Abstract
B cell lineage acute lymphoblastic leukemia is the most common leukemia occurring in children and young adults and is the leading cause of cancer related deaths. The 5 year overall survival outcome in children with B-ALL has improved significantly in the last few decades. In the past, the discovery of various genetic alterations and targeted therapy have played a major role in decreasing disease-related deaths. In addition, numerous advances in the pathogenesis of B-ALL have been found which have provided better understanding of the genes involved in disease biology with respect to diagnostic and prognostic implications. Present review will summarize current understanding of risk stratification, genetic factors including cytogenetics in diagnosis and prognosis of B-ALL.
Keywords: Cytogenetic risk groups , Copy number alterations, Targeted therapy
Introduction
B cell lineage acute lymphoblastic leukaemia is the most common neoplasm in children and young adults occurring due to clonal proliferation of lymphoid progenitor cells. Majority of children with B-ALL are characterized by distinct constellations of structural and submicroscopic genetic alterations simultaneously. Each clone evolves due to the accumulation of mutations within a cell [1, 2].
B-ALL is the leading cause of cancer related death in children and young adults. It can occur in adults as well, though with lesser frequency and poor survival outcome. In 2016, World health organization (WHO) reported the annual incidence of ALL worldwide as 1–4.75 cases/100,000 population with 80–85% being B-ALL [3].
The 5 year overall survival outcome in children with B-ALL has improved significantly from 10% to up to 90%, in last 60 years [4]. The outcome of the disease is dependent on treatment and risk stratification. In the past, the discovery of genetic alterations like BCR-ABL1 and subsequent development of risk stratification strategies and targeted therapy have played a major role in decreasing disease-related deaths.
There have been numerous advances in the pathogenesis of B-ALL which have provided better understanding of the genes involved in disease biology with respect to diagnostic and prognostic implications.
Present review will summarize current understanding of risk stratification, genetic factors including cytogenetics in diagnosis and prognosis of B-ALL.
WHO Classification-What’s New?
In 2016, world health organization, added two provisional subcategories to the existing classification of 2008 which included B-ALL, not otherwise specified (NOS) and B-ALL with recurrent genetic abnormalities (B-ALL with hyperdiploidy, B-ALL with hypodiploidy, B-ALL with BCR-ABL1 translocation, B-ALL with ETV6-RUNX1 and B-ALL with IL3-IGH translocation). The categories include intrachromosomal amplification of chromosome 21 (iAMP21) and BCR-ABL1 like ALL (Table 1).
Table 1.
Cytogenetic alterations in childhood B-acute lymphoblastic leukemia
| Risk Group | Alteration | Frequency | References | |
|---|---|---|---|---|
| India | Western Countries | |||
| Good risk | High hyper diploidy | 12–27 | 20–30% | [4, 26] |
| t(12;21)(p13;q22) | 6–12% | 25–30% | ||
| Intermediate risk | t(1;19)(q23;p13)/TCF3-PBX1 | 2–7 | 5–6 | [3, 26] |
| t(5;14)(q31;q32)/IL3-IgH | – | < 1% | ||
| Poor risk | Hypoploidy | Upto 8% | 2–5% | [26, 27] |
| t(9;22)(q34;q11) | 6–10% | 2–5% | ||
| iAMP21 | – | 2% | ||
| t(17;19)(q22;p13)/TCF3-HLF | 0.6% | 0.1% | ||
| T(v;11q23)/KMT2A rearrangements | 1–3% | 5–8% | ||
Hyperdiploidy
Hyperdiploidy (> 47 chromosomes) is the most frequently occurring genetic alteration in B-ALL, seen in up to 20–30% of children and 5% of adults worldwide. High hyperdiploidy (50–67 chromosomes) is characterized by nonrandom gains of chromosomes 21, X, 14, 6, 18, 4, 17, and 10, in the order of their frequency of occurrence. Presence of additional chromosome 4 and 10 is reported to be associated with better prognosis [5, 6]. The probable underlying mechanisms for causation of hyperdiploidy include endoreduplication due to silencing of cell cycle controlling genes and weakness of the mitotic spindle due to some unknown mechanism. Children with ALL with hyperdiploidy respond better to chemotherapy [7]. The plaussible mechanisms for better response include, decreased apoptotic threshold, upregulation of cell death pathways through increased gene copy numbers and increased immunosurveillance due to constitutive endoplasmic reticulum stress response [8, 9].
Hypodiploidy
Hypodiploid ALL is associated with poor prognostic outcome [10] and is seen in 6–7% of children with ALL [11]. There are predominantly three subtypes of hypodiploid ALL: Near-haploid ALL (NH ALL) with 24–31 chromosomes, low-hypodiploid ALL (LH ALL) with 32–39 chromosomes, High hypodiploid ALL (HH ALL) with 40–43 chromosomes and a distinct group, near diploid ALL (ND ALL) with 44 or 45 chromosomes [12]. The possible genetic basis of poor prognostic outcome with hypodiploid ALL is based on the premise that loss of heterozygosity resulting from hypodiploidy leads to unmasking of recessive alleles.
NH ALL is characterized by Ras activating mutations, including those of NF1, NRAS, KRAS, and PTPN11 and inactivation of IKZF3. LH ALL is characterized by biallelic alteration of TP53, deletions of CDKN2A/B and RB1, and inactivation of IKZF2. NH ALL and LH ALL are seen in 0.5% each of childhood ALL [13]. Near diploid ALL are frequently associated with ETV6-RUNX1 fusion or rearrangements forming dicentric chromosomes [14]. Children with near diploid ALL do not have poor prognostic outcome. NH ALL and LH ALL may undergo doubling of the hypodiploid clone and the chromosome number may fall in the hyperdiploid or triploid range leading to masking of hypodiploidy. It needs to be distinguished from hyperdiploidy during risk stratification as the outcome may be very different [15].
BCR-ABL1; t(9;22)(q34.1;q11.2) Translocation
BCR-ABL1; t(9;22)(q32;q11) translocation was the first alteration to be identified in any leukemia. In 1959, Peter Nowell and David A Hungerford demonstrated abnormally small chromosome 22 and referred to this chromosome as ‘a minute chromosome’ in their classical paper entitled ‘A minute chromosome in human chronic granulocytic leukemia [16]. The t(9;22)(q32;q11) translocation is due to reciprocal exchange of chromosomal material between chromosomes 9 and 22. The breakpoints within the BCR gene are found in three defined regions. Major breakpoint cluster region (M-bcr), minor breakpoint cluster region (m-bcr) and micro breakpoint cluster region (µ-bcr) which encode for three main BCR-ABL fusion oncoproteins of distinct molecular weights, p210, p190 and p230, respectively [17].
The p210 isoform is expressed in up to 33% of adult Ph + B-ALL, while 66% of adult Ph + B-ALL express p190 isoform. Approximately, 90% of childhood Ph + B-ALL express p190. The Ph chromosome in B-ALL, in particular in childhood ALL, arises in lymphoid lineage precursors. p210BCR-ABL and p190BCR-ABL are pleiotropic molecules with many qualitatively similar activities [18]. p190BCR-ABL has higher tyrosine kinase activity leading to more aggressive phenotype while p210BCR-ABL is more associated with more indolent chronic leukemia phenotype [19].
BCR-ABL1 translocation is associated with poor survival outcome and is more prevalent in Indian children (upto 15%) with B-ALL [20–22] as compared to that reported in western literature (2–5%) [4].
ETV6-RUNX1; t(12;21)(p13.2;q22.1) Translocation
The ETV6-RUNX1 t(12;21)(p13.1;q22) translocation, is the most common genetic lesion seen in childhood ALL. It is seen in 20–30% of pediatric and ∼ 3% of adults B-ALL patients [23].
In this translocation, the ETV6 (or TEL) gene on chromosome 12p joins RUNX1 (AML1) gene on chromosome 21. It is a karyocryptic translocation, discovered accidentally on chromosome painting in 1994. t(12;21)(p13;q22) originates from microclustered breakpoint regions of ETV6 intron 5 and RUNX1 intron 1 or 2, resulting in a ETV6-RUNX1 fusion gene [24]. This translocation is seen in up to 1% of unselected population which signifies that the frequency of this translocation is 100 times the incidence rate of ALL with ETV6-RUNX1, thereby suggesting that generation of a functional ETV6-RUNX1 fusion is insufficient to generate overt ALL and additional secondary mutation is necessary [25].
ETV6-RUNX1 t(12;21)(p13.1;q22) translocation is a good risk cytogenetic alteration and is less frequent in Indian children (6–12%) [20–22, 26] with ALL as compared to that reported in western literature (25–30%) [4].
KMT2A rearrangements; t(11q23.3;v) Translocation
The KMT2A (lysine methyl transferase 2A) gene, previously known as MLL or mixed leukemia lymphoma gene rearrangements are potent leukemia precursors and require few cooperating lesions to induce leukemia transformation. The concordance rate for KMT2A rearrangements is close to 100% in identical twins [27]. The common KMT2A partners in ALL include t(4;11)(q21;23);(KMT2A-AF4), t(9;11) (p23;q23);(KMT2A-AF9) and t(11;19)(q23;p13.3);(KMT2A-ENL). The t(4;11)(q21;23); (KMT2A-AF4) is the most common translocation in ALL with major peak in infancy (up to 70%). It has poor prognosis with low overall survival (median survival 10 months). t(9;11) (p23;q23);(KMT2A-AF9) and t(11;19)(q23;13.3);( KMT2A -ENL) occur more frequently in infants than children and adults. Children with KMT2A gene rearrangements have better prognosis in older children (1–9 years) as compared to that in infants. KMT2A rearrangements are less frequent in Indian children with B-ALL (1–3%) [26].
TCF3-PBX1, t(1;19)(q23;p13.3) Translocation
The TCF3-PBX1, t(1;19)(q23;p13.3) translocation, more common in children than adults, is seen in approximately 6% of childhood B-ALL. TCF3 (E2A gene) forms chimeric gene after fusion either with PBX1 in TCF3-PBX1, t(1;19)(q23;p13.3) translocation or with TCF3-HLF (E2A-HLF), t(17;19)(q22;p13) in ALL. TCF3 gene encodes E12 and E47, produced by differential splicing. TCF3 is required at the earliest detectable stage of B cell commitment and regulates Ig class switch recombination in peripheral mature B cells at later stages of B cell maturation.
t(17;19)(q22;p13); TCF3-HLF translocation is predominantly found in 1% of childhood B-ALL and is rarely observed in adults. TCF3-HLF translocation is associated with poor prognostic outcome despite intensive chemotherapy [28]. Two different genomic rearrangements that generate TCF3-HLF have been described. Both the rearrangements involve intron 3 of HLF gene. In type I rearrangement, second breakpoint is in intron 13 of TCF3 gene while in type II rearrangements it is in intron 12. Clinically, type 1 rearrangements are associated with disseminated intravascular coagulation (DIC) while type II rearrangements are present with hypercalcemia [29].
IgH-IL3, t(5;14)(q31.1; q32.1) Translocation
IgH-IL3 fusion due to t(5;14)(q31.1; q32.1) translocation leads to regulation of IL3 gene by enhancer region of IgH chain gene, resulting in overexpression of IL3. Patients with this translocation clinically present with reactive eosinophilia [3].
Intrachromosomal Amplification of Chromosome 21(iAMP21)
Intrachromosomal amplification of chromosome 21(iAMP21) shows single copy of chromosome 21 comprising multiple regions of gain, amplification, inversion and deletion. The gain of 3 or more copies of part of chromosome 21 incorporating RUNX1 (5 or more signals of RUNX1) is diagnostic of iAMP21. It is seen in up to 2% of B-ALL. iAMP is postulated to arise due to multiple break fusion bridge cycles, secondary to an initiating event, most commonly proposed to be loss or dysfunction of telomeric region [30–32]. The common region of amplification ranges from 32.8 to 37.9 Mb with the highest level identified to be spanning 5.1 Mb of chromosome 21, within which the RUNX1 gene is located [33]. iAMP 21 is associated with poor outcome [34]. Though iAMP21 is usually exclusive of recurrent chromosomal translocation, it is seen in small number of ALL with ETV6-RUNX1, BCR-ABL1 or high hyperdiploidy. The risk of iAMP21 is increased in individuals with constitutional Robertsonian t(15;21) translocations [35]. Children with iAMP can be detected using fluorescent in situ hybridization probe for RUNX1 on chromosome 21. Presence of five or more signals of the gene or three or more extra copies on single chromosome 21 will imply intrachromosomal amplification of chromosome 21.
BCR-ABL1 Like ALL
BCR-ABL1 like ALL (Ph like ALL) is a complex subgroup of BCR-ABL1 negative ALL with range of genomic alterations that activate cytokine receptor genes and tyrosine kinase signaling pathways. BCR-ABL1 like ALL is seen in up to 10–15% of children and 20–27% of young adults and they requently harbor IKZF1 alterations (30%) [36]. Children with BCR-ABL1 like ALL have higher relapse rate.
There are five distinct subgroups of BCR-ABL1 like ALL based on the type of cytokine receptor or kinase fusion present including rearrangements of CRLF2, ABL-class gene rearrangements, JAK2 and EPOR rearrangements, sequence mutations or deletions activating JAK-STAT- or MAPK signaling pathways, and other rare kinase alterations.
Rearrangements of CRLF2 are the most frequent alterations in BCR-ABL1 like ALL and are seen in upto 42% of children with B-ALL. Half of patients with CRLF2 have JAK1 or JAK2 alterations. ABL-class gene rearrangements are seen in 14% of BCR-ABL1 like ALL and involves rearrangements of ABL1, ABL2, PDGFRB and CSF1R. ABL class rearrangements are sensitive to kinase inhibitors like imatinib while JAK2 mutations respond to ruxolitinib [37]. JAK2 and EPOR rearrangements together constitute 11% of BCR-ABL1 like ALL and lead to constitutive activation of JAK-STAT signaling pathways. JAK2 mutations are more prevalent in adults with ten different fusion partners as compared to children. EPOR rearrangements have been identified in ~ 4% of BCR-ABL1 like ALL [38]. Sequence mutations and focal deletions of JAK2 genes leading to activation of the JAK-STAT pathway are present in 13% of BCR-ABL1 like ALL. Kinase-activating lesions were not identified in the remaining 5% of BCR-ABL1 like ALL [39].
Newer Genetic Alterations
In addition to the cytogenetic alterations, alterations in the many genes have been identified which encode proteins with key roles in lymphoid development and differentiation (eg, PAX5, IKZF1, EBF1, and LMO2), cell-cycle regulation and tumour suppression (CDKN2A/B, PTEN, and RB1), lymphoid signaling (BTLA, CD200, TOX, and the glucocorticoid receptor NR3C1), and transcriptional regulation and co-activation (ETV6 and ERG) [2].
IKAROS Zink Finger Family 1 (IKZF1) Gene
IKZF1 gene, located on chromosome 7p12.2 is 101 kb in size and consists of eight exons [40). It encodes for IKAROS protein. It is expressed in hematopoietic progenitor populations, pluripotent and self-propagating hematopoietic stem cells IKZF1 gene activates signaling molecules (FLT3 and IL7R), lymphoid associated transcription factors (EBF1) and suppresses myeloid associated transcription factors (Sfpi 1and Gfi 1) in hematopoietic stem cells. IKZF1 increases activation threshold for mature B cells and regulates isotype selection during class switch recombination beyond pre-B cell stage [41].
IKZF1 alterations are found in up to 10–15% of B-ALL and 4% of T-ALL population. Deletions are responsible for up to 90% of cases of IKZF1 alterations leading to acute lymphoblastic leukemia, rest being point mutations [1]. Haploinsufficiency with exon 2–7 deletions or complete absence of IKZF1 gene is the most common functional group and is seen in up to 55% of B-ALL with IKZF1 deletions. Exon 4–7 deletions, leading to the formation of functionally dominant negative Ik6 isoform, comprise of 33% of IKZF1 deletions. It lacks DNA binding domain and nuclear localization sequences, leading to accumulation in cytoplasm. Since dimerization domain is intact, it can heterodimerize with other proteins of the same family but cannot bind to DNA, leading to dominant negative effect. It leads to more severe phenotype than haploinsufficiency. There can also be biallelic deletions, leading to complete absence of IKAROS protein [40].
IKZF1 alterations are present at a much higher frequency in high-risk BCR-ABL1 positive B-ALL (66–75%) in childhood ALL and are associated with increased frequency of relapse and resistance to chemotherapy [42]. Haploinsufficiency accelerates the onset of B cell leukemia in p190 BCR-ABL1 B-ALL [43]. IKZF1 gene alterations may occur in association with activating JAK2 point mutations that have high expression of the CRLF2 cytokine receptor, as a result of CRLF2 gene rearrangements. A large fraction of the remaining IKZF1 mutated B-ALL, display a BCR-ABL1 like gene-expression signature, characterized by other variants of ABL1 and JAK2 translocation or gene lesions in IL7R. Alterations of IKZF1 are associated with poor clinical outcome and act as a strong predictor of relapse [44]. Yeoh et al.recommended intensification of treatment for children with IKZF1 deletion [45].
Cytokine Receptor Like Factor-2 Gene
Cytokine Receptor Like Factor 2 (CRLF2), a type I cytokine receptor subunit, also known as thymic stromal lymphopoietin receptor (TSLPR). CRLF2 gene is located in the pseudoautosomal regions1 (PAR1) of chromosomes X and Y at Xp22.3/Yp11.3.and include SHOX, CSF2RA, IL3RA and P2RY8 [45] genes. TSLP promotes early B cell development and overexpression is known to stimulate the growth of human B-ALL [46].
CRLF2 overexpression is noted in 7% of patients with B-ALL, 15% of B-ALL that lack recurrent rearrangements, 53% of ALL children with down syndrome 27–50% of children with BCR-ABL1 like ALL and in 25% patients with iAMP 21 [47]. CRLF2 overexpression can result from a CRLF2-P2RY8 fusion due to 320-kb interstitial deletion centromeric of CRLF2 gene which places CRLF2 close to promoter region of G-protein-coupled purinergic receptor P2RY8 gene, translocation with highly active IgH chain gene at chromosome 14q32.33 and Phe232Cys, gain-of-function mutation. Phe232Cys mutation promotes constitutive dimerization. CRLF2 expresses independently without dimerizing with IL7R [47]. CRLF2 overexpression is also seen in those without above mentioned alterations.
CRLF2 overexpression is associated with JAK2 mutations in 50% of patients but all cases with JAK2 mutations overexpress CRLF2. Activation of JAK-STAT signaling by CRLF2 mutation, CRLF2 with mutant JAK2, or CRLF2 with another partner mimics BCR-ABL1 signaling [48].
Cyclin Dependent Kinase inhibitor 2A/B Genes
The deletions of chromosomal region 9p have been documented in 18–45% children with acute lymphoblastic leukemia [49]. The chromosomal region 9p21 harbours CDKN2A and CDKN2B genes. CDKN2A gene (also known as CDKN2, MTS1 and CDK4I), a tumour suppressor gene on 9p21.3, encodes p16INK4a and ARF transcripts. P16INK4a competes with cyclin D for binding to CDK4 and inhibits Cyclin D- CDK4 complex from phosphorylation of RB gene, causing cell cycle arrest at G1-S phase while ARF causes inhibition of E3 ubiquitin ligase HDM2 in turn causing activation of p53 gene. Loss of heterozygosity (LOH) of chromosome arm 9p, is one of the most frequent genetic events in childhood ALL. Haploinsufficiency of CDKN2A, has been shown to be adequate to promote tumour progression. Homozygous deletion of CDKN2A is the dominant mechanism of its inactivation in leukemogenesis. The incidence of CDKN2A/B gene alteration was found to be significantly higher in T-ALL as compared to B lineage ALL, more so for biallelic deletions [50].
Some of these studies have found significant association of CDKN2A/B gene studies with poor survival outcome while others have failed to show such association [51, 52].
Recently, pharmacological inhibitor of cyclin dependent kinase 4/6 (CDK4/6) have been developed (palbociclib, ribociclib and abemaciclib). Palbociclib has been approved by Food and Drug Administration for use in breast carcinoma. However, use of palbociclib in acute lymphoblastic leukemia are under clinical trials (www.cancer.gov) [53].
PAX5 Gene
PAX5 gene, located on 9p13, is involved in the hematopoietic system and cell differentiation [54]. The expression of PAX5 gene is initiated in pre-pro-B cells and maintained throughout subsequent stages of B cell development and down-regulated in plasma cells. PAX5 deletions have been identified in over 30% of children with B-ALL. However, PAX5 deletions are shown not to be associated with outcome. PAX5 deletions are commonly associated with rearrangements like ETV6 (12p13) and JAK2 (9p24) in about 2.5% of patients. PAX5 deletions are shown to increase the inherited susceptibility of B-ALL [55, 56].
RB1 gene
RB1 gene located on chromosome 13q14.12, is the predominant regulatory gene for cell cycle regulation during G1-S phase [57]. RB protein is a chromatin-associated protein which limits transcription of genes required for cell proliferation primarily via E2F transcription factor. The transcription factor E2F binds to the promoter region of DNA polymerase subunits, cyclin A and cyclin E genes and expression of genes takes place. RB1 gene is also implicated in apoptosis, DNA repair, chromatin modification, and differentiation. Inactivation of Rb gene function disrupts the cell cycle regulation and compromises the ability of the cell to exit cell cycle making it susceptible to oncogenic proliferation. RB1 gene deletions are known in 2–4% children with ALL [58]. RB1 gene is frequently deleted (39%) in children with iAMP21 and LHALL.
BTG1 Gene
BTG1 (B cell translocation) gene, located on chromosome 12q21.33, is an anti-proliferative gene family that regulates cell growth and differentiation. Expression of this gene is highest in the G0/G1 phases of the cell cycle and down-regulated when cells enter the cell cycle. The BTG protein functions as a coactivator of cell differentiation. BTG1 gene deletions are seen in up to 10% children with ALL and are known to be clustered with ETV6-RUNX1 (15%) translocations in children with ALL [59].
EBF1 Gene
EBF1 (Early B cell factor) gene is located on chromosome 5q33.3, is a tissue-specific and differentiation stage-specific DNA-binding protein that participates in the regulation of the pre-B and B lymphocyte-specific MB1. The translocation involving EBF1 with PDGFRB is seen associated with BCR-ABL1 like ALL in the absence of BCR-ABL1 translocation [60].
Risk Stratification in ALL
Presently, risk stratification is based on certain factors including age, leukocyte count at presentation, CNS/testicular infiltration, chromosomal alterations, response to prednisolone therapy and presence of minimal residual disease. Among cytogenetic alterations, children with Hyperdiploidy and ETV6-RUNX1 translocation are risk stratified with good risk cytogenetics while those with, BCR-ABL1 translocation, hypoploidy, KMT2A rearrangements, TCF3-HLF translocation, iAMP21 and BCR-ABL1 like rearrangements are stratified as high risk ALL.
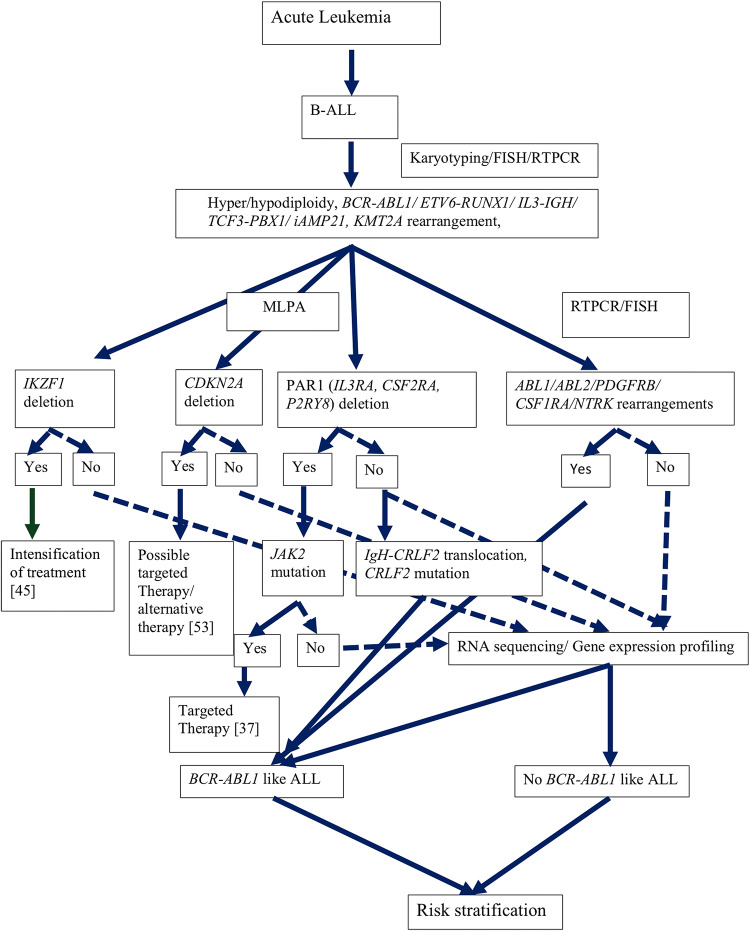
Bone marrow karyotyping should be done for all the children for chromosomal analysis for ploidy as well as recurrent and random translocations. Apart from karyotyping, ploidy status can also be detected using flow cytometry and fluorescent in situ hybridization (FISH). By flow cytometry DNA quantity is measured as DNA index which is a ratio of sample peak channel to reference peak channel. A normal diploid cell will have DNA index of 1.0. DNA index in high hyperdiploidy is more than 1.16 [61]. In the absence of karyotype, FISH can be also be used for hyperdiploidy using centromeric probes for common chromosome gains [62]. However, for recurrent chromosomal rearrangements, FISH is the method of choice. Reverse transcriptase PCR (RT-PCR) can also be performed for recurrent translocations. Patients negative for conventional translocations (BCR-ABL1, MLL rearrangement, TCF3-PBX1, ETV6-RUNX1, iAMP21) should be investigated for BCR-ABL1 like translocations. Some studies have recommended that all patients should be tested for at least IKZF1 and CDKN2A gene deletions and overexpression of CRLF2 gene. Deletions of these genes as well as those of PAR1, may be tested with multiplex ligation dependent probe amplification assay (MLPA). PAR1 gene (IL3RA, CSF2RA and P2RY8) gene deletions lead to overexpression of CRLF2 gene. IgH-CRLF2 alterations may be tested using FISH while mutation of CRLF2 gene can be identified by ARMS-PCR. CRLF2 is a surface membrane receptor and expression can be detected by flow cytometry and by mRNA quantification. Patients with CRLF2 rearrangement should be tested for JAK2 mutations as these are seen in upto 50% of patients with CRLF2 rearrangements. Those negative for CRLF2 gene rearrangements should be tested for other genes involved in BCR-ABL1 like alterations including ABL1, ABL2, CSF1R, JAK2, NTRK3 and PDGFRB these can be tested by RT-PCR and confirmed by FISH. Kinase fusion gene rearrangements can be tested using break apart probes (e.g. ABL1/ABL2/CSF1R/JAK2/NTRK3/PDGFRB) rearrangements and specific translocations can be demonstrated using locus specific probes (JAK2-ETV6/ JAK2-PAX5). The patients negative for these common gene alterations may tested by RNA sequencing followed by exome/genome sequencing after segregation of blasts (Fig. 1) [36].
Fig. 1.
Approach to molecular diagnosis in B-ALL (continuous line represents definite responses; dotted lines represent response requiring further investigations
Children with acute lymphoblastic leukemia are monitored for response to treatment with response to prednisolone therapy and minimal residual disease assessment. Response to prednisolone therapy is assessed at day 8 as good prednisolone response (peripheral blasts < 1000 blasts/µL and poor prednisolone response (> 1000 blast/µL).
MRD is the single most powerful predictor of relapse in childhood ALL and assessed at the end of induction chemotherapy [63]. MRD can be measured by three methods including i) ASO-PCR MRD method which is, based on the principle that Immunoglobulin genes (V, D, and J segment) and TCR gene rearrangements occur during B and T cell development. Deletion and random insertion of nucleotides at the junctional sites generate unique gene sequences for each cell and its progeny. T cell receptor gene rearrangements are found in up to 90% of B-lineage ALL patients, and immunoglobulin gene rearrangements can be found in about 20% of T-lineage ALL due to recombination activity [64]. ii). Real time PCR amplification of oncogenic fusion transcripts which is a sensitive (10−4–10−6) technique but can be used where recurrent cytogenetic alterations were found at initial presentation, hence, limiting it’s utility. The number of target gene copies must be normalized using a ubiquitously expressed housekeeping gene as a reference, with the number of chimeric transcripts expressed as ratio of the number of copies of the reference gene transcripts and iii). Flowcytometric assessment is based on CD markers expression on cell surface and is used for assessing clonal origin. Positive MRD or presence of residual disease is considered if blasts are ≥ 0.01% (10–4) of total mononuclear cells. This method is technically simple, rapid and cost effective though less sensitive than PCR based tests and requires fresh sample with viable cells [65].
Novel Targeted Therapy
Recently, targeted therapy has occupied a central stage in the treatment of leukemia. Newer drugs have also been suggested in the treatment of leukemia (Table 2).
Table 2.
Targeted therapy in acute lymphoblastic leukemia
| Group | Drug | Mechanism of action | usage |
|---|---|---|---|
| Kinase inhibitors [62] | Imatinib [63] | Binds to ATP binding site of ABL tyrosine kinase | Ph + ALL |
| Dasatinib [63] | Binds to ATP binding site of ABL tyrosine kinase. Also inhibits Serine kinase inhibitor | Ph + ALL. Useful in Imatinib resistant ALL. Also inhibits cKIT and PDGFRB | |
| MK0457 [64] | Binds to Aurora family serine-threonine kinases | Phase II study for T315I mutant CML and Ph + ALL | |
| Fms-like tyrosine kinase inhibitors (FLT3) inhibitors | Lestaurtinib (CEP-701) [65] | Inhibits FLT3, JAK2 and tropomyosin receptor kinases | FDA approved (Orphan drug) in AML |
| Sunitinib [66] | Targets receptors for PDGF and VEGF | FDA approved for Renal cell Carcinoma and imatinib resistant GIST | |
| Proteasome inhibitor [67] | Bortezomib (Velcade) [68] | Induces G2–M cell cycle arrest and apoptosis by causing Bcl-2 phosphorylation and cleavage | FDA approved for multiple myeloma |
| Carfilzomib [69] | Inhibits MAPK signaling, increased sensitivity to chemotherapy | FDA approved for multiple myeloma | |
| JAK2 inhibitor [70] | Ruxolitinib [71] | Binds JAK2 kinase domain in both wild and mutated JAK2 | FDA approved for intermediate & high risk myelofibrosis |
| Monoclonal antibodies [72] | Rituximab (Rituxan) [73] | Monoclonal Antibody against CD20. Direct signaling, complement dependent cellular cytotoxicity and antibody dependent cellular cytotoxicity | FDA approved in NHL |
| Epratuzumab [74] | Monoclonal Antibody against CD22. Antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity and direct induction of apoptosis | Under clinical trial for ALL | |
| Blinatumomab [75] | Bispecific Ab (BiTE), T cell engager Ab, Directs CD3+ against CD19+ B-ALL | US FDA approved drug for B-ALL in first or second complete remission with MRD ≥ 0.1% | |
| Histone deacetylase inhibitors [76] | Depsipeptide (FK228) [77] | Synergistic with DNA methyl transferase inhibitors; Upregulates CDKN2A (p21). Down regulates cyclin D1 and D2 | FDA approved for CTCL |
| Vorinostat (Zolinza) [78] | Chelator for Zinc ions in active site of histone deacetylases | FDA approved for CTCL | |
| Nucleoside analogue [79] | Forodesine [80] | Purine nucleoside phosphorylase (PNP) inhibitor | Orphan drug designation for use in T-ALL |
| Clofarabine [81] | Deoxyadenosine analogue. Direct inhibition of DNA synthesis | US FDA approved to treat patients age 1 to 21, relapsed or refractory B-ALL | |
| Nelarabine [82] | Pro drug of ara-G | Approved third line treatment for T-ALL | |
| Gamma secretase inhibitors [83] | MK-0752 [84] | NOTCH1 inhibitor; in T-ALL | Phase I clinical trials for T-ALL |
| mTOR inhibitors | Sirolimus [85] | Induce cell cycle arrest | US FDA approved for lymphangioleiomyomatosis (LAM) |
| CDk4/6 inhibitors | Palbociclib [51] | Ensures inhibition of cyclin D1-CDK4/6 complex formation | FDA approved for HR+, Her2− breast carcinoma |
Conclusion
To conclude, there is remarkable advancement in the diagnosis and risk allocation in patients with childhood B cell lineage acute lymphoblastic leukemia, however, deeper understanding into disease biology specially with emphasis on ethnic variability is needed. Gene expression profiling and genome wide association studies have given impetus to the understanding of genomic involvement in the pathogenesis of the disease, association of individual genes with survival outcome in different ethnic populations is yet to be seen. It is pertinent to explore the role of novel targeted therapies against each candidate gene.
Acknowledgements
The name of the department(s) and institution(s) to which the work should be attributed: Department of Laboratory sciences and Molecular medicine, Army Hospital (R&R), New Delhi, India.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manisha Agarwal, Email: manishagrwl079@gmail.com.
Rachna Seth, Email: drrachnaseth1967@gmail.com.
Tathagata Chatterjee, Email: ctathagat@hotmail.com.
References
- 1.Mullighan CG. The molecular genetic makeup of acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:389–396. doi: 10.1182/asheducation-2012.1.389. [DOI] [PubMed] [Google Scholar]
- 2.Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. The Lancet. 2013;381(9881):1943–1955. doi: 10.1016/S0140-6736(12)62187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med. 2015;373(16):1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 5.Secker-Walker LM, Lawler SD, Hardisty RM. Prognostic implications of chromosomal findings in acute lymphoblastic leukaemia at diagnosis. Br Med J. 1978;2(6151):1529–1530. doi: 10.1136/bmj.2.6151.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dastugue N, Suciu S, Plat G, Speleman F, Cave H, Girard S, et al. Hyperdiploidy with 58–66 chromosomes in childhood B-acute lymphoblastic leukemia is highly curable: 58951 CLG-EORTC results. Blood. 2013;121(13):2415–2423. doi: 10.1182/blood-2012-06-437681. [DOI] [PubMed] [Google Scholar]
- 7.Paulsson K, Johansson B. High hyperdiploid childhood acute lymphoblastic leukemia. Genes Chromosom Cancer. 2009;48(8):637–660. doi: 10.1002/gcc.20671. [DOI] [PubMed] [Google Scholar]
- 8.Carroll WL. Safety in numbers: hyperdiploidy and prognosis. Blood. 2013;121(13):2374–2376. doi: 10.1182/blood-2013-02-480350. [DOI] [PubMed] [Google Scholar]
- 9.Moorman AV, Chilton L, Wilkinson J, Ensor HM, Bown N, Proctor SJ. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood. 2010;115(2):206–214. doi: 10.1182/blood-2009-07-232124. [DOI] [PubMed] [Google Scholar]
- 10.Nachman JB, Heerema NA, Sather H, Camitta B, Forestier E, Harrison CJ, et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood. 2007;110(4):1112–1115. doi: 10.1182/blood-2006-07-038299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raimondi SC, Zhou Y, Mathew S, Shurtleff SA, Sandlund JT, Rivera GK, et al. Reassessment of the prognostic significance of hypodiploidy in pediatric patients with acute lymphoblastic leukemia. Cancer. 2003;98(12):2715–2722. doi: 10.1002/cncr.11841. [DOI] [PubMed] [Google Scholar]
- 12.Harrison CJ, Moorman AV, Broadfield ZJ, Cheung KL, Harris RL, Reza Jalali G, et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br J Haematol. 2004;125(5):552–559. doi: 10.1111/j.1365-2141.2004.04948.x. [DOI] [PubMed] [Google Scholar]
- 13.Safavi S, Paulsson K. Near-haploid and low-hypodiploid acute lymphoblastic leukemia: two distinct subtypes with consistently poor prognosis. Blood. 2017;129(4):420–423. doi: 10.1182/blood-2016-10-743765. [DOI] [PubMed] [Google Scholar]
- 14.Holmfeldt L, Wei L, Diaz-Flores E, Walsh M, Zhang J, Ding L, et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat Genet. 2013;45(3):242–252. doi: 10.1038/ng.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pui CH, Williams DL, Raimondi SC, Rivera GK, Look AT, Dodge RK, et al. Hypodiploidy is associated with a poor prognosis in childhood acute lymphoblastic leukemia. Blood. 1987;70(1):247–253. [PubMed] [Google Scholar]
- 16.Nowell PC. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;142:1497. [Google Scholar]
- 17.Eisenberg A, Silver R, Soper L, Arlin Z, Coleman M, Bernhardt B, et al. The location of breakpoints within the breakpoint cluster region (bcr) of chromosome 22 in chronic myeloid leukemia. Leukemia. 1988;2(10):642–647. [PubMed] [Google Scholar]
- 18.Reckel S, Hantschel O (2017) Bcr-Abl: one kinase, two isoforms, two diseases. Oncotarget [Internet]. 8(45). Available from: https://www.oncotarget.com/fulltext/20874. Cited 8 Jun 2018 [DOI] [PMC free article] [PubMed]
- 19.Junmei Z, Fengkuan Y, Yongping S, Baijun F, Yuzhang L, Lina L et al (2015) Coexistence of P190 and P210 BCR/ABL transcripts in chronic myeloid leukemia blast crisis resistant to imatinib. SpringerPlus [Internet]. 4(1). Available from: http://www.springerplus.com/content/4/1/170. Cited 8 Jun 2018 [DOI] [PMC free article] [PubMed]
- 20.Gupta SK, Bakhshi S, Kumar L, Kamal VK, Kumar R. Gene copy number alteration profile and its clinical correlation in B cell acute lymphoblastic leukemia. Leukemia Lymphoma. 2017;58(2):333–342. doi: 10.1080/10428194.2016.1193855. [DOI] [PubMed] [Google Scholar]
- 21.Singh M, Bhatia P, Trehan A, Varma N, Sachdeva MS, Bansal D, et al. High frequency of intermediate and poor risk copy number abnormalities in pediatric cohort of B-ALL correlate with high MRD post induction. Leuk Res. 2018;66:79–84. doi: 10.1016/j.leukres.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 22.Siddaiahgari S, Awaghad M, Latha M. Clinical, immunophenotype and cytogenetic profile of acute lymphoblastic leukemia in children at tertiary health care centre in India. Muller J Med Sci Res. 2015;6(2):112. [Google Scholar]
- 23.Pui C-H, Robison LL, Look AT. Acute lymphoblastic leukaemia. The Lancet. 2008;371(9617):1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 24.Wiemels JL, Cazzaniga G, Daniotti M, Eden OB, Addison GM, Masera G, et al. Prenatal origin of acute lymphoblastic leukaemia in children. Lancet. 1999;354(9189):1499–1503. doi: 10.1016/s0140-6736(99)09403-9. [DOI] [PubMed] [Google Scholar]
- 25.Pui C-H, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amare PSK, Jain H, Kabre S, Deshpande Y, Pawar P, Banavali S, et al. Cytogenetic Profile in 7209 Indian patients with de novo acute leukemia: a single centre study from India. J. Cancer Ther. 2016;07(07):530–544. [Google Scholar]
- 27.Ziemin-van der Poel S, McCabe NR, Gill HJ, Espinosa R, Patel Y, Harden A, et al. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA. 1991;88(23):10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 29.Safavi M, Safaei A, Lotfi M. A rare variant of t(17;19) in a case of Philadelphia positive adult acute lymphoblastic leukemia presenting with disseminated intravascular coagulation. Blood Res. 2018;53(1):92. doi: 10.5045/br.2018.53.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.On behalf of the Ponte di Legno International Workshop in Childhood Acute Lymphoblastic Leukemia. Harrison CJ, Moorman AV, Schwab C, Carroll AJ, Raetz EA, et al. An international study of intrachromosomal amplification of chromosome 21 (iAMP21): cytogenetic characterization and outcome. Leukemia. 2014;28(5):1015–1021. doi: 10.1038/leu.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moorman AV, Ensor HM, Richards SM, Chilton L, Schwab C, Kinsey SE, et al. Prognostic effect of chromosomal abnormalities in childhood B cell precursor acute lymphoblastic leukaemia: results from the UK Medical Research Council ALL97/99 randomised trial. Lancet Oncol. 2010;11(5):429–438. doi: 10.1016/S1470-2045(10)70066-8. [DOI] [PubMed] [Google Scholar]
- 32.Moorman AV, Richards SM, Robinson HM, Strefford JC, Gibson BES, Kinsey SE, et al. Prognosis of children with acute lymphoblastic leukemia (ALL) and intrachromosomal amplification of chromosome 21 (iAMP21) Blood. 2007;109(6):2327–2330. doi: 10.1182/blood-2006-08-040436. [DOI] [PubMed] [Google Scholar]
- 33.Rand V, Parker H, Russell LJ, Schwab C, Ensor H, Irving J, et al. Genomic characterization implicates iAMP21 as a likely primary genetic event in childhood B cell precursor acute lymphoblastic leukemia. Blood. 2011;117(25):6848–6855. doi: 10.1182/blood-2011-01-329961. [DOI] [PubMed] [Google Scholar]
- 34.Robinson HM, Harrison CJ, Moorman AV, Chudoba I, Strefford JC. Intrachromosomal amplification of chromosome 21 (iAMP21) may arise from a breakage–fusion–bridge cycle. Genes Chromosom Cancer. 2007;46(4):318–326. doi: 10.1002/gcc.20412. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Schwab C, Ryan SL, Papaemmanuil E, Robinson HM, Jacobs P, et al. Constitutional and somatic rearrangement of chromosome 21 in acute lymphoblastic leukaemia. Nature. 2014;508(7494):98–102. doi: 10.1038/nature13115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roberts KG, Li Y, Payne-Turner D, Harvey RC, Yang Y-L, Pei D, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran TH, Loh ML. Ph-like acute lymphoblastic leukemia. Hematology. 2016;2016(1):561–566. doi: 10.1182/asheducation-2016.1.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts KG, Gu Z, Payne-Turner D, McCastlain K, Harvey RC, Chen I-M, et al. High frequency and poor outcome of Philadelphia chromosome-like acute lymphoblastic leukemia in adults. J Clin Oncol. 2017;35(4):394–401. doi: 10.1200/JCO.2016.69.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reshmi SC, Harvey RC, Roberts KG, Stonerock E, Smith A, Jenkins H et al (2017) Targetable kinase gene fusions in high risk B-ALL: a study from the Children’s Oncology Group. Blood. blood-2016–12–758979. [DOI] [PMC free article] [PubMed]
- 40.Kastner P, Dupuis A, Gaub M-P, Herbrecht R, Lutz P, Chan S. Function of Ikaros as a tumor suppressor in B cell acute lymphoblastic leukemia. Am J Blood Res. 2013;3(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- 41.Tijchon E, Havinga J, van Leeuwen FN, Scheijen B. B-lineage transcription factors and cooperating gene lesions required for leukemia development. Leukemia. 2013;27(3):541–552. doi: 10.1038/leu.2012.293. [DOI] [PubMed] [Google Scholar]
- 42.van der Veer A, Zaliova M, Mottadelli F, De Lorenzo P, te Kronnie G, Harrison CJ, et al. IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood. 2014;123(11):1691–1698. doi: 10.1182/blood-2013-06-509794. [DOI] [PubMed] [Google Scholar]
- 43.Mi J-Q, Wang X, Yao Y, Lu H-J, Jiang X-X, Zhou J-F, et al. Newly diagnosed acute lymphoblastic leukemia in China (II): prognosis related to genetic abnormalities in a series of 1091 cases. Leukemia. 2012;26(7):1507–1516. doi: 10.1038/leu.2012.23. [DOI] [PubMed] [Google Scholar]
- 44.Iacobucci I, Storlazzi CT, Cilloni D, Lonetti A, Ottaviani E, Soverini S, et al. Identification and molecular characterization of recurrent genomic deletions on 7p12 in the IKZF1 gene in a large cohort of BCR-ABL1-positive acute lymphoblastic leukemia patients: on behalf of Gruppo Italiano Malattie Ematologiche dell’Adulto Acute Leukemia Working Party (GIMEMA AL WP) Blood. 2009;114(10):2159–2167. doi: 10.1182/blood-2008-08-173963. [DOI] [PubMed] [Google Scholar]
- 45.Yeoh AEJ, Lu Y, Chin WHN, Chiew EKH, Lim EH, Li Z, et al. Intensifying treatment of childhood B lymphoblastic leukemia with IKZF1 deletion reduces relapse and improves overall survival: results of Malaysia–Singapore ALL 2010 study. J Clin Oncol. 2018;36(26):2726–2735. doi: 10.1200/JCO.2018.78.3050. [DOI] [PubMed] [Google Scholar]
- 46.Harvey RC, Mullighan CG, Chen I-M, Wharton W, Mikhail FM, Carroll AJ, et al. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115(26):5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B cell precursor acute lymphoblastic leukemia. Blood. 2009;114(13):2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 48.Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, et al. Functional screening identifies CRLF2 in precursor B cell acute lymphoblastic leukemia. Proc Natl Acad Sci. 2010;107(1):252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Usvasalo A, Savola S, Räty R, Vettenranta K, Harila-Saari A, Koistinen P, et al. CDKN2A deletions in acute lymphoblastic leukemia of adolescents and young adults: an array CGH study. Leuk Res. 2008;32(8):1228–1235. doi: 10.1016/j.leukres.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 50.Mirebeau D, Acquaviva C, Suciu S, Bertin R, Dastugue N, Robert A, et al. The prognostic significance of CDKN2A, CDKN2B and MTAP inactivation in B-lineage acute lymphoblastic leukemia of childhood. Results of the EORTC studies 58881 and 58951. Haematologica. 2006;91(7):881–885. [PubMed] [Google Scholar]
- 51.Bertin R, Acquaviva C, Mirebeau D, Guidal-Giroux C, Vilmer E, Cavé H. CDKN2A, CDKN2B, and MTAP gene dosage permits precise characterization of mono- and bi-allelic 9p21 deletions in childhood acute lymphoblastic leukemia: CDKN2A, CDKN2B, and MTAP Dosage in Leukemia. Genes Chromosom Cancer. 2003;37(1):44–57. doi: 10.1002/gcc.10188. [DOI] [PubMed] [Google Scholar]
- 52.Heerema NA, Sather HN, Sensel MG, Liu-Mares W, Lange BJ, Bostrom BC, et al. Association of chromosome arm 9p abnormalities with adverse risk in childhood acute lymphoblastic leukemia: a report from the Children’s Cancer Group. Blood. 1999;94(5):1537–1544. [PubMed] [Google Scholar]
- 53.Kasner MT, Wilde L, Keiffer G, Palmisiano ND, Calabretta B. A phase I trial of palbociclib in combination with dexamethasone in relapsed or refractory adult B cell acute lymphoblastic leukemia (ALL) J Clin Oncol. 2019;37(15):TPS7065. doi: 10.1016/j.leukres.2023.107075. [DOI] [PubMed] [Google Scholar]
- 54.Zhou Y, You MJ, Young KH, Lin P, Lu G, Medeiros LJ, et al. Advances in the molecular pathobiology of B lymphoblastic leukemia. Hum Pathol. 2012;43(9):1347–1362. doi: 10.1016/j.humpath.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 55.Cobaleda C, Schebesta A, Delogu A, Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol. 2007;8(5):463–470. doi: 10.1038/ni1454. [DOI] [PubMed] [Google Scholar]
- 56.Öfverholm I, Tran AN, Heyman M, Zachariadis V, Nordenskjöld M, Nordgren A, et al. Impact of IKZF1 deletions and PAX5 amplifications in pediatric B cell precursor ALL treated according to NOPHO protocols. Leukemia. 2013;27(9):1936–1939. doi: 10.1038/leu.2013.92. [DOI] [PubMed] [Google Scholar]
- 57.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2(2):103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 58.Studniak E, Maloney E, Ociepa T, Urasiński T, Skonieczka K, Haus O, et al. Allelic loss of selected tumor suppressor genes in acute lymphoblastic leukemia in children. Pol J Pathol. 2013;2:121–128. doi: 10.5114/pjp.2013.36199. [DOI] [PubMed] [Google Scholar]
- 59.Waanders E, Scheijen B, van der Meer LT, van Reijmersdal SV, van Emst L, Kroeze Y, et al. The origin and nature of tightly clustered BTG1 deletions in precursor B cell acute lymphoblastic leukemia support a model of multiclonal evolution. PLoS Genet. 2012;8(2):e1002533. doi: 10.1371/journal.pgen.1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwab C, Ryan SL, Chilton L, Elliott A, Murray J, Richardson S, et al. EBF1-PDGFRB fusion in pediatric B cell precursor acute lymphoblastic leukemia (BCP-ALL): genetic profile and clinical implications. Blood. 2016;127(18):2214–2218. doi: 10.1182/blood-2015-09-670166. [DOI] [PubMed] [Google Scholar]
- 61.Nunez R. DNA measurement and cell cycle analysis by flow cytometry. Curr Issues Mol Biol. 2001;3(3):67–70. [PubMed] [Google Scholar]
- 62.Parihar M, Singh MK, Islam R, Saha D, Mishra DK, Saha V, et al. A triple-probe FISH screening strategy for risk-stratified therapy of acute lymphoblastic leukaemia in low-resource settings. Pediatr Blood Cancer. 2018;65(12):e27366. doi: 10.1002/pbc.27366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Campana D. Minimal residual disease in acute lymphoblastic leukemia. Semin Hematol. 2009;46(1):100–106. doi: 10.1053/j.seminhematol.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brüggemann M, Kotrova M. Minimal residual disease in adult ALL: technical aspects and implications for correct clinical interpretation. Blood Adv. 2017;1(25):2456–2466. doi: 10.1182/bloodadvances.2017009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J. 2017;7(6):e577. doi: 10.1038/bcj.2017.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mathisen MS, O’Brien S, Thomas D, Cortes J, Kantarjian H, Ravandi F. Role of tyrosine kinase inhibitors in the management of Philadelphia chromosome-positive acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2011;6(3):187–194. doi: 10.1007/s11899-011-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leoni V, Biondi A. Tyrosine kinase inhibitors in BCR-ABL positive acute lymphoblastic leukemia. Haematologica. 2015;100(3):295–299. doi: 10.3324/haematol.2015.124016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giles FJ, Swords RT, Nagler A, Hochhaus A, Ottmann OG, Rizzieri DA, et al. MK-0457, an Aurora kinase and BCR–ABL inhibitor, is active in patients with BCR–ABL T315I leukemia. Leukemia. 2013;27(1):113–117. doi: 10.1038/leu.2012.186. [DOI] [PubMed] [Google Scholar]
- 69.Chiarini F, Lonetti A, Evangelisti C, Buontempo F, Orsini E, Evangelisti C, et al. Advances in understanding the acute lymphoblastic leukemia bone marrow microenvironment: from biology to therapeutic targeting. Biochim Biophys Acta (BBA) Mol Cell Res. 2016;1863(3):449–463. doi: 10.1016/j.bbamcr.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 70.Ling Y, Xie Q, Zhang Z, Zhang H (2018) Protein kinase inhibitors for acute leukemia. Biomarker Research [Internet]. 6(1). Available from: https://biomarkerres.biomedcentral.com/articles/10.1186/s40364-018-0123-1. Cited 17 Dec 2019 [DOI] [PMC free article] [PubMed]
- 71.Lionel D, Christophe L, Marc A, Jean-Luc C. Oral mucositis induced by anticancer treatments: physiopathology and treatments. Ther Clin Risk Manag. 2006;2(2):159–168. doi: 10.2147/tcrm.2006.2.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Du X-L, Chen Q. Recent advancements of bortezomib in acute lymphocytic leukemia treatment. Acta Haematol. 2013;129(4):207–214. doi: 10.1159/000345260. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi K, Inukai T, Imamura T, Yano M, Tomoyasu C, Lucas DM, et al. Anti-leukemic activity of bortezomib and carfilzomib on B cell precursor ALL cell lines. PLoS ONE. 2017;12(12):e0188680. doi: 10.1371/journal.pone.0188680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Degryse S, Cools J (2015) JAK kinase inhibitors for the treatment of acute lymphoblastic leukemia. Journal of Hematology & Oncology [Internet]. 8(1). Available from: https://www.jhoonline.org/content/8/1/91. Cited 17 Jun 2018 [DOI] [PMC free article] [PubMed]
- 75.Harrison C, Vannucchi AM. Ruxolitinib: a potent and selective Janus kinase 1 and 2 inhibitor in patients with myelofibrosis. An update for clinicians. Ther Adv Hematol. 2012;3(6):341–354. doi: 10.1177/2040620712459746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Novel HD, Leukemia A-B. Hematology. 2011;2011(1):243–249. [Google Scholar]
- 77.Levato L, Molica S. Rituximab in the management of acute lymphoblastic leukemia. Expert Opin Biol Ther. 2018;18(2):221–226. doi: 10.1080/14712598.2018.1425389. [DOI] [PubMed] [Google Scholar]
- 78.Raetz EA, Cairo MS, Borowitz MJ, Lu X, Devidas M, Reid JM, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): Phase II results from Children’s Oncology Group (COG) study ADVL04P2: re-Induction With Epratuzumab in Relapsed ALL. Pediatr Blood Cancer. 2015;62(7):1171–1175. doi: 10.1002/pbc.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hathaway L, Sen JM, Keng M. Impact of blinatumomab on patient outcomes in relapsed/refractory acute lymphoblastic leukemia: evidence to date. Patient Relat Outcome Measures. 2018;9:329–337. doi: 10.2147/PROM.S149420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mummery A, Narendran A, Lee K-Y. Targeting epigenetics through histone deacetylase inhibitors in acute lymphoblastic leukemia. Curr Cancer Drug Targets. 2011;11(7):882–893. doi: 10.2174/156800911796798922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Masetti R, Serravalle S, Biagi C, Pession A. The role of HDACs inhibitors in childhood and adolescence acute leukemias. J Biomed Biotechnol. 2011;2011:1–9. doi: 10.1155/2011/148046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jing B, Jin J, Xiang R, Liu M, Yang L, Tong Y, et al (2018) Vorinostat and quinacrine have synergistic effects in T cell acute lymphoblastic leukemia through reactive oxygen species increase and mitophagy inhibition. Cell Death Disease [Internet]. 9(6). Available from: https://www.nature.com/articles/s41419-018-0679-6. Cited 17 Dec 2019 [DOI] [PMC free article] [PubMed]
- 83.Ravandi F, Gandhi V. Novel purine nucleoside analogues for T cell-lineage acute lymphoblastic leukaemia and lymphoma. Expert Opin Investig Drugs. 2006;15(12):1601–1613. doi: 10.1517/13543784.15.12.1601. [DOI] [PubMed] [Google Scholar]
- 84.Robak P, Robak T. Older and new purine nucleoside analogs for patients with acute leukemias. Cancer Treat Rev. 2013;39(8):851–861. doi: 10.1016/j.ctrv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 85.Bonate PL, Arthaud L, Cantrell WR, Stephenson K, Secrist JA, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5(10):855–863. doi: 10.1038/nrd2055. [DOI] [PubMed] [Google Scholar]
- 86.Kadia TM, Gandhi V. Nelarabine in the treatment of pediatric and adult patients with T cell acute lymphoblastic leukemia and lymphoma. Expert Rev Hematol. 2017;10(1):1–8. doi: 10.1080/17474086.2017.1262757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golde TE, Koo EH, Felsenstein KM, Osborne BA, Miele L. γ-Secretase inhibitors and modulators. Biochim Biophys Acta (BBA) Biomembr. 2013;1828(12):2898–2907. doi: 10.1016/j.bbamem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Greene LM, Nathwani SM, Zisterer DM. Inhibition of γ-secretase activity synergistically enhances tumour necrosis factor-related apoptosis-inducing ligand induced apoptosis in T cell acute lymphoblastic leukemia cells via upregulation of death receptor 5. Oncol Lett. 2016;12(4):2900–2905. doi: 10.3892/ol.2016.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evangelisti C, Evangelisti C, Chiarini F, Lonetti A, Buontempo F, Bressanin D, et al. Therapeutic potential of targeting mTOR in T cell acute lymphoblastic leukemia (Review) Int J Oncol. 2014;45(3):909–918. doi: 10.3892/ijo.2014.2525. [DOI] [PubMed] [Google Scholar]