Abstract
Maize and its products are most often prone to fungal contamination especially during cultivation and storage by toxigenic fungi. Aflatoxicosis still persist in Ghana despite the numerous education on several ways of its prevention at the farm as well as its adverse health implications which are food safety concerns. A random assessment and human risk analysis was conducted on 90 maize (72 white and 18 colored) samples from markets across all the regions of Ghana. Total aflatoxins (AFtotal) and the constitutive aflatoxins (AFB1, AFB2, AFG1, and AFG2) were analyzed by High-Performance Liquid Chromatography (HPLC). Out of a total of ninety (90) samples investigated, 72 (80%) tested positive for AFB1 and the contamination levels ranged from 0.78 ± 0.04 to 339.3 ± 8.6 µg kg−1. Similarly, AFG2 was detected in only 14 (15.5%) samples, and their values ranged between 1.09 ± 0.03 and 5.51 ± 0.26 µg kg−1 while AF total ranged between 0.78 ± 0.04 and 445.01 ± 8.9 µg kg−1 constituting approximately 72 (80%). Limits of AFB1 and total aflatoxins (AFtotal) for the Ghana Standards Authority (GSA) (5 and 10 µg kg−1) and the European Food Safety Authority (EFSA) (2 and 4 µg kg−1), were used as checks. A total of 33 (41.25%) samples were above the limits for both. Risk assessments recorded for Estimated Daily Intake (EDI), Hazard Quotient (H.Q), Hazard Index (H.I), Margin of Exposure (MOE), av. Potency, and population risks ranged 0.087–0.38 μg kg−1 bw day−1, 1.5–6.9, 0.0087–0.38, 3.64–12.09, 0–0.0396 ng Aflatoxins kg−1 bw day−1 and, 3.5 × 10–1–0.015 respectively for total aflatoxins. While ranges for aflatoxins B1 (AFB1) recorded were 0.068–0.3 μg Kg bw−1 day−1, 2.43–10.64, 0.0068–0.030, 4.73–20.51, 0–0.0396 ng Aflatoxins kg−1 bw day−1 and, 2.69 × 10–3–0.012 for Estimated Daily Intake (EDI), Hazard Quotient (H.Q), Hazard Index (H.I), Margin of Exposure (MOE), Av. potency, and population risks respectively. It was deduced that although there was some observed contamination of maize across the different ecological zones, the consumption of maize (white and colored) posed no adverse health effects on the population of Ghana since computed H.I was less than 1 (< 1).
Subject terms: Biotechnology, Microbiology, Environmental sciences, Natural hazards
Introduction
Zea mays (Maize) is a principal cereal and staple for people living in warm climates throughout Asia, Africa, and the Americas who are predisposed to the effects of climate change1 in terms of production, consumption and income generation. Forming part of everyday meals, maize, and its products since time immemorial, has been part of the African culture, and so form part of an everyday meal in most homes2. It is commonly consumed fresh or processed into cooked or fermented, milled and beverage products3–5. It is also extensively used to prepare delectable dishes either singly or in combination with other staples particularly groundnuts or legumes or animal sources of protein to complement each other to combat malnutrition since its protein content is inadequate23.
In Ghana, maize is broadly appreciated as a stable crop since it is grown in all agro-ecological zones. More than 50% of rural households cultivate it traditionally under rainfed conditions. Besides, also 16% of urban households are involved in its production. However, there is a yield gap especially in the northern and upper regions. This has ultimately created an imbalance between its production and consumption6. Intake levels of approximately 43–46 kg person−1 day−1 of household consumption of maize in rural subsistence farming communities in Ghana have been reported7,8.
Maize is susceptible to fungal infections mainly from Fusarium and Aspergillus species and consequent contamination with their mycotoxins; fumonisins and aflatoxins9 respectively throughout its growth, harvest, transport, and storage10,11. A change in climate simultaneously impacts the complex communities of Aflatoxin (AF)-producing fungi by altering the number of AF-producers to change its fungal community’s structure.
Aflatoxins are secondary metabolites, which are naturally occurring contaminants of food and elaborate the toxins under auspicious conditions of temperature, relative humidity, and poor storage conditions. They are now known to be mainly produced by A. flavus, A. parasiticus, Aspergillus nomius and two different Emericella species12.
Biochemically, aflatoxins are difurano-coumarin derivatives with a bifuran group joined to the coumarin nucleus and a pentanone ring (in case of AFBs) or a lactone ring (in case of AFGs)13,14. AFB1, AFB2, AFG1, and AFG2 are the four most significant AFs among the identified 20. The B-types are produced by A. flavus while G-types are produced by A. parasiticus15. Yu et al.16, as well as Yabe and Nakajimam17 identified approximately 18 enzymatic steps with at least 25 genes answerable for producing the enzymes and regulating the biosynthesis of aflatoxins process. All the aflatoxins-producing fungi exhibit a great variation in terms of qualitative and quantitative differences in the toxicology abilities that are noticeable attributes by different strains within each fungal species.
Consequently, in sub-Saharan Africa, mycotoxin studies have focused mostly on aflatoxins (in maize and groundnuts) and fumonisins (in maize) while the other potentially dangerous mycotoxins in other foods have received less attention. This is possibly so because maize is a staple food with extensive use that complements groundnuts to combat protein-energy malnutrition typically used in complementary feeding18 and so naturally any microorganism and toxins that affect it directly, will be of critical concern. All valuations of exposure implicate maize or groundnuts as the main source of aflatoxins or fumonisins.
The total maize harvest in Africa according to FAO (2017), was estimated at 40 million hectares, with Nigeria being the top producer (16%) followed by Tanzania. Worldwide maize consumption is estimated to be more than 116 million tons with 30% and 21% of the consumption occurring globally and in Sub-Saharan African (SSA), respectively. Approximately 14 countries in SSA consume 85–95% of white maize as their staple food19. In most of the developing countries from Africa, there is an increased risk of hepatocellular carcinoma in the presence of hepatitis B virus infection and esophageal cancer being linked to aflatoxins contamination of food18.
As emphasized by some previous researchers20–23, mycotoxins especially aflatoxins toxicity has always been a topic of contentious interest in the international market and economic development of a country, many of agricultural products are often rejected due to excessive contaminations (beyond specific thresholds of host countries). This is evidenced in previously published works on aflatoxins and cereals in Ghana which revealed some tenacity and unsatisfactory trend of contamination24–28.
To overcome this problem, many countries have set standard safety levels of aflatoxins in food and food products and animal feed to ensure quality. Furthermore, several attempts have been made to educate the populace and stakeholders on the preventive practices and impacts of these mycotoxins on health29 chiefly attributable to non-compliance to Good Management Practices (GMP), Good Agricultural Practices (GAP) and Good Hygienic Practice (GHP).
It was hypothesized that maize grains meant for consumption and sold on markets across Ghana did not contain aflatoxins. The objective of this study was, therefore, to assess the potential exposure to aflatoxin through consumption of commercial maize products (market maize), we conducted a cross-sectional assessment of market maize contamination.
Materials and methods
Study area
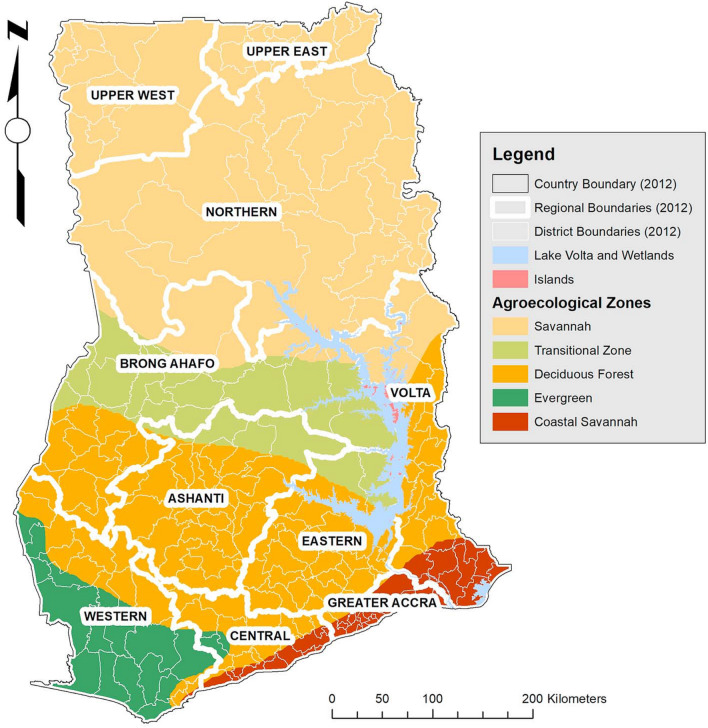
On the Gulf of Guinea in West Africa, is located in Ghana. It covers about 23,884,245 ha of land and water area between latitudes 4°N and 11°N and longitudes 4°W and 2°E30,31. The country is demarcated into 10 regions and 216 districts, categorized into five main agro-ecological (Coastal Savannah, Evergreen, Deciduous Forest, Transitional, and Savannah) zones (Fig. 1). An estimated 24,658,832 people were counted across the whole country during the 2010 Ghana Population and Housing Census32.
Figure 1.
Map of Ghana showing regions of sampling (Adapted from Abbam et al.33).
The three northern regions (Upper West, Upper East, and Northern) are predominantly agro-ecologically Savannah. Ashanti, Brong-Ahafo, and Eastern regions are mainly transitional and deciduous forest areas. The western region is mostly Evergreen and Deciduous Forest and highly economically active. Greater Accra (predominantly Coastal) and Ashanti (predominantly Deciduous Forest) are the most developed and urbanized regions and the lowest in terms of agricultural activity. The Volta region cuts across three agro-ecological (coastal, deciduous forest, and savannah) zones. The central region, primarily deciduous forest and coastal, is the fourth poorest region. Fishing and agriculture are the main economic activities. The five principal maize growing areas are in the Northern, Brong‐Ahafo, Ashanti, Central and Eastern Regions.
Sample collection
To collect a representative data set, we first obtained the list of villages in each district from the Regional Directorate of the Ministry of Agriculture. From each district, an average of 9 villages (Table 1) were then randomly selected. The maize sellers in each market were conveniently sampled where about one kilogram (1 kg) of raw maize samples were purchased concurrently within the period of February to September 2018 and grouped into 2 categories (white and colored maize) (Tables 3, 4, 5, 6 and 7) Twenty (20) grams each of maize samples were fetched and kept in sterile bags in ice chests and sent to the laboratory within the same day in a vehicle where they were stored in a deep freezer at − 20 °C until ready for chemical analysis35.
Table 1.
Geographical locations and some attributes of the origin of samples.
| Region | No. of samples | Agro-ecological zones | Rainfall (mm) | Temperature (°C) | Coordinates |
|---|---|---|---|---|---|
| Greater Accra | 9/90 | Coastal Savannah | 800–1000 | 26.6 | 5.8143° N, 0.0747° E |
| Central | 9/90 | Deciduous Forest | 1400–1600 | 26.7 | 5.5608° N, 1.0586° W |
| Western | 8/90 | Evergreen | 1800–2000 | 25.9 | 5.3902° N, 2.1450° W |
| Eastern | 12/90 | Deciduous Forest | 1400–1900 | 25.9 | 6.2374° N, 0.4502° W |
| Ashanti | 11/90 | Deciduous Forest | 1200–1400 | 26.3 | 6.7470° N, 1.5209° W |
| Brong-Ahafo | 9/90 | Transitional zone | 1400–1600 | 23.9 | 7.9559° N, 1.6761° W |
| Volta | 7/90 | Coastal Savannah/Deciduous forest | 1000–1400 | 26.2 | 6.5781° N, 0.4502° E |
| Northern | 10/90 | Savannah | 1000–1200 | 27.9 | 9.5439° N, 0.9057° W |
| Upper East | 7/90 | Savannah | 800–1000 | 28.3 | 10.7082° N, 0.9821° W |
| Upper West | 8/90 | Savannah | 1000–1200 | 27.8 | 10.2530° N, 2.1450° W |
Table 3.
Concentration of aflatoxin types in different maize samples from different locations of Ghana.
| Category | Food sample | Concentrations of Aflatoxins (µg kg−1) | ||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||
| White | MzHalf- Assini | 59.5 ± 0.99a | 10.9 ± 0.4a | n.d | n.d | 70.4 ± 1.8a |
| MzDzodze | 8.77 ± 0.47 g | 2.40 ± 0.3d | 0.98 ± 0.03d | n.d | 12.15 ± 1.1 h | |
| MzKaneshie | 16.02 ± 1.2e | 2.53 ± 0.15dc | n.d | n.d | 18.55 ± .0.9 g | |
| MzKsi cm | 9.65 ± 0.25 g | 2.98 ± 0.92c | 2.71 ± 0.91bc | n.d | 15.34 ± 0.9gh | |
| MzSuhum | 16.27 ± 0.39e | 3.11 ± 0.5c | 1.89 ± 0.05d | 1.97 ± 0.06a | 23.24 ± 0.32f. | |
| MzKintamp | 20.4 ± 0.8d | 4.93 ± 0.27bc | 3.51 ± 0.85b | 1.66 ± 0.06a | 30.5 ± 2.15dc | |
| MzMoree | 8.03 ± 1.7 g | 0.93 ± 0.04e | n.d | n.d | 8.96 ± 0.22i | |
| MzKorle G | n.d | n.d | n.d | n.d | n.d | |
| MzNsawam | 4.92 ± 0.6 h | 0.69 ± 0.03e | n.d | n.d | 5.61 ± 0.26i | |
| MzHo cm | n.d | n.d | n.d | n.d | n.d | |
| MzWa | n.d | n.d | n.d | n.d | n.d | |
| MzKotokraba | 5.31 ± 0.26 h | 0.86 ± 0.03e | n.d | n.d | 6.17 ± 0.8i | |
| MzAowin | 47.75 ± 2.25b | 5.23 ± 0.25b | 2.98 ± 0.9b | n.d | 55.96 ± 1.2b | |
| MzSunyani | 34.32 ± 2.1c | 5.2 ± 0.22b | n.d | n.d | 39.55 ± 2.3dc | |
| MzMadina | 14.92 ± 0.5ef | 1.5 ± 0.07de | 2.31 ± 0.91bc | n.d | 18.73 ± 0.65 g | |
| MzAsafo | 42.07 ± 1.5b | 3.17 ± 0.15c | n.d | n.d | 45.24 ± 1.45c | |
| MzBerekum | 5.18 ± 0.25 h | 0.67 ± 0.02e | n.d | n.d | 5.85 ± 0.26i | |
| MzTema | 11.17 ± 1.22f. | 10.24 ± 1.02a | 5.6 ± 0.55a | n.d | 27.01 ± 0.4e | |
Mean ± Standard Deviation that do not share a letter are significantly different (p > 0.05).
n.d not detected.
Table 4.
Concentration of aflatoxin types in different maize samples from different locations of Ghana.
| Category | Food sample | Concentrations of Aflatoxins (µg/kg) | ||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||
| White | MzKoforidua | n.d | n.d | n.d | n.d | n.d |
| MzWalewale | 12.87 ± 1.4c | 0.61 ± 0.05e | n.d | n.d | 13.48 ± 0.35d | |
| MzNsawkaw | 5.29 ± 0.24de | n.d | 2.51 ± 0.14b | n.d | 7.8 ± 0.45e | |
| MzAsamankese | 7.44 ± 1.2d | 4.86 ± 0.44b | 4.22 ± 0.2a | 2.06 ± 0.17a | 18.58 ± 1.1c | |
| MzHohoe | 14.4 ± 1.26c | 0.56 ± 0.03e | n.d | n.d | 14.96 ± 1.3d | |
| MzAbutia | 7.07 ± 0.32d | 0.52 ± 0.02e | n.d | n.d | 7.53 ± 0.50e | |
| MzSogakope | 12.92 ± 1.2c | 5.0 ± 0.42b | 4.2 ± 0.26a | n.d | 22.12 ± 0.45c | |
| MzKintampo | n.d | n.d | n.d | n.d | n.d | |
| MzTamale | 44.20 ± 0.51a | 7.7 ± 0.50a | 1.6 ± 0.05bc | n.d | 53.5 ± 0.95a | |
| MzKayera | n.d | n.d | n.d | n.d | n.d | |
| MzNanton1 | 10.63 ± 1.2c | n.d | n.d | n.d | 10.63 ± 1.20d | |
| MzNanton 2 | 11.42 ± 1.22c | 1.19 ± 0.1d | n.d | n.d | 12.61 ± 1.20d | |
| MzSavelugu | 22.33 ± 0.35b | 4.75 ± 0.48bc | n.d | n.d | 27.08 ± 1.4b | |
| MzAssin fosu | 20.73 ± 0.3b | 4.75 ± 0.48bc | n.d | n.d | 25.08 ± 1.4b | |
| MzAdeamra | n.d | n.d | n.d | n.d | n.d | |
| MzAfrancho | n.d | n.d | n.d | n.d | n.d | |
| MzEjisu.mkt | 8.55 ± 0.60d | n.d | n.d | n.d | 8.55 ± 0.60e | |
| MzAfrmso | 4.96 ± 0.43e | n.d | 2.09 ± 0.13b | n.d | 7.05 ± 0.50e | |
Mean ± Standard Deviation that do not share a letter are significantly different (p > 0.05).
n.d not detected.
Table 5.
Concentration of aflatoxin types in different maize samples from different locations of Ghana.
| Category | Food sample | Concentrations of Aflatoxins (µg/kg) | ||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||
| White | MzDonkork | 60.51 ± 1.65c | 17.66 ± 1.5c | 10.21 ± 1.1b | 2.7 ± 0.6c | 91.08 ± 2.1b |
| MzKwaekese | 7.86 ± 0.45 h | 0.54 ± 0.04 g | n.d | n.d | 8.4 ± 0.85 g | |
| MzPedu | n.d | n.d | n.d | n.d | n.d | |
| MzAfrm.pl | 14.28 ± 1.20 g | 1.58 ± 0.06 g | 13.19 ± 0.9a | n.d | 29.05 ± 3.50f. | |
| MzKayeru | n.d | n.d | n.d | n.d | n.d | |
| MzKamina | 2.5 ± 0.12i | n.d | 4.53 ± 0.22d | n.d | 7.03 ± 0.4 g | |
| MzAgbogbloshi | 45.13 ± 0.61d | 16.32 ± 1.3c | 11.5 ± 1.22b | 2.8 ± 0.12c | 75.75 ± 1.84c | |
| MzNima | 39.5 ± 3.01de | 13.77 ± 0.9d | 4.99 ± 0.26d | 5.5 ± 0.27a | 63.76 ± 1.71d | |
| MzMakola | 94.42 ± 2.24b | n.d | n.d | n.d | 94.42 ± 2.24b | |
| MzZuo | 1.88 ± 0.06i | n.d | n.d | n.d | 1.88 ± 0.06 h | |
| MzZebilla | 2.26 ± 0.6i | n.d | n.d | n.d | 2.26 ± 0.6 h | |
| MzMankesim | 23.11 ± 0.31f. | 8.3 ± 0.5f. | 4.67 ± 0.47d | n.d | 36.08 ± 2.5e | |
| MzSandema | 43.7 ± 2.25d | 21.6 ± 0.33b | 13.75 ± 1.2a | n.d | 79.05 ± 1.82c | |
| MzNkwatia | 232.47 ± 8.33a | 15.77 ± 1.4c | n.d | n.d | 248.24 ± 8.45a | |
| MzDromkma | n.d | n.d | n.d | n.d | n.d | |
| MzFunsi | 36.4 ± 2.5de | 20.5 ± 0.35b | 6.8 ± 0.33 cd | 1.11 ± 0.05d | 64.8 ± 1.67d | |
| MzAbliri | 25.2 ± 0.35f. | 11.7 ± 1.2de | n.d | n.d | 36.9 ± 2.14e | |
| MzBielepong | 51.73 ± 0.3d | 24.1 ± 0.4a | 14.5 ± 1.2a | 3.5 ± 0.6b | 93.83 ± 5.2b | |
Mean ± Standard Deviation that do not share a letter are significantly different (p > 0.05).
n.d not detected.
Table 6.
Concentration of aflatoxin types in different maize samples from different locations of Ghana.
| Category | Food Sample | Concentrations of Aflatoxins (µg/kg) | ||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||
| White | MzBakano | n.d | n.d | n.d | n.d | n.d |
| MzUCC | 2.2 ± 0.15i | n.d | n.d | n.d | 2.2 ± 0.15j | |
| MzAdu1 | n.d | n.d | n.d | n.d | n.d | |
| MzAdu2 | n.d | n.d | n.d | n.d | n.d | |
| MzEjura | 339.3 ± 8.6a | 103 ± 2.5a | 2.71 ± 0.12 cd | n.d | 445.01 ± 8.9a | |
| MzBonwire | 102.1 ± 2.5c | 0.88 ± 0.03de | n.d | n.d | 102.98 ± 2.5d | |
| MzSefw. W | 11.5 ± 0.3 g | 0.62 ± 0.03de | n.d | n.d | 12.12 ± 1.4 g | |
| MzBibiani | 5.21 ± 0.32 h | 1.5 ± 0.06d | n.d | n.d | 6.71 ± 0.08i | |
| MzSefw.Bk | 185.17 ± 2.15b | 9.37 ± 0.25b | n.d | n.d | 194.54 ± 2.5b | |
| MzAtwim | 18.21 ± 1.31f. | 2.66 ± 0.13c | n.d | n.d | 20.87 ± 0.35 g | |
| MzT’di | 3.93 ± 0.22 h | 0.79 ± 0.03de | n.d | n.d | 4.72 ± 0.28i | |
| MzNsaw | n.d | n.d | n.d | n.d | n.d | |
| MzTumu | 75.4 ± 1.85d | 40.3 ± 0.55a | 7.62 ± 0.33b | 2.57 ± 0.3b | 125.89 ± 2.5c | |
| MzAzuguyeri | 24.7 ± 0.35e | 6.77 ± 0.30bc | 2.61 ± 0.92 cd | n.d | 34.08 ± 3.01f. | |
| MzBolga | 5.18 ± 0.25 h | 2.91 ± 0.9c | 1.02 ± 0.02d | n.d | 9.11 ± 0.22 h | |
| MzKayeru | 25.96 ± 0.31e | 7.89 ± 0.32b | 1.22 ± 0.06d | n.d | 35.07 ± 2.82f. | |
| MzChogu | 10.91 ± 1.22 g | 4.23 ± 0.23c | 2.5 ± 0.92 cd | 1.88 ± 0.06bc | 19.52 ± 0.31 g | |
| MzOffinso | 27.78 ± 0.52e | 9.47 ± 0.46b | 5.41 ± 0.25ab | 5.51 ± 0.26a | 48.17 ± 0.55e | |
Mean ± Standard Deviation that do not share a letter are significantly different (p > 0.05).
n.d not detected.
Table 7.
Concentration of aflatoxin types in different maize samples from different locations of Ghana.
| Category | Food Sample | Concentrations of Aflatoxins (µg/kg) | ||||
|---|---|---|---|---|---|---|
| AFB1 | AFB2 | AFG1 | AFG2 | Total | ||
| Colored | MzLawra | 2.69 ± 0.12e | 1.55 ± 0.05ab | 1.01 ± 0.02b | 1.09 ± 0.03a | 6.29 ± 0.81 cd |
| MzDambai | 5.35 ± 0.7d | 1.67 ± 0.05ab | 1.05 ± 0.11b | n.d | 8.07 ± 0.85c | |
| MzGbawe | 2.37 ± 0.12e | n.d | n.d | n.d | 2.37 ± 0.12e | |
| MzKintampo | 16.95 ± 0.84a | 1.04 ± 0.06ab | 3.67 ± 0.22a | n.d | 21.66 ± 0.32a | |
| MzGurugu | 1.77 ± 0.06e | n.d | n.d | n.d | 1.77 ± 0.06e | |
| MzKeta | 1.61 ± 0.06e | n.d | n.d | n.d | 1.61 ± 0.06e | |
| MzTech jxn | 0.88 ± 0.02ef | 0.67 ± 0.02ab | n.d | n.d | 1.55 ± 0.06e | |
| MzGwolu | 1.78 ± 0.06e | 0.80 ± 0.03ab | n.d | n.d | 2.66 ± 0.15e | |
| MzDormaa | 3.01 ± 0.5e | 2.55 ± 0.14a | n.d | n.d | 5.56 ± 0.26 cd | |
| MzTechiman | 17.43 ± 0.81a | 3.2 ± 0.22a | n.d | n.d | 20.63 ± 0.33a | |
| MzBame | 5.2 ± 0.5c | 0.77 ± 0.04ab | 1.02 ± 0.05b | n.d | 6.99 ± 0.82c | |
| MzKwadaso | 8.41 ± 0.85b | 2.85 ± 0.14a | 1.01 ± 0.05b | n.d | 11.26 ± 1.22b | |
| MzLapaz | 4.5 ± 0.27d | 1.6 ± 0.07ab | 1.07 ± 0.06b | n.d | 7.17 ± 0.45c | |
| MzJuaboso | 1.89 ± 0.06e | 0.82 ± 0.04ab | n.d | n.d | 2.71 ± 0.15e | |
| MzNavrongo-ue | 0.78 ± 0.04ef | n.d | n.d | n.d | 0.78 ± 0.04f. | |
| MzMkt circle | n.d | n.d | 1.1 ± 0.02b | n.d | 1.1 ± 0.02e | |
| MzAnomabo | n.d | n.d | n.d | n.d | n.d | |
| MzNadowli | n.d | n.d | n.d | n.d | n.d | |
Mean ± Standard Deviation that do not share a letter are significantly different (p > 0.05).
n.d not detected.
Extraction of samples
AFB1, AFB2, AFG, and AFG2 were extracted from samples according to the European Committee for Standardization (CEN) official method EN1412334 for aflatoxin extraction. Methanol in water (200 ml) (8 + 2) and 5 g NaCl were used to extract 25 g of sample. Hexane (100 ml) was added to samples containing more than 50% fat. Mixture was homogenized for 3 min at 3000 rpm (2 min) and 3500 rpm (1 min). The extracts were filtered and 10 ml of filtrate added to 60 ml of phosphate buffer saline (PBS) for solid-phase extraction using a pre-conditioned immune-affinity columns specific for AFB1, AFB2, AFG1, and AFG2. The 70 ml filtrate-PBS mixture was loaded onto the pre-conditioned column and allowed to elute by gravity at a flow rate of 1 ml min−1. This was followed by a cleanup with 15 ml distilled water at a flow rate of 5 ml min−1. Aflatoxins were eluted in two steps into a 5 ml volumetric flask with 0.5 ml followed by 0.75 ml of methanol (HPLC grade) and allowed to elute by gravity. Deionized water was used to make up volume of eluates to 5 ml and eluate vortexed and 2 ml pipetted into HPLC vials for quantification35.
HPLC parameters
Injection volume: 10 μl flow rate: 1 ml min−1, column temperature: 35 °C, excitation wavelength: 360 nm, emission wavelength: 440 nm, mobile phase composition: water/acetonitrile/MeOH (65:15:20 v/v/v), post-column derivatization: Kobra cells. HPLC Column Specification Spherisorb ODS1- Excel (4.6 mm × 25 cm), 5 μm particle size, 250 A pore size.
LOD = Limit of detection.
LOQ = Limit of quantification.
ACN = Acetonitrile.
MeOH = Methanol.
LOD calculation = 3 * standard deviation/slope.
LOQ calculation = 3 × LOD.
Supplier of Column R- Biopharm, Block 10 campus, West Scotland.
Science Park, Acre Road, Glasgow, Scotland G20 OXA35.
Analysis of samples
The aflatoxins (by Aspergillus flavus and A. parasiticus) levels in the samples were determined by High-Performance Liquid Chromatography HPLC (Agilent 1260 Series, OpenLab software, X-bridge column) (250 mm × 4.6 mm, i.d., 5 μm), USA with fluorescence detector and post-column derivatization using Kobra cells to generate bromine electrochemically at the CSIR- Food Research Institute, Ghana. LOD for AFB1, AFB2, AFG1 and AFG2 were 0.20 μg kg−1, 0.17 μg kg−1, 0.26 μg kg−1 and 0.36 μg kg−1 respectively (Table 2)35.
Table 2.
Limits of Detection and Quantification (LOD & LOQ) of aflatoxins AFB1, AFB2, AFG1, AFG2 and Total aflatoxins (µg/kg) measured by HPLC.
| Aflatoxin | Limits | Amount (µg/kg) |
|---|---|---|
| AFB1 | LOD | 0.20 |
| LOQ | 0.60 | |
| AFB2 | LOD | 0.17 |
| LOQ | 0.51 | |
| AFG1 | LOD | 0.26 |
| LOQ | 0.78 | |
| AFG2 | LOD | 0.36 |
| LOQ | 1.08 |
LOD limit of detection, LOQ limit of quantification.
Limit of detection/quantification (LOD/LOQ)
Limit of detection and quantification (LOD/LOQ) of the HPLC were estimated by making a calibration curve around the least standard used for spiking, 5 µ kg−1 (lowest concentration range of calibration curve). Blank did not produce any signal, so the LOD and LOQ were calculated as;
| 1 |
| 2 |
Measurement accuracy
Spiking of pure aflatoxin standard solution was done according to method described by Kortei et al.35 to ensure measurement accuracy of analysis. Three levels spiking were done at the lower, mid and upper concentration range of the calibration curve concentrations (5 ppb, 15 ppb and 30 ppb). Spike volumes of pure standards were calculated as;
| 3 |
Spike volumes were distributed evenly on aflatoxins free sample (blank) and spiked sample analysed for percentage recovery which was calculated as;
| 4 |
Measurement precision
Repeatability and intermediate precision analyses of an internal reference material (IRM) was used to ensure measurement precision of the method. For repeatability analysis, 10 parallel extractions of the IRM was done by the same analyst at the same time using the same HPLC and the relative standard deviation among results calculated. For intermediate precision, 10 extractions of the IRM were done at different days by different analysts and the relative standard deviation among results calculated35. The relative standard deviations were calculated as; [Standard deviation/mean] * 100.
Required performance criteria for accuracy and precision
Repeatability
Relative standard deviation among repeatable results should be less than 15%.
Intermediate precision
Relative standard deviation among results obtained under intermediate precision conditions should be less than 20%.
Recovery
Percent recovery of measurement procedure should be in a range of 80–120%.
Limit of detection
The limit of detection should be less than 1 ug kg−1 for all aflatoxins.
Limit of quantification
The limit of Quantification should be less than 3 ug kg−1 for all aflatoxins.
Linearity
Linearity from regression curve should be 0.99 (B1, B2, G1) and 0.98 (G2)35.
Experimental data
Repeatability
Relative standard deviation was; B1 = 5.5%; B2 = 6.7%; G1 = 7.4%; G2 = 12.1% and Total aflatoxins = 5.2%.
Intermediate Precision (Reproducibility): Relative standard deviation was; B1 = 13.2%; B2 = 13.4%; G1 = 13.7%; G2 = 12.2% and Total aflatoxins = 11.9%.
Recovery: Percent recovery of measurement procedure was; Low concentration: B1 = 107%; B2 = 87.2%; G1 = 113.4%; G2 = 112.8% and Total aflatoxins = 108.2%.
High concentration: B1 = 102.6%; B2 = 101.6%; G1 = 104.2%; G2 = 104.4% and Total aflatoxins = 103.3%.
Linearity: Linearity from regression curve was; B1 = 0.991; B2 = 0.997, G1 = 0.994; G2 = 0.995.
Human health risk assessment of exposure to total aflatoxins via consumption of cereals.
Exposure estimation
Calculation of the Estimated Daily Intake (EDI) was done by using the mean levels of aflatoxins obtained in maize samples, the daily intakes of the same samples6, and the average body weight. The EDI for mean aflatoxins was calculated according to the following formula and expressed in μg kg−1 of body weight/day (μg kg bw−1day−1)36.
| 5 |
Daily intake of maize in Ghana according to MoFA6 is 42.5 kg day−1.
Estimation of hazard quotient (HQ)
Hazard Quotient (HQ) is otherwise referred to as the Non-Carcinogenic Effect of the toxin. The non-carcinogenic effect of the individual toxin is designated by hazard quotient (HQ) as described by Kortei et al.25. The HQ was estimated using Eq. (6):
| 6 |
where EDI and rfD are average daily dose and reference dose respectively.
Estimation of Hazard Index (HI)
The Hazard Index (HI) was calculated according to the below-mentioned formula, by dividing the EDI by TD50, divided by a safety factor of 50,000. TD50 is the dose (ng kg−1 body weight−1 day−1) required to induce tumors in half of the test animals that would have remained tumor-free at zero doses as described by Ismail et al.37 and Ishikawa et al.38.
| 7 |
Population risk characterization for aflatoxins
Risk characterization for genotoxic and carcinogenic compounds such as aflatoxins is based on the margin of exposures (MOEs), which was calculated by dividing the Benchmark dose lower limit (BMDL) for aflatoxins- 400 ngkg−1 bw day−1 by toxin exposure39
| 8 |
In cases where MOEs were lower than 10,000, a public health concern is indicated which implied that aflatoxin exposures above 0.04 ngkg−1 bw day−1 (as obtained by dividing 400 ngkg−1 bw day−1 by 10,000) represented a risk of public health concern40.
Estimated liver cancer risk due to consumption of maize
The estimated liver cancer risk for Ghanaian adult consumers was calculated for aflatoxins because the ingestion of the toxin can be traced to the development of liver cancer11,39. This involved estimating the population cancer risk per 100,000 which was obtained by multiplying the EDI value with the average hepatocellular carcinoma (HCC) potency figure from individual potencies of HBsAg-positive and for HBsAg negative groups.
The JECFA estimated potency values for AFB1 which corresponded to 0.3 cancers year−1 100,000−1 population/ng kg−1 bw day−1 (uncertainty range: 0.05–0.5) in HBs Ag positive individuals and 0.01 cancers year−1 100,000−1 population/ng kg−1 bw day−1 (uncertainty range: 0.002–0.03) in HBsAg-negative individuals11,39 were adopted for this calculation. Also, the HBsAg + prevalence rate of 10.2% for Ghana41 was adopted and 89.8% (100–10.2%) was extrapolated for HBsAg-negative groups. Hence the average potency for cancer in Ghana was estimated as follows:
| 9 |
Thus the population risk was estimated using the following formula:
| 10 |
Statistical analysis
The aflatoxins concentrations were calculated using regression analysis from the curves derived from the standards of the aflatoxins with Excel for Microsoft Windows (version 10). Means and standard deviations of results were subjected to analyses of variance (one-way ANOVA) at the significant difference (p < 0.05) and separation of means were determined via post-hoc test using Duncan’s multiple range test DMRT with SPSS 22 (Chicago, USA). Means and standard deviations were computed and graphical representations were used appropriately.
Results
Good linearity or coefficients of correlations (R2 > 0.990) within the tested range was obtained for most of the food samples tested. For the recovery analysis, one maize and rice samples were previously analyzed to assure the absence of studied mycotoxins, were used in the validation procedure. The Limits of Detection for AFB1 and AFB2 likewise AFG1 and AFG2 ranged between 0.13 and 0.15 while Limits of Quantification ranged between 0.26 and 0.30 respectively for both (Table 2).
The number of maize samples contaminated with AFB1, AFB2, AFG1, AFG2 and AF Total (Total Aflatoxins) are presented in Tables 3, 4, 5, 6 and 7. The order of toxicity was AFB1 > AFB2 > AFG1 > AFG2. As explained by Quinto et al.42 the terminal furan moiety of AFB1 is the critical point for determining the degree of biological activity of this group of fungal toxins. Out of a total of ninety (90) samples investigated, 72 (80%) tested positive for AFB1 and the contamination levels ranged from 0.78 ± 0.04 to 339.3 ± 8.6 µg kg−1 for MzNavrongo and MzEjura respectively. For AFB2, 59 (65.5%) samples tested positive and had levels ranging from 0.52 ± 0.02 to 103 ± 2.5 µg kg−1 for MzSefwi-Wiawso and MzEjura respectively. AFG1 was present in 35 (38.8%) samples of range 0.98 ± 0.03–14.5 ± 1.2 µg kg−1 for MzDzodze and MzBielepong respectively while, AFG2 was detected in only 14 (15.5%) samples, and their values ranged between 1.09 ± 0.03–5.51 ± 0.26 µg kg−1 for MzLawra and MzOffinso respectively. Lastly, a total ranged between 0.78 ± 0.04- 445.01 ± 8.9 µg kg−1 for MzNavrongo and MzEjura respectively. The total aflatoxin determinations were obtained from 72 (80.2%) samples.
The greatest aflatoxin yields of 445.01 ± 8.9 and 339.3 ± 8.6 µg kg−1 for AFTotal and AFB1 respectively, were significantly (p < 0.05) higher than all other samples studied in the various categories.
Toxin quantity limits prescribed by the Ghana Standards Authority which are slightly higher and more flexible than the European Union (EFSA) are 5, 10 µg kg−1 and 2, 4 µg kg−1 respectively for AFB1 and Total aflatoxins.
Results from the study were compared to the European Food Safety Authority (EFSA) and Ghana Standards Authority (GSA) regulatory concentration limits for total aflatoxins (AF Total) and Aflatoxins B1 (AFB1). From Table 8, a majority of 41/72 (56.9%) of the 72 samples analyzed for total aflatoxins in group one (white maize samples) exceeded the limits of GSA. These maize samples had AFTotal ranging from 12.12 ± 1.4 to 445.01 ± 8.9 µg kg−1. Only 3/18 (16.67%) out of colored maize samples (Group two) was found to exceed the GSA limit which also ranged 11.26 ± 1.22–21.66 ± 0.32 µg kg−1.
Table 8.
Proportions of samples that exceeded AFTotal and AFB1 and limits of Ghana Standard Authority (GSA) and the European Food Safety Authority (EFSA).
| Samples | Total samples | Exceeding GSA regulation | Exceeding EFSA regulation | ||
|---|---|---|---|---|---|
| Yes (%) | Range | Yes (%) | Range | ||
| AFTotal | |||||
| Group 1 | 72 | 41 (56.9%) | 12.12 ± 1.4–445.01 ± 8.9 | 54 (75%) | 4.72 ± 0.28–445.01 ± 8.9 |
| Group 2 | 18 | 3 (16.67%) | 11.26 ± 1.22–21.66 ± 0.32 | 8 (44.4%) | 5.56 ± 0.26–21.66 ± 0.32 |
| Total | 90 | 47 (52.2%) | 11.26 ± 1.22–445.01 ± 8.9 | 62 (68.89%) | 4.72 ± 0.28–445.01 ± 8.9 |
| AFB1 | |||||
| Group 1 | 72 | 50 (69.44%) | 5.18 ± 0.25–339.3 ± 8.6 | 56 (77.78%) | 2.2 ± 0.15–339.3 ± 8.6 |
| Group 2 | 18 | 5 (27.78%) | 5.2 ± 0.5–17.43 ± 0.81 | 9 (50.0%) | 2.37 ± 0.12–16.95 ± 0.84 |
| Total | 90 | 55 (60.85%) | 5.18 ± 0.25–339.3 ± 8.6 | 65 (72.2%) | 2.2 ± 0.15–339.3 ± 8.6 |
European Union Food Safety (EFSA) limit for AFTotal = 4 µg kg−1.
European Union Food Safety (EFSA) limit for AFB1 = 2 µg kg−1.
Ghana Standards Authority (GSA) limit = 10 µg kg−1.
Ghana Standards Authority (GSA) limit = 5 µg kg−1.
Overall, 47/90 (52.2%) of the maize recorded values above the set limit and ranged 11.26 ± 1.22–445.01 ± 8.9 µg kg−1.
About 54/72 (75%) of white maize and 8/18 (44.4%) colored maize corresponding to ranges of 4.72 ± 0.28–445.01 ± 8.9 and 5.56 ± 0.26–21.66 ± 0.32 µg kg−1 respectively exceeded the tolerable limit of the EFSA. Overall for EFSA, 62/90 (68.89%) of white maize samples of range 4.72 ± 0.28–445.01 ± 8.9 µg kg−1 exceeding limits were recorded. Whereas 70.4 and 11.5% of samples respectively from the two groups exceeded EFSA for AFB1 (Table 8).
For AFB1, GSA limits exceeded were 50/72 (69.44%) and ranged 5.18 ± 0.25–339.3 ± 8.6 µg kg−1 while for the colored maize samples, 5/18 (27.78%) of range 5.2 ± 0.5–17.43 ± 0.81 µg kg−1 were recorded. For the overall, 55/90 (60.85%) of range, 5.18 ± 0.25–339.3 ± 8.6 µg kg−1 were recorded.
Values of 56/72 (77.78%) with range 2.2 ± 0.15–339.3 ± 8.6 µg kg−1 and 9/18 (50.0%) with range 2.37 ± 0.12–16.95 ± 0.84 µg kg−1 were recorded for white and colored maize samples respectively. Finally, as the overall, 65/90 (72.2%) maize samples of range 2.2 ± 0.15–339.3 ± 8.6 µg kg−1 exceeded limits for EFSA.
Risk assessment
The Estimated Daily Intakes (EDI) of the total aflatoxins in the maize samples were 109.7, 58.8, 33.08 and 25.2 μg Kg bw−1 day−1 for infants, children, adolescents, and adults respectively. For the Hazard Quotient (HQ), values of 5.5 × 105, 2.94 × 105, 1.65 × 105, and 1.26 × 105 were recorded respectively. A range of 2.5–10.97 was recorded for H.I. Margin of Exposure (MOE) values recorded were 3.64, 6.80, 12.09 and 6.75 respectively. The average potency of the aflatoxins was 0.0396 ng Aflatoxins kg−1 bw day−1 and produced a population risks of 4.344, 2.3, 1.31 and 1.0 respectively (Table 9).
Table 9.
Evaluation of risk for Total Aflatoxins via consumption of maize.
| Av.body wgt.(kg) | References (weight) | Estimated daily intake (EDI) (μg Kg bw−1 day−1) | Hazard quotient (H.Q) | hazard index (h.i) | MOE | Av. potency (ng Aflatoxins kg−1 bw day−1) | Population risk | |
|---|---|---|---|---|---|---|---|---|
| Infants (6–52mths) | 7 |
Glover-Amengor et al.43 Abeshu et al.44 |
109.7 | 5.5 × 105 | 10.97 | 3.64 | 0.0396 | 4.344 |
| Children (5–11 years) | 26 (24–28) |
Biritwum et al.45 WHO46 |
58.8 | 2.94 × 105 | 5.88 | 6.80 | 0.0396 | 2.3 |
| Adolescents (12–18 years) | 46.25 (38.5–54) | Afrifa-Anane et al.47 | 33.08 | 1.65 × 105 | 3.30 | 12.09 | 0.0396 | 1.31 |
| Adults (18–60 years) | 60.7 | Walpole et al.48 | 25.2 | 1.26 × 105 | 15.87 | 6.75 | 0.0396 | 1.0 |
Margin of Exposure-MOE.
*Mean aflatoxins-36.13 µg kg−1.
*Daily intake of maize for infants was halved (0.5 × 42.5 kg).
*Daily intake of 42.5 kg was used for children, adolescents, and adults.
TD50 = 0.2 ng Kg bw−149.
1 ng = 0.001 μg.
For AFB1, the EDIs for infants, children, adolescents, and adults were 84.5, 45.5, 25.6 and 19.5 μg Kg bw−1 day−1 respectively. HQ values recorded were 5.5 × 105, 2.94 × 105, 1.65 × 105 and 1.26 × 105 respectively. Margin of Exposure (MOE) values recorded were 4.73, 8.79, 15.63 and 20.51 respectively. The average potency was the same as total aflatoxins while the population risks were respectively 3.35, 1.80, 1.01 and 0.77 (Table 10).
Table 10.
Evaluation of risk for Aflatoxins B1 (AFB1) via consumption of maize.
| Av.Body weight (kg) | References (weight) | Estimated daily intake (EDI) (μg Kg bw−1 day−1) | Hazard quotient (H.Q) | Hazard Index (H.I) | MOE | Av. potency (Aflatoxins ng kg−1 Bw day−1) | Population risk | |
|---|---|---|---|---|---|---|---|---|
| Infants (6–52mths) | 7.08 (2.5–11.65) |
Glover-Amengor et al.43 Abeshu et al.44 |
84.5 | 4.2 × 105 | 8.5 | 4.733 | 0.0396 | 3.35 |
| Children (5–11 years) | 26 (24–28) |
Biritwum et al.45 WHO46 |
45.5 | 2.3 × 10–3 | 4.6 × 10–3 | 8.79 | 0.0396 | 1.80 |
| Adolescents (12–18 years) | 46.25 (38.5–54) | Afrifa-Anane et al.47 | 25.6 | 1.3 × 105 | 2.6 | 15.63 | 0.0396 | 1.01 |
| Adults (18–60 years) | 60.7 | Walpole et al.48 | 19.5 | 9.7 × 104 | 1.95 | 20.51 | 0.0396 | 0.77 |
The margin of Exposure- MOE.
TD50 = 0.2 ng kg bw−149.
1 ng = 0.001 μg.
*Mean of AFB1 27.85 µg kg−1.
*Daily intake of maize for infants was halved (0.5 × 42.5).
*Daily intake of 42.5 kg was used for children, adolescents, and adults.
Discussion
Aflatoxin contamination of market maize is a significant public health concern. Our findings demonstrated widespread aflatoxin contamination of maize within the regional market distribution system. The different maize samples obtained from the different markets across the country had varying quantities of AFB1 and AFtotal. The greatest quantity of aflatoxins recorded from this study (445.01 ± 8.9 µg kg−1) was, by and large, lower than values of 692 and 945 ng g−1 from maize samples obtained from Fumesua and Ejura in Ghana respectively by Dadzie et al.24.
Kpodo50 in earlier surveys reported aflatoxin levels in the range of 20–355 ng g−1 maize samples from silos and warehouses in Ejura while fermented corn dough collected from major processing sites also contained aflatoxin levels of 0.7–313 ng g−1. James et al.51 also found high average aflatoxin levels in maize samples collected from North Kwahu (153 ng g−1), Ejura Sekyere-Dumasi (121 ng g−1) and Nkoranza (134 ng g−1).
Agbetiameh et al.26 reported values of range 1–341 ppb in maize from different ecological zones in Ghana. Likewise, Blankson et al.28 also reported a range of 1.77 ± 0.01–24.58 ± 0.05 μg kg−1 in maize-based samples in locally prepared cereals for consumption in Ghana.
From Kenya, Nduti et al.52 reported values of range 7.92 ± 1.57–22.54 ± 4.94 ppb from maize flour samples obtained from three regions.
Lewis et al.8 reported greater values of aflatoxin quantities of > 1000 ppb from maize samples as they investigated aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in Eastern and Central Kenya.
AFB1 was present in seven of eight samples ranging from 30 to 6127 μg kg−1 and two of eight samples were found positive for AFB2 ranging from 53 to 1738 μg kg−1 as reported by Sewram et al.53 from Brazil as they investigated corn-based infant food sold on different markets. In their study in Ghana, aflatoxins were reported to markedly contaminate local food (weanimix) from cereal-legume blend for children and reported aflatoxin B1 levels in local weanimix of range 7.9–500 ppb (ug kg−1)54.
The reasonably high levels of aflatoxins detected in this study, put forward that aflatoxins can continue to persist in food even after the inactivation of the fungi in spite of all the rigorous processing methods because of their ability to resist chemical and thermal changes55. A danger looms hereafter, since there is a high likelihood of detecting aflatoxins in processed cereal foods more so if the ingredients used for the food are initially contaminated before processing and subsequent consumption. Thus, the incidence of aflatoxins in processed cereal-based food might indicate contamination of the raw cereals at a point in the value chain (either on the farm or during storage). It is also noteworthy that the not detected (n.d) status recorded in some samples may not necessarily mean total absence of aflatoxins but were simply below detectable limits of the equipment.
Aflatoxins contamination occurs via an initial phase during crop development and a second phase during crop maturation. The contamination is greater in warm, humid, and even hot deserts and drought conditions56 since mycotoxins are optimally produced in adverse periods.
Atoxigenic biocontrol of aflatoxins promises a safe, economical, ecosystem friendly, cost-effective method of aflatoxin mitigation throughout the value chain57–61. Implementing aflatoxin biocontrol management strategies to reduce aflatoxin contamination in the field and throughout storage would result in improved health, enhanced trade, increased income, and the welfare of farmers and consumers in Ghana.
Climatic variations of the different agro-ecological zones might be an explanation to the seasonal occurrences of types and levels of toxins in the food material62. Planning interventions against dietary exposure to aflatoxins form an important basis for these current findings since the rainfall pattern in major parts of the country is categorized bimodally by long and short rainy seasons intermingled with brief dry spells62. A general mycotoxicosis risk is signaled through a seasonal assessment of aflatoxin residues in food specifically given climatic changes triggering hot and dry influences linked to increased contamination56,63,64. It was also observed in this study that colored maize recorded lower quantities of aflatoxins as compared with levels of the white maize samples. Presumably, the biochemical substances present in the maize accounting for the colorations such as beta carotene, zeaxanthins, lutein etc. may have inhibited the propagation of Aspergillus species.
In Africa, of which Ghana is no exception, smallholder farmers produce most of the maize of which most of them use poor harvest techniques such as insufficient drying and storage of their crops. Additionally, the cultivation of local maize varieties which are susceptible to both insect damage and diseases, less drought-tolerant, expose the maize to infection by encouraging the growth of aflatoxin-producing fungi during crop development, maturation and harvest in the field65.
Despite the availability of improved varieties, local varieties are still planted by a significant fraction of maize farmers. Even though there is knowledge of the occurrence of aflatoxin accumulation in maize collected in markets and farmer stores across Ghana26, little is known of the aflatoxin levels when the maize is still in the field (prior-to-harvest maize) and the composition of community structures of Aspergillus section flavor associated with the maize in Ghana.
Conclusion
It can be surmised from the results of this study that from a total of ninety (90) maize samples investigated, 65 (72.2%) tested positive for AFB1 and ranged from 2.2 ± 0.15–339.3 ± 8.6 µg kg−1. A similar proportion of 62 (68.89%) was also recorded for total aflatoxins (AFTotal) and ranged between 4.72 ± 0.28–445.01 ± 8.9 µg kg−1 and these were above the limits set by the Ghana Standards Authority (GSA) (5 and 10–15 µg kg−1) and the European Food Safety Authority (EFSA) (2 and 4 µg kg−1). Human health risk assessment from aflatoxins exposure through maize consumption from the markets by infants, children, adolescents, and adults showed a significant non-carcinogenic adverse health risk to humans since all calculated values for Hazard Quotient (HQ) were > 1 while there was no observed adverse health effect (HI < 1). Conversely, MOE values obtained in this study were all greater than 0.04 ngkg−1 bw day−1 which implied a potential health risk as suggested by the World Health Organization (2008).
Government institutions, private and non-governmental organizations, as well as national media networks need to play a key role in raising consciousness of the public health impacts of aflatoxin. Bearing in mind the necessity of attaining food security and food safety for vulnerable people in these areas, there is also a need for data and risk management capacity tools for locally driven policy reform.
Limitations of the study
The human health risk assessments were arrived at using deterministic methods (based on assumptions) as opposed to using probabilistic approaches.
The primary data obtained from the laboratory, are usually presented as means + standard deviation from three determinations which statistically, do not represent the true average of the concentrations.
Acknowledgements
Authors are grateful to the University of Health and Allied Sciences and the CSIR-Food Research Institute for their immense support and the successful completion of this research work. Our heartfelt thanks to the families of the authors for their undying support.
Author contributions
N.K.K, T.A, P.T.A, and R.A.S contributed to the conception and design of the experiment, analysis of the data and writing the manuscript. N.K.K., F.A., P.G.A, P.E.A and T.A. did the sample collection. V.K-B. and H.A.A. analyzed the samples.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis L, Onsongo M, Njapau H, Schurz-Rogers H, Luber G, Kieskaz S, Nyamongo J, Backer L, Dahiye AM, Misore A, DeCock K, Rubin C. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in Eastern and Central Kenya. J. Environ. Health Perspect. 2005;113(12):1763–1767. doi: 10.1289/ehp.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohajan HK. Food and nutrition scenario of Kenya. Am. J. Food Nutr. 2014;2(2):28–38. [Google Scholar]
- 3.Matumba L, Sulyok M, Njoroge SM, Njumbe Ediage E, Van Poucke C, De Saeger S, Kriska R. Uncommon occurrence ratios of aflatoxin B1, B2, G1, and G2 in maize and groundnut from Malawi. Mycotoxin Res. 2015;31:57–62. doi: 10.1007/s12550-014-0209-z. [DOI] [PubMed] [Google Scholar]
- 4.Shephard GS, Rheeder JP, van der Westhuizen L. Effect of the traditional cooking practice on fumonisin content of maize porridge consumed in the former Transkei region of South Africa. World Mycotoxin J. 2012;5(4):405–407. doi: 10.3920/WMJ2012.1445. [DOI] [Google Scholar]
- 5.Hove M, Boevre MD, Lachat C, Jacxsens L, Nyanga LK, DeSaeger S. Occurrence and risk assessment of mycotoxins in subsistence farmed maize from Zimbabwe. Food Control. 2016;69:36–44. doi: 10.1016/j.foodcont.2016.04.038. [DOI] [Google Scholar]
- 6.Quiñones, E. J. & Diao, X. Assessing Crop Production and Input Use Patterns in Ghana–What Can We Learn from the Ghana Living Standards Survey (GLSS5); Ghana Strategy Support Program (GSSP) Working Paper No. 0024. (International Food Policy Research Institute: Accra, Ghana, 2011).
- 7.Food Balance Sheets-FAOSTAT. http://www.fao.org/faostat/en/#data/FBS/visualize (2018/2019).
- 8.MoFA. Food and Agriculture Sector Development Policy (FASDEP I) 55 (2000).
- 9.Kimanya ME, De Meulenaer B, Tiisekwa B, Ndomondo- Sigonda M, Devlieghere F, Van Camp J, Kolsteren P. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Addit. Contam. 2008;25:1353–1364. doi: 10.1080/02652030802112601. [DOI] [PubMed] [Google Scholar]
- 10.Mutegi C, Kimani J, Otieno G, Wanyama R, Christie ME, Mallikarjunan K, Kaaya A. Market attributes and their effect on levels of aflatoxin in peanuts (Arachis hypogaea L.) from Nairobi and western Kenya. East Afr. Agric. For. J. 2010;77:95–103. [Google Scholar]
- 11.Shephard GS. Risk assessment of aflatoxins in food in Africa. AFood Addit. Contam. A. 2008;25(10):1246–1256. doi: 10.1080/02652030802036222. [DOI] [PubMed] [Google Scholar]
- 12.Frisvad JC, Thrane U, Samson RA, Pitt JI. Important mycotoxins and the fungi which produce them. In: Hocking AD, Pitt J, Samson RA, Thrane U, editors. Advances in Food Mycology Advances in Experimental Medicine and Biology. New York: Springer Science and Business Media; 2006. [DOI] [PubMed] [Google Scholar]
- 13.Schuda PF. Syntheses of Natural Products. Berlin: Springer; 1980. Aflatoxin chemistry and syntheses; pp. 75–111. [Google Scholar]
- 14.Mahato DK, Lee KE, Kamle M, Devi S, Dewangan KN, Kumar P, Kang SG. Aflatoxins in food and feed: An overview on prevalence detection and control strategies. Front. Microbiol. 2019;10:2266. doi: 10.3389/fmicb.2019.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar P, Mahato DK, Kamle M, Mohanta TK, Kang SG. Aflatoxins: A global concern for food safety, human health, and their management. Front. Microbiol. 2017;7:2170. doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu J, Bhatnagar D, Ehrlich KC. Aflatoxin biosynthesis. Rev. Iberoam. Micol. 2002;19:191–200. [PubMed] [Google Scholar]
- 17.Yabe K, Nakajima H. Enzyme reactions and genes in aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2004;64:745–755. doi: 10.1007/s00253-004-1566-x. [DOI] [PubMed] [Google Scholar]
- 18.Kimanya ME. The health impacts of mycotoxins in the eastern Africa region. Curr. Opin. Food Sci. 2015;6:7–11. doi: 10.1016/j.cofs.2015.11.005. [DOI] [Google Scholar]
- 19.Food and Agriculture Organization of the United Nations (FAO) (2017). Crop Prospects and Food Situation. GIEWS Reports http://www.fao.org/3/a-i8278e.pdf. Accessed 17/07/2020.
- 20.Odamtten GT. Natural Occurrence, Economic Impact and Control of Aflatoxins in Africa. Brazaville: WHO Expert Committee Meeting on Aflatoxins and Health; 2005. [Google Scholar]
- 21.Awuah RT, Agyemang KO, Fialor SC, Jolly CM. Are Ghanaians aware of the aflatoxin menace? In: Leslie JF, Bandyopadhyay R, Visconti A, editors. Mycotoxins: Detection Methods, Management, Public Health, and Agricultural Trade. Wallingford: CABI Publishing; 2008. pp. 327–334. [Google Scholar]
- 22.Kpodo KA, Bankole SA. Mycotoxins: Detection Methods, Management, Public Health and Agricultural Trade. Wallingford: CABI Publishing; 2008. Mycotoxin contamination in foods in West and Central Africa; pp. 103–116. [Google Scholar]
- 23.Achaglinkame MA, Opoku N, Amagloh FK. Aflatoxin contamination in cereals and legumes to reconsider usage as complimentary food ingredients for Ghanaian infants: A review. J. Nutr. Intermed. Metab. 2017;10:1–7. doi: 10.1016/j.jnim.2017.09.001. [DOI] [Google Scholar]
- 24.Dadzie MA, Oppong A, Ofori K, Eleblu JS, Ifie EB, Blay E, Obeng-Bio E, Appiah-Kubi Z, Warburton ML. Distribution of Aspergillus flavus and aflatoxin accumulation in stored maize grains across three agro-ecologies in Ghana. Food Control. 2019;104:91–98. doi: 10.1016/j.foodcont.2019.04.035. [DOI] [Google Scholar]
- 25.Kortei NK, Agyekum AA, Akuamoa F, Kyei-Baffour V, Alidu HW. Risk assessment and levels of naturally occurring aflatoxins in packaged cereals and cereal-based foods on the Ghanaian market. Toxicol. Rep. 2019;6:34–41. doi: 10.1016/j.toxrep.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agbetiameh D, Ortega-Beltran A, Awuah RT, Atehnkeng J, Cotty PJ, Bandyopadhyay R. Prevalence of aflatoxin contamination in maize and groundnut in Ghana: Population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 2018;102:764–772. doi: 10.1094/PDIS-05-17-0749-RE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ofosu IW, Anka-Brewoo G, Lutterodt HE, Tetteh LPM, Benefo EO, Ellis WO. Aflatoxin exposures and risks in maize-based foods consumed by university students. Acta Sci. Nutr. Health. 2019;3(2):110–119. [Google Scholar]
- 28.Blankson GK, Mills-Robertson FC, Ofosu IW. Survey of occurrence levels of Aflatoxins in selected locally processed cereal-based foods for human consumption from Ghana. Food Control. 2019;95:170–175. doi: 10.1016/j.foodcont.2018.08.005. [DOI] [Google Scholar]
- 29.GSA. Ghana Intensifies Bid for the Eradication of Aflatoxins. https://www.gsa.gov.gh/2019/04/ghana-intensifies-bid-for-eradication-of-aflatoxin-related-losses-from-agricultural-value-chains/ (2019)
- 30.MOFA (Ministry of Food and Agriculture) Agriculture in Ghana. Facts and Figures. Accra: Statistics, Research and Information Directorate (SRID), MoFA; 2014. [Google Scholar]
- 31.Nkrumah F, Klutse NAB, Adukpo DC, Owusu K, Quagraine KA, Owusu A, Gutowski W. Rainfall variability over Ghana: Model versus rain gauge observation. Int. J. Geosci. 2014;5(7):673–683. doi: 10.4236/ijg.2014.57060. [DOI] [Google Scholar]
- 32.Ghana Statistical Service. 2010 population and housing census, 2010 Popul. Hous. Census Natl. Anal. Rep., 305–337 (2013).
- 33.Abbam T, Johnson FA, Dash J, Padmadas SS. Spatiotemporal variations in rainfall and temperature in Ghana over the twentieth century, 1900–2014. Earth Space Sci. 2018;5:120–132. doi: 10.1002/2017EA000327. [DOI] [Google Scholar]
- 34.European Committee for Standardization (CEN). Foodstuffs: Determination of Aflatoxin B1 and the Sum of Aflatoxin B1, B2, G1 and G2 in Hazelnuts, Peanuts, Pistachios, Figs, and Paprika Powder: High Performance Liquid Chromatographic Method with Post-column Derivatisation and Immunoaffinity Column Cleanup. https://www.cen.eu/cen/Sectors/TechnicalCommitteesWorkshops/CENTechnicalCommittees/Pages/Standards.aspx?param=6256&title=CEN/TC%20275 (2007).
- 35.Kortei NK, Annan T, Akonor PT, Seidu AR, Annan HA, Wiafe-Kwagyan M, Ayim-Akonor M, Akpaloo PG. Aflatoxins in randomly selected groundnuts (Arachis hypogaea) and its products from some local markets across Ghana: Human risk assessment and monitoring. Toxicol. Rep. 2021;8:186–195. doi: 10.1016/j.toxrep.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dos Santos JS, Souza TM, Ono EYS, Hashimoto EH, Bassoi MC, de Miranda MZ, Itano EN, Kawamura O, Hirooka EY. Natural occurrence of deoxynivalenol in wheat from Paraná State Brazil and estimated daily intake by wheat products. Food Chem. 2013;138:90–95. doi: 10.1016/j.foodchem.2012.09.100. [DOI] [PubMed] [Google Scholar]
- 37.Ismail A, Riaz M, Levin RE, Akhtar S, Gong YY, Hameed A. Seasonal prevalence level of aflatoxin M1 and its estimated daily intake in Pakistan. Food Control. 2016;60:461–465. doi: 10.1016/j.foodcont.2015.08.025. [DOI] [Google Scholar]
- 38.Ishikawa AT, Takabayashi-Yamashita CR, Ono E, Bagatin AK, Rigobello FF, Kawamura O, Hirooka EY, Itano EN. Exposure assessment of infants to Aflatoxin M1 through consumption of breast milk and infant powdered milk in Brazil. Toxins. 2016;8:246. doi: 10.3390/toxins8090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.EFSA European Food Safety Authority Opinion of the scientific panel on contaminants in the food chain on a request from the commission related to the potential increase of consumer health risk by a possible increase of the existing maximum levels for aflatoxins in almonds, hazelnuts, and pistachios, and derived products. EFSA J. 2007;446:1–127. [Google Scholar]
- 40.WHO. http://www.who.int/healthinfo/globalburdendisease/2004reportupdate/en/index.html (2008).
- 41.Ofori-Asenso R, Agyeman AA. Hepatitis B in Ghana: A systematic review& meta-analysis of prevalence studies (1995–2015) BMC Infect. Dis. 2016;16:130. doi: 10.1186/s12879-016-1467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinto M, Spadaccino G, Palermo C, Centonze D. Determination of aflatoxins in cereal flours by solid-phase microextraction coupled with liquid chromatography and post-column photochemical derivatization-fluorescence detection. J. Chromatogr. A. 2009;1216:8636–8641. doi: 10.1016/j.chroma.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 43.Glover-Amengor M, et al. Nutritional status of children 0–59 months in selected intervention communities in northern Ghana from the Africa RISING project in 2012. Arch. Public Health. 2016;74:12. doi: 10.1186/s13690-016-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abeshu MA, Lelisa A, Geleta B. Complementary Feeding: Review of recommendations, feeding practices, and adequacy of homemade complementary food preparations in developing countries: Lessons from Ethiopia. Front. Nutr. 2016;3:41. doi: 10.3389/fnut.2016.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biritwum RB, Gyapong J, Mensah G. The epidemiology of Obesity in Ghana. Ghana Med. J. 2005;39(3):82–85. [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. World Health Organization. “The global burden of disease: 2004 update,” 2008 (2006). https://www.who.int/childgrowth/standards/Technical_report.pdf (2006).
- 47.Afrifa-Anane E, Agyemang C, Codjoe NA, Ogedegbe G, De-Graft Aikins A. The association of physical activity, body mass index and the blood pressure levels among urban poor youth in Accra, Ghana. BMC Public Health. 2015;15(269):1–9. doi: 10.1186/s12889-015-1546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walpole SC, Prieto-Merino D, Edwards P, Cleland J, Stevens G, Roberts I, et al. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. doi: 10.1186/1471-2458-12-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuiper-Goodman T. Uncertainties in the risk assessment of three mycotoxins: Aflatoxin, ochratoxin, and zearalenone. Can. J. Physiol. Pharmacol. 1990;68:1017–1024. doi: 10.1139/y90-155. [DOI] [PubMed] [Google Scholar]
- 50.Kpodo KA, Sørensen A, Jakobsen M. The occurrence of mycotoxins in fermented maize products. Food Chem. 1996;56:147–153. doi: 10.1016/0308-8146(95)00155-7. [DOI] [Google Scholar]
- 51.James B, Cardwell K, Adda C, Annang D, Hell K, Korie S, Edorh M, Gbeassor F, Nagatey K, Houenou G. Public information campaign on aflatoxin contamination of maize grains in market stores in Benin, Ghana and Togo. Food Addit. Contam. B Surveill. 2007;24(11):1283–2129. doi: 10.1080/02652030701416558. [DOI] [PubMed] [Google Scholar]
- 52.Nduti NN, Njeru PN, Mwaniki M, Reid G. Aflatoxin variations in maize flour and grains collected from various regions of Kenya. Afr. J. Food Agric. Nutr. Dev. 2017;17(1):11743–11756. doi: 10.18697/ajfand.77.16875. [DOI] [Google Scholar]
- 53.Sewram V, Shephard GS, Marasas WFO, Castro MF. Improving extraction of Fumonisin mycotoxins from Brazilian corn-based infant foods. J. Food Prot. 2003;66(5):854–859. doi: 10.4315/0362-028X-66.5.854. [DOI] [PubMed] [Google Scholar]
- 54.Kumi J, Mitchell NJ, Asare GA, Dotse E, Kwaa F, Phillips TD, et al. Aflatoxins and fumonisins contamination of home-made food (weanimix) from cereal-legume blends for children. Ghana Med. J. 2014;3:48. doi: 10.4314/gmj.v48i3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yazdanpanah H, Mohammadi T, Abouhossain G, Cheraghali AM. Effect of roasting on degradation of aflatoxins in contaminated pistachio nuts. Food Chem. Toxicol. 2005;43:1135–1139. doi: 10.1016/j.fct.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Cotty PJ, Jaime-Garcia R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007;119:109–115. doi: 10.1016/j.ijfoodmicro.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 57.Atehnkeng J, Ojiambo PS, Cotty PJ, Bandyopadhyay R. Field efficacy of a mixture of toxigenic Aspergillus flavus Link: Fr vegetative compatibility groups in preventing aflatoxin contamination in maize (Zea mays L.) Biol. Control. 2014;72:62–70. doi: 10.1016/j.biocontrol.2014.02.009. [DOI] [Google Scholar]
- 58.Wu F, Liu Y, Bhatnagar D. Cost-effectiveness of aflatoxin control methods: Economic incentives. Toxin Rev. 2008;27:203–225. doi: 10.1080/15569540802393690. [DOI] [Google Scholar]
- 59.Mehl HL, Jaime R, Callicott KA, Probst C, Garber NP, Ortega-Beltran A, Grubisha LC, Cotty PJ. Aspergillus flavus diversity on crops and in the environment can be exploited to reduce aflatoxin exposure and improve health. Ann. N. Y. Acad. Sci. 2012;1273:7–17. doi: 10.1111/j.1749-6632.2012.06800.x. [DOI] [PubMed] [Google Scholar]
- 60.Bandyopadhyay R, Ortega-Beltran A, Akande A, Mutegi C, Atehnkeng J, Kaptoge L, Senghor LA, Adhikari BN, Cotty PJ. Biological control of aflatoxins in Africa: current status and potential challenges in the face of climate change. World Mycotoxin J. 2016;9:771–789. doi: 10.3920/wmj2016.2130. [DOI] [Google Scholar]
- 61.Bandyopadhyay R, Atehnkeng J, Ortega-Beltran A, Akande A, Falade TDO, Cotty PJ. “Ground-truthing” efficacy of biological control for aflatoxin mitigation in farmers fields in Nigeria: From field trials to commercial usage. Front. Microbiol. 2019 doi: 10.3389/fmicb.2019.02528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mugo RMJ, Ininda M, Okoola RE. Interannual variability of onset and cessation of the long rains in Kenya. J. Meteorol. Relat. Sci. 2016;9:30–47. doi: 10.20987/jmrs.3.05.2016. [DOI] [Google Scholar]
- 63.Kebede H, Abbas HK, Fisher DK, Bellaloui N. Relationship between aflatoxin contamination and physiological responses of corn plants under drought and heat stress. Toxins. 2012;4(11):1385–1403. doi: 10.3390/toxins4111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith LE, Stasiewicz M, Hestrin R, Morales L, Mutiga S, Nelson RJ. Examining environmental drivers of spatial variability in aflatoxin accumulation in Kenyan maize: Potential utility in risk prediction models. Afr. J. Food Agric. Nutr. Dev. 2016;16(3):11086–11105. [Google Scholar]
- 65.Sserumaga JP, Ortega- Beltran A, Wagacha JM, Mutegi CK, Bandyopadhyay R. Aflatoxin-producing fungi associated with pre-harvest maize contamination in Uganda. Int. J. Food Microbiol. 2020;313:108376. doi: 10.1016/j.ijfoodmicro.2019.108376. [DOI] [PubMed] [Google Scholar]