Abstract
Purpose
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare and poor prognostic hematological malignancy. There is still no standard treatment established for BPDCN patients. We aim to summarize the main clinical, biological features and treatment of 9 BPDCN patients.
Methods
Nine patients with BPDCN who had been diagnosed between July 2008 and December 2018 in Ankara University School of Medicine, were retrospectively evaluated.
Results
All patients (n = 9) were male, median age was 64 (21–80). Five patients (55.6%) had bone marrow infiltration, 5 patients (55.6%) cutaneous lesions, 6 patients (66.7%) lymph node involvement, 2 patients (22.2%) central nervous system involvement and 2 patients (22.2%) spleen involvement at time of diagnosis. Complex karyotype was observed in 2 patients. CHOP was given to 5 patients (55.6%), hyper-CVAD to 2 patients (22.2%), fludarabine, cyclophosphamide and mitoxantrone to 1 patient (11.1%) and cyclophosphamide, etoposide, methylprednisolone to 1 patient (11.1%) as first line chemotherapy. Four patients (44.4%) underwent allogeneic hematopoietic stem cell transplantation (AHSCT) in complete remission (CR) 1. Venetoclax was given to a transplant ineligible patient who had skin and lymph node involvement, with the off-label use. The median follow-up time was 15.9 months (3–48.6 months). Estimated median overall survival was 15.9 + 1.6 (95% CI 12.7–19.1) months.
Conclusion
Intensive induction therapies followed by AHSCT in CR seems to be best approaches for patients with BPDCN. Thus, more effective treatment strategies particularly targeted therapies should be warranted to improve the survival of patients with this rare disease.
Keywords: Blastic plasmacytoid dendritic cell neoplasm, Clinical and biological features, Intensive chemotherapy regimens, Allogeneic hematopoietic stem cell transplantation
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, clinically aggressive hematological malignancy derived from the precursor of plasmacytoid dendritic cells involved in innate immunity. Although it was named previously as agranular CD4 + NK cell leukemia, blastic NK cell leukemia/lymphoma, agranular CD4 + CD56 + hematodermic neoplasm or tumor due to uncertainty of histogenesis, it has been defined as ‘blastic plasmacytoid dendritic cell neoplasm’ in 2008 World Health Organization (WHO) classification of acute myeloid leukemia and related neoplasms [ [1], [32] ].
However clonal evolution in the development of BPDCN is not clear yet, current data suggest that BPDCN arises from clonal hematopoiesis with SRSF2, TET2 and NPM1 mutations like other myeloid neoplasms [2].
The neoplastic blastic cells in BPDCN are expected to express CD4, HLA-DR, CD56, TCL1, CD123, BDCA2, BDCA4, CD2AP. The other immunophenotypic characteristics are CD34−, CD3−, MPO−, CD45RA+/RO−, CD11c−, CD116low, CD36+ [3]. In addition to presence of CD4 and CD56 expression, demonstration of CD123 and TCL1 expressions are important for making the diagnosis of the disease [4].
Previous reports indicate that overall incidence of BPDCN is extremely low, accounting for less than 1% of all hematologic malignancies [5] and 0.7% of cutaneous lymphomas [6]. The exact incidence of BPDCN is unknown however, the overall incidence is thought to be 0.04 cases per 100,000 population [7]. BPDCN generally occurs in the elderly, with the median age ranging from 60 to 70 years. There is a male predominance with a male to female ratio of approximately 3:1 [8–10].
The disease common manifestation is cutaneous involvement with or without bone marrow leukemic infiltration. Unfortunately there is no consensus on treatment for patients with this highly aggressive neoplasia [11].
Here, we report the clinicopathological features and the treatment outcomes of 9 BPDCN cases at a single institution in Turkey.
Materials and Methods
We retrospectively evaluated 9 BPDCN patients who had been diagnosed between July 2008 and December 2018 in Ankara University School of Medicine. The diagnosis of BPDCN was based on clinical, morphological, and immunophenotypically features identified by the 2008 revision of the WHO classification of acute myeloid leukemia and related neoplasms [12]. The medical records of Ankara University School of Medicine were reviewed in terms of age, sex, clinical presentation, complete blood count, peripheral blood smear, bone marrow aspiration and biopsy, cytogenetic data, radiological imaging including CT and PET, as well as pathologic findings of the skin lesions. SPSS22 was used for statistical analysis and overall survival was assessed by of Kaplan–Meier method.
Results
Clinical and Laboratory Characteristics
All our patients (n = 9) were male, median age was 64 (21–80). At the time of diagnosis, 5 (55.6%) patients had bone marrow infiltration, 5 (55.6%) patients had cutaneous lesions, 6 (66.7%) patients had lymph node (LN) involvement, 2 (22.2%) patients had central nervous system (CNS) involvement and 2 (22.2%) patients had spleen involvement. No liver involvement was observed. The cytogenetics revealed complex karyotype in 2 patients and del 13q in 1 patient (Table 1).
Table 1.
Clinical and laboratory features of the patients at diagnosis
| Patients no. | Gender/age (yr) | Skin involvement | Bone marrow involvement | Involved other tissues | Peripheral blood (109/L) | Cytogenetics | Flow cytometry (bone marrow) | Antigenic profile of the neoplastic BPD cells by IHC on tissue biopsies |
|---|---|---|---|---|---|---|---|---|
| 1 | M/20 | − | + | − | WBC:2.3 × 109/L HB:4.9 g/dl HTC:13.7% PLT:28 × 109/L | NV | CD45 + , CD19, HLADR + , CD33 + , MPO − , CD13 − CD14 − CD15 − NG2 ± CD16 − CD11b − CD2, CD7CD4 + , CD56bright + , CD123 + , CD38 + , CD34 − CD117− | CD45, CD56, CD123, CD4, TCL1 |
| 2 | M/58 | nodules | + | Spleen, LN | WBC:5.9 × 109/L HB:13.6 g/dl HTC:39.9% PLT:95 × 109/L | 46, XY[20] del 13q +[17/200] | CD45 weak + , HLA DR + , CD19 − , CD13 − CD33 −/weak + CD117∓, MPO − , CD2 + , CD4 + CD7 + , CD5 − CD16 − CD56∓, CD64 − CD71 + CD34 − , CD24 − CD123 + , tdt + CD43+ | TCL1, CD123, CD4, CD56 |
| 3 | M/76 | patches | + | Spleen, LN | WBC:15.7 × 109/L HB:12.7 g/dl HTC:39.9% PLT:87 × 109/L | 45, 49, XY, +X, der (5), 9, der(9), 10, 12, der(13), +20, +21, +mar1, +mar2, +mar3, inc[cp5]/46, XY[4] | CD45 weak + , HLA DR +CD19, CD33weak ± MPO − CD13 − , CD34, CD123 + , CD15 − , CD71 − CD117 − CD11b, CD24 −CD36 + CD64 −, CD9 −CD4 + CD2 − CD3 − , CD43 + CD7 weak ± , NG2 −CD56 bright + | TCL1, CD123, CD4, CD56 |
| 4 | M/70 | − | + | − | WBC:116 × 109 HB:15 g/dl HTC:43% PLT: 70 × 109 | NV | CD45 +, HLA DR ± , CD19 −, CD33 ± , MPO − CD34 − , CD123 + , CD56 +, CD15 − , CD7 − , CD5 − CD8 −, CD4 + CD2 + , CD3 − , TCR− | CD123, TCL1, CD56 |
| 5 | M/67 | − | + | LN, CNS | WBC:3.4 × 109 HB:10 g/dl HTC:31.2% PLT: 30 × 109 | 47, XY, der(1) add(1)(p), der(9) add (9)(q), der(12), der(15), der(7) [cp9]/46, XY[1] | CD45 + HLA DR + CD33 + MPO − CD19 − CD2 + CD4 + CD56 + CD123 + sCD3 − cCD3 − CD8 − CD7 + CD5 − TCRab − TCRgd − CD36 + CD11b − CD64 − CD24− | CD45, CD56, CD123, TCL1 |
| 6 | M/47 | patches | − | LN, CNS | WBC:6.6 × 109 HB:15.7 g/dl HTC:44.3% PLT: 201 × 109 | 46, XY | No involvement | TCL1, CD56, CD4, CD123 |
| 7 | M/64 | nodules and patches | − | − | WBC:3.9 × 109 HB:14.1 g/dl HTC:43.3% PLT: 203 × 109 | 46, XY | No involvement | TCL1, CD56, CD4, CD123 |
| 8 | M/37 | − | − | LN | WBC:4.2 × 109 HB:14.1 g/dl HTC:44.3% PLT: 272 × 109 | 46, XY | No involvement | CD4, CD45, CD123, CD56, TCL1 |
| 9 | M/80 | nodules and patches | − | LN | WBC:4.9 × 109 HB:13.1 g/dl HTC:43.3% PLT: 376 × 109 | 46, XY | No involvement | CD4, CD123, CD56, TCL1 |
M male, yr year, Bx: biopsy, WBC white blood cell, HB hemoglobin, HTC hematocrit, PLT platelet, LN lymph node, CNS central nervous system, IHC immunohistochemistry, LN Lymph node, NV not valid
Two patients had hypercellular diffuse bone marrow infiltration with atypical round shaped cells (Fig. 1) and others had hypercellular bone marrow with various amount of atypical blastic cell infiltration on bone marrow aspiration and biopsies. Five of 9 patients were presented with cutaneous involvement including purple nodular lesions, bruise-like brown to violaceous infiltrated multiple patches and mixed lesions (Fig. 2; Table 1). Skin biopsies revealed BPDCN (Fig. 3). Immunohistochemistry (IHC) of skin biopsies were summarized in Table 1.
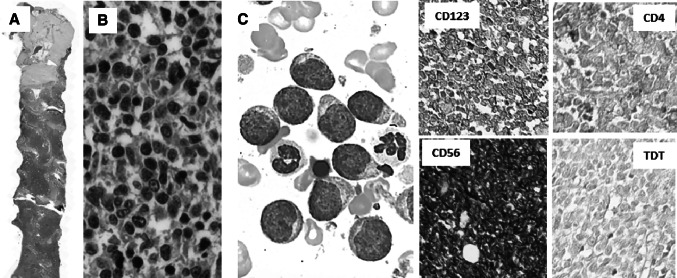
Fig. 1.
Case number 1: a Diffiuse bone marrow (BM) involvement with atypical blastic cells. b The neoplastic cells’ nuclei have fine granular chromatin and the cytoplasm does not have granules or vacuoles. The characteristic phenotype as CD123, CD56 and CD4 expression and TdT negativity was helpful for making the diagnosis
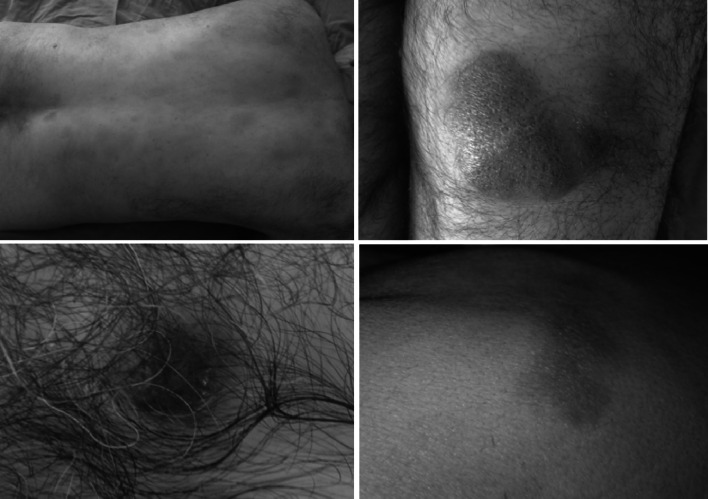
Fig. 2.
Bruise-like brown to violaceous infiltrated patches and erythematous nodules
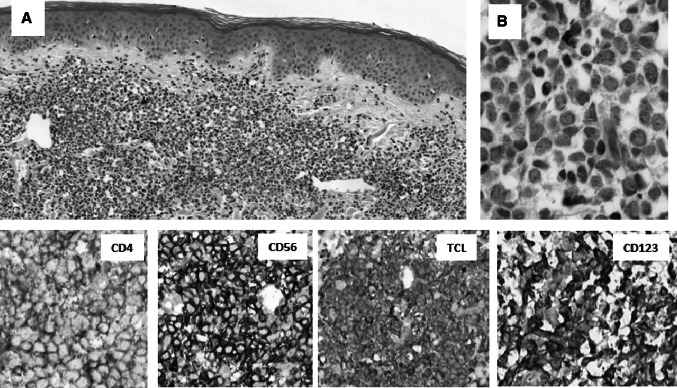
Fig. 3.
The skin involvement is characterised by diffuse infiltration of dermis (a), by the neoplastic immature cells round and oval nucleated cells with the fine granulated chromatin and invisible cytoplasm (b). The characteristic phenotype with CD4, CD56, TCL and CD123 expressions are the most helpfu findings for making the diagnosis
Treatment Protocols and Outcomes
Two patients were treated with Hyper-CVAD chemotherapy regimen including cyclophosphamide, doxorubicin, vincristine, dexamethasone, methotrexate, cytosine arabinoside and folinic acid. AHSCT with full match sibling donor was performed to patient no.1 in first complete remission (CR1). Patient no.2 underwent AHSCT with 9/10 mismatch unrelated donor (MUD) in CR1.
Both was conditioned with cyclophosphamide and total body irradiation regimen (Cy-TBI). Anti-thymocyte globin (ATG) was added to conditioning regimen of patient no.2 due to MUD. Third patient was ineligible for AHSCT. Six cycles of CHOP chemotherapy including cyclophosphamide, doxorubicin, vincristine and methylprednisolone was administered to him. Partial remission (PR) was achieved. He refused follow up and further treatments. Patient no.4 was first patient diagnosed with BPDCN. Treatments involved fludarabine, cyclophosphamide and mitoxantrone (FCM) were given to him. He died due to progressive disease (PD). Patient no.5 who had CNS involvement, was treated with CHOP chemotherapy and intrathecal (IT) treatment with methotrexate, cytosine arabinoside, dexamethasone. PD was observed. Patients no.6,7 and 8 were given 6 cycles of CHOP chemotherapy and CR was obtained as response to first line therapy in all patients. Patients 6 and 8 had full match sibling donors so proceeded with AHSCT in CR1. One of them was conditioned with thiotepa—fludarabine-busulfan—ATG due to CNS involvement and other one with Cy-TBI. Patient no.9 was 80 years-old gentlemen so he was treated with cyclophosphamide, etoposide and methylprednisolone (CEP). CR was achieved after first line treatment but, he relapsed a year later. Venetoclax at a dose of 400 mg daily was initiated with the approval of the health authority and CR was provided 6 months after venetoclax onset (Table 2).
Table 2.
Treatment protocols and responses
| Patients no. | Induction therapy regimen | Response to first-line therapy | Relapse | Salvage treatment | Pre-AHSCT disease status | Donor type | Conditioning regimen | Response to AHSCT | Disease status at last visit |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Hyper-CVAD | CR | No | – | CR1 | Full match sibling | Cy-TBI | CR | PD |
| 2 | Hyper-CVAD | CR | No | – | CR1 | 9/10 mismatch unrelated | Cy-TBI-ATG | CR | PD |
| 3 | CHOP | PR | Yes | Denied further treatment | – | tx ineligible | – | – | PD |
| 4 | FCM | PD | – | Palliative care | – | tx ineligible | – | – | PD |
| 5 | CHOP | PD | – | Palliative care | – | tx ineligible | – | – | PD |
| 6 | CHOP | CR | No | – | CR1 | Full match sibling | THIO + FLU + BU + ATG | CR | CR |
| 7 | CHOP | CR | No | – | – | tx ineligible | – | – | CR |
| 8 | CHOP | CR | No | – | CR1 | Full match sibling | Cy-TBI | CR | CR |
| 9 | CEP | CR | Yes | Venetoclax | – | tx ineligible | – | – | CR |
CR, complete remission, CR1, first complete remission, PR, partial remission, PD, progressive disease, AHSCT, allogeneic hematopoietic stem cell transplant, tx, transplantation, FCM, fludarabine–cyclophosphamide–mitoxantrone, Cy-TBI, cyclophosphamide-total body irradiation, ATG, anti-thymocyte globin, THIO + FLU + BU, thiotepa–fludarabine–busulfan, CEP, cyclophosphamide–etoposide–methylprednisolone
As first line therapy, 2 (22.2%) patients received hyper-CVAD regimen due to leukemic presentation, 5 (55.6%) patients treated with CHOP chemotherapy like lymphoma, 1 (11.1%) patient treated with FCM and 1 patient received CEP chemotherapy. After first line therapy, CR was achieved in 6 (66.6%) patients and PR in 1 (11.1%) patient, while PD was observed in 2 (22.2%) patients. Four (44.4%) patients underwent AHSCT, 3 patients with HLA full match sibling donor, 1 patient with HLA 9/10 MUD in CR1. Three of them conditioned with Cy-TBI, 1 of them with thiotepa based regimen due to CNS involvement. CR was achieved in all patients after AHSCT. Venetoclax was given to a patient with relapsed disease, who was not eligible for transplant. At last visit, PD was observed in 5 (55.5%) patients and 4 (44.5%) patients were in CR (Table 2).
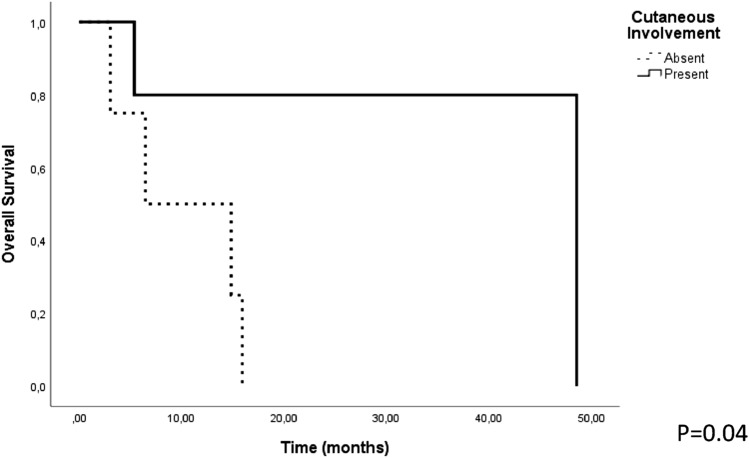
The median follow-up time was 15.9 months (3–48.6 months). Estimated median overall survival (OS) was 15.9 + 1.6 (95% CI 12.7–19.1) months. Factors such as age (≥ 60 years), consolidative AHSCT, bone marrow, skin, lymph node, CNS and spleen involvement that could affect OS and progression free survival (PFS) were analyzed. Cutaneous involvement was associated with better OS (p = 0.04) but had no effect on the PFS (p = 0.3). All other factors had no effect on the OS and PFS (p > 0.05) (Fig. 4).
Fig. 4.
The relationship between OS and cutaneous involvement
Discussion
In this report, main clinical and biological characteristics, management and outcomes of 9 BPDCN patients were retrospectively analyzed. Clinical features of our patients were similar with the previous reports. All our patients were male, median age was 64 (21–81). Elderly male predominance was observed as mentioned in previous reports except one of 20 years old patient [13].
A small number of cases are presented with leukemia without skin involvement [9, 14]. In our patient group, 3 patients had BPDCN with leukemic presentation, lacking cutaneous involvement. Two patients had bruise-like brown to violaceous infiltrated patches, 1 patient had purple nodular lesions and 2 patients had mixed lesions. A retrospectively analysis of 90 patients revealed that 73% of the patients had brown or purple nodular lesions, 12% of the patients had bruise-like brown to violaceous infiltrated patches, and %14 of the patients had disseminated and mixed lesions. [15]. Atalay et al. reported conjunctival and gingival infiltration in one of 3 cases [16] which was not seen in our series.
In our cohort, 1 patient had 13q deletion (34% of the cells) with FISH and 2 patients had complex cytogenetic abnormalities in chromosome 7, 9, 12, 13 and 15. Lucioni et al. reported that the deletion of 9p21.3 locus was the most recurrent event in their series and homozygous loss of locus 9p21.3 was as a basically independent adverse prognostic factor [17]. Karyotype analysis frequently demonstrate complex cytogenetic abnormalities, but diagnostic or pathogenic value of these abnormalities is still unknown [3]. Leroux et al. reported the results of conventional cytogenetic and FISH analyses in 21 patients whereas 66% had complex cytogenetic abnormalities with predominance of genomic losses. They revealed six major recurrent aberrations affecting 5q, 12p, 13q,6q, 15q, and 9, which were involved in 72% (5q), 64% (12p and 13q), 50% (6q), 43% (15q), and 28% (monosomy 9) of their cases [18].
BPDCN is rare aggressive neoplastic disease which involves most frequently skin, bone marrow and lymphoid tissues. When the skin is the first involved area, the diagnosis may be challenging especially for the pathologists who are not experienced in hematopathology [33]. The neoplastic cells characteristically express CD4, CD56, CD123, TCL1 and CD303. Except CD303 none of these markers are specific for BPDC and at least 4 of them is expected to be positive for the confident diagnosis [8]. CD303 is recently described as a lectin type cell membrane receptor selectively expressed on normal BPDC involved in antigen capture and IFN production [19]. Skin biopsies are characterized by diffuse, monotonous dermal infiltration by medium-sized cells with round nuclei and narrow cytoplasm [20]. At the initial presentation bone marrow may not be involved as it is seen four of our patients. When the bone marrow is involved, flow cytometry helps for the diagnosis.
No consensus on treatment of the patients with BPDCN has been published yet. Although patients respond to intensive chemotherapy-based induction regimens, relapse is common and OS ranges from 8 to 14 months [21, 22]. The rarity of disease does not allow prospective clinical trials to define well-established treatment strategies.
In Italian cohort CR was achieved in 7 of 26 patients after AML-type regimens and 10 of 15 patients after ALL/lymphoma type regimens. ALL/lymphoma-type chemotherapy was significantly better than AML like therapies (p = 0.02). The median OS of the AHSCT recipients was significantly longer compared to the non-transplanted patients with median 22.7 months (range 12–32.9) [9]. ALL like induction chemotherapy including Hyper-CVAD yielded higher response rate as 90% but remission duration was short with median OS rates ranged between 12 and 16 months [23].
In our cohort, AHSCT was performed to 4 (44.4%) patients in CR1. Consolidative AHSCT was not associated with better OS and PFS and estimated median overall survival was 15.9 +1.6 (95% CI 12.7–19.1) months. In a review including 97 patients, the authors reported that age ≥ 60 years was a negative prognostic factor, skin restricted disease was not associated with better OS compared with advance disease and median OS was only 13 months for all patients [24]. We found that cutaneous involvement was associated with better OS (p = 0.04) but had no effect on the PFS (p = 0.3).
Two of our patients had CNS disease without neurological symptoms at diagnosis. One of them had very poor prognosis despite treated with both IT treatment and CHOP regimen. Martín et al. reported occult CNS involvement in 6/10 cases at diagnosis despite none of the patients presented with neurological symptoms. They suggest that treatment of occult CNS involvement may lead to a dramatically improved outcome of BPDCN [25].
New targeted agents have been developed recently with early encouraging results in BPDCN. Tagraxofusp, a CD123-directed cytotoxin, was the first drug to be specifically approved for the treatment of BPDCN [26, 27]. Venetoclax, a BCL-2 inhibitor, is another promising drug in patients with relapsed refractory BPDCN [27]. BCL-2 is overexpressed in most blastic plasmacytoid dendritic-cell neoplasms, however it is not expressed in normal plasmacytoid dendritic cells [28, 29]. There are reports about successful outcome of venetoclax in relapsed refractory BPDCN patients who were not provided to AHSCT [30, 31]. In our series venetoclax was given to a relapsed refractory 81 years old patient who was presented with cutaneous lesions. The patient continues to do well at 6 months follow-up, with no new skin lesions or evidence of recurrence.
Conclusion
Clinical presentation of BPDCN can be ambiguous and diagnosis of BPDCN needs experienced hematopathologist. There is still no consensus on the treatment of these cases but chemotherapy regimens for aggressive lymphoproliferative diseases followed by AHSCT in CR1 still remains to be the most appropriate choice. New targeted therapies are emerging with promising outcomes in BPDCN, however patients eligible for transplantation still should be offered AHSCT.
Funding
There was no external funding.
Compliance with ethical standards
Conflict of interest
On behalf of all authors, there was no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethica standards of the insititutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained form all individual participants included in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv Anat Pathol. 2009;16(6):392–404. doi: 10.1097/PAP.0b013e3181bb6bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suma S, et al. Blastic plasmacytoid dendritic cell neoplasm arising from clonal hematopoiesis. Int J Hematol. 2018;108(4):447–451. doi: 10.1007/s12185-018-2461-z. [DOI] [PubMed] [Google Scholar]
- 3.Laribi K, et al. Blastic plasmacytoid dendritic cell neoplasm: from origin of the cell to targeted therapies. Biol Blood Marrow Transplant. 2016;22(8):1357–1367. doi: 10.1016/j.bbmt.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Angelot-Delettre F, Garnache-Ottou F. Blastic plasmacytoid dendritic cell neoplasm. Blood. 2012;120(14):2784. doi: 10.1182/blood-2012-03-417030. [DOI] [PubMed] [Google Scholar]
- 5.Bueno C, et al. Incidence and characteristics of CD4(+)/HLA DRhi dendritic cell malignancies. Haematologica. 2004;89(1):58–69. [PubMed] [Google Scholar]
- 6.Ng AP, et al. Primary cutaneous CD4+/CD56+ hematodermic neoplasm (blastic NK-cell lymphoma): a report of five cases. Haematologica. 2006;91(1):143–144. [PubMed] [Google Scholar]
- 7.Guru Murthy GS, Pemmaraju N, Atallah E. Epidemiology and survival of blastic plasmacytoid dendritic cell neoplasm. Leuk Res. 2018;73:21–23. doi: 10.1016/j.leukres.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Sweet K. Blastic plasmacytoid dendritic cell neoplasm: diagnosis, manifestations, and treatment. Curr Opin Hematol. 2020;27(2):103–107. doi: 10.1097/MOH.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 9.Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239–246. doi: 10.3324/haematol.2012.072645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagano L, et al. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188–202. doi: 10.1111/bjh.14146. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan JM, Rizzieri DA. Treatment of blastic plasmacytoid dendritic cell neoplasm. Hematol Am Soc Hematol Educ Program. 2016;2016(1):16–23. doi: 10.1182/asheducation-2016.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vardiman JW, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 13.Petrella T, et al. Blastic NK-cell lymphomas (agranular CD4+ CD56+ hematodermic neoplasms): a review. Am J Clin Pathol. 2005;123(5):662–675. doi: 10.1309/GJWNPD8HU5MAJ837. [DOI] [PubMed] [Google Scholar]
- 14.Rauh MJ, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation, lacking cutaneous involvement: case series and literature review. Leuk Res. 2012;36(1):81–86. doi: 10.1016/j.leukres.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Julia F, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169(3):579–586. doi: 10.1111/bjd.12412. [DOI] [PubMed] [Google Scholar]
- 16.Atalay F, et al. Blastic plasmacytoid dendritic cell neoplasm: skin and bone marrow infiltration of three cases and the review of the literature. Indian J Hematol Blood Transfus. 2015;31(2):302–306. doi: 10.1007/s12288-014-0464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucioni M, et al. Twenty-one cases of blastic plasmacytoid dendritic cell neoplasm: focus on biallelic locus 9p21.3 deletion. Blood. 2011;118(17):4591–4594. doi: 10.1182/blood-2011-03-337501. [DOI] [PubMed] [Google Scholar]
- 18.Leroux D, et al. CD4(+), CD56(+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Francais de Cytogenetique Hematologique. Blood. 2002;99(11):4154–4159. doi: 10.1182/blood.V99.11.4154. [DOI] [PubMed] [Google Scholar]
- 19.Montes-Moreno S, et al. SPIB, a novel immunohistochemical marker for human blastic plasmacytoid dendritic cell neoplasms: characterization of its expression in major hematolymphoid neoplasms. Blood. 2013;121(4):643–647. doi: 10.1182/blood-2012-08-447599. [DOI] [PubMed] [Google Scholar]
- 20.Cota C, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010;34(1):75–87. doi: 10.1097/PAS.0b013e3181c5e26b. [DOI] [PubMed] [Google Scholar]
- 21.Feuillard J, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99(5):1556–1563. doi: 10.1182/blood.V99.5.1556. [DOI] [PubMed] [Google Scholar]
- 22.Kim HS, et al. Clinical features and treatment outcomes of blastic plasmacytoid dendritic cell neoplasm: a single-center experience in Korea. Korean J Intern Med. 2017;32(5):890–899. doi: 10.3904/kjim.2015.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riaz W, et al. Blastic plasmacytoid dendritic cell neoplasm: update on molecular biology, diagnosis, and therapy. Cancer Control. 2014;21(4):279–289. doi: 10.1177/107327481402100404. [DOI] [PubMed] [Google Scholar]
- 24.Reimer P, et al. What is CD4+ CD56+ malignancy and how should it be treated? Bone Marrow Transplant. 2003;32(7):637–646. doi: 10.1038/sj.bmt.1704215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin-Martin L, et al. Blastic plasmacytoid dendritic cell neoplasm frequently shows occult central nervous system involvement at diagnosis and benefits from intrathecal therapy. Oncotarget. 2016;7(9):10174–10181. doi: 10.18632/oncotarget.7101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syed YY. Tagraxofusp: first global approval. Drugs. 2019;79(5):579–583. doi: 10.1007/s40265-019-01087-z. [DOI] [PubMed] [Google Scholar]
- 27.Economides MP, Konopleva M, Pemmaraju N. Recent developments in the treatment of blastic plasmacytoid dendritic cell neoplasm. Ther Adv Hematol. 2019;10:2040620719874733. doi: 10.1177/2040620719874733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sapienza MR, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia. 2014;28(8):1606–1616. doi: 10.1038/leu.2014.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzankov A, et al. Plasmacytoid dendritic cell proliferations and neoplasms involving the bone marrow: summary of the workshop cases submitted to the 18th Meeting of the European Association for Haematopathology (EAHP) organized by the European Bone Marrow Working Group, Basel 2016. Ann Hematol. 2017;96(5):765–777. doi: 10.1007/s00277-017-2947-4. [DOI] [PubMed] [Google Scholar]
- 30.Agha ME, Monaghan SA, Swerdlow SH. Venetoclax in a patient with a blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2018;379(15):1479–1481. doi: 10.1056/NEJMc1808354. [DOI] [PubMed] [Google Scholar]
- 31.Grushchak S, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore) 2017;96(51):e9452. doi: 10.1097/MD.0000000000009452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swerdlow SH, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: International Agency for Research on Cancer; 2008. [Google Scholar]
- 33.Maus GC, et al. Clinical and pathological features and differential diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN): a series of cases. Blood. 2016;128(22):5183. doi: 10.1182/blood.V128.22.5183.5183. [DOI] [Google Scholar]