement and ΔF = Ft–F0, where Ft is the fluorescence intensity at time t.
(C) 50 μM of CPA was added to initiate a release of Ca2+ from the ER in Cos-7 cells. n = 30 cells.
(D) 500 μM of ATP was added to initiate a release of Ca2+ from the ER in C2C12 cells. n = 9 cells.
(E) C2C12 cells plotted as normalized ΔF/F0 in response to 1 mM 4-cmc showing overall release of whole cells is similar where each color circle matches each cell in inset.
(F) Pixel by pixel plot of ΔF/F0 response from the six cells with R = 0.42.
(G) ΔF/F0 intensity map of the six cells analyzed in E&F. Scale bars, 20 μm.
See also Figure S3.
Table 1.
Epifluorescence and HILO imaging responses of G-CatchER+ to multiple reagents and cell types
| Cell Type | Drug Type | Epifluorescence Imaging |
HILO Imaging |
||||||
|---|---|---|---|---|---|---|---|---|---|
| [Drug] (μM) | ΔF/F0 | Cell # | Trial # | [Drug] (μM) | ΔF/F0 | Cell # | Trial # | ||
| C2C12 | 4-cmc | 200 | −0.19 ± 0.01 | 43 | 9 | 1000 | −0.46 ± 0.01 | 32 | 11 |
| Cos-7 | 4-cmc | 200 | −0.15 ± 0.08 | 24 | 6 | 500 | −0.12 ± 0.01 | 36 | 7 |
| HEK293 | 4-cmc | 200 | −0.24 ± 0.07 | 27 | 5 | 500 | −0.22 ± 0.02 | 40 | 6 |
| HeLa | 4-cmc | ND | ND | ND | ND | 1000 | −0.24 ± 0.01 | 10 | 2 |
| Hip. Neurons | 4-cmc | ND | ND | ND | ND | 500 | −0.38 ± 0.04 | 9 | 3 |
| C2C12 | CPA | 15 | −0.24 ± 0.02 | 35 | 5 | 50 | −0.26 ± 0.02 | 18 | 7 |
| Cos-7 | CPA | 15 | −0.26 ± 0.02 | 10 | 3 | 50 | −0.17 ± 0.01 | 31 | 6 |
| HEK293 | CPA | 15 | −0.23 ± 0.01 | 32 | 5 | 50 | −0.23 ± 0.01 | 18 | 3 |
| Hip. Neurons | CPA | ND | ND | ND | ND | 50 | −0.10 ± 0.04 | 8 | 3 |
| C2C12 | ATP | 100 | −0.10 ± 0.03 | 26 | 8 | 500 | −0.14 ± 0.01 | 12 | 7 |
| Cos-7 | ATP | 100 | −0.15 ± 0.02 | 24 | 9 | 500 | −0.12 ± 0.02 | 13 | 3 |
| HEK293 | ATP | 100 | −0.10 ± 0.02 | 19 | 4 | 500 | −0.16 ± 0.01 | 24 | 3 |
| HeLa | Histamine | ND | ND | ND | ND | 100 | −0.13 ± 0.01 | 33 | 9 |
| Hip. Neurons | DHPG | ND | ND | ND | ND | 100 | −0.07 ± 0.01 | 8 | 8 |
Data represent mean ± SEM error. ND, no data; ΔF/F0, change in fluorescence signal over initial fluorescence in response to drugs. Data collected at room temperature; Hip. Neurons, primary hippocampal neurons that have been cultured for 13 days in vitro. See also Figures S3 and S7.
G-CatchER+ responses to multiple ER-Ca2+ modulators were assessed using HILO and epifluorescence. C2C12 cells, which express high levels of RyRs, had the strongest peak (−0.46 ± 0.01) following addition of 1 mM 4-cmc (Figure 2B and Table 1). On the other hand, Cos-7 and HEK293 cells had a smaller release following addition of 0.5 mM 4-cmc (−0.12 ± 0.01 and −0.22 ± 0.02, respectively) because higher concentrations of 4-cmc negatively affected these cell types (Table 1). In addition, HeLa cells treated with 1 mM 4-cmc still had a markedly lower response (−0.24 ± 0.01) (Table 1). Intriguingly, 0.5 mM ATP treatment to activate P2YR and subsequent ER Ca2+ release via the IP3 receptor in all three cell types (C2C12, Cos-7, and HEK293 cells) resulted in a smaller amplitude change than 4-cmc but was in a similar range to each other (Figure 2D and Table 1). We also observed similar changes (−0.13 ± 0.01) in HeLa cells following treatment of histamine, which activates the histamine receptor (H1R) to trigger PLC-mediated ER Ca2+ release (Table 1). Similarly, 50 μM treatment of the SERCA pump inhibitor CPA, which prevents the refilling of ER Ca2+ stores until washout, led to a reduction in ER Ca2+ levels in these three cell types with more variable amplitude peak responses in comparison to ATP (Table 1). The vehicle control for CPA, DMSO, showed a subtle yet insignificant effect in C2C12 cells (Figure S3J). We observed consistent ER Ca2+ release using epifluorescence microscopy and further monitored changes in other compounds, thapsigargin and caffeine, that cause extrusion of Ca2+ from the ER using G-CatchER+ (Figure S3 and Table 1).
G-CatchER+ captures spatially confined ER calcium dynamics in neurons
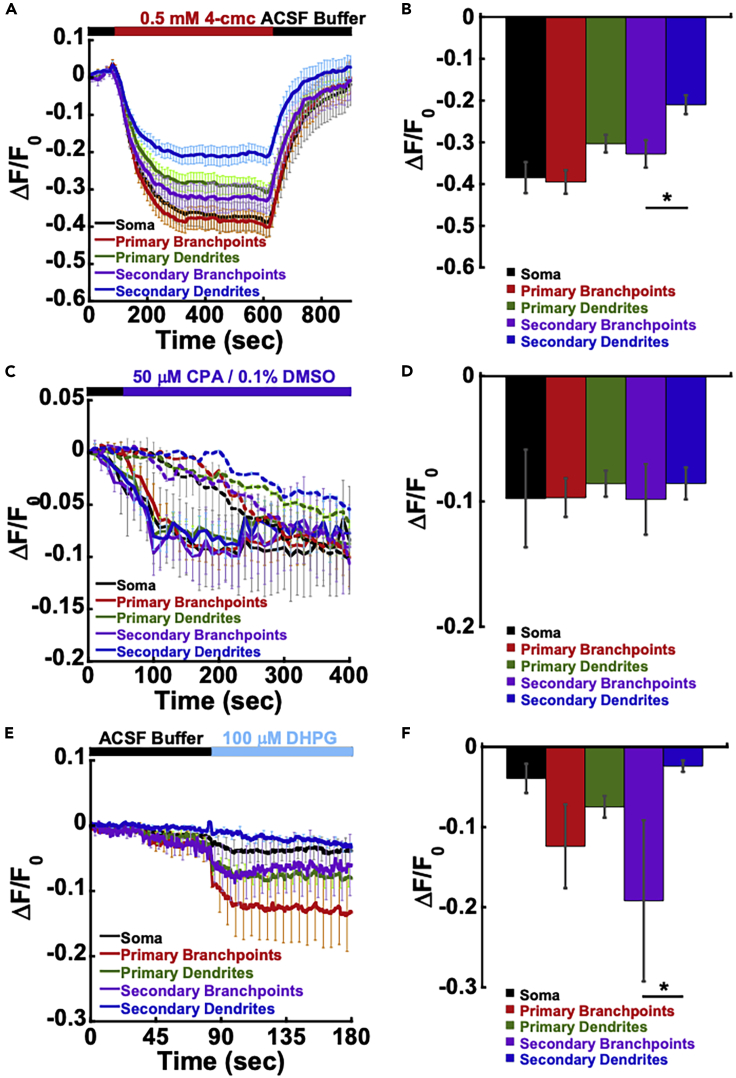
We next determined if G-CatchER+ could report accurate changes in ER Ca2+ levels in neurons, which have elaborate and complex ER morphologies (Terasaki et al., 1994; Spacek and Harris, 1997). G-CatchER+ was expressed in primary hippocampal neurons with no overt effects on overall health (Figure S4). Similar to immortalized cells, G-CatchER+ highly colocalized with ER markers (data not shown). With the addition of 0.5 mM 4-cmc, we identified a significant difference in the G-CatchER+ response between secondary branchpoints and secondary dendrites (Figures 3A and 3B). Treatment with 50 μM CPA resulted in a smaller change in amplitude with no significant differences between different neuronal regions (Figures 3C and 3D). Subcellular effects with refilling were also less apparent with CPA, which was in part contributed by a DMSO effect (Figures 3C and 3D). We determined the maximum fluorescence of G-CatchER+ in different regions of neurons by applying 50 μM of ionomycin and 10 mM Ca2+ (ΔF/F; soma: 0.58 ± 0.13; primary dendrites: 0.35 ± 0.05; primary branchpoints: 0.56 ± 0.13; secondary dendrites: 0.21 ± 0.04; secondary branchpoints: 0.42 ± 0.10; p = 0.06) (Figure S4). Basal [Ca2+]ER in different neuronal regions was calculated using established methods (soma: 167.8 ± 45.5 μM; primary dendrites: 308.2 ± 56.9 μM; primary branchpoints: 192.3 ± 45.7 μM; secondary dendrites: 458.8 ± 88.5 μM; secondary branchpoints: 253.0 ± 42.9 μM) (Figure S4E) (de Juan-Sanz et al., 2017). We found that the estimated ER Ca2+ concentration in secondary dendrites was significantly higher than the soma and primary branchpoints (p = 0.02), indicating that ER Ca2+ concentrations vary in hippocampal neuronal regions.
Figure 3.
G-CatchER+ response in hippocampal neurons
(A) 0.5 mM of 4-cmc was added to initiate a RyR-dependent release of Ca2+ from the ER in mouse primary hippocampal neurons.
(B) Corresponding bar graph of 4-cmc activated Ca2+ release (in ΔF/F0) from the ER grouped by neuron regions. Error bars are ±SEM, ∗p = 0.05, one-way ANOVA, Tukey's multiple comparisons.
(C) Inhibition of SERCA with 50 μM of CPA initiated a release of Ca2+ from the ER.
(D) Corresponding bar graph of CPA inhibited Ca2+ release (in ΔF/F0) from the ER grouped by neuron regions. Error bars are ±SEM, one-way ANOVA, Tukey's multiple comparisons.
(E) 100 μM of DHPG was added to initiate the release of Ca2+ from the ER via mGluR1/5 activation in hippocampal neurons.
(F) Corresponding bar graph of DHPG-induced Ca2+ release (in ΔF/F0) from the ER grouped by neuron regions. Error bars are ±SEM, ∗p = 0.02, one-way ANOVA, Tukey's multiple comparisons.
See also Figure S4.
The concerted actions of spatially confined Ca2+ flux provide the appropriate threshold response for various forms of synaptic plasticity (Citri and Malenka, 2008). Long-term depression (LTD) via activation of group I metabotropic glutamate receptors (mGluR-LTD) is a type of synaptic plasticity that results in IP3-mediated release of ER Ca2+ stores and is associated with an increase in the removal of Ca2+ permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Abe et al., 1992; Aramori and Nakanishi, 1992; Palmer et al., 1997; Snyder et al., 2001). Addition of the group I mGluR agonist DHPG led to a release of ER Ca2+, with a peak amplitude of −0.07 ± 0.01 (Figure 3E and Table 1). Intriguingly, similar to 4-cmc, we also observed a significant difference in the G-CatchER+ response between secondary branchpoints and secondary dendrites (−0.19 ± 0.1 versus −0.02 ± 0.01) (Figure 3F). Taken together, these findings suggest a possible selective barrier or filtering mechanism of RyR- and mGluR-dependent ER Ca2+ release in distal dendrites of hippocampal neurons.
G-CatchER+ reports minute regional calcium-mediated events
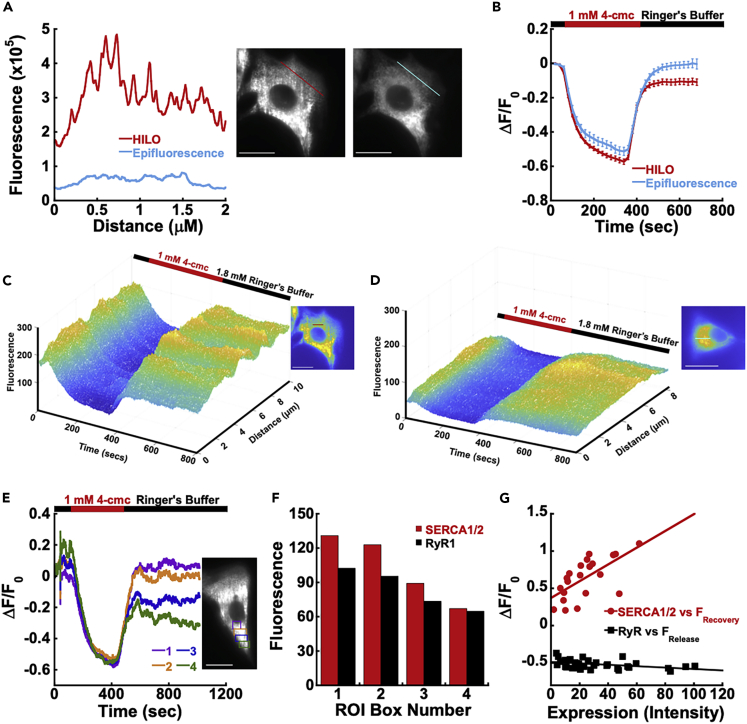
Next, we determined if G-CatchER+ could detect regional subcellular changes in Ca2+ signaling in the cell throughout the ER/SR network. The improved imaging resolution in HILO microscopy allows us to monitor the local Ca2+ responses at specific regions of interest (ROIs) throughout the entire ER/SR network. Indeed, cross-section analysis of the same region in the same cell (Figures 4A, 4C, and 4D) uncovers larger heterogeneities and variations through the ER/SR network in HILO imaging compared with that in epifluorescence imaging. When further examining two individual C2C12 cells for whole cell responses versus individual ROIs throughout the cell, we observed a variance in responses (Figure S6). When the ROIs for these two cells under HILO imaging were examined, there was a release variability of ±9.6% and a recovery variability of ±18.9% in comparison to the whole cell (Figure S6). Even when the data were normalized to initiation (t = 0 s) or to release (t = 300 s) there was still a large variance for the recovery at ±12.5% and ±13.7%, respectively.
Figure 4.
G-CatchER+ in combination with HILO reports regional calcium-mediated events
(A) Cross-section along the same region of the cell under epifluorescence (blue) or HILO imaging (red).
(B) Average response to 1 mM 4-cmc over the same time points using HILO (red, n = 13) and epifluorescence (blue, n = 10) imaging.
(C and D) Cross section of C2C12 cells in response to 1 mM 4-cmc using HILO (C) and epifluorescence (D) imaging techniques.
(E) Release from the RyR in C2C12 cells in response to 1 mM 4-cmc in regions of interest covering the perinuclear ER (1, purple), proximal to the perinuclear ER (2, orange), proximal ER (3, blue), and distal ER (4, green).
(F) The expression of SERCA1/2 (red) and RyR1 (black) in the regions from (E).
(G) Scatterplot of SERCA1/2 (R = 0.66, n = 7) and RyR1 (R = 0.42, n = 11) expression in the perinuclear ER, proximal ER, and distal ER in each cell as a function of ΔF/F recovery and release values, respectively, in each of these regions. Scale bars, 20 μm.
The observed heterogeneous Ca2+ release and recovery responses at different ROIs of cells by HILO microscopy prompted us to seek the origin of these “hotspots” of Ca2+ and receptor expression potentially causing this variability. It has long been speculated that “hotspots” or localized signaling regions, in the SR of skeletal muscle cells, such as C2C12 cells, are created by differential high expression of channel proteins with the capability to create Ca2+ concentration differences along the junctional SR, which communicate with the plasma membrane for E-C coupling (Tang et al., 2011; Launikonis et al., 2005; Rudolf et al., 2006).
To confirm whether these differences are influenced by differential ER protein distribution differences, we mapped the cellular distribution of key ER channel proteins, RyR and SERCA, using immunocytochemistry and high-resolution confocal microscopy. C2C12 cells transfected with G-CatchER+ were labeled with RyR1 or SERCA1 and SERCA2 together (SERCA1/2) to examine the subcellular distribution of these key ER regulators. We observed the differential expression of RyR1 in C2C12 cells that contained regions of higher expression throughout the cell with some cells having more uniform distribution and others having pockets of enhanced RyR expression (Figures S5A and S5B), whereas the distribution of SERCA1/2 were found to be highly enriched in and around the ER perinuclear region and diminishing toward the distal ER (Figures S5C and S5D).
To unambiguously verify whether reported minute Ca2+ dynamics is dictated by key ER-associated receptor expression, we developed a methodology that allowed us to co-register minute local Ca2+ dynamics by G-CatchER+ with regional ER protein expression. C2C12 cells were treated with 1 mM 4-cmc to mimic a full release of Ca2+ from the ER. We did not observe a significant correlation (R = 0.42) between RyR1 expression and the ΔF/F0 release (300–425 s) (Figure 4G), as the response plateaued throughout the cell (Figure 2F). In contrast, we observed a linear correlation (R = 0.66) of minute Ca2+ refilling dynamics with SERCA1/2 expression (Figure 4G). The expression levels of SERCA1/2 are directly related to the recovery capability of the ER in C2C12 cells. ROIs 1 (perinuclear ER) and 2 (proximal ER) have a full recovery (ΔF/F0 recovery (600–625 s)) and higher SERCA1/2 expression (Figure 4G). On the other hand, ROIs 3 and 4 (distal ER) have incomplete recovery that corelates with low expression of SERCA1/2 (Figures 4E and 4F). Taken together, the detection of minute ER Ca2+ dynamics dictated by localized key ER-expressed receptors is likely to play a major role in the regulation of Ca2+ dynamics. Targeting of G-CatchER+ to regions that exhibit expression differences most likely results in response differences of key ER proteins and Ca2+ microdomains.
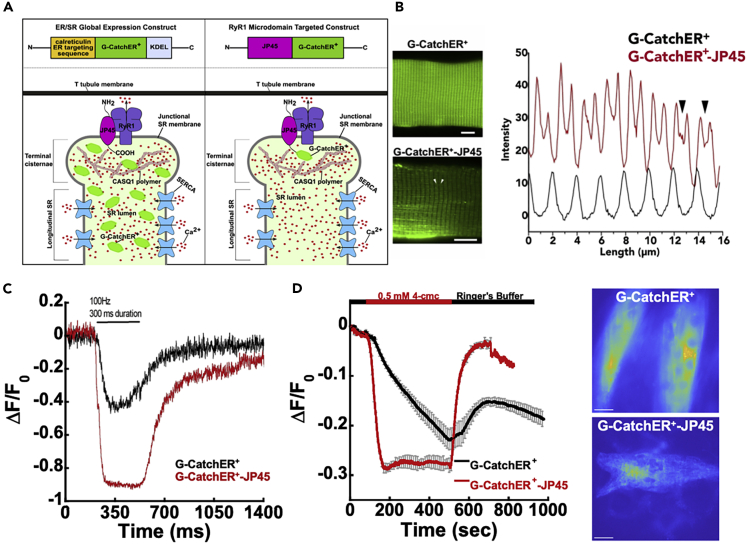
JP45-tethered G-CatchER+ reports local calcium release
The existence of local Ca2+ microdomains in proximity to the RyR and its contributions to E-C coupling have remained controversial (Sztretye et al., 2011a, 2011b). When we examined the RyR distribution in C2C12 cells exhibiting differential formation of the myotubule (≥3 distinct nuclei) as well as myoblasts (1 nuclei), we found that RyR predominantly localized in myotubules, clustering on the edges of the ER (orange) (Figure S5E). Consistently, myotubule-localized RyR was significantly higher than single nuclei myoblasts by 2.8-fold, and the mature tubule was 1.9-fold higher than the forming tubule (three indistinct nuclei) (Figure S5F). JP45 is an integral protein constituent of the skeletal muscle sarcoplasmic reticulum junctional face membrane. The C terminus of JP45 is localized in the junctional SR lumen and interacts with calsequestrin (CASQ), whereas its 130-amino acid residue-long cytoplasmic domain interacts with the α-interacting domain within the loop connecting repeat I and repeat II of Cav1.1 (Anderson et al., 2003, 2006; Mosca et al., 2013; Bayley et al., 1984). To investigate the signaling dynamics in these enriched RyR regions, we fused G-CatchER+ to the C terminus of JP45 to obtain the probe G-CatchER+-JP45 (Figure 5). Because of its sublocalization, G-CatchER+-JP45 can detect Ca2+ signals occurring in a domain adjacent to the junctional SR membrane encompassing the RyR calcium release channel. Wild-type mice were electroporated with G-CatchER+-JP45 and G-CatchER+. G-CatchER+-JP45 localized to the luminal side of junctional face membrane of the terminal cisternae. In contrast, G-CatchER+ localized throughout the whole lumen of the SR (Figures 5B and 5C). FDB expressing CatchER+-JP45 and G-CatchER+ were isolated and stimulated with a 100 Hz electrical pulse for 300 ms, and the resulting Ca2+ transients were recorded (Figure 5C). Fibers expressing G-CatchER+-JP45 (red trace) exhibited a more rapid and larger decrease in fluorescence compared with FDB fibers expressing G-CatchER+ (black trace) (Figure 5C), indicating that in the area adjacent to the lumenal domain of the RyR1, the kinetics and size of the Ca2+ transient are larger compared with those occurring in the whole SR. The fall time (90%–10%) of the transients for G-CatchER+ and G-CatchER+-JP45 are 181.8 ± 66.3 ms and 144.3 ± 81.7 ms, respectively. The peak amplitude ΔF/F0 for G-CatchER+ and G-CatchER+-JP45 are −0.49 ± 0.19 (n = 10 fibers from three mice) and −0.79 ± 0.18∗ (n = 16 fibers from three mice ∗p < 0.05, Mann-Whitney test).
Figure 5.
Monitoring global and microdomain changes in SR Ca2+ with globally expressed and targeted G-CatchER+
(A) Representative plasmid construction and expression of globally expressed G-CatchER+ and targeted G-CatchER+-JP45 in the skeletal muscle SR. (Left panel) Global expression of G-CatchER+. G-CatchER+ contains the calreticulin signal peptide at the N terminus and the KDEL ER/SR retention sequence at the C-terminus. Resulting expression of the sensor follows a uniform distribution pattern throughout the SR monitoring global Ca2+. (Right panel) RyR1 microdomain targeted G-CatchER+ with JP45. G-CatchER+ resides at the C-terminus of JP45. Resulting expression of the sensor positions it near the lumenal opening of RyR1 in proximity to CASQ1 polymers that establish a large proximate Ca2+ pool in the TC necessary for E-C coupling.
(B) Wild-type FDB fibers were transfected with G-CatchER+ and G-CatchER+-JP45. Images reveal differential expression patterns for the targeted and un-targeted probe. Intensity changes from G-CatchER+ and G-CatchER+-JP45 fibers plotted against fiber length. Arrows correspond to arrows on G-CatchER+-JP45. A clear distinction is seen in the localization of G-CatchER+ and G-CatchER+-JP45.
(C) Ca2+ transients in FDB fibers were recorded upon stimulation with a 100-Hz electrical pulse for 300 ms. Black trace is fluorescence intensity monitored with G-CatchER+ expressed globally, and the red trace is G-CatchER+-JP45 located at the lumenal side of the junctional face membrane of the terminal cisternae.
(D) HILO imaging in C2C12 myotubule cells for G-CatchER+ (black, n = 6) and G-CatchER+-JP45 (red, n = 3) in response to 500-μM 4-cmc with representative cells for imaging. Scale bars, 10 μm in panel (B) and 20 μm in panel (D).
See also Figure S5.
Similar results were obtained when immature fibers in the form of C2C12 myotubules were stimulated with 0.5 mM 4-cmc to directly activate Ca2+ release via RyR (Figure 5D). G-CatchER+-JP45 exhibited a significantly large peak amplitude (−0.28 ± 0.01) and had a fast (88 s) Ca2+ release compared with those detected with G-CatchER+ (−0.23 ± 0.02 and 392 s, respectively). The rise time and fall time of G-CatchER+-JP45 (88 and 105 s) was 4.5-fold faster than G-CatchER+.
Transgenic G-CatchER+in vivo analyses reveal cold-evoked Ca2+ release dynamics
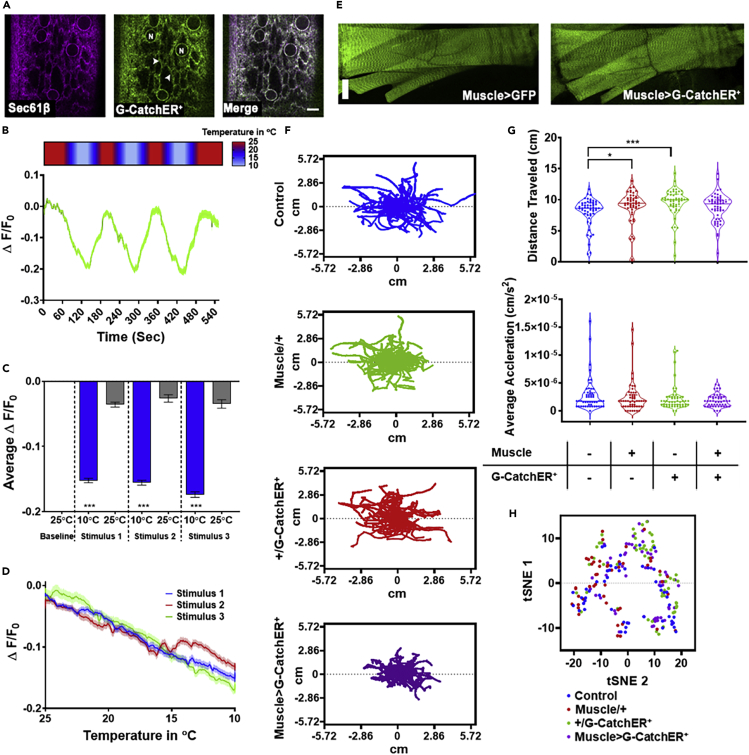
To demonstrate the utility of G-CatchER+ for in vivo imaging of ER/SR Ca2+ dynamics, we generated genetically encoded, inducible UAS-G-CatchER+ transgenic strains in Drosophila melanogaster. As proof-of-principle, we investigated in vivo G-CatchER+ sensor expression and dynamics in third instar larval muscles using Mef2GAL4 to drive pan-muscle expression of UAS-G-CatchER+. Contraction of striated skeletal muscle is initiated by a stimulus-evoked depolarization-induced influx of Ca2+ ions into muscle fibers. This influx of Ca2+ triggers a Ca2+-induced Ca2+ release (CICR) mechanism that is critical to muscle contraction and involves Ca2+ release from intracellular stores (e.g. ER/SR) via the action of RyR intracellular Ca2+ channels (Rios, 2018). Insect studies have demonstrated that exposure to cold stimuli leads to muscle depolarization and under extended exposure, paralysis in adults referred to as chill coma (Macmillan and Sinclair, 2011; MacMillan et al., 2014). In Drosophila larvae, acute exposure to noxious cold stimuli elicits a nocifensive full-body bilateral coordinated contraction behavior along the anteroposterior axis (Turner et al., 2016). This behavior is dependent upon neural activity of class III multi-dendritic somatosensory neurons that function as peripheral cold nociceptors (Turner et al., 2016); however it is unknown if cold-evoked larval contraction behavior correlates with Ca2+ release dynamics from intracellular stores in larval muscle. We hypothesized that cold exposure would elicit temperature-dependent CICR from ER/SR intracellular muscle stores. To address this hypothesis, we performed a series of in vivo imaging and behavioral studies using live, intact third instar Drosophila larvae expressing G-CatchER+.
To confirm that G-CatchER+ is appropriately targeted to ER/SR in vivo, we co-expressed G-CatchER+ and the ER Sec61β translocon complex protein tagged with tdTomato in larval muscles. Consistent with C2C12 cells (Figure 2A), we observed tight co-localization of G-CatchER+ with Sec61β in perinuclear regions and reticular networks of Drosophila larval muscles (Figure 6A). We next performed in vivo imaging of cold-evoked Ca2+ dynamics from intracellular ER/SR stores by expressing G-CatchER+ in Drosophila larval muscles. Drosophila larvae were subjected to repeated cold temperature ramps from 25°C down to 10°C (noxious) and back to 25°C coupled with live imaging of G-CatchER+ fluorescence changes in muscle. Cold stimulation evoked robust Ca2+ release as measured by a significant reduction of ~0.15 ΔF/F0 at 10°C stimulus relative to baseline 25°C and recovery to 25°C. ER/SR Ca2+ levels recovered consistently with repeated cold 10°C stimulus to ~0.035 ΔF/F0 (Figures 6B and 6C), suggesting that sensor integrity is not notably impacted by the acute cold stimulation regimen. Moreover, analyses of G-CatchER+ sensor dynamics across the full temperature ramp revealed temperature-dependent increases in Ca2+ release with the greatest change in fluorescence occurring at the lowest temperature (10°C) (Figure 6D). Furthermore, as G-CatchER+ muscle expression is initiated by Mef2GAL4 starting from the mid-embryonic stage of development (~10 h after egg laying) and continues throughout larval development up to the end of the third instar stage (~120 h after egg laying), the observed cold-evoked Ca2+ release suggests long-term G-CatchER+ expression does not interfere with detection of stimulus-generated intracellular Ca2+ store dynamics.
Figure 6.
In vivo G-CatchER+ validation in Drosophila melanogaster larval muscles
(A) In vivo validation of transgenic G-CatchER+ expression in muscle ER/SR. The ER translocon complex protein Sec61β tagged with tdTomato (left, magenta) and G-CatchER+ (middle, green) exhibit tight co-localization in larval muscle characterized by perinuclear (nucleus marked by “N”) and reticular network subcellular distributions (arrowheads). Genotype: Mef2>G-CatchER+, Sec61β::tdTomato. Scale bar, 10μm. Representative image from N = 10 larvae.
(B and C) Assessing cold-evoked ER/SR Ca2+ dynamics in muscle using G-CatchER+. Heatmap (B, top) shows stimulus temperature, where warmer temperatures are in red and cooler temperatures are in light blue. (B, bottom) Ca2+ levels reduce in response to cold stimulus, where percent change in fluorescence of G-CatchER+ plotted against time in seconds. Stimulus regimen details in the Methods. N = 20 animals. Error bars ±SEM. (C) Average percent change in fluorescence of G-CatchER+ at 25°C and 10°C, where there is a significant decrease in G-CatchER+ fluorescence at 10°C when compared with 25°C. Genotype: Mef2>G-CatchER+. N = 20 animals. Error bars ±SEM, ∗∗∗p < 0.0001. Kruskal-Wallis test.
(D) Analyses of G-CatchER+ sensor dynamics reveal temperature-dependent increases in Ca2+ release across the three cold ramp stimulations with the greatest change in fluorescence at the lowest temperature (10°C).
(E) Expression of G-CatchER+ does not grossly alter muscle morphology. (Left) Muscle cells expressing GFP and (right) muscle cells expressing G-CatchER+. Genotype: Mef2>GFP and Mef2>G-CatchER+. Scale bar, 50 μm. Representative image from N = 10 larvae.
(F–H) Drosophila larval locomotion assay to assess potential impacts of extended G-CatchER+ expression on larval crawling behavior. (F) Larval locomotion tracks for individual animals during 1-min assay across various genotypes. (G, top) Distance traveled in cm and (G, bottom) average acceleration in cm/s2. N = 48–52 animals; ∗p < 0.05, ∗∗∗p < 0.001. Kruskal-Wallis test. (H) t-SNE analysis of multiple metrics including average distance traveled, average velocity, average acceleration, average bending, and total moving time. Iterations = 1000, perplexity = 20. (F–H) Genotype: Control (w1118), Muscle/+ (Mef2-GAL4 (x) w1118), +/G-CatchER+ (UAS-G-CatchER+ (x) w1118), and Muscle > G-CatchER+ (Mef2-GAL4 (x) UAS-G-CatchER+). N = 48–52 animals.
To further investigate potential cytotoxic effects of extended G-CatchER+ expression on overall muscle architecture or tissue function, we performed morphological imaging and behavioral assays. We detected no gross morphological defects in Drosophila larval muscles expressing G-CatchER+ relative to muscles expressing membrane-tagged GFP (Figure 6E). We then performed larval locomotion assays to assess whether extended muscle expression of G-CatchER+ alters larval peristaltic crawling behavior that is dependent upon waves of muscle contraction. In general, we found no significant impairments in locomotion for G-CatchER+ expressing larvae relative to wild-type and genetic background control larvae (Figures 6F–6H). No significant difference in average distance traveled or average acceleration were discovered for larvae expressing G-CatchER+ compared with relevant controls (Figure 6G). Lastly, t-distributed stochastic neighbor embedding (tSNE) analysis of multiple locomotion assay variables including average distance traveled, average velocity, average acceleration, average bending, and total moving time did not reveal any distinct clusters representing any specific genotype (Figure 6H). Collectively, these in vivo studies reveal proper targeting of transgenically expressed G-CatchER+ and identify cold-evoked ER/SR Ca2+ dynamics in muscle without gross impairments in muscle architecture or function based upon extended expression.
Discussion
Several genetically encoded Ca2+ probes have been created to monitor changes in ER/SR Ca2+ including the Cameleon, D1ER (Palmer et al., 2004), red genetically encoded indicators for optical imaging (GECOs) such as the low-affinity sensors LAR-GECO1 (Suzuki et al., 2014b), G-CEPIA1er based on cfGCaMP2 (Suzuki et al., 2014b), GCaMP6-150 (de Juan-Sanz et al., 2017), and GCaMP-ER2 (Sun et al., 2019). Many of these sensors rely on CaM that contains multiple Ca2+ binding sites to sense Ca2+, requiring a subsequent conformational change upon binding to the CaM targeting peptide such as M13. Conceptually differing from all other reported strategies, we used our innovative platform to develop next-generation classes of Ca2+ sensors by designing a Ca2+ binding site in the enhanced green fluorescent protein (EGFP) and targeting it to the ER (Tang et al., 2011). Here, our newly developed ER Ca2+ probe G-CatchER+ exhibits improved folding and fluorescence at 37°C, which is advantageous to mammalian cells and for use in vivo. The introduction of mutations also increases the Kd from 0.3 mM to 1.1 mM. Indeed, G-CatchER+ also exhibits a much greater on-rate compared with the Ca2+ sensor G-CEPIA1er (Figures 1F and S2) and reports linear Ca2+ responses to allow a quantitative estimation of ER Ca2+ and dynamic changes without obscurity/limitations due to cooperativity among coupled Ca2+ binding sites in most reported GECIs. Using both epifluorescence and HILO microscopy, G-CatchER+ quantitatively reports both channel- and receptor-mediated ER/SR Ca2+ transients in different cell lines and primary cells following addition of stimulatory or inhibitory agents (Figure S7). Interestingly, large variations in amplitude of 4-cmc triggered Ca2+ release via RyR channels in different cell types, consistent to the differential expression of RyR. On the other hand, the IP3R-mediated Ca2+ release have equivalent amplitudes, which is independent of cell type. Blocking ER Ca2+ reuptake by inhibiting SERCAs also has a small variation in the cell types analyzed (Table 1).
Using HILO microscopy to image Ca2+ release and recovery from small ROIs, we have revealed the 3D heterogeneous distribution of minute ER Ca2+ release and refilling dynamics in C2C12 cells. Such heterogeneous release and recovery are in agreement with the expression level of RyR and SERCA1/2 concentrations (Figures 4G and S5). With simultaneous HILO imaging and co-staining of the same regions of these key proteins, we conclude that the heterogeneous distribution of ER Ca2+ release and refilling originates from the hetero-positioning and clustering of these key channel/pump proteins to form “hotspots” of Ca2+ dynamics. A localized ER Ca2+ microdomain related to stromal interaction molecule 1 (STIM1) distribution was reported using a G-CEPIA1 with SNAPER tag (Luo et al., 2019). However, the expression of RyR, IP3R, and SERCA and local Ca2+ kinetics were not reported.
To further map the “hotspots” Ca2+ dynamics, we created a G-CatchER+-JP45 Ca2+ sensor to localize it to the junctional region close to RyR1 at the lumenal site. Through analysis of the Ca2+ release kinetics visualized by locally expressed G-CatchER+-JP45 compared with globally expressed G-CatchER+ in the SR/ER of FDB muscle fibers upon electrostimulation, we demonstrate the local Ca2+ release at the membrane compartment encompassing RyR1 channel is 2.1-fold greater than global SR/ER release with much faster kinetics (Figure 5C). Such rapid Ca2+ release dynamics likely results from calsequestrin depolymerization caused by the drop of the lumenal SR Ca2+ concentration (Manno et al., 2017; Park et al., 2004). Sztretye et al. (2011b) reported a Ca2+ sensor D4cpv-Casq1 by fusing cameleon D4cpv with the cDNA of calsequestrin 1 or a variant that binds less Ca2+. Our created sensor G-CatchER+-JP45 eliminates the potential alteration of the ER Ca2+ dynamics due to expression of the Ca2+ buffering protein calsequestrin with multiple Ca2+ binding sites in the targeting sequence. Thus, it does allow unambiguous determination of the intrinsic Ca2+ kinetics and ER concentration that are controlled by calsequestrin.
Our direct visualization of Ca2+ hotspots and local microdomains close to the RyR without introducing Ca2+ binding proteins in a GECI has several important implications. First, the molecular mechanism of Ca2+ signaling is dependent on the isoforms of RyRs and SERCAs along with their cellular distributions. RyR1 is expressed in skeletal muscle and is enriched in the cerebellum, hippocampus, extraocular muscle (EOM), and diaphragm (Martin et al., 1998; Sonnleitner et al., 1998; Sekulic-Jablanovic et al., 2015). RyR2 is expressed in cardiac muscle, cerebellum, and hippocampus, as well as in human myometrial tissue (Martin et al., 1998; Marx et al., 2000; Awad et al., 1997). RyR3 is expressed more broadly than RyR1 and RyR2, having been found in such regions as liver, kidney, brain, placenta, and skeletal muscle. RyR3 in the brain is found to be most highly expressed in regions such as the hippocampus, cerebellum, caudate nucleus, and amygdala, with lower expression in the thalamus, corpus callosum, and substantia nigra (Nakashima et al., 1997; Martin et al., 1998). SERCA pumps are expressed from three main genes encoding SERCA type 1–3, with SERCA1 mainly expressed in skeletal muscle and fast twitch muscle fibers and SERCA2 expressed in all tissue types, whereas SERCA3 is found mainly in non-muscle cells with minor expression in muscle cells (Hovnanian, 2007; Chemaly et al., 2018). Our developed calcium sensor will allow us to specifically probe the roles of differential subcellular Ca2+ dynamics in the regulation of rapid biological and pathological processes. Second, tethering our developed sensor to the ER local microdomains, which is proximal to the channel proteins, will enable us to understand molecular basis of diseases associated with ER dysfunction. Malignant hyperthermia (MH), central core disease of muscle (CCD), multiminicore disease with external ophthalmoplegia (MMDO), and catecholaminergic polymorphic ventricular tachycardia (CPVT) have all been found to be related to RyR mutation and dysfunction leading to a loss in muscular and respiratory function (Kushnir et al., 2018; Santulli et al., 2018). Both SERCA1 and 2 have disease mutations that cause Brody myopathy (BRM), acrokeratosis verruciformis (AKV), and Darier disease (DD), disorders affecting the skin and causing lesions (Hovnanian, 2007; Chemaly et al., 2018).
We found that G-CatchER+ was effective in measuring ER-Ca2+ dynamics in primary hippocampal neurons. It is well established that the neuronal ER has a heterogeneous distribution, with its morphology becoming more complex at dendritic branchpoints (Cui-Wang et al., 2012; Spacek and Harris, 1997) that varies along the dendritic arbor (Cui-Wang et al., 2012; Terasaki et al., 1994). The presence of ribosome studded ER in distal dendrites to allow for local protein synthesis suggests that ER-Ca2+ dynamics might be differentially regulated in these regions (Krijnse-Locker et al., 1995). Based on the data from our Fmax experiments (Figure S4E), we calculated a significant difference in concentrations of basal [Ca2+] in select neuronal regions. Interestingly, treatment of neurons with the RyR agonist 4-cmc and the group I mGluR agonist DHPG yielded a significant decrease in ER Ca2 release between secondary branchpoints and secondary dendrites. We did not observe this effect upon treatment with CPA. The causes of the differential basal [Ca2+] in neuronal subregions and whether or not these also drive the heterogeneous Ca2+ release observed with RyR and DHPG treatment warrant future investigation. Stimulation of neurons with DHPG is known to modify ER morphology, altering the mobility of ER export and cargo (Cui-Wang et al., 2012; Aridor et al., 2004). Moreover, differential expression and neurotransmitter sensitivities of gamma aminobutyric acid (GABA) and AMPA receptors have been observed in hippocampal CA1 distal dendrites (Andrasfalvy and Magee, 2001; Pettit and Augustine, 2000), which appears to correlate with our observed differential ER-Ca2+ dynamics. More importantly, the distribution of key ER receptors themselves is not uniform in neurons (Blaustein and Golovina, 2001). RyRs are broadly distributed in apical dendrites, dendritic shafts, and dendritic spines (Walton et al., 1991; Segal and Korkotian, 2014), and IP3Rs are localized in cell bodies, dendritic shafts, and proximal dendrites (Sharp et al., 1993). Our observation of a decrease in ER-Ca2+ responses in distal dendrites following DHPG is in agreement with the reported distribution pattern of the IP3R. However, the significance of a RyR-mediated decrease in ER Ca2+ release in secondary branchpoints remains unknown. It is possible that it may be affected by the RyR isoforms that are expressed in hippocampal neurons (Sharp et al., 1993; Vlachos et al., 2009; Galeotti et al., 2008). Future experiments would be focused on determining the spatial relationships between mGluRs, RyR isoforms, and key ER-associated proteins within select neuronal regions to establish correlations with the differential spatial ER-Ca2+ dynamics that we observed.
We found that transgenically expressed G-CatchER+ reports temperature-dependent ER/SR Ca2+ release/refilling in Drosophila larval muscles in vivo. Acute exposure of Drosophila larvae to noxious cold stimuli is known to elicit a nocifensive full-body contraction behavior (Turner et al., 2016); however, prior to this study it was unknown how cold stimuli influence Ca2+ dynamics from intracellular stores in muscle to promote contractile function. We observed that cold stimulation induced significant reductions in G-CatchER+ fluorescence, which recovers to near baseline levels upon cycling back to room temperature. Although future studies will be necessary to systematically interrogate the molecular mechanisms regulating cold-evoked ER/SR Ca2+ dynamics, these studies provide proof-of-principle evidence for the utility of G-CatchER+ as an in vivo sensor of local dynamic Ca2+ transients and suggest that noxious cold-evoked larval contraction behavior may be dependent upon CICR mechanisms operating in muscle that regulate contractile function. Furthermore, we document that extended in vivo expression of G-CatchER+ does not appear to impair stimulus-evoked Ca2+ dynamics nor impacts gross tissue morphology or function. Based on the vast array of tissue- and cell-type-specific GAL4 driver transgenic strains already available, our newly reported UAS-G-CatchER+ transgenic strains can be used to study local ER/SR Ca2+ dynamics in a broad range of cellular and tissue contexts. For example, G-CatchER+ transgenic expression could be combined with cell- or tissue-type-specific genetic disruption of putative regulators of processes such as CICR for molecular dissection. Similarly, G-CatchER+ expression could be multiplexed with red fluorescent GECIs such as R-GECO or RCaMP at cell- or tissue-type specific levels to enable simultaneous high-resolution visualization of global cytosolic and subcellular compartment (e.g. ER/SR) Ca2+ dynamics, which could provide critical insights into spatially and/or temporally resolved Ca2+ signaling events.
Importantly, ER-mediated Ca2+ release is altered in multiple neurological disease states (Glaser et al., 2019). The IP3R has three isoforms, IP3R type 1–3 (Mikoshiba, 2015; Ivanova et al., 2014; Tada et al., 2016). Differential mutations in IP3Rs cause multiple neurological disorders, which include spinocerebellar ataxia 15 (SCA15), spinocerebellar ataxia 29 (SCA29) (Mikoshiba, 2015; Ivanova et al., 2014; Tada et al., 2016), and Gillespie syndrome (Gerber et al., 2016; McEntagart et al., 2016). ER-mediated Ca2+ dysfunction has also been linked to many neurodegenerative disorders, which include Alzheimer disease (AD), Huntington disease (HD), and Parkinson disease (PD) (Bezprozvanny and Hayden, 2004; Cali et al., 2014; Magi et al., 2016; Glaser et al., 2019). The sensitivity of G-CatchER+ will give researchers the ability to monitor minute changes in ER-Ca2+ flux in disease processes both in vitro and in vivo and will enhance our understanding of the role of regional dysfunctional ER-Ca2+ in neurological disease states.
Limitations of the study
Although single fluorophore fluorescence-based calcium sensors have many reported advantages over synthetic calcium dyes and FRET-based probes, there are inherent limitations that exist within this sensor that include pH sensitivity and photobleaching. This may be a limitation with G-CatchER+. Future work will focus on mitigating these technical limitations. Moreover, our studies did not use this sensor in a disease model of ER dysfunction. Future work will focus on applications of this newly developed calcium probe in uncovering the mechanism of ER dysfunction related to diseases such as central core disease, multiminicore disease, and tubular aggregate myopathy.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Jenny J. Yang (jenny@gsu.edu).
Materials availability
All plasmids used in this study are available upon reasonable requests from the lead contact.
Data and code availability
All data generated or analyzed during this study will be available from the lead contact upon request.
Methods
All methods can be found in the accompanying Transparent methods supplemental file.
Acknowledgments
We thank Michael Kirberger for critical review and editing of this manuscript. We thank Anita Randon, Oluwatosin Y. Ibhagui, Rakshya Gorkhali, Sheng Tan, and Kuangcai Chen for their helpful discussion, training, and contribution and Zachary Allen for technical assistance. We would also like to thank Michael Kovach from Horiba Scientific for technical support. This work was supported in part by a NIH grant 1R01GM081749 to JJY and its supplemental grant to F.R.; Brain and Behavior fellowship to C.L.M. and A.A.P.; CDT fellowship to X.D.; an NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation Research Partners Program (P&S Fund Investigator) and a Whitehall Foundation (Grant 2017-05-35) to A.M.M.; NIH grants R01NS115209 and R01NS086082 to D.N.C.; NSF grants CBET-1604612 and NIH grants R01GM115763 to N.F.; a Georgia State University Neurogenomics 2CI Fellowship to M.A.G. and A.A.P.; and a Kenneth W. and Georgeanne F. Honeycutt Fellowship to M.A.G. and A.A.P.
Author contributions
J.J.Y., F.N.R., C.L.M., X.D., B.D., N.F., G.G., M.A.G., A.M.M., A.A.P., D.N.C., S.T., and F.Z. designed research; F.N.R., C.L.M., X.D., B.M., B.D., A.A.P., M.A.G., C.M., G.G., S.W., T.A.R., K.M.S., R.C.T., Y.Z., J.Q., K.H., and J.L. performed research; F.N.R., C.L.M., X.D., B.M., B.D., A.A.P., M.A.G., K.M.S., Y.Z., analyzed data, and F.N.R., C.L.M., X.D., B.D., A.A.P., D.N.C., M.A.G., A.M.M., F.Z., and J.J.Y. wrote the paper.
Declaration of interests
J.J.Y. is the shareholder of InLighta Biosciences. J.J.Y. is a named inventor on an issued patent (US10371708). The rest of the authors declare no competing interests.
Inclusion and diversity
We worked to ensure sex balance in the selection of non-human subjects. We worked to ensure diversity in experimental samples through the selection of the cell lines. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Published: March 19, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102129.
Supplemental information
References
- Abe T., Sugihara H., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J. Biol. Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Anderson A.A., Altafaj X., Zheng Z., Wang Z.M., Delbono O., Ronjat M., Treves S., Zorzato F. The junctional SR protein JP-45 affects the functional expression of the voltage-dependent Ca2+ channel Cav1.1. J. Cell Sci. 2006;119:2145–2155. doi: 10.1242/jcs.02935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.A., Treves S., Biral D., Betto R., Sandona D., Ronjat M., Zorzato F. The novel skeletal muscle sarcoplasmic reticulum JP-45 protein. Molecular cloning, tissue distribution, developmental expression, and interaction with alpha 1.1 subunit of the voltage-gated calcium channel. J. Biol. Chem. 2003;278:39987–39992. doi: 10.1074/jbc.M305016200. [DOI] [PubMed] [Google Scholar]
- Andrasfalvy B.K., Magee J.C. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J. Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramori I., Nakanishi S. Signal transduction and pharmacological characteristics of a metabotropic glutamate receptor, mGluR1, in transfected CHO cells. Neuron. 1992;8:757–765. doi: 10.1016/0896-6273(92)90096-v. [DOI] [PubMed] [Google Scholar]
- Aridor M., Guzik A.K., Bielli A., Fish K.N. Endoplasmic reticulum export site formation and function in dendrites. J. Neurosci. 2004;24:3770–3776. doi: 10.1523/JNEUROSCI.4775-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad S.S., Lamb H.K., Morgan J.M., Dunlop W., Gillespie J.I. Differential expression of ryanodine receptor RyR2 mRNA in the non-pregnant and pregnant human myometrium. Biochem. J. 1997;322:777–783. doi: 10.1042/bj3220777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley P., Ahlstrom P., Martin S.R., Forsen S. The kinetics of calcium-binding to calmodulin - Quin-2 and ans stopped-flow fluorescence studies. Biochem. Biophys. Res. Commun. 1984;120:185–191. doi: 10.1016/0006-291x(84)91431-1. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium. 2002;32:235–249. doi: 10.1016/s0143416002001823. [DOI] [PubMed] [Google Scholar]
- Berridge M.J., Bootman M.D., Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Hayden M.R. Deranged neuronal calcium signaling and Huntington disease. Biochem. Biophys. Res. Commun. 2004;322:1310–1317. doi: 10.1016/j.bbrc.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Blaustein M.P., Golovina V.A. Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- Bourne J.N., Harris K.M. Nanoscale analysis of structural synaptic plasticity. Curr. Opin. Neurobiol. 2012;22:372–382. doi: 10.1016/j.conb.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T., Ottolini D., Brini M. Calcium signaling in Parkinson's disease. Cell Tissue Res. 2014;357:439–454. doi: 10.1007/s00441-014-1866-0. [DOI] [PubMed] [Google Scholar]
- Chemaly E.R., Troncone L., Lebeche D. SERCA control of cell death and survival. Cell Calcium. 2018;69:46–61. doi: 10.1016/j.ceca.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A., Malenka R.C. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33:18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Cui-Wang T., Hanus C., Cui T., Helton T., Bourne J., Watson D., Harris K.M., Ehlers M.D. Local zones of endoplasmic reticulum complexity confine cargo in neuronal dendrites. Cell. 2012;148:309–321. doi: 10.1016/j.cell.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Juan-Sanz J., Holt G.T., Schreiter E.R., De Juan F., Kim D.S., Ryan T.A. Axonal endoplasmic reticulum Ca(2+) content controls release Probability in CNS nerve terminals. Neuron. 2017;93:867–881 e6. doi: 10.1016/j.neuron.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti N., Vivoli E., Bartolini A., Ghelardini C. A gene-specific cerebral types 1, 2, and 3 RyR protein knockdown induces an antidepressant-like effect in mice. J. Neurochem. 2008;106:2385–2394. doi: 10.1111/j.1471-4159.2008.05581.x. [DOI] [PubMed] [Google Scholar]
- Gerber S., Alzayady K.J., Burglen L., Bremond-Gignac D., Marchesin V., Roche O., Rio M., Funalot B., Calmon R., Durr A. Recessive and dominant de novo ITPR1 mutations cause Gillespie syndrome. Am. J. Hum. Genet. 2016;98:971–980. doi: 10.1016/j.ajhg.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T., Arnaud Sampaio V.F., Lameu C., Ulrich H. Calcium signalling: a common target in neurological disorders and neurogenesis. Semin. Cell Dev. Biol. 2019;95:25–33. doi: 10.1016/j.semcdb.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Hovnanian A. SERCA pumps and human diseases. Subcell Biochem. 2007;45:337–363. doi: 10.1007/978-1-4020-6191-2_12. [DOI] [PubMed] [Google Scholar]
- Ivanova H., Vervliet T., Missiaen L., Parys J.B., De Smedt H., Bultynck G. Inositol 1,4,5-trisphosphate receptor-isoform diversity in cell death and survival. Biochim. Biophys. Acta. 2014;1843:2164–2183. doi: 10.1016/j.bbamcr.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Jimenez-Moreno R., Wang Z.M., Messi M.L., Delbono O. Sarcoplasmic reticulum Ca2+ depletion in adult skeletal muscle fibres measured with the biosensor D1ER. Pflugers Arch. 2010;459:725–735. doi: 10.1007/s00424-009-0778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara A.A., Allen D.G. The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad. J. Physiol. 2001;534:87–97. doi: 10.1111/j.1469-7793.2001.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J., Parton R.G., Fuller S.D., Griffiths G., Dotti C.G. The organization of the endoplasmic reticulum and the intermediate compartment in cultured rat hippocampal neurons. Mol. Biol. Cell. 1995;6:1315–1332. doi: 10.1091/mbc.6.10.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir A., Wajsberg B., Marks A.R. Ryanodine receptor dysfunction in human disorders. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:1687–1697. doi: 10.1016/j.bbamcr.2018.07.011. [DOI] [PubMed] [Google Scholar]
- Landolfi B., Curci S., Debellis L., Pozzan T., Hofer A.M. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J. Cell Biol. 1998;142:1235–1243. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis B.S., Zhou J., Royer L., Shannon T.R., Brum G., Rios E. Confocal imaging of [Ca2+] in cellular organelles by SEER, shifted excitation and emission ratioing of fluorescence. J. Physiol. 2005;567:523–543. doi: 10.1113/jphysiol.2005.087973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C., Wang H., Liu Q., He W., Yuan L., Xu P. A genetically encoded ratiometric calcium sensor enables quantitative measurement of the local calcium microdomain in the endoplasmic reticulum. Biophys. Rep. 2019;5:31–42. [Google Scholar]
- MacMillan H.A., Findsen A., Pedersen T.H., Overgaard J. Cold-induced depolarization of insect muscle: differing roles of extracellular K+ during acute and chronic chilling. J. Exp. Biol. 2014;217:2930–2938. doi: 10.1242/jeb.107516. [DOI] [PubMed] [Google Scholar]
- Macmillan H.A., Sinclair B.J. Mechanisms underlying insect chill-coma. J. Insect Physiol. 2011;57:12–20. doi: 10.1016/j.jinsphys.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Magi S., Castaldo P., Macri M.L., Maiolino M., Matteucci A., Bastioli G., Gratteri S., Amoroso S., Lariccia V. Intracellular calcium dysregulation: implications for alzheimer's disease. Biomed. Res. Int. 2016;2016:6701324. doi: 10.1155/2016/6701324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno C., Figueroa L.C., Gillespie D., Fitts R., Kang C., Franzini-Armstrong C., Rios E. Calsequestrin depolymerizes when calcium is depleted in the sarcoplasmic reticulum of working muscle. Proc. Natl. Acad. Sci. U S A. 2017;114:E638–E647. doi: 10.1073/pnas.1620265114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Chapman K.E., Seckl J.R., Ashley R.H. Partial cloning and differential expression of ryanodine receptor/calcium-release channel genes in human tissues including the hippocampus and cerebellum. Neuroscience. 1998;85:205–216. doi: 10.1016/s0306-4522(97)00612-x. [DOI] [PubMed] [Google Scholar]
- Marx S.O., Reiken S., Hisamatsu Y., Jayaraman T., Burkhoff D., Rosemblit N., Marks A.R. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- McEntagart M., Williamson K.A., Rainger J.K., Wheeler A., Seawright A., De Baere E., Verdin H., Bergendahl L.T., Quigley A., Rainger J. A restricted repertoire of de novo mutations in ITPR1 cause Gillespie syndrome with evidence for dominant-negative effect. Am. J. Hum. Genet. 2016;98:981–992. doi: 10.1016/j.ajhg.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Rios E., Schneider M.F. A general procedure for determining the rate of calcium release from the sarcoplasmic reticulum in skeletal muscle fibers. Biophys. J. 1987;51:849–863. doi: 10.1016/S0006-3495(87)83413-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba K. Role of IP3 receptor signaling in cell functions and diseases. Adv. Biol. Regul. 2015;57:217–227. doi: 10.1016/j.jbior.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Miyawaki A., Llopis J., Heim R., Mccaffery J.M., Adams J.A., Ikura M., Tsien R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature. 1997;388:882–887. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Mosca B., Delbono O., Laura Messi M., Bergamelli L., Wang Z.M., Vukcevic M., Lopez R., Treves S., Nishi M., Takeshima H. Enhanced dihydropyridine receptor calcium channel activity restores muscle strength in JP45/CASQ1 double knockout mice. Nat. Commun. 2013;4:1541. doi: 10.1038/ncomms2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Nishimura S., Maeda A., Barsoumian E.L., Hakamata Y., Nakai J., Allen P.D., Imoto K., Kita T. Molecular cloning and characterization of a human brain ryanodine receptor. FEBS Lett. 1997;417:157–162. doi: 10.1016/s0014-5793(97)01275-1. [DOI] [PubMed] [Google Scholar]
- Palmer A.E., Jin C., Reed J.C., Tsien R.Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl. Acad. Sci. U S A. 2004;101:17404–17409. doi: 10.1073/pnas.0408030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer M.J., Irving A.J., Seabrook G.R., Jane D.E., Collingridge G.L. The group I mGlu receptor agonist DHPG induces a novel form of LTD in the CA1 region of the hippocampus. Neuropharmacology. 1997;36:1517–1532. doi: 10.1016/s0028-3908(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Papp S., Dziak E., Michalak M., Opas M. Is all of the endoplasmic reticulum created equal? The effects of the heterogeneous distribution of endoplasmic reticulum Ca2+-handling proteins. J. Cell Biol. 2003;160:475–479. doi: 10.1083/jcb.200207136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Park I.Y., Kim E., Youn B., Fields K., Dunker A.K., Kang C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: a proposed mechanism for coupled calcium binding and protein polymerization. J. Biol. Chem. 2004;279:18026–18033. doi: 10.1074/jbc.M311553200. [DOI] [PubMed] [Google Scholar]
- Pedelacq J.D., Cabantous S., Tran T., Terwilliger T.C., Waldo G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006;24:79–88. doi: 10.1038/nbt1172. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Tepikin A., Park M.K. The endoplasmic reticulum: one continuous or several separate Ca2+ stores? Trends Neurosci. 2001;24:271–276. doi: 10.1016/s0166-2236(00)01787-2. [DOI] [PubMed] [Google Scholar]
- Pettit D.L., Augustine G.J. Distribution of functional glutamate and GABA receptors on hippocampal pyramidal cells and interneurons. J. Neurophysiol. 2000;84:28–38. doi: 10.1152/jn.2000.84.1.28. [DOI] [PubMed] [Google Scholar]
- Rios E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 2018;150:521–537. doi: 10.1085/jgp.201711959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf R., Magalhaes P.J., Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J. Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santulli G., Lewis D., Des Georges A., Marks A.R., Frank J. Ryanodine receptor structure and function in health and disease. Subcell Biochem. 2018;87:329–352. doi: 10.1007/978-981-10-7757-9_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.F., Simon B.J., Szucs G. Depletion of calcium from the sarcoplasmic reticulum during calcium release in frog skeletal muscle. J. Physiol. 1987;392:167–192. doi: 10.1113/jphysiol.1987.sp016775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M., Korkotian E. Endoplasmic reticulum calcium stores in dendritic spines. Front. Neuroanat. 2014;8:64. doi: 10.3389/fnana.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekulic-Jablanovic M., Palmowski-Wolfe A., Zorzato F., Treves S. Characterization of excitation–contraction coupling components in human extraocular muscles. Biochem. J. 2015;466:29–36. doi: 10.1042/BJ20140970. [DOI] [PubMed] [Google Scholar]
- Sharp A.H., Mcpherson P.S., Dawson T.M., Aoki C., Campbell K.P., Snyder S.H. Differential immunohistochemical localization of inositol 1,4,5-trisphosphate- and ryanodine-sensitive Ca2+ release channels in rat brain. J. Neurosci. 1993;13:3051–3063. doi: 10.1523/JNEUROSCI.13-07-03051.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemering K.R., Golbik R., Sever R., Haseloff J. Mutations that suppress the thermosensitivity of green fluorescent protein. Curr. Biol. 1996;6:1653–1663. doi: 10.1016/s0960-9822(02)70789-6. [DOI] [PubMed] [Google Scholar]
- Snyder E.M., Philpot B.D., Huber K.M., Dong X., Fallon J.R., Bear M.F. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat. Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Sonnleitner A., Conti A., Bertocchini F., Schindler H., Sorrentino V.J.T.E.J. Functional properties of the ryanodine receptor type 3 (RyR3) Ca2+ release channel. EMBO J. 1998;17:2790–2798. doi: 10.1093/emboj/17.10.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spacek J., Harris K.M. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J. Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C., Shui B., Zhao W., Liu H., Li W., Lee J.C., Doran R., Lee F.K., Sun T., Shen Q.S. Central role of IP3R2-mediated Ca(2+) oscillation in self-renewal of liver cancer stem cells elucidated by high-signal ER sensor. Cell Death Dis. 2019;10:396. doi: 10.1038/s41419-019-1613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J., Kanemaru K., Ishii K., Ohkura M., Okubo Y., Iino M. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat. Commun. 2014;5:4153. doi: 10.1038/ncomms5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Kuenen J.G., Schipper K., Van Der Velde S., Ishii S., Wu A., Sorokin D.Y., Tenney A., Meng X., Morrill P.L. Physiological and genomic features of highly alkaliphilic hydrogen-utilizing Betaproteobacteria from a continental serpentinizing site. Nat. Commun. 2014;5:3900. doi: 10.1038/ncomms4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztretye M., Yi J., Figueroa L., Zhou J., Royer L., Allen P., Brum G., Rios E. Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca(2+) release in skeletal muscle. J. Gen. Physiol. 2011;138:231–247. doi: 10.1085/jgp.201010592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztretye M., Yi J., Figueroa L., Zhou J., Royer L., Rios E. D4cpv-calsequestrin: a sensitive ratiometric biosensor accurately targeted to the calcium store of skeletal muscle. J. Gen. Physiol. 2011;138:211–229. doi: 10.1085/jgp.201010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M., Nishizawa M., Onodera O. Roles of inositol 1,4,5-trisphosphate receptors in spinocerebellar ataxias. Neurochem. Int. 2016;94:1–8. doi: 10.1016/j.neuint.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Tang S., Deng X., Jiang J., Kirberger M., Yang J.J. Design of calcium-binding proteins to sense calcium. Molecules. 2020;25:2148. doi: 10.3390/molecules25092148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Reddish F., Zhuo Y., Yang J.J. Fast kinetics of calcium signaling and sensor design. Curr. Opin. Chem. Biol. 2015;27:90–97. doi: 10.1016/j.cbpa.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S., Wong H.C., Wang Z.M., Huang Y., Zou J., Zhuo Y., Pennati A., Gadda G., Delbono O., Yang J.J. Design and application of a class of sensors to monitor Ca(2+) dynamics in high Ca(2+) concentration cellular compartments. Proc. Natl. Acad. Sci. U S A. 2011;108:16265–16270. doi: 10.1073/pnas.1103015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Slater N.T., Fein A., Schmidek A., Reese T.S. Continuous network of endoplasmic reticulum in cerebellar Purkinje neurons. Proc. Natl. Acad. Sci. U S A. 1994;91:7510–7514. doi: 10.1073/pnas.91.16.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga M., Imamoto N., Sakata-Sogawa K. Highly inclined thin illumination enables clear single-molecule imaging in cells. Nat. Methods. 2008;5:159–161. doi: 10.1038/nmeth1171. [DOI] [PubMed] [Google Scholar]
- Turner H.N., Armengol K., Patel A.A., Himmel N.J., Sullivan L., Iyer S.C., Bhattacharya S., Iyer E.P.R., Landry C., Galko M.J., Cox D.N. The TRP channels Pkd2, NompC, and trpm act in cold-sensing neurons to mediate unique aversive behaviors to noxious cold in Drosophila. Curr. Biol. 2016;26:3116–3128. doi: 10.1016/j.cub.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A., Korkotian E., Schonfeld E., Copanaki E., Deller T., Segal M. Synaptopodin regulates plasticity of dendritic spines in hippocampal neurons. J. Neurosci. 2009;29:1017–1033. doi: 10.1523/JNEUROSCI.5528-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton P.D., Airey J.A., Sutko J.L., Beck C.F., Mignery G.A., Sudhof T.C., Deerinck T.J., Ellisman M.H. Ryanodine and inositol trisphosphate receptors coexist in avian cerebellar Purkinje neurons. J. Cell Biol. 1991;113:1145–1157. doi: 10.1083/jcb.113.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y., Solntsev K.M., Reddish F., Tang S., Yang J.J. Effect of Ca(2)(+) on the steady-state and time-resolved emission properties of the genetically encoded fluorescent sensor CatchER. J. Phys. Chem. B. 2015;119:2103–2111. doi: 10.1021/jp501707n. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study will be available from the lead contact upon request.