Abstract
The objective of the present study was to prepare valencene (sesquiterpene) containing invasomes for itraconazole (ITZ) transungual delivery using central composite design. The phospholipid (X1) and valencene (X2) were selected as an independent variables, while vesicles size (Y1), entrapment efficiency (Y2) and in vitro drug release (Y3) were chosen as dependent variables. The antifungal activity of optimized formulation was screened against Trichophyton rubrum, a common causative onychomycosis pathogen, by cup plate method. The optimized ITZ-loaded invasomes formulation presented vesicles size of 176.8 ± 6.03 nm, entrapment efficiency of 83.21 ± 4.11% and in vitro drug release of 75.22 ± 5.03%. The ITZ-loaded invasomes gel formulation showed good homogeneity, pH 6.5 ± 0.23, viscosity 7.33 ± 0.67 Pa s and drug content 94.13 ± 1.13%. The spreadability and extrudability of developed ITZ-loaded invasomes gel were found to be 7.85 ± 0.24 gcm/s and 162 ± 2.74 g, respectively. The ITZ-loaded invasomes gel presented 71.11 ± 3.65% cumulative permeation of drug via goat hooves. The in vitro antifungal activity depicted that the ITZ-loaded invasomes gel and marketed preparation were presented zone of inhibition of 21.42 mm and 10.64 mm against T. rubrum respectively. Hence the prepared ITZ-loaded invasomes formulation could therefore be a promising topical dosage to mitigate the indications and hasten the cure for onychomycosis than conventional available therapies.
Keywords: Central composite design, Itraconazole, Onychomycosis, Transungual, Valencene
Introduction
Nails can undergo a numeral of disorders that’s lead to discoloration of nails, they become brittle. Such disorder can induce chronic injury contributing to thicken and ingrowth of nails. The tainted nail plate could lead to its separation from the nail bed. It was reported that about 50% of the nail infections was due to the onychomycosis. Onychomycosis is the nail disorder that affects nail unit parts, which includes nail bed, the nail matrix and/or the nail plate (Thatai and Sapra 2017). Toenails are much more susceptible to infection than the fingernails (4:1) (Gupta et al. 2001).
Around 14% of the total human population is affected by Onychomycosis, with more preponderance in elders and in diabetic patients (Thatai and Sapra 2014). It is documented that, only 2.6% of children under 18 years carry nail infection, whereas approximate 90% of elderly people suffer from onychomycosis (Pal et al. 2015).
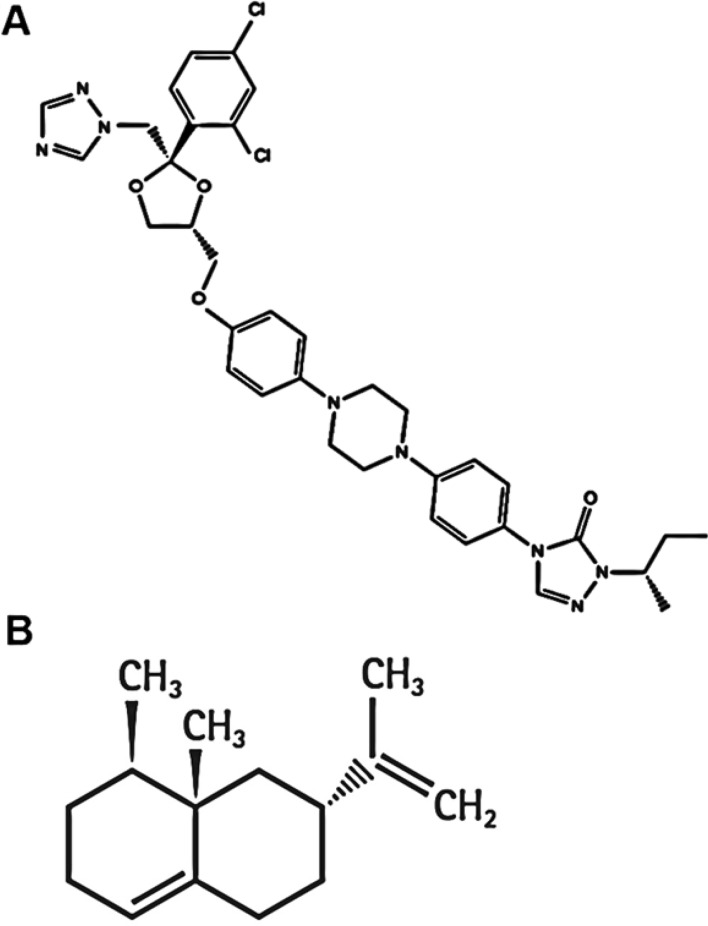
The management of onychomycosis extensively demands the long-time oral/topical antifungal treatment that is usually related with severe drug interactions, side effects, and high recurrence rates. Previously, itraconazole (ITZ) was found effective for the treatment of onychomycosis (Gupta et al. 1998; Gupta and Stec 2019; Jain and Sehgal 2003; Zhang et al. 2018). Recently, Khater and Khattab assessed and compare the effectiveness of ITZ alone and ITZ in combination with long-pulsed Nd-Yag laser for the treatment of onychomycosis nails (Hamed Khater and Khattab 2020). Attempts have been made previously for the transungual delivery of ITZ. Antecedently, Kushwaha et al. (2015) concluded that permeation enhancement technique, such as iontophoresis could be used to deliver ITZ-HCL into and across the nail plate effectively. In another report, authors have investigated the effect of monoterpenes on ITZ transungual permeation (Abdollahi et al. 2020). Although in another study, ITZ-loaded microemulsion-based gel was investigated for the treatment of onychomycosis nails (Barot et al. 2012). Based on the previous reports, transungual treatment is considered an appealing route because it is noninvasive, produced local action and avoids systemic drug interactions and untoward effects of drug. Hence in the present study transungual delivery system of ITZ (Fig. 1a) was envisaged that could noticeably ameliorate the permeability of the drug through the nail for the effective management of onychomycosis.
Fig. 1.
The chemical structure of a ITZ and b valencene
Recently, vesicular systems have been extensively studied as drug carriers for topical administration of drug molecules. Among the vesicles-based drug delivery systems, invasomes are the encouraging drug delivery systems and have been recently investigated in formulation development. The main benefits of invasomes are their potentials to improve the permeability of the drug(s) across the barrier and reduced its systemic absorption; hence, restraining the action of drug(s) at the site of application (Babaie et al. 2020). Both hydrophobic and hydrophilic drugs have been delivered using invasomes (Shah et al. 2015). In comparison to liposomes, invasomes are elastic vesicles comprising of phospholipids, ethanol and terpene (or mixture of terpenes) (Dragicevic-Curic et al. 2009; Kamran et al. 2016; Qadri et al. 2017). Presence of ethanol could be the reason for the soft nature of the vesicles as compared to the liposomes. The elastic nature of vesicles could contribute its improved penetration ability as compared to liposomes (Bommannan et al. 1991). In addition to ethanol, the presences of terpene could also improve its penetration ability via skin by interrupting the constricted arrangement of the stratum corneum lipids (Ahad et al. 2016, 2009, 2011a, b; Aqil et al. 2007). Invasomes are also morphologically different from vesicles, such as transfersomes; transmission electron microscopy images demonstrated that the transfersomes were “unilamellar to multilamellar” while the liposomes and invasomes were unilamellar (Babaie et al. 2020). We stress the high potential of invasomes in topical therapeutic applications on the basis of available reports.
In this study, we adopt central composite design to optimize ITZ-loaded invasomes for transungual delivery for the treatment of onychomycosis. The chosen independent variables for the preparation of invasomes formulations were phospholipid (X1) and valencene (X2) while vesicles size (Y1), entrapment efficiency (EE) (Y2) and in vitro drug release (Y3) were selected as dependent variables. Further, the antifungal activity of optimized formulation was evaluated against T. rubrum, a common causative onychomycosis pathogen, by cup plate method. In the present study, valencene (Fig. 1b, sesquiterpene) was used in the composition of the preparation of invasomes. We have previously reported the penetration enhancement ability of valencene for both hydrophilic and lipophilic drugs. the melting point of valencene is 274 °C and a log partition coefficient of 6.285 (Ahad et al. 2011a, b).
Materials and method
“Itraconazole was procured from Kusum Healthcare Pvt Ltd, India”. Valencene was procured from “Sigma-Aldrich Chemicals Private Limited Mumbai, India”. “Ethanol was procured from Changshu Yangyuan Chemical Co. Ltd., China”. “Methanol (HPLC grade) was bought from Spectrochem Pvt. Ltd., Mumbai, India”. Carbopol 934 and triethanolamine were received from S. D. Fine Chemical, Mumbai, India”.
Preparation of ITZ invasomes
ITZ-loaded invasomes formulation was prepared by previously published method (Kamran et al. 2016; Qadri et al. 2017; Shah et al. 2015). In brief, phospholipid, ITZ and valencene was transferred to a clean, dry, round bottom flask and dissolved in methanol: chloroform mixture in the ratio 1:2. The organic solvent was evaporated using rotary evaporator under low pressure. The remaining dried lipid film was rehydrated with ethanolic-water mixture by rotation at 60 rpm at ambient temperature to obtained coarse dispersion. The coarse dispersion was probe sonicated for 10 min (5 min gap after 5 min) to get nano-sized invasomes (Kamran et al. 2016). All the formulations were prepared to 10 ml that contained 5 mg of ITZ.
Central composite design for the optimization of ITZ invasomes
ITZ-loaded invasomes formulation was optimized using CCD, using Design-Expert 9.0.3.1 software (Stat-Ease Inc., Minneapolis, USA). The selected independent variables were phospholipid (X1) and valencene (X2), while vesicles size (Y1), EE (Y2) and in vitro drug release (Y3) were chosen as dependent responses (Table 1). The amount of drug (5 mg) and volume of dispersion (10 ml) were kept as constant in all the prepared formulations. Total thirteen formulations were generated and their responses were noted.
Table 1.
Variables and response selected for central composite design for the optimization of ITZ-loaded invasomes formulation
| Variables | Level | ||||
|---|---|---|---|---|---|
| − 1.414 | −1 | 0 | + 1 | + 1.414 | |
| Independent variables | |||||
| X1 = Phospholipid (mg) | 23.43 | 40 | 80 | 120 | 136.57 |
| X2 = Valencene (%) | 0.29 | 0.5 | 1 | 1.5 | 1.71 |
| Dependent variables | |||||
| Y1 = Vesicles size (nm) | |||||
| Y2 = EE (%) | |||||
| Y3 = In vitro release (%) | |||||
EE entrapment efficiency, ITZ Itraconazole
Measurement of vesicles size, polydispersity index and zeta potential of ITZ invasomes
The vesicles size, polydispersity index were determined by zeta sizer (Malvern Zetasizer, Malvern instrument, UK with DTS -nano Software®) at 25 ± 1 °C and at a scattering angle of 90°. The zeta potential was also determined using the same instrument based on the electrophoretic mobility. The invasomes formulation was diluted with distilled water and then was placed in quartz cuvette and analyzed. The experiments were conducted in triplicate (Ahad et al. 2018a, b).
Determination of EE of ITZ invasomes
For determination of EE of ITZ-loaded invasomes, free drug was separated from vesicles by ultracentrifugation at 14,000 rpm for 60 min (Ammar et al., 2018; El-Nabarawi et al. 2018). After centrifugation, supernatant was separated and mixed with suitable solvent and analyzed by “UV spectrophotometer” at 263 nm (Parikh et al. 2011). The EE was quantified using following Eq. 1.
| 1 |
Evaluation of in vitro drug release of ITZ invasomes
The experiment was completed using previously published method (Thapa et al. 2018). The dialysis bag “(molecular weight cut off 12,000 Da; Sigma-Aldrich, St. Louis, MO, USA)” was soaked in dissolution media [phosphate buffered saline pH 7.4 (PBS): ethanol, 7:3] for 8 h before use. The invasomes formulation was poured in the dialysis bag which was then placed in the 100 ml dissolution media kept in a beaker. The dissolution media was agitated at a speed of 200 rpm and the ambient temperature was maintained. Two ml sample were taken at different regular time intervals and same amount of dissolution media was changed to maintain sink conditions. The sample was examined by UV spectrophotometer at λmax 263 nm (Parikh et al. 2011).
Morphological evaluation of ITZ invasomes
For transmission electron microscopy optimized invasomes formulation was diluted with prefiltered distilled water and then sample was treated with 1% of phosphotungustic acid for negative staining and placed on copper grids (Polysciences, Warrington, PA, USA), followed by sample drying. The formulation was then examined for vesicles surface morphology by Tecnai S transmission electron microscope (FEI Tecnai S Twin, Netherlands) at an accelerating voltage of 100 kV.
Preparation and evaluation of ITZ invasomes gel
The gel formulation of optimized ITZ invasomes was prepared by dispersing the 1%, 1.5% and 2% Carbopol 934 in a required quantity of distilled water in a separate beaker with continuous stirring at 300 rpm. On complete dispersion of carbopol in water, it swelled overnight then sonicated for 15 min to remove air entrapment. Then, triethanolamine (neutralizing agent) was added with gentle stirring to obtain a homogeneous gel base. Finally, ITZ-loaded invasomes was added drop wise into this preformed gel base with continuous agitation until homogeneous gel formulation was obtained.
Assessment of homogeneity of invasomes gel
The ITZ-loaded invasomes gel was characterized for homogeneity by visual observation after the gel has been set in the container. They were analyzed for their appearance and presence of any aggregates and grades were considered as +++ Good, ++ Fair, + Poor (Abdellatif and Tawfeek 2016).
Measurement of invasomes gel viscosity
The viscosity of formulated gel was analyzed at ambient temperature using Brookfield viscometer with spindle number C50-1 (Brookfield Engineering Laboratories Inc., Middleboro, MA, USA), at 50 rpm of spindle of rheometer for 50 s. All determinations were carried out in triplicate (Ahad et al. 2014).
Evaluation of invasomes gel pH
The pH of the ITZ-loaded invasomes gel formulation was measured at 25 ± 1 °C by digital pH meter (AccumentAB 15, Fisher scientific, USA) electrode in contact with the gel and wait for 1 min to equilibrate.
Determination of extrudability and spreadability of invasomes gel
A simple method was used for estimation of extrudability in terms of weight in gram required to extrude a 0.5 cm ribbon of gel in 10 s from a collapsible tube (Bachhav and Patravale 2009). Spreadability was characterized on the basis of slip and drag methods (Chaudhary et al. 2011). A modified apparatus composed of two glass slides having gel in between, the lower side was fixed to a wooden plate and upper one attached to a balance by a hook was used to analyse spreadability, which was determined using the following Eq. 2:
| 2 |
where S denotes the spreadability (g/s), m represents the weight in pan (g), l is the fixed distance moved by the slide and t is the time.
Determination of drug content of invasomes gel
A specific quantity (1 g) of invasomes gel was mixed into 100 ml methanol and the sample was sonicated, filtered and was determined by UV spectrophotometer at λ max 263 nm after suitable dilution (Parikh et al. 2011).
In vitro goat hooves permeation study of invasomes gel
In vitro permeation of invasomes gel was studied using Franz diffusion cell assembly (Logan instrument Corp., NJ, USA). Franz cell having a surface area of 0.636 cm2 and 4 ml receiver cell volume was used for the permeation study. The goat hooves were kept in the deep freezer at − 20 °C till used. Before starting permeation study, hooves were brought at room temperature and then were placed in PBS for 60 min for hydration. After this, hooves were placed between two compartments of the Franz diffusion cells. The posterior part of the hooves was facing the donor cell and the anterior side was facing the receiver cell. The receiver cell was filled with PBS: ethanol (7:3) and agitated with a magnetic stirrer at 100 rpm and at 37 ± 1 °C temperature. The samples were taken at different time points (0, 0.25, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8 h) and the samples were then estimated for drug content by UV spectrophotometry at λmax of 263 nm (Parikh et al. 2011).
Assessment of antifungal activity of invasomes gel
The antifungal activity was determined by cup plate method. The fungal strain of Trychophyton rubrum was distributed over nutrient agar media in a Petridish and then aseptically, the ITZ-loaded invasomes gel (1%) and the ITZ marketed gel (Azrith 1% gel) were placed in respective marked cavity of plates. The plates were transferred in refrigerator at 4 °C for 1 to 2 h; plates were incubated at 28 °C temperature in incubator for 24 h and after that the completion of incubation period, the zone of inhibition in millimeter was measured (Mahtab et al. 2016).
Results and discussion
Invasomes mainly consists of three components i.e. terpene, ethanol and phospholipid. Ethanol and terpene were used as permeation enhancers and phospholipid of endogenous origin was used as a carrier of the drug (Dragicevic-Curic et al. 2008, 2011).
Ethanol interacts with lipid moiety in the polar head group region, resulting in a decrease in the transition temperature of the nail lipids thus improved their fluidity. The interaction of ethanol into the polar head group region can improved in the membrane permeability (Qadri et al. 2017). Ethanol may also provide the vesicles with soft flexible features which are easily penetrate into deeper layer of the nails. Terpenes improve drug permeation by mitigating lipid packaging and/or distributing the mounding of the bilayers in the nails (Kamran et al. 2016; Shah et al. 2015).
Optimization of ITZ-loaded invasomes using central composite design
A CCD was used for the optimization of ITZ-loaded invasomes. The independent variables chosen were the phospholipid (X1) and valencene (X2); while vesicles size (Y1), EE (Y2) and in vitro drug release (Y3) were the selected dependent variables. The results of 13 prepared formulations and the regression analysis for responses Y1, Y2, Y3 are shown in Table 2. Three-dimensional plots and counter plot were optimized for all the three for variables Y1, Y2 and Y3 are shown in Figs. 2, 3, and 4. The polynomial equations (Eqs. 3, 4 and 5) for responses Y1, Y2, Y3 are presented below. The coefficient values and standard error for different coefficients for variables Y1, Y2, Y3 presented in Table 3.
| 3 |
| 4 |
| 5 |
Table 2.
Observed responses in central composite design for optimization of ITZ-loaded invasomes formulation and summary of results of regression analysis for responses Y1, Y2 and Y3 for fitting to quadratic model
| Formulations | Independent variables | Dependent variables | |||
|---|---|---|---|---|---|
| X1 | X2 | Y1 | Y2 | Y3 | |
| F1 | 23.43 | 1.00 | 46.51 ± 3.01 | 41.43 ± 2.85 | 51.12 ± 4.43 |
| F2 | 80.00 | 1.00 | 163.41 ± 9.56 | 78.23 ± 7.86 | 69.22 ± 5.31 |
| F3 | 80.00 | 1.71 | 189.23 ± 9.31 | 66.15 ± 6.31 | 74.15 ± 5.02 |
| F4 | 80.00 | 1.00 | 159.20 ± 8.61 | 76.73 ± 2.01 | 68.34 ± 3.41 |
| F5 | 120.00 | 0.50 | 201.02 ± 8.43 | 68.35 ± 2.43 | 47.24 ± 3.10 |
| F6 | 80.00 | 1.00 | 176.80 ± 6.03 | 83.21 ± 4.11 | 75.22 ± 5.03 |
| F7 | 40.00 | 1.50 | 87.18 ± 5.10 | 58.42 ± 3.32 | 81.42 ± 6.35 |
| F8 | 40.00 | 0.50 | 49.34 ± 2.01 | 53.21 ± 6.20 | 50.31 ± 6.21 |
| F9 | 136.57 | 1.00 | 196.41 ± 7.12 | 86.23 ± 3.16 | 42.19 ± 2.04 |
| F10 | 80.00 | 1.00 | 158.27 ± 8.11 | 79.02 ± 4.32 | 70.26 ± 3.17 |
| F11 | 80.00 | 0.29 | 121.12 ± 8.18 | 64.73 ± 4.01 | 52.12 ± 3.26 |
| F12 | 120.00 | 1.50 | 214.31 ± 7.25 | 85.15 ± 7.31 | 52.41 ± 2.23 |
| F13 | 80.00 | 1.00 | 164.12 ± 6.17 | 81.42 ± 5.14 | 71.52 ± 5.16 |
| Quadratic model | R2 | Adjusted R2 | Predicted R2 | SD | %CV |
|---|---|---|---|---|---|
| Y1 | 0.9723 | 0.9525 | 0.8355 | 12.15 | 8.20 |
| Y2 | 0.9381 | 0.8938 | 0.6252 | 4.47 | 6.30 |
| Y3 | 0.9517 | 0.9172 | 0.7365 | 3.72 | 6.00 |
X1 phospholipid (mg), X2 valencene (%), Y1 vesicles size (nm), Y2 EE (%), Y3 In vitro release (%), CV coefficient of variation, EE entrapment efficiency, ITZ itraconazole, SD standard deviation
Fig. 2.
a Three-dimensional plot and b Contour plot showing effect of variables on ITZ-loaded invasomes vesicles size (nm). ITZ Itraconazole
Fig. 3.
a Three-dimensional plot and b contour plot showing effect of variables on EE (%) of ITZ-loaded invasomes. EE entrapment efficiency, ITZ Itraconazole
Fig. 4.
a Three-dimensional plot and b contour plot showing effect of variables on in vitro release (%) of ITZ from invasomes formulation. ITZ itraconazole
Table 3.
Quadratic model and the coefficients for the vesicles size, EE and in vitro release of ITZ loaded invasomes formulation
| Terms | Y1-Vesicles size (nm) | Y2-EE (%) | Y3-In vitro release (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | Rangea | Coefficient | SE | Rangea | Coefficient | SE | Rangea | |
| X1 | 61.35 | 5.43 | 51.19 to 71.51 | 13.15 | 1.58 | 9.42 to 16.89 | − 5.59 | 1.31 | (− 8.70) to (− 2.48) |
| X2 | 18.43 | 4.30 | 8.28 to 28.59 | 3.00 | 1.58 | (− 0.73) to 6.74 | 8.43 | 1.31 | 5.32 to 11.54 |
| X1X2 | − 6.14 | 6.07 | (− 20.50) to 8.23 | 2.90 | 2.24 | (− 2.39) to 8.18 | − 6.48 | 1.86 | (− 10.88) to(− 2.09) |
| X21 | − 21.54 | 4.61 | (− 32.43) to (10.65) | − 7.53 | 1.69 | (− 11.54) to (− 3.53) | − 11.39 | 1.41 | (− 14.47) to(− 8.06) |
| X22 | − 4.68 | 4.61 | (− 15.57) to 6.21 | − 6.73 | 1.69 | (− 10.74) to (− 2.72) | − 3.15 | 1.41 | (− 6.49) to0.18 |
X1 Phospholipid (mg), X2 Valencene (%), CV coefficient of variation, EE entrapment efficiency, ITZ itraconazole, SE standard error
aThe range indicates the lower and upper value of coefficients at 95% confidence interval
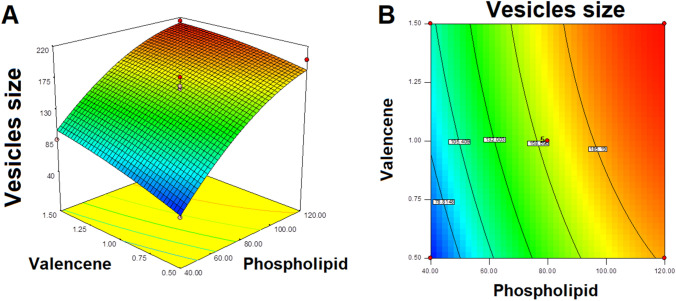
Response Y1: effect of independent variables on vesicles size of ITZ invasomes
Vesicles size is an important variable for evaluating the formulation. Smaller the vesicles size increased the interfacial surface area for drug permeation. The vesicles size of different ITZ-loaded formulations are shown in the Table 2. The noted model F value of 49.10 exhibited that the model is significant (Table 4). The least vesicles size (46.51 ± 3.01 nm) was determine for formulation F1 while the large vesicles size was observed as 121.12 ± 4.18 nm for formulation F11.
Table 4.
Analysis of variance for response surface quadratic model
| Terms | Y1-Vesicles size (nm) | Y2-EE (%) | Y3-In vitro release (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F values | P values | Remarks | F values | P values | Remarks | F values | P values | Remarks | |
| Quadratic model | 49.10 | < 0.0001 | Significant | 21.20 | 0.0004 | Significant | 27.60 | 0.0002 | Significant |
| X1 | 204.03 | < 0.0001 | 69.27 | < 0.0001 | 18.07 | 0.0038 | |||
| X2 | 18.42 | 0.0036 | 3.61 | 0.0992 | 41.10 | 0.0004 | |||
| X1X2 | 1.02 | 0.3459 | 1.68 | 0.2359 | 12.16 | 0.0102 | |||
| X21 | 21.87 | 0.0023 | 19.76 | 0.0030 | 65.27 | < 0.0001 | |||
| X22 | 1.03 | 0.3433 | 15.76 | 0.0054 | 4.99 | 0.0605 | |||
| Lack of Fit | 4.94 | 0.0783 | Not significant | 5.65 | 0.0639 | Not significant | 3.14 | 0.1487 | Not significant |
X1 phospholipid (mg), X2 valencene (%), EE entrapment efficiency
It was observed that occurrence of phospholipid in formulation showed positive effect on vesicles size, it was obtained that on higher the phospholipid concentration, the size of the vesicles was increased (Fig. 2 and Table 2). For example, formulation F1 taking 23.43 mg of phospholipid obtainable vesicles size of 46.51 ± 3.01 nm while the formulation 9 contain higher concentration of phospholipid (136.57 mg) observed vesicles size of 196.41 ± 7.12 nm Table 2.
Similar outcomes were obtained with formulation F8 containing 40 mg phospholipid observed vesicles size of 49.34 ± 2.01 nm while the formulation F5 taking 120 mg of phospholipid obtained vesicles size of 201.02 ± 8.43 nm.
In other words, the presence of valencene in the invasomes also showed positive effect of vesicles size. It was observed that the vesicles size of invasomes was increased on increasing the valencene concentration in the formulation. For example, formulation F8 containing valencene 0.5% presented vesicles size of 49.34 ± 2.01 nm, while the formulation F7 containing valencene 1.5% showed vesicles size of 87.18 ± 5.10 nm Table 2.
Similar outcomes were also analyzed for formulation 11 (valencene 0.29%) presented vesicles size of 121.12 ± 8.18 nm while the formulation 3 (valencene 1.70%) showed the vesicles size of 189.23 ± 9.31 nm.
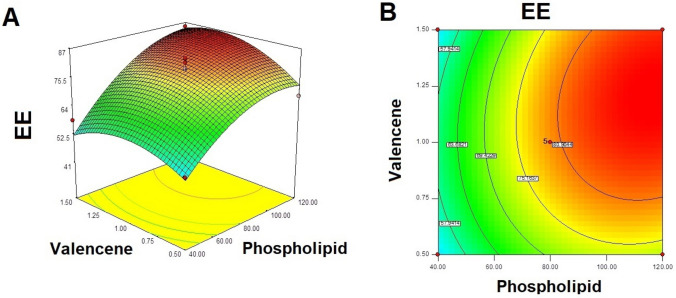
Response Y2: effect of independent variables on EE of ITZ invasomes
The noted model F value of 21.20 displayed that the model is significant (Table 4). The minimum EE of 41.43 ± 2.85% was obtained with formulation F1 while the formulation F9 showed maximum EE of 86.23 ± 3.16% Table 2.
The phospholipid concentration produced a positive effect on the EE; it was shown in response graph (Fig. 3) that on high in phospholipid concentration, the EE of ITZ in invasomes also higher. Formulation F8 (phospholipid 40 mg) observed the EE of 58.42 ± 3.32% while the formulation F5 (phospholipid 120 mg) showed entrapment effect of 85.15 ± 7.31%. Similarly, formulation F7 (phospholipid 40 mg) presented entrainment efficiency of 53.21 ± 6.20% while the formulation F12 taking phospholipid 120 mg showed EE of 68.35 ± 2.43%. Similar results were also obtained for formulation F1 (phospholipid 23.43 mg; EE 41.43 ± 2.85%) and formulation F9 (phospholipid 136.57 mg; EE 86.23 ± 3.16%) Table 2.
Similarly, valencene also exhibited positive effect on entrapment efficiency; it was observed that on increasing the valencene concentration the EE of ITZ also increased in invasomes. For example, F8 (valencene 0.5%) showed EE of 53.21 ± 6.20% while the F7 (valencene 1.5%) showed EE of 58.42 ± 3.32%. Likewise, formulation F5 (valencene 0.5%) presented EE of 68.35 ± 2.43% while formulation F12 (valencene 1.5%) presented EE of 85.15 ± 7.31%.
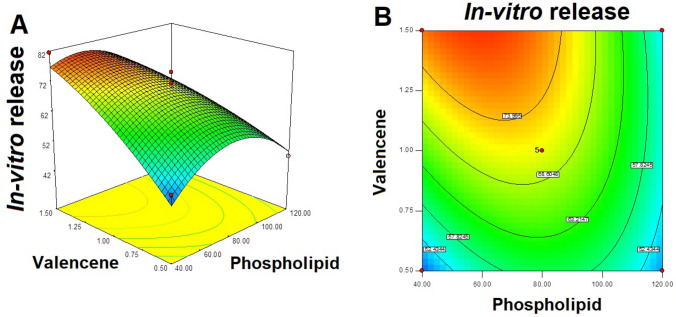
Response Y3: effect of independent variables on in vitro drug release of ITZ invasomes
In vitro release of ITZ from prepared invasomes is selected response for the optimization of invasomes formulation. The noted model F value of 27.60 displayed that the model is significant (Table 4). It was observed that initial increasing in phospholipid concentration (from 23.43 to 80 mg), the in vitro release of ITZ from formulation was increased but furthers higher in phospholipid concentration (from 80 to 136.57 mg) the in vitro drug release decreased. For example, formulation F1 (phospholipid of 23.43 mg) showed 51.12 ± 4.43% drug release while formulation F2 (phospholipid 80 mg) presented in vitro drug release of 69.22 ± 5.31% (Table 2). On further increasing the phospholipid concentration to 136.57 mg in formulation F9, the in vitro drug release of ITZ from formulation dropped to 42.19 ± 2.04% (Table 2).
Valencene showed positive impact on in vitro release of drug from formulation (Fig. 4). It was showed that in vitro drug release was increased on increasing the valencene concentration. Formulation F7 and F12 (valencene 1.5%) presented higher in vitro drug release as comparison to the formulation F8 and F5 (valencene 0.5%), respectively.
Similarly, formulation F3 (valencene 1.7%) presented higher in vitro drug release (74.15 ± 5.02%) in comparison to the formulation F11 (valencene 0.29%) which showed in vitro drug release of 52.12 ± 3.26% (Table 2).
The best formulation of ITZ invasomes system was chose based on the conditions of getting the minimum value of vesicles size and having maximal level of EE and in vitro drug release by applying optimization method of central composite design. The formulation ingredients with phospholipid (80 mg), ethanol (10%) and valencene (1%) were obtained to fulfill requisites of an optimized ITZ-loaded invasomes formulation.
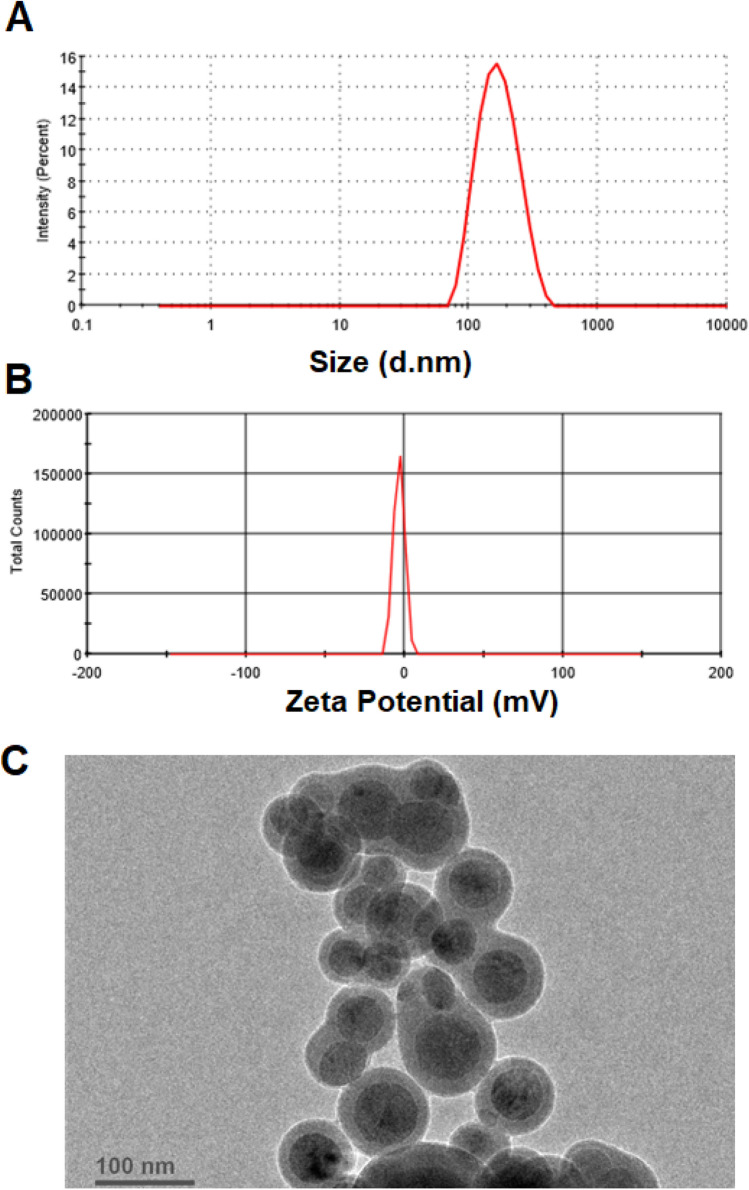
The optimized formulation observed vesicles size of 176.8 ± 6.03 nm (Fig. 5a), EE of 83.21 ± 4.11% and in vitro drug release of 75.22 ± 5.03%. These values are found in close to the predicted values of vesicles size (160.28 nm), EE (79.72%) and in vitro drug release (70.91%) generated by the point predicted method of central composite design. The optimized formulation presented PDI of 0.121 and zeta potential of − 3.29 mV (Fig. 5b).
Fig. 5.
Image showing a vesicles size, b zeta potential and c TEM image of optimized ITZ-loaded invasomes formulation. ITZ itraconazole, TEM transmission electron microscopy
Transmission electron microscopy of ITZ invasomes
The invasomes were demonstrated as sealed, spherical and well identified structure in TEM image (Fig. 5c). ITZ-loaded invasomes were in the nanometric size range as observed by TEM, which is in corroboration with the size measurements data.
ITZ invasomes gel formulation characterization
ITZ-loaded invasomes gel containing 1% carbopol observed good homogeneity with absence of lumps and showed sufficient viscosity (7.33 ± 0.67 Pa s). The pH and drug content of ITZ-loaded invasomes gel were found to be 6.5 ± 0.23 and 94.13 ± 1.13%, respectively. The spreadability and extrudability of developed ITZ-loaded invasomes gel were found to be 7.85 ± 0.24 gcm/s and 162 ± 2.74 g, respectively.
In vitro goat hooves permeation study of ITZ invasomes gel
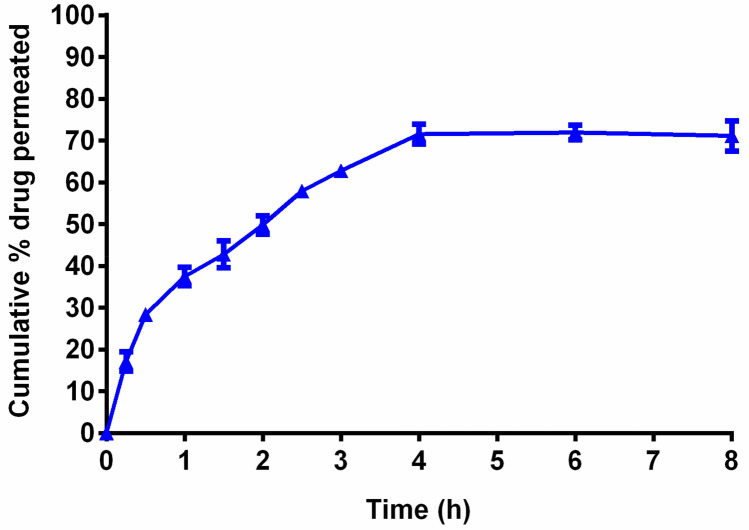
In vitro permeation study of invasomes gel was performed via goat hooves. The ITZ-loaded invasomes gel presented 71.11 ± 3.65% cumulative permeation of drug via goat hooves. The permeation profile of ITZ-loaded invasomes gel was shown in Fig. 6. The permeation of drug could be due to elastic nature of vesicles which allows them to pass efficiently across the goat hooves.
Fig. 6.
Plot of cumulative % drug permeated versus time for ITZ-loaded invasomes gel formulation. ITZ itraconazole
Antifungal activity of ITZ invasomes gel
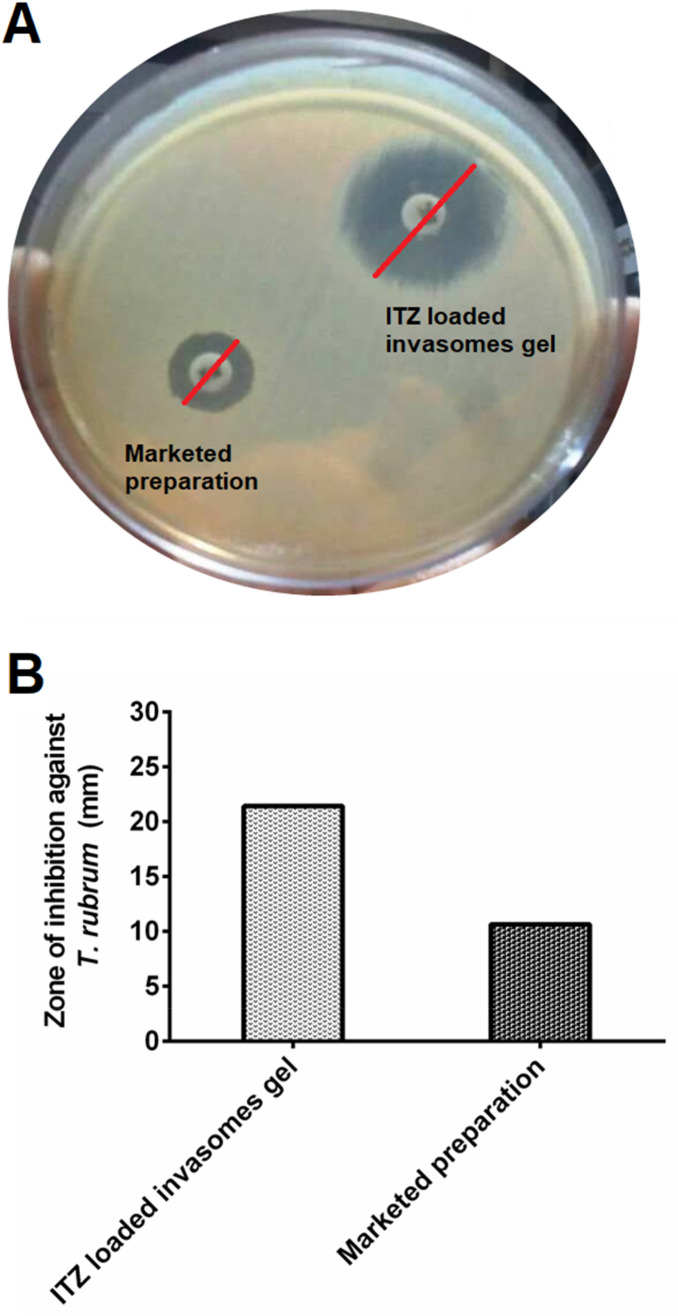
The in vitro antifungal activity which is depicted by the zone of inhibition produced by ITZ-loaded invasomes gel (1%) and marketed (Azrith 1% gel) formulation was found to be 21.42 mm and 10.64 mm respectively (Fig. 7). The improved in vitro antifungal activity of ITZ-loaded invasomes gel formulation may be attributed to improved diffusion of vesicles containing ITZ through fungal cell walls to inhibit ergosterol synthesis. Hence in this study the ITZ-loaded invasomes gel observed maximum inhibitory activity with respect to marketed preparation.
Fig. 7.
a Antifungal activity of optimized ITZ-loaded invasomes gel and marketed preparation (Azrith 1% gel) against T. rubrum. b Zone of inhibition of optimized ITZ-loaded invasomes gel formulation in comparison with marketed preparation against T. rubrum. ITZ itraconazole
Conclusions
The aim of the current study was to prepare itraconazole-loaded invasomes for transungual delivery using central composite design. Prepared invasomes were evaluated for vesicles size, entrapment efficiency, in vitro drug release and vesicles shape. The optimized itraconazole-loaded invasomes was loaded into gel formulation and characterized for homogeneity, viscosity, pH, extrudability, spreadability, drug content, in vitro goat hooves permeation study and antifungal activity. Results of current study exhibited that the central composite design was successfully used for the preparation and optimization of ITZ-loaded invasomes formulation. The optimized itraconazole-loaded invasomes formulation presented vesicles size of 176.8 ± 6.03 nm, entrapment efficiency of 83.21 ± 4.11% and in vitro drug release of 75.22 ± 5.03%. The itraconazole-loaded invasomes gel formulation presented good homogeneity, pH 6.5 ± 0.23, viscosity 7.33 ± 0.67 Pa.s and drug content 94.13 ± 1.13%. The spreadability and extrudability of itraconazole-loaded invasomes gel were found to be 7.85 ± 0.24 gcm/s and 162 ± 2.74 g, respectively. The invasomes gel presented 71.11 ± 3.65% cumulative percent itraconazole permeation via goat hooves. The in vitro antifungal activity which is shown by the zone of inhibition produced by ITZ-loaded invasomes gel (1%) and marketed (Azrith 1% gel) formulation was found to be 21.42 mm and 10.64 mm respectively against Trichophyton rubrum. In current study the itraconazole-loaded invasomes gel displayed better antifungal activity as compared to marketed preparation. The improved in vitro antifungal activity of itraconazole-loaded invasomes gel formulation may be attributed to improved diffusion of itraconazole-loaded invasomes vesicles through fungal cell walls to inhibit ergosterol synthesis. It was; therefore, concluded that the prepared formulation of itraconazole invasomes could be a successful topical dosage form to alleviate the indications of onychomycosis nails.
Author contributions
QH: Investigation, formulation, methodology. MA: Ideas, supervision, conceptualization. AA: Software, writing-original draft preparation. SSI: Software, interpreting the data. AP: Data collection. AQ: Review and editing. ZI: Ideas, design of methodology
Compliance with ethical standards
Conflict of interest
The authors report no declarations of interest.
Contributor Information
Mohd. Aqil, Email: aqilmalik@yahoo.com, https://scholar.google.com/citations?user=eIGSFGsAAAAJ&hl=en
Abdul Ahad, Email: abdulahad20@yahoo.com, Email: aahad@ksu.edu.sa, https://scholar.google.com/citations?user=qKsiOo0AAAAJ&hl=en.
References
- Abdellatif AA, Tawfeek HM. Transfersomal Nanoparticles for Enhanced Transdermal Delivery of Clindamycin. AAPS PharmSciTech. 2016;17:1067–1074. doi: 10.1208/s12249-015-0441-7. [DOI] [PubMed] [Google Scholar]
- Abdollahi D, Jafariazar Z, Afshar M. Effect of monoterpenes on ex vivo transungual delivery of itraconazole for the management of onychomycosis. J Cosmet Dermatol. 2020;19:2745–2751. doi: 10.1111/jocd.13317. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Kohli K, Chaudhary H, Sultana Y, Mujeeb M, Talegaonkar S. Chemical penetration enhancers: a patent review. Expert Opin Ther Pat. 2009;19:969–988. doi: 10.1517/13543770902989983. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. Role of novel terpenes in transcutaneous permeation of valsartan: effectiveness and mechanism of action. Drug Dev Ind Pharm. 2011;37:583–596. doi: 10.3109/03639045.2010.532219. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M, Ali A. Interactions between novel terpenes and main components of rat and human skin: mechanistic view for transdermal delivery of propranolol hydrochloride. Curr Drug Deliv. 2011;8:213–224. doi: 10.2174/156720111794479907. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. Design, formulation and optimization of valsartan transdermal gel containing iso-eucalyptol as novel permeation enhancer: preclinical assessment of pharmacokinetics in Wistar albino rats. Expert Opin Drug Deliv. 2014;11:1149–1162. doi: 10.1517/17425247.2014.914027. [DOI] [PubMed] [Google Scholar]
- Ahad A, Aqil M, Ali A. The application of anethole, menthone, and eugenol in transdermal penetration of valsartan: enhancement and mechanistic investigation. Pharm Biol. 2016;54:1042–1051. doi: 10.3109/13880209.2015.1100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahad A, Raish M, Ahmad A, Al-Jenoobi FI, Al-Mohizea AM. Development and biological evaluation of vesicles containing bile salt of telmisartan for the treatment of diabetic nephropathy. Artif Cells Nanomed Biotechnol. 2018;46:532–539. doi: 10.1080/21691401.2018.1430700. [DOI] [PubMed] [Google Scholar]
- Ahad A, Raish M, Ahmad A, Al-Jenoobi FI, Al-Mohizea AM. Eprosartan mesylate loaded bilosomes as potential nano-carriers against diabetic nephropathy in streptozotocin-induced diabetic rats. Eur J Pharm Sci. 2018;111:409–417. doi: 10.1016/j.ejps.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Ammar HO, Mohamed MI, Tadros MI, Fouly AA. Transdermal delivery of ondansetron hydrochloride via bilosomal systems. In vitro, ex vivo, and in vivo characterization studies. AAPS PharmSciTech. 2018;19:2276–2287. doi: 10.1208/s12249-018-1019-y. [DOI] [PubMed] [Google Scholar]
- Aqil M, Ahad A, Sultana Y, Ali A. Status of terpenes as skin penetration enhancers. Drug Discov Today. 2007;12:1061–1067. doi: 10.1016/j.drudis.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Babaie S, Bakhshayesh ARD, Ha JW, Hamishehkar H, Kim KH. Invasome: a novel nanocarrier for transdermal drug delivery. Nanomaterials (Basel) 2020;10:341. doi: 10.3390/nano10020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhav YG, Patravale VB. Microemulsion based vaginal gel of fluconazole: formulation, in vitro and in vivo evaluation. Int J Pharm. 2009;365:175–179. doi: 10.1016/j.ijpharm.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Barot BS, Parejiya PB, Patel HK, Mehta DM, Shelat PK. Microemulsion-based antifungal gel delivery to nail for the treatment of onychomycosis: formulation, optimization, and efficacy studies. Drug Deliv Transl Res. 2012;2:463–476. doi: 10.1007/s13346-012-0109-8. [DOI] [PubMed] [Google Scholar]
- Bommannan D, Potts RO, Guy RH. Examination of the effect of ethanol on human stratum corneum in vivo using infrared spectroscopy. J Control Release. 1991;16:299–304. doi: 10.1016/0168-3659(91)90006-Y. [DOI] [Google Scholar]
- Chaudhary H, Kohli K, Amin S, Rathee P, Kumar V. Optimization and formulation design of gels of Diclofenac and Curcumin for transdermal drug delivery by Box–Behnken statistical design. J Pharm Sci. 2011;100:580–593. doi: 10.1002/jps.22292. [DOI] [PubMed] [Google Scholar]
- Dragicevic-Curic N, Grafe S, Albrecht V, Fahr A. Topical application of temoporfin-loaded invasomes for photodynamic therapy of subcutaneously implanted tumours in mice: a pilot study. J Photochem Photobiol B. 2008;91:41–50. doi: 10.1016/j.jphotobiol.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Dragicevic-Curic N, Scheglmann D, Albrecht V, Fahr A. Development of different temoporfin-loaded invasomes-novel nanocarriers of temoporfin: characterization, stability and in vitro skin penetration studies. Colloids Surf B Biointerfaces. 2009;70:198–206. doi: 10.1016/j.colsurfb.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Dragicevic-Curic N, Friedrich M, Petersen S, Scheglmann D, Douroumis D, Plass W, Fahr A. Assessment of fluidity of different invasomes by electron spin resonance and differential scanning calorimetry. Int J Pharm. 2011;412:85–94. doi: 10.1016/j.ijpharm.2011.04.020. [DOI] [PubMed] [Google Scholar]
- El-Nabarawi MA, Shamma RN, Farouk F, Nasralla SM. Dapsone-loaded invasomes as a potential treatment of acne: preparation, characterization, and in vivo skin deposition assay. AAPS PharmSciTech. 2018;19:2174–2184. doi: 10.1208/s12249-018-1025-0. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Stec N. Emerging drugs for the treatment of onychomycosis. Expert Opin Emerg Drugs. 2019;24:213–220. doi: 10.1080/14728214.2019.1685493. [DOI] [PubMed] [Google Scholar]
- Gupta AK, De Doncker P, Scher RK, Haneke E, Daniel CR, 3rd, Andre J, Baran R. Itraconazole for the treatment of onychomycosis. Int J Dermatol. 1998;37:303–308. doi: 10.1046/j.1365-4362.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- Gupta AK, Albreski D, Del Rosso JQ, Konnicov N. The use of the new oral antifungal agents, itraconazole, terbinafine, and fluconazole, to treat onychomycosis and other dermatomycoses. Curr Probl Dermatol. 2001;13:220–246. doi: 10.1067/mdm.2001.108128. [DOI] [Google Scholar]
- Hamed Khater M, Khattab FM. Combined long-pulsed Nd-Yag laser and itraconazole versus itraconazole alone in the treatment of onychomycosis nails. J Dermatol Treat. 2020;31:406–409. doi: 10.1080/09546634.2019.1623861. [DOI] [PubMed] [Google Scholar]
- Jain S, Sehgal VN. Itraconazole versus terbinafine in the management of onychomycosis: an overview. J Dermatol Treat. 2003;14:30–42. doi: 10.1080/09546630305541. [DOI] [PubMed] [Google Scholar]
- Kamran M, Ahad A, Aqil M, Imam SS, Sultana Y, Ali A. Design, formulation and optimization of novel soft nano-carriers for transdermal olmesartan medoxomil delivery: in vitro characterization and in vivo pharmacokinetic assessment. Int J Pharm. 2016;505:147–158. doi: 10.1016/j.ijpharm.2016.03.030. [DOI] [PubMed] [Google Scholar]
- Kushwaha A, Jacob M, Shiva Kumar HN, Hiremath S, Aradhya S, Repka MA, Murthy SN. Trans-ungual delivery of itraconazole hydrochloride by iontophoresis. Drug Dev Ind Pharm. 2015;41:1089–1094. doi: 10.3109/03639045.2014.927481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtab A, Anwar M, Mallick N, Naz Z, Jain GK, Ahmad FJ. Transungual delivery of ketoconazole nanoemulgel for the effective management of onychomycosis. AAPS PharmSciTech. 2016;17:1477–1490. doi: 10.1208/s12249-016-0488-0. [DOI] [PubMed] [Google Scholar]
- Pal P, Thakur RS, Ray S, Mazumder B. Design and development of a safer non-invasive transungual drug delivery system for topical treatment of onychomycosis. Drug Dev Ind Pharm. 2015;41:1095–1099. doi: 10.3109/03639045.2014.931966. [DOI] [PubMed] [Google Scholar]
- Parikh SK, Patel AD, Dave JB, Patel CN, Sen DJ. Development and validation of UV spectrophotometric method for estimation of itraconazole bulk drug and pharmaceutical formulation. Int J Drug Dev Res. 2011;3:324–328. [Google Scholar]
- Qadri GR, Ahad A, Aqil M, Imam SS, Ali A. Invasomes of isradipine for enhanced transdermal delivery against hypertension: formulation, characterization, and in vivo pharmacodynamic study. Artif Cells Nanomed Biotechnol. 2017;45:139–145. doi: 10.3109/21691401.2016.1138486. [DOI] [PubMed] [Google Scholar]
- Shah SM, Ashtikar M, Jain AS, Makhija DT, Nikam Y, Gude RP, Steiniger F, Jagtap AA, Nagarsenker MS, Fahr A. LeciPlex, invasomes, and liposomes: a skin penetration study. Int J Pharm. 2015;490:391–403. doi: 10.1016/j.ijpharm.2015.05.042. [DOI] [PubMed] [Google Scholar]
- Thapa C, Ahad A, Aqil M, Imam SS, Sultana Y. Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using Box–Behnken design. J Drug Deliv Sci Technol. 2018;44:431–439. doi: 10.1016/j.jddst.2018.02.003. [DOI] [Google Scholar]
- Thatai P, Sapra B. Transungual delivery: deliberations and creeds. Int J Cosmet Sci. 2014;36:398–411. doi: 10.1111/ics.12142. [DOI] [PubMed] [Google Scholar]
- Thatai P, Sapra B. Transungual gel of terbinafine hydrochloride for the management of onychomycosis: formulation, optimization, and evaluation. AAPS PharmSciTech. 2017;18:2316–2328. doi: 10.1208/s12249-017-0711-7. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xu H, Shi Y, Yu J, Tao Y, Li X. An exploration of the optimum dosage and number of cycles of itraconazole pulse therapy for severe onychomycosis. Mycoses. 2018;61:736–742. doi: 10.1111/myc.12799. [DOI] [PubMed] [Google Scholar]