Abstract
Spontaneous intracerebral hemorrhage (sICH) is one of the deadliest subtypes of stroke, and no treatment is currently available. One of the major risk factors is tobacco use. In this article, we review literature on how tobacco use affects the risk of sICH and also summarize the known effects of tobacco use on outcomes following sICH. Several studies demonstrate that the risk of sICH is higher in current cigarette smokers compared to non-smokers. The literature also establishes that cigarette smoking not only increases the risk of sICH but also increases hematoma growth, results in worse outcomes, and increases the risk of death from sICH. This review also discusses potential mechanisms activated by tobacco use which result in an increase in risk and severity of sICH. Exploring the underlying mechanisms may help alleviate the risk of sICH in tobacco users as well as may help better manage tobacco user sICH patients.
Keywords: Cerebral hemorrhage, Tobacco, Smoking, Hemorrhagic stroke, Hematoma, Risk factors
Introduction
Hemorrhagic stroke occurs following blood vessel rupture-induced bleeding into the brain [1]. In parenchymal hemorrhage, a hematoma forms with or without extension into the ventricles [2]. Spontaneous intracerebral hemorrhage (sICH) accounts for about 10% of all strokes [1,3]. The incidence rate varies by geographical location. The sICH percentage of total strokes is higher in Japan and Korea than in the USA, UK, and Australia [1,4]. A systematic review of population-based studies and comparison of incidence rates of sICH in high income and low to middle income countries observed that the rates of primary sICH in low to middle income countries are almost double of that in high income countries [5]. The incidence rate in the developed world has remained stable over the last three decades [4,6]. A population-based study that compared changes in incidence and etiology of sICH in Oxfordshire, UK between 1981 and 2006 observed that the incidence of sICH is decreasing in the population aged <75 years while increasing in the population aged >75 years without any overall change in the incidence rate [7].
sICH results from the rupture of parenchymal blood vessels mainly within the basal ganglia, thalamus, pons, or cerebellum [8]. Characteristic symptoms for sICH diagnosis include rapidly progressive neurological signs and symptoms, headache, vomiting, seizures, and reduced consciousness [9]. sICH is the deadliest stroke sub-type with a 30-day mortality of over 40% [10-12]. The 1-month mortality rate varies depending on the location of the hemorrhage [8]. Less than half of sICH patients survive 1 year post-ictus, and about one-third survive for 5 years [13]. The median case fatality is lower in Japan (16.7%) compared to the rest of the world (42.3%) [11]. Only 21% of sICH patients are able to lead an independent life at 6 months [14]. Currently, there is no proven therapy to prevent hematoma expansion in sICH patients, and thus clinicians are unable to offer more than supportive care.
Hematoma expansion
Hematoma growth early after ictus is common in sICH patients [10]. The majority of hematoma expansion occurs during the first 4 hours post-symptom onset; however, hematoma expansion with a wide degree of variability continues in patients during the first day [10,15]. This is mainly due to variable duration between baseline and a follow-up scan as well as in the threshold used for expansion. Hematoma expansion is defined as an increase of hematoma volume on a repeat computed tomography (CT) by 33% to 50% (or an absolute change in hematoma volume of 12.5 to 20 mL) [16]. An earlier review article noted that about 73% of patients experience some expansion in hematoma volume and about 33% of them experience a larger than 33% growth in hematoma volume [8].
Early hematoma growth is also associated with neurological deterioration when evaluated using Glasgow Coma Scale and National Institutes of Health Stroke Scale scores [10]. The hemorrhage volume is considered a powerful predictor of 1-month mortality and morbidity in sICH patients [17]. An earlier study reported that for deep hemorrhages, a smaller hematoma volume (<30 mL) resulted in a lower 1-month mortality (23%), while a larger hematoma volume (>60 mL) resulted in a higher 1-month mortality (93%) [17]. Davis et al. [18] performed a meta-analysis of three prior clinical studies to determine if subsequent hematoma growth further increases the risk of poor outcomes in sICH patients. They observed that each mL increase in hematoma volume resulted in a 1% increase in the risk of death. They also observed that every 10% increase in hematoma growth resulted in a 5% increase in the risk of death, and that hematoma growth is also an independent determinant of poor functional outcome [18]. These clinical studies indicate that preventing hematoma growth in sICH patients may result in improved outcomes in these patients.
Mechanisms of injury
An artery rupture results in initial bleeding. This initial bleeding and related hypertension results in hematoma expansion leading to physical damage to the surrounding cells and cellular architecture. This phenomenon is described as “mass effect.” [19] Larger hematoma expansion results in a midline shift, causing physical damage in areas remote from the bleeding site. Hematoma and surrounding edema lead to an increase in intracranial pressure (ICP). This increase in ICP may also cause a decrease in cerebral perfusion pressure causing ischemia/oligemia injury to the brain [20]. These are primary mechanisms of injury following sICH.
Pathways activated by the primary injury and hematoma cause secondary brain injury following sICH. Once the hematoma is stable, thrombin levels increase as a part of hemostasis. This increase in thrombin levels activates several cell-damaging pathways including infiltration of inflammatory cells, proliferation of mesenchymal cells, scar formation, brain edema, seizures, microglial activation, and both astrocytic proliferation and death [19,21,22]. There is also a breakdown of the blood-brain barrier (BBB), which also contributes to secondary brain injury [23]. BBB breakdown is believed to support activation of the complement system and subsequent erythrocyte lysis [19]. The iron from hemoglobin and carbonic anhydrase 1 released during hematoma resolution also contribute to secondary brain injury [19]. BBB breakdown and blood components can also activate microglia [21,24]. Microglial activation is one of the major sources of secondary brain damage after sICH, as microglia release various inflammatory mediators and activate astrocytes to release proinflammatory cytokines [21,24,25]. In a rat model of intracerebral hemorrhage (ICH), microglial activation was seen within 1 hour after collagenase-induced ICH and remained at increased levels up to 3 to 4 weeks afterwards [24]. Furthermore, other cell death mechanisms such as excitotoxicity, seizures, spreading depression, and activation of cell death pathways also play a role in secondary brain injury [19].
Etiology
Risk factors for sICH include comorbidities such as hypertension, cerebral amyloid angiopathy, and chronic liver disease. Medication-associated risk factors for sICH are antiplatelet therapy, anticoagulant treatment, and selective serotonin reuptake inhibitors [1,4,6,9]. Non-modifiable risk factors for sICH include older age and male sex. Modifiable risk factors include alcohol consumption, cigarette smoking, and cholesterol/diet [1,4,6,9]. An earlier study observed that older (≥55 years of age) sICH patients with low and high body mass index (BMI) are associated with deep sICH when compared with those with normal BMI [26]. The same study did not find any effect of BMI on lobar sICH [26]. Although the relative risk (RR) of sICH is higher in men compared to women, the severity of sICH is greater in women compared to men [6,27,28]. The risk ratio for sICH is also higher in blacks and Hispanics [27]. Apolipoprotein E 2 and 4 alleles are also risk factors for sICH [6]. Besides, Genome Wide Association Studies identified several genetic loci that are associated with the risk of sICH [29]. Hypercholesterolemia and a high intake of fruits and vegetables are associated with a reduced risk of sICH [6].
Although the overall incidence rate of sICH has not changed over the past 30 years, the risk factor profile has changed [6]. An earlier study identified that the incidence of hypertension-associated sICH decreased over time; however, the incidence of sICH associated with the use of antithrombotic medication increased over the same time period [7]. With an increase in the incidence of antithrombotic use-associated sICH in older individuals and an expected increase in the prevalence of amyloid angiopathy with a growing aging population, the overall number of cases of sICH is expected to increase in the future [7].
Relationship between tobacco use and ICH
One risk factor of major concern is cigarette smoking as seen in Figure 1. Tobacco use is one of the leading causes of preventable death in the world [3]. Although the smoking rate has decreased (from 41.2% in 1980 to 31.1% in 2012), owing to population growth, there has been an increase in the number of current cigarette smokers in the world (from 721 million in 1980 to 967 million in 2012) [30]. Nicotine, the major toxic agent in the complex chemical mixture of cigarette smoke which contains more than 9,000 different chemicals, increases the risk of cardio-/cerebrovascular disease [31]. As mentioned, tobacco use is well-recognized as one of the major risk factors for sICH [32-40]. Table 1 summarizes the results of several original studies evaluating the risk of sICH in current tobacco users worldwide [33,37,41-52]. Only studies that included greater than 40 sICH cases and that specified confirmation of ICH with either cranial CT imaging, CT angiography, or magnetic resonance imaging were included in the list [53]. These studies clearly indicate that tobacco use increases the risk of sICH across various ethnicities and populations, although this risk is not uniform. Tobacco use is one of the most frequent risk factors for sICH in young people, and smoking is most prevalent in young sICH patients [54-56]. This is of particular concern as greater than 30% of men and 20% of women between ages 20 to 49 years smoke daily [30]. The risk of sICH recurrence in survivors of sICH is about 1% to 3% per year [57]. An earlier study evaluating factors for recurrence of sICH identified tobacco use as one of the risk factors [58].
Figure 1.
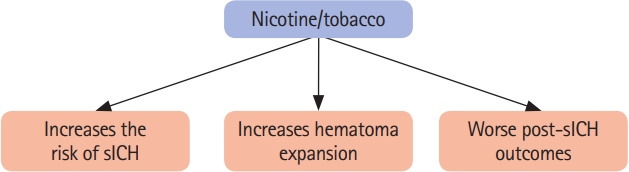
A schematic diagram summarizing the impact of nicotine/tobacco use on the risk of spontaneous intracerebral hemorrhage (sICH) and outcomes following sICH.
Table 1.
Risk of ICH in current tobacco users
| Study | Study population | Results (95% CI) |
|---|---|---|
| Abbott et al. (1986) [46] | Cohort of 8,006 men of Japanese ancestry (Honolulu Heart Program) (75 ICH cases) | RR, 2.8 (1.7–4.8) |
| Fogelholm et al. (1993) [47] | Case-control study of 155 Finnish ICH patients and 155 matched controls | OR, 1.40 (0.7–2.8) |
| Juvela et al. (1995) [48] | Case-control study with 156 ICH patients aged 16–60 years and 332 matched controls in Helsinki | RR, 1.6 (1.1–2.3) |
| Thrift et al. (1996) [45] | Case-control study of 331 ICH cases aged 18–80 years and 342 matched controls from the Melbourne Risk Factor Study (MERFS Group) | OR, 1.17 (0.72–1.89) |
| Kubota et al. (1997) [49] | Case-control study in Japan with 158 ICH patients aged 20–70 years and 158 matched controls | OR, 0.91 (0.57–1.44) |
| Woo et al. (2002) [50] | Case-control study in the United States of 188 ICH cases and 366 control subjects | OR, 1.3 (0.8–2.0) |
| Feldmann et al. (2005) [33] | Case-control study of 217 sICH patients aged 18–49 years and 419 matched controls | OR, 1.58 (1.02–2.44) |
| Lu et al. (2008) [44] | Cohort of 45,449 Swedish women aged 30–50 years (Swedish Women’s Lifestyle and Health Cohort Study) (47 ICH cases) | RR, 1.5 (0.7–3.1) |
| O’Donnell et al. (2010) [51] | Case-control study in 22 countries of 6,000 participants, including control pairs (INTERSTROKE Study Phase 1) (663 ICH) | OR, 1.45 (1.07–1.96) |
| Xu et al. (2013) [37] | Cohort of 66,820 Chinese participants aged >65 years (530 ICH cases) | HR, 1.80 (1.35–2.40) |
| Ferket et al. (2014) [52] | Pooled cohort of 27,493 participants from the Atherosclerosis Risk in Communities Study (ARIC), Cardiovascular Health Study (CHS), and Rotterdam Study (325 ICH cases) | HR, 1.53 (1.17–2.00) |
| Pujades-Rodriguez et al. (2015) [43] | Cohort of 1,937,360 participants aged 30 or more years from the CALIBER program (2,388 ICH cases) | HR, 1.61 (1.37–1.89) |
| O’Donnell et al. (2016) [41] | Case-control study in 32 countries of 26,919 participants (INTERSTROKE Study Phase 2) (3,059 ICH cases) | OR, 1.14 (0.95–1.36) |
| Price et al. (2018) [42] | Cohort of 712,433 British women of the Million Women Study (1,010 ICH cases) | RR, 1.91 (1.68–2.19) |
ICH, intracerebral hemorrhage; CI, confidence interval; RR, relative risk; OR, odds ratio; sICH, spontaneous intracerebral hemorrhage; HR, hazard ratio; CALIBER, ClinicAl disease research using LInked Bespoke Studies and Electronic health Records.
Several studies also looked at the difference between the risk of sICH in currently smoking men and women (Table 2) [34,43,48,59,60]. Two clinical studies found that incidence of sICH is higher in males compared to females [61,62]. Another study reported that males have a higher risk for hematoma expansion (odds ratio [OR] of 1.7) following sICH than females [63]. The Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Disease (JPHC) Study followed smokers without a history of stroke, coronary heart disease, or cancer from the early 90s to the end of 2001 and observed that the RR of parenchymal hemorrhage was higher in smoking women compared to smoking men [59]. Another study that performed a pooled analysis of three large-scale cohort studies in Japan reported that the hazard ratio (HR) for sICH was 1.27 and 1.87 in male and female current smokers, respectively [60]. On the contrary, a study by the Midland (England) Cerebrovascular Research Group observed that compared to non-smokers, the adjusted RR for sICH was 1.82 and 1.3 in male and female smokers, respectively [34]. It is important to note that tobacco use affects the hormones estrogen and testosterone. An earlier study found that estrogen has a protective effect on intracranial aneurysm rupture, which is commonly associated with subarachnoid hemorrhage (SAH) [64]. Because cigarette smoking has anti-estrogenic effects, it is plausible that cigarette smoking may cause an increased risk of sICH in women by removing this protection [65]. The effect of tobacco use on testosterone levels in men are inconclusive [65]. Several studies reported that tobacco use increases testosterone levels in men, and one study found that increased testosterone may worsen behavioral outcome in a rodent model of sICH [66-69]. However, further confirmatory studies in a large population are needed to reach a definite conclusion in this regard. Although a general increase in the risk of sICH is seen in all current tobacco users, whether tobacco use has a sex-specific effect with respect to increasing the risk for sICH is inconclusive so far.
Table 2.
Relationship between sex and risk of sICH in smokers
| Study | Study population | Results (95% CI) |
|---|---|---|
| Gill et al. (1989) [34] | Cohort of 621 stroke patients (52 ICH cases in men and 36 ICH cases in women) and 573 controls (270 men and 303 women) (most cases were verified by CT) | RR (men), 1.82 (0.9–3.7) |
| RR (women), 1.30 (0.5–3.4) | ||
| Juvela et al. (1995) [48] | Case-control study in Helsinki with 156 ICH patients aged 16–60 years (96 men and 60 women) and 332 matched controls (192 men and 140 women) | RR (men), 1.3 (0.8–2.1) |
| RR (women), 2.1 (1.1–3.9) | ||
| Mannami et al. (2004) [59] | Cohort of 27,063 and 27,435 Japanese men and women aged 40–59 years from the Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Disease (JPHC Study Cohort I) (219 ICH cases in men and 129 ICH cases in women) | RR (men), 0.90 (0.65–1.25) |
| RR (women), 1.53 (0.86–4.25) | ||
| Honjo et al. (2010) [60] | Cohort of 140,026 and 156,810 Japanese men and women aged 40–79 years (363 ICH cases in men and 313 ICH cases in women) | HR (men), 1.27 (1.00–1.62) |
| HR (women), 1.87 (1.34–2.60) | ||
| Pujades-Rodriguez et al. (2015) [43] | Cohort of 1,937,360 participants aged 30 or more years from the CALIBER program (1,098 ICH cases in men and 1,290 ICH cases in women) | HR (men), 1.46 (1.16–1.84) |
| HR (women), 1.76 (1.41–2.21) |
sICH, spontaneous intracerebral hemorrhage; CI, confidence interval; ICH, intracerebral hemorrhage; CT, computed tomography; RR, relative risk; HR, hazard ratio; CALIBER, ClinicAl disease research using LInked Bespoke Studies and Electronic health Records.
To assess if there is a potential dose-response relationship between tobacco consumption and the risk of sICH, several studies evaluated the risk of sICH based on the number of cigarettes smoked. As can be seen from Table 3, an increasing number of cigarettes consumed daily mostly correlate with an increased risk of sICH [35,36,59,70-73]. In some of the studies, it appears that the risk of sICH decreases with increasing number of cigarettes consumed daily [41,59,74]. One potential explanation is ethnic differences. In Table 3, the first three studies were conducted in Caucasian majority countries, while the latter four studies were conducted in Asian countries. Differences in the rate of passive smoking in Asian and Caucasian majority countries may also provide an additional explanation for these differences. It is plausible that a higher rate of passive smoking in Asian countries may lead to increased baseline risk of sICH, and this may result in a potential ceiling effect, which may prevent a further dose-dependent increase in the risk of sICH in smokers. This conjecture is also supported by an earlier meta-analysis by Price et al. [42]. They observed that the risk of sICH in current cigarette smokers was higher in European and North American studies when compared to Asian studies. Also, because cigarette smoking is a risk factor for several other conditions, it is plausible that this decreased incidence rate in greater tobacco consumption group may be due to death from other conditions [75]. However, this remains to be investigated via an appropriate clinical study. Overall, clinical studies identify tobacco use as one of the major risk factors for sICH. Studies also establish a dose-response relationship between tobacco consumption (i.e., cigarette smoking) and the risk of sICH. Despite several clinical studies indicating the deleterious effects of smoking/tobacco use in sICH patients, there are no confirmatory systematic preclinical studies evaluating the effects of smoking on the risk of sICH. Future studies investigating the effect of tobacco use on the risk of sICH with adequate power are needed in the field.
Table 3.
Relationship between smoking frequency and risk of sICH
| Study | Study population | Results (95% CI) |
|---|---|---|
| Jamrozik et al. (1994) [71] | Case-control study of 536 stroke cases in Perth, Western Australia matched with control subjects (59 ICH cases, 279 controls) | OR (1–20 cigarettes/day), 3.17 (0.92–11.0) |
| OR (≥21 cigarettes/day), 9.84 (2.09–46.4) | ||
| Kurth et al. (2003) [36] | Cohort of 22,022 United States male physicians from the Physicians’ Health Study (108 ICH cases) | RR (<20 cigarettes/day), 1.60 (0.50–5.07) |
| RR (≥20 cigarettes/day), 2.06 (1.08–3.96) | ||
| Kurth et al. (2003) [35] | Cohort of 39,783 United States women from the Women’s Health Study (40 ICH cases) | RR (<15 cigarettes/day), 2.15 (0.62–7.43) |
| RR (≥15 cigarettes/day), 2.67 (1.04–7.52) | ||
| Yamagishi et al. (2003) [72] | Cohort of 3,626 Japanese men aged 40–69 years (50 ICH cases) | RR (1–20 cigarettes/day), 0.6 (0.2–1.7) |
| RR (>20 cigarettes/day), 1.3 (0.5–3.1) | ||
| Mannami et al. (2004) [59] | Cohort of 27,063 Japanese men from the Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Disease (JPHC Study Cohort I) (219 ICH cases) | RR (1–19 cigarettes/day), 0.77 (0.49–1.21) |
| RR (20–39 cigarettes/day), 1.01 (0.71–1.44) | ||
| RR (≥40 cigarettes/day), 0.68 (0.34–1.35) | ||
| Kelly et al. (2008) [73] | Cohort of 169,871 Chinese persons aged >40 years (2,353 ICH cases) | RR (1–9 cigarettes/day), 1.19 (1.05–1.36) |
| RR (10–19 cigarettes/day), 1.14 (0.98–1.32) | ||
| RR (≥20 cigarettes/day), 1.20 (1.04–1.37) | ||
| Lawlor et al. (2008) [70] | Cohort of 648,346 Korean men aged 30–64 years old (2,380 ICH cases) | HR (<10 cigarettes/day), 1.16 (0.95–1.41) |
| HR (10–19 cigarettes/day), 1.17 (0.97–1.41) | ||
| HR (≥20 cigarettes/day), 1.03 (0.84–1.27) |
sICH, spontaneous intracerebral hemorrhage; CI, confidence interval; ICH, intracerebral hemorrhage; OR, odds ratio; RR, relative risk; HR, hazard ratio.
Smokers are known to have other comorbidities which may further predispose them to an increased risk of sICH. For example, tobacco use is shown to further increase the risk of sICH when in combination with other genetic risk factors and conditions such as hypertension, alcohol use, and pregnancy [76-78]. Tian et al. [78] observed an increased risk of sICH in hypertensive sICH patients with COL1A2 rs42524 polymorphism, especially when accompanied by alcohol consumption and tobacco use. One study found that cigarette smoking more than doubles the risk of sICH in hypertensive persons [79]. Another study found a 19% increase in the risk of sICH with a 10 mm Hg increase in systolic blood pressure level in smokers than in never smokers [77]. A Finnish study observed a U-shaped dose-response relationship between alcohol consumption in male smokers and sICH risk, with a 82% increased risk of sICH in heavy drinkers (>5 drinks per day) when compared to non-drinkers [80]. One British study also found that aspirin use on the previous night combined with current smoking increased the risk of sICH by 40% [81]. Furthermore, cigarette smoking is an important risk factor for developing type 2 diabetes [82], and several studies demonstrated that diabetes is also another risk factor for sICH [42,83-85]. Smoking may have a synergistic effect on diabetes patients, which may further increase the risk of sICH. These studies highlight that tobacco use itself directly and indirectly increases the risk of sICH.
Outcomes following sICH in tobacco users
Besides increasing the risk of sICH, earlier clinical studies also established that tobacco use also worsens outcomes following sICH (Figure 1). Smoking is considered as an important predictor of substantial hematoma expansion (SHE), short- and long-term mortality, and poor outcome in sICH patients [86-88]. As mentioned previously, hematoma expansion occurs in the majority of sICH patients following ictus. The risk of post-ictus hematoma expansion is higher in tobacco-using sICH patients. An earlier study that evaluated SHE (an absolute increase in ICH volume >6 mL or an increase >33% on the follow-up CT) in sICH patients demonstrated that the risk of SHE between 12 and 72 hours is significantly higher (OR, 2.527; P=0.023) in currently smoking sICH patients than in non-smoking sICH patients [88]. For this reason, the study included current smoking as one parameter to calculate the Hematoma Expansion Prediction Score [88]. A linear regression analysis found a significant correlation between daily cigarette smoking consumption and intracerebral hemorrhagic volumes in sICH patients [89].
As cigarette smoking increases the risk of hematoma expansion, it also increases the risk of poorer outcomes and death from sICH (Figure 1). Based on the Johns Hopkins clinical stroke database and the Nationwide Inpatient Sample database, Faigle et al. [86] observed that black (OR, 3.31; P=0.074) and white (OR, 7.42; P=0.010) smoker sICH patients had a higher risk of in-hospital mortality. Another study by Saloheimo et al. [87] evaluated risk factors for death in sICH patients (n=140) that survived the acute phase (3 months) and compared their long-term prognosis with the control population (n=206). They observed that during their 7-year follow-up, RR of death was significantly (P=0.043) higher in smoker sICH patients (RR, 3.09) than in the control population (RR, 2.73) [87]. Furthermore, one prospective study found that cigarette smoking was associated with an increased risk of death from sICH (RR, 1.9) [90]. This accumulation of evidence suggests that tobacco use increases hematoma volume and is linked to worse outcomes in sICH patients (Figure 1).
Former tobacco users and sICH
Most of the clinical studies merged former smokers into one non-smoker category, so limited studies are available that investigated the risk of sICH in former smokers. Some of these studies are summarized in Table 4 [42,45,59,60,70,71,91]. A cohort study in Korean men identified that quitters (HR, 0.75) and sustained (smoking status consistent from beginning to end of study period) ex-smokers (HR, 0.70) had a lower multivariable-adjusted risk of hemorrhagic stroke compared to non-reducing heavy smokers [92]. A British study similarly observed that ex-smokers were able to reduce their risk of sICH by at least 38% compared to current smokers. This risk reduction was even seen in former smokers that had quit less than 2 years prior to the study [43]. One Swedish study of women aged 30 to 50 years found an increased age-adjusted risk of sICH in former smokers (RR, 1.6) compared to non-smokers, although this study had a limited number of subjects [44]. Furthermore, a 10-year multicenter prospective study from China observed that the risk of hemorrhagic stroke remains higher in former male smokers (HR, 1.77). However, this study also reported that the risk of hemorrhagic stroke is lower in former female smokers (HR, 0.84). Owing to limited cases of SAH and to improve the statistical power, this study combined sICH and SAH cases in a single hemorrhagic stroke group [93]. A British study found a slight but significant increase in the risk of sICH of former smokers [42]. On the other hand, several studies found no difference between former smokers and never smokers, indicating that risk reduction in former smokers returns to that of never smokers [42,45,70,71,91]. However, owing to their limited number of former smokers, many of these studies do not differentiate former smokers based on how long they previously smoked, how much they previously smoked, and how long it has been since they quit smoking. Furthermore, the studies in Table 4 either did not specify how former smokers were defined, or those that did had varying definitions. Overall, these studies are inconclusive and a more detailed study in a larger population may help evaluate the risk of sICH in former smokers.
Table 4.
Relationship between former smoking and risk of sICH
| Study | Study population | Results (95% CI) |
|---|---|---|
| Jamrozik et al. (1994) [71] | Case-control study of 536 stroke cases in Perth, Western Australia matched with control subjects (59 ICH cases, 279 controls) | HR, 1.11 (0.43–2.85) |
| Thrift et al. (1996) [45] | Case-control study of 331 ICH cases aged 18–80 years and 342 matched controls from the Melbourne Risk Factor Study (MERFS Group) (85 ICH cases in former smokers, 68 controls) | OR, 1.09 (0.70–1.70) |
| Mannami et al. (2004) [59] | Cohort of 19,782 Japanese men aged 40–59 years from the Japan Public Health Center-based Prospective Study on Cancer and Cardiovascular Disease (JPHC Study Cohort I) (45 ICH cases in former smokers, 59 ICH cases in never smokers) | RR, 0.72 (0.49–1.07) |
| Sturgeon et al. (2007) [91] | Pooled cohort of 21,680 American men and women aged >45 years from the Atherosclerosis Risk in Communities Study (ARIC) and the Cardiovascular Health Study (CHS) (43 ICH cases in former smokers, 63 ICH cases in never smokers) | RR, 0.86 (0.59–1.27) |
| Lawlor et al. (2008) [70] | Cohort of 648,346 Korean men aged 30–64 years (515 ICH cases in former smokers, 483 ICH cases in never smokers) | HR, 0.90 (0.75–1.08) |
| Honjo et al. (2010) [60] | Pooled cohort of 140,026 and 156,810 Japanese men and women aged 40–79 years (117 ICH cases in male former smokers, 92 ICH cases in male never smokers, 14 ICH cases in female former smokers, 272 ICH cases in female never smokers) | HR (men), 0.94 (0.71–1.23) |
| HR (women), 1.62 (0.94–2.78) | ||
| Price et al. (2018) [42] | Cohort of 712,433 British women of the Million Women Study (468 ICH cases in former smokers, 780 ICH cases in never smokers) | RR, 1.10 (1.01–1.21) |
sICH, spontaneous intracerebral hemorrhage; CI, confidence interval; ICH, intracerebral hemorrhage; HR, hazard ratio; OR, odds ratio; RR, relative risk.
Secondhand smoking/passive smoking and sICH
Although the field lacks extensive studies investigating the effects of secondhand smoking on sICH, its effects are similar to the effects of chronic active smoking, and there is a significant morbidity and mortality associated with secondhand smoking [94,95]. This is of particular concern as over a third of the world population is exposed to passive smoking [95]. Furthermore, nicotine has been found in the urine of non-smokers that share an immediate environment with smokers [96]. Nicotine in non-smokers, owing to the potential effect of passive smoking, may be responsible for undercalculating sICH risk and dampening the benefits of smoking cessation [60]. This is especially important in countries such as China, Japan, and South Korea, where there is a significant gap in smoking rates between men and women and where secondhand smoking may have a bigger impact [30,59,97,98].
Several studies found an increased risk of death from hemorrhagic stroke in passive smokers [99-101]. A Japanese study found a significant increased risk (35%) of death from sICH in never-smoking women exposed to secondhand smoking than never-smoking women without secondhand smoking exposure [100]. This risk increased to 64% when the women (aged 40 to 79 years) lived with a smoking spouse and another smoking family member. One Chinese study observed a significantly increased risk (22%) of death from sICH in passive smokers and an increasing risk of death from hemorrhagic stroke with an increasing quantity of cigarettes smoked by spouses [101]. In contrast, other studies did not find any association between passive smoking and hemorrhagic stroke [102,103]. However, these studies had a limited number of participants, combined sICH cases and SAH cases together as hemorrhagic stroke cases, and/or differed in how they measured secondhand smoking (i.e., exposure from home vs. workplace, exposure from spouse vs. family member, number of years exposed, and number of cigarettes smoked by spouse). Furthermore, none of these studies measured the nicotine content in the passive smokers’ systems. In general, these studies are inconclusive, and a more detailed, larger study is needed to identify any risk of sICH in passive smokers.
Potential mechanisms by which tobacco use affects the risk of sICH and outcome following sICH
Earlier clinical/epidemiological studies convincingly established that: (1) tobacco use/smoking substantially increases the risk of sICH [32-40], (2) smoker sICH patients have a higher risk of in-hospital mortality and poor outcome [86,87], (3) current smokers have an increased risk of hematoma expansion [88], and (4) there is a correlation between daily cigarette smoking consumption and intracerebral hemorrhagic volumes in sICH [89]. However, the underlying mechanisms are not well understood.
Tobacco use is known to activate multiple mechanisms that may contribute to an increased risk of sICH as well as worse outcomes following sICH. Figure 2 highlights some possible mechanisms by which tobacco or nicotine use may increase the risk of and worsen outcomes following sICH. Although chronic tobacco use is not associated with increased blood pressure, it is well established that nicotine transiently increases blood pressure when a person is smoking [31]. Activation of the sympathetic nervous system leads to the activation of nicotinic acetylcholine receptors, which in turn leads to an increase in heart rate and also blood pressure [31]. Increased risk of hypertensive heart disease, renal failure, and intestinal ischemia in smokers indicate poor vascular function [104]. It is plausible that this transient increase in blood pressure and impaired vascular function in tobacco users may increase the risk of sICH (Figure 2).
Figure 2.
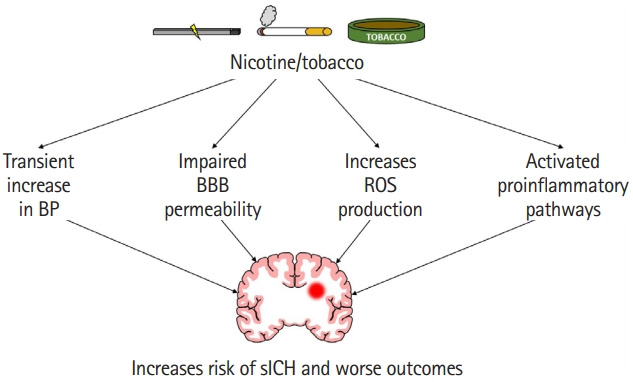
A cartoon highlighting various pathways responsible for increased risk of spontaneous intracerebral hemorrhage (sICH) and worse outcomes following sICH in nicotine/tobacco users. BP, blood pressure; BBB, blood-brain barrier; ROS, reactive oxygen species.
However, one contradiction is that cigarette smoking is well known to activate platelets, increase procoagulant activity, elevate fibrinogen levels, and cause plaque formation and rupture [105-108]. In that case, theoretically, this should result in decreased hematoma expansion and a better prognosis for the smoker. One Japanese group found some improvement in animal models, although nicotine treatment was given after sICH induction and no improvement was seen in hematoma volume [109,110]. A small Korean study found that cigarette smokers had better functional outcomes than non-smokers [111]. However, cigarette smoking and nicotine had a detrimental effect in sICH patients in clinical studies (Table 1) and in animal models from our laboratory (data not published). Some clinical studies also confirmed that no beneficial effect was seen after 3 months in sICH smoking patients [112,113]. The procoagulant benefit of cigarette smoking does not seem to be enough to overcome its detrimental effects.
In contrast, exposure to cigarette smoke causes denser fibrin clots and impairs fibrinolysis [107]. One study found depleted tissue plasminogen activator (tPA), a protease responsible for fibrinolysis and thrombolysis, in the brain capillaries of nicotine-treated rats compared to the control group [114]. Other studies showed increased levels and activity of plasminogen activator inhibitor-1, an inhibitor of tPA [107]. This could possibly lead to slower hematoma resolution, resulting in more damage and worse long-term outcomes in smoking sICH patients.
Cigarette smoking also weakens vessel wall integrity (Figure 2). Several studies found increased circulating endothelial cells and/or anuclear carcasses in the blood of humans right after smoking or inhaling cigarette smoke [115,116]. Another study found an increase in anuclear endothelial cells in the blood of rats following nicotine (equivalent to smoking of one cigarette) administration [117]. A study found increased OR of cerebral microbleeds in current smokers compared to non-smokers [118]. Cerebral microbleeds increase the risk of sICH, especially in Asian populations and in recurring ICH patients [119,120], which may explain the increased risk of sICH in the Asian population [1,6]. Another possible pathway could be through the effect of tobacco use on the risk of intracranial aneurysms. Some studies found that cigarette smoking and female sex increases the risk of multiple and larger aneurysms [121,122]. Arterial vessel damage and an acute increase in blood pressure could lead to an increased risk of rupture and intracerebral hemorrhage [123].
One of the potential mechanisms by which tobacco use increases hematoma expansion is by increasing BBB permeability (Figure 2). An earlier study observed that chronic treatment with smokeless tobacco products increases circulating levels of proinflammatory cytokine tumor necrosis factor α (TNF–α) [124]. Furthermore, chronic exposure of young mice to nicotine for 2 weeks resulted in increased expression of proinflammatory cytokines, chemokines, and adhesion molecules in brain microvessels [125]. This study reported that the levels of TNF–α increased by 12- and 14-fold in brain microvessels and brain tissue of animals exposed to chronic nicotine treatment than in control animals. Increased levels of TNF–α impairs barrier properties of the endothelium by affecting multiple pathways [126]. One of the pathways by which TNF–α impairs BBB properties is by affecting the dispersion of platelet-endothelial cell adhesion molecule-1 (PECAM-1) potentially via increasing PECAM-1 phosphorylation [127,128]. An earlier review article identified TNF–α stimulation-induced altered PECAM-1 dispersion as one of the major pathways by which TNF–α increases barrier permeability [126]. Overall, the literature indicates that chronic nicotine exposure weakens the integrity of BBB via increased levels of TNF–α (Figure 2).
Furthermore, chronic nicotine treatment is also shown to impair BBB function as evident by increased sucrose permeability, lower expression of the tight junction protein zona occludens 1 (ZO-1), and lower reactivity of claudin-3 in endothelial cells [129,130]. Increased BBB permeability is associated with an increased risk of bleeding in the brain. For example, early increase in parenchymal gadolinium enhancement, an indicator of compromised BBB, after thrombolytic therapy to ischemic stroke patients is significantly associated with an increased risk of hemorrhagic transformation [131]. This weakened BBB integrity may lead to a larger hematoma following rupture of cerebral vessels. Given that a small increase in hematoma volume significantly increases mortality in sICH patients, it is plausible that observed higher mortality and poorer outcomes in smoker ICH patients may be due to increased hematoma expansion/volume in this patient population [17].
Although nicotine is the most frequently studied component of cigarette smoking, cigarette smoke exposure also compromises BBB integrity (Figure 2). This is of particular concern as cigarette smoke affects not only firsthand smokers but also secondhand smokers. One study found that when brain microvascular endothelial cells (HBMEC: a model of human BBB) are exposed to tobacco smoke, cell viability decreased in a dose-specific manner and there was an increase in proinflammatory adhesion molecules [132]. This proinflammatory state could cause disruption of the BBB [133]. Furthermore, tobacco smoke contains high amounts of nitric oxide, which could result in increased BBB permeability [133-135].
Another harmful effect of tobacco smoke is its effect on secondary brain injury, especially in terms of microglial and astrocytic activation and oxidative injury (Figure 2). One study found that treatment with nicotine-derived nitrosamine ketone, a major component of tobacco smoke, caused microglia and astrocyte activation in mouse brain [136]. Increased levels before sICH could result in a greater proinflammatory state after sICH in cigarette smokers compared to non-smokers. Earlier studies also established the role of cigarette smoke exposure on reactive oxygen species (ROS) production. Several studies demonstrated that the exposure of HBMEC to tobacco products leads to an increased release of ROS and reactive nitrogen species (RNS) [132,137]. Naik et al. [137] also established that tobacco smoke exposure leads to the release of proinflammatory cytokines (TNF–α and interleukin 6). Acute smoking is shown to reversibly inhibit mitochondrial complex IV activity in human peripheral blood mononuclear cells [138]. This increase in oxidative stress and increased release of proinflammatory cytokines in the brain have also been seen in animal models treated with nicotine-derived nitrosamine ketone [136,139]. Increased ROS/RNS release and increased activation of proinflammatory pathways and mitochondrial dysfunction are known to play a role in brain damage following hemorrhagic stroke [140-146]. Others also established the role of free radicals in sICH-induced brain damage [147-149]. Because nicotine exposure increases the activation of pathways involved in post-sICH brain damage, it is plausible that elevated activation of those pathways is enhanced postsICH, which in turn leads to increased brain damage.
Conclusions
Several clinical studies identify tobacco use as a risk factor for sICH both in women and men. There is a dose-response relationship between tobacco use and the risk of sICH. Tobacco use also worsens outcomes following sICH. Tobacco use activates several mechanisms that increases the risk of sICH as well as increases post-sICH damage. Further studies are required to conclusively evaluate whether quitting tobacco use reverses the increased risk of sICH and passive smoking increases the risk of sICH.
Acknowledgments
This work was sponsored by the James and Esther King Biomedical Research Grant (9JK08). Authors are grateful to Dr. Brant Watson for critical reading of this manuscript.
Footnotes
Disclosure
The authors have no financial conflicts of interest.
References
- 1.Unnithan AKA, Mehta P. Abai B. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. Hemorrhagic stroke. [Google Scholar]
- 2.Rajashekar D, Liang JW. Abai B. StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. Intracerebral hemorrhage. [Google Scholar]
- 3.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 4.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 6.Poon MT, Bell SM, Al-Shahi Salman R. Epidemiology of intracerebral haemorrhage. Front Neurol Neurosci. 2015;37:1–12. doi: 10.1159/000437109. [DOI] [PubMed] [Google Scholar]
- 7.Lovelock CE, Molyneux AJ, Rothwell PM; Oxford Vascular Study. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol. 2007;6:487–493. doi: 10.1016/S1474-4422(07)70107-2. [DOI] [PubMed] [Google Scholar]
- 8.Burchell SR, Tang J, Zhang JH. Hematoma expansion following intracerebral hemorrhage: mechanisms targeting the coagulation cascade and platelet activation. Curr Drug Targets. 2017;18:1329–1344. doi: 10.2174/1389450118666170329152305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392:1257–1268. doi: 10.1016/S0140-6736(18)31878-6. [DOI] [PubMed] [Google Scholar]
- 10.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 11.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 12.Murthy SB, Biffi A, Falcone GJ, Sansing LH, Torres Lopez V, Navi BB, et al. Antiplatelet therapy after spontaneous intracerebral hemorrhage and functional outcomes. Stroke. 2019;50:3057–3063. doi: 10.1161/STROKEAHA.119.025972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poon MT, Fonville AF, Al-Shahi Salman R. Long-term prognosis after intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2014;85:660–667. doi: 10.1136/jnnp-2013-306476. [DOI] [PubMed] [Google Scholar]
- 14.Counsell C, Boonyakarnkul S, Dennis M, Sandercock P, Bamford J, Burn J, et al. Primary intracerebral haemorrhage in the Oxfordshire Community Stroke Project. Cerebrovasc Dis. 1995;5:26–34. [Google Scholar]
- 15.Gulati D, Dua D, Torbey MT. Hemostasis in intracranial hemorrhage. Front Neurol. 2017;8:80. doi: 10.3389/fneur.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. 2012;11:101–118. doi: 10.1016/S1474-4422(11)70264-2. [DOI] [PubMed] [Google Scholar]
- 17.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage: a powerful and easy-touse predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 18.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 19.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11:720–731. doi: 10.1016/S1474-4422(12)70104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prabhakaran S, Naidech AM. Ischemic brain injury after intracerebral hemorrhage: a critical review. Stroke. 2012;43:2258–2263. doi: 10.1161/STROKEAHA.112.655910. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Tsirka SE. Contribution of extracellular proteolysis and microglia to intracerebral hemorrhage. Neurocrit Care. 2005;3:77–85. doi: 10.1385/NCC:3:1:077. [DOI] [PubMed] [Google Scholar]
- 22.Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17:5316–5326. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keep RF, Zhou N, Xiang J, Andjelkovic AV, Hua Y, Xi G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS. 2014;11:18. doi: 10.1186/2045-8118-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J. Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol. 2017;54:1874–1886. doi: 10.1007/s12035-016-9785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tschoe C, Bushnell CD, Duncan PW, Alexander-Miller MA, Wolfe SQ. Neuroinflammation after intracerebral hemorrhage and potential therapeutic targets. J Stroke. 2020;22:29–46. doi: 10.5853/jos.2019.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biffi A, Cortellini L, Nearnberg CM, Ayres AM, Schwab K, Gilson AJ, et al. Body mass index and etiology of intracerebral hemorrhage. Stroke. 2011;42:2526–2530. doi: 10.1161/STROKEAHA.111.617225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005;65:518–522. doi: 10.1212/01.wnl.0000172915.71933.00. [DOI] [PubMed] [Google Scholar]
- 28.Appelros P, Stegmayr B, Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 29.Wahab KW, Tiwari HK, Ovbiagele B, Sarfo F, Akinyemi R, Traylor M, et al. Genetic risk of spontaneous intracerebral hemorrhage: systematic review and future directions. J Neurol Sci. 2019;407:116526. doi: 10.1016/j.jns.2019.116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer-Lindgren L, Thomson B, et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: implications for electronic cigarette use. Trends Cardiovasc Med. 2016;26:515–523. doi: 10.1016/j.tcm.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ariesen MJ, Claus SP, Rinkel GJ, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34:2060–2065. doi: 10.1161/01.STR.0000080678.09344.8D. [DOI] [PubMed] [Google Scholar]
- 33.Feldmann E, Broderick JP, Kernan WN, Viscoli CM, Brass LM, Brott T, et al. Major risk factors for intracerebral hemorrhage in the young are modifiable. Stroke. 2005;36:1881–1885. doi: 10.1161/01.STR.0000177480.62341.6b. [DOI] [PubMed] [Google Scholar]
- 34.Gill JS, Shipley MJ, Tsementzis SA, Hornby R, Gill SK, Hitchcock ER, et al. Cigarette smoking: a risk factor for hemorrhagic and nonhemorrhagic stroke. Arch Intern Med. 1989;149:2053–2057. doi: 10.1001/archinte.149.9.2053. [DOI] [PubMed] [Google Scholar]
- 35.Kurth T, Kase CS, Berger K, Gaziano JM, Cook NR, Buring JE. Smoking and risk of hemorrhagic stroke in women. Stroke. 2003;34:2792–2795. doi: 10.1161/01.STR.0000100165.36466.95. [DOI] [PubMed] [Google Scholar]
- 36.Kurth T, Kase CS, Berger K, Schaeffner ES, Buring JE, Gaziano JM. Smoking and the risk of hemorrhagic stroke in men. Stroke. 2003;34:1151–1155. doi: 10.1161/01.STR.0000065200.93070.32. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Schooling CM, Chan WM, Lee SY, Leung GM, Lam TH. Smoking and hemorrhagic stroke mortality in a prospective cohort study of older Chinese. Stroke. 2013;44:2144–2149. doi: 10.1161/STROKEAHA.113.001500. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Y, Zhao YC. Association between the nicotinamide adenine dinucleotide phosphate oxidase p22phox gene -A930G polymorphism and intracerebral hemorrhage. Mol Med Rep. 2015;11:3511–3516. doi: 10.3892/mmr.2015.3154. [DOI] [PubMed] [Google Scholar]
- 39.Smajlović D, Salihović D, Ibrahimagić OC, Sinanović O, Vidović M. Analysis of risk factors, localization and 30-day prognosis of intracerebral hemorrhage. Bosn J Basic Med Sci. 2008;8:121–125. doi: 10.17305/bjbms.2008.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qureshi AI, Suri MA, Safdar K, Ottenlips JR, Janssen RS, Frankel MR. Intracerebral hemorrhage in blacks: risk factors, subtypes, and outcome. Stroke. 1997;28:961–964. doi: 10.1161/01.str.28.5.961. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, et al. Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet. 2016;388:761–775. doi: 10.1016/S0140-6736(16)30506-2. [DOI] [PubMed] [Google Scholar]
- 42.Price AJ, Wright FL, Green J, Balkwill A, Kan SW, Yang TO, et al. Differences in risk factors for 3 types of stroke: UK prospective study and meta-analyses. Neurology. 2018;90:e298–e306. doi: 10.1212/WNL.0000000000004856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pujades-Rodriguez M, George J, Shah AD, Rapsomaniki E, Denaxas S, West R, et al. Heterogeneous associations between smoking and a wide range of initial presentations of cardiovascular disease in 1937360 people in England: lifetime risks and implications for risk prediction. Int J Epidemiol. 2015;44:129–141. doi: 10.1093/ije/dyu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu M, Ye W, Adami HO, Weiderpass E. Stroke incidence in women under 60 years of age related to alcohol intake and smoking habit. Cerebrovasc Dis. 2008;25:517–525. doi: 10.1159/000131669. [DOI] [PubMed] [Google Scholar]
- 45.Thrift AG, McNeil JJ, Forbes A, Donnan GA. Risk factors for cerebral hemorrhage in the era of well-controlled hypertension. Melbourne Risk Factor Study (MERFS) Group. Stroke. 1996;27:2020–2025. doi: 10.1161/01.str.27.11.2020. [DOI] [PubMed] [Google Scholar]
- 46.Abbott RD, Yin Y, Reed DM, Yano K. Risk of stroke in male cigarette smokers. N Engl J Med. 1986;315:717–720. doi: 10.1056/NEJM198609183151201. [DOI] [PubMed] [Google Scholar]
- 47.Fogelholm R, Murros K. Cigarette smoking and risk of primary intracerebral haemorrhage: a population-based case-control study. Acta Neurol Scand. 1993;87:367–370. doi: 10.1111/j.1600-0404.1993.tb04119.x. [DOI] [PubMed] [Google Scholar]
- 48.Juvela S, Hillbom M, Palomäki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995;26:1558–1564. doi: 10.1161/01.str.26.9.1558. [DOI] [PubMed] [Google Scholar]
- 49.Kubota M, Yamaura A, Ono J, Itani T, Tachi N, Ueda K, et al. Is family history an independent risk factor for stroke? J Neurol Neurosurg Psychiatry. 1997;62:66–70. doi: 10.1136/jnnp.62.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo D, Sauerbeck LR, Kissela BM, Khoury JC, Szaflarski JP, Gebel J, et al. Genetic and environmental risk factors for intracerebral hemorrhage: preliminary results of a population-based study. Stroke. 2002;33:1190–1195. doi: 10.1161/01.str.0000014774.88027.22. [DOI] [PubMed] [Google Scholar]
- 51.O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- 52.Ferket BS, van Kempen BJ, Wieberdink RG, Steyerberg EW, Koudstaal PJ, Hofman A, et al. Separate prediction of intracerebral hemorrhage and ischemic stroke. Neurology. 2014;82:1804–1812. doi: 10.1212/WNL.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. 1999;6:207–211. doi: 10.1177/204748739900600403. [DOI] [PubMed] [Google Scholar]
- 54.Hauer AJ, Ruigrok YM, Algra A, van Dijk EJ, Koudstaal PJ, Luijckx GJ, et al. Age-specific vascular risk factor profiles according to stroke subtype. J Am Heart Assoc. 2017;6:e005090. doi: 10.1161/JAHA.116.005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koivunen RJ, Satopää J, Meretoja A, Strbian D, Haapaniemi E, Niemelä M, et al. Incidence, risk factors, etiology, severity and short-term outcome of non-traumatic intracerebral hemorrhage in young adults. Eur J Neurol. 2015;22:123–132. doi: 10.1111/ene.12543. [DOI] [PubMed] [Google Scholar]
- 56.Ruíz-Sandoval JL, Cantú C, Barinagarrementeria F. Intracerebral hemorrhage in young people: analysis of risk factors, location, causes, and prognosis. Stroke. 1999;30:537–541. doi: 10.1161/01.str.30.3.537. [DOI] [PubMed] [Google Scholar]
- 57.Kase CS, Kurth T. Prevention of intracerebral hemorrhage recurrence. Continuum (Minneap Minn) 2011;17:1304–1317. doi: 10.1212/01.CON.0000410037.64971.e3. [DOI] [PubMed] [Google Scholar]
- 58.Pelhate M, Sattelle DB. Synthetic saxitoxin selectively inhibits sodium currents in the cockroach giant axon [proceedings] J Physiol. 1978;284:89P–90P. [PubMed] [Google Scholar]
- 59.Mannami T, Iso H, Baba S, Sasaki S, Okada K, Konishi M, et al. Cigarette smoking and risk of stroke and its subtypes among middle-aged Japanese men and women: the JPHC Study Cohort I. Stroke. 2004;35:1248–1253. doi: 10.1161/01.STR.0000128794.30660.e8. [DOI] [PubMed] [Google Scholar]
- 60.Honjo K, Iso H, Tsugane S, Tamakoshi A, Satoh H, Tajima K, et al. The effects of smoking and smoking cessation on mortality from cardiovascular disease among Japanese: pooled analysis of three large-scale cohort studies in Japan. Tob Control. 2010;19:50–57. doi: 10.1136/tc.2009.029751. [DOI] [PubMed] [Google Scholar]
- 61.Zou Y, Zhang C, Ge H, Li H, Fang X, Zhong J, et al. Comparison of epidemiological and clinical features between two chronological cohorts of patients with intracerebral hemorrhage. J Clin Neurosci. 2020;72:169–173. doi: 10.1016/j.jocn.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 62.George J, Rapsomaniki E, Pujades-Rodriguez M, Shah AD, Denaxas S, Herrett E, et al. How does cardiovascular disease first present in women and men? Incidence of 12 cardiovascular diseases in a contemporary cohort of 1,937,360 people. Circulation. 2015;132:1320–1328. doi: 10.1161/CIRCULATIONAHA.114.013797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marini S, Morotti A, Ayres AM, Crawford K, Kourkoulis CE, Lena UK, et al. Sex differences in intracerebral hemorrhage expansion and mortality. J Neurol Sci. 2017;379:112–116. doi: 10.1016/j.jns.2017.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tada Y, Wada K, Shimada K, Makino H, Liang EI, Murakami S, et al. Estrogen protects against intracranial aneurysm rupture in ovariectomized mice. Hypertension. 2014;63:1339–1344. doi: 10.1161/HYPERTENSIONAHA.114.03300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jandíková H, Dušková M, Stárka L. The influence of smoking and cessation on the human reproductive hormonal balance. Physiol Res. 2017;66:S323–S331. doi: 10.33549/physiolres.933724. [DOI] [PubMed] [Google Scholar]
- 66.Svartberg J, Jorde R. Endogenous testosterone levels and smoking in men: the fifth Tromsø study. Int J Androl. 2007;30:137–143. doi: 10.1111/j.1365-2605.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- 67.Dai WS, Gutai JP, Kuller LH, Cauley JA. Cigarette smoking and serum sex hormones in men. Am J Epidemiol. 1988;128:796–805. doi: 10.1093/oxfordjournals.aje.a115033. [DOI] [PubMed] [Google Scholar]
- 68.Wang W, Yang X, Liang J, Liao M, Zhang H, Qin X, et al. Cigarette smoking has a positive and independent effect on testosterone levels. Hormones (Athens) 2013;12:567–577. doi: 10.14310/horm.2002.1445. [DOI] [PubMed] [Google Scholar]
- 69.Chen Z, Xi G, Mao Y, Keep RF, Hua Y. Effects of progesterone and testosterone on ICH-induced brain injury in rats. Acta Neurochir Suppl. 2011;111:289–293. doi: 10.1007/978-3-7091-0693-8_48. [DOI] [PubMed] [Google Scholar]
- 70.Lawlor DA, Song YM, Sung J, Ebrahim S, Smith GD. The association of smoking and cardiovascular disease in a population with low cholesterol levels: a study of 648,346 men from the Korean national health system prospective cohort study. Stroke. 2008;39:760–767. doi: 10.1161/STROKEAHA.107.494823. [DOI] [PubMed] [Google Scholar]
- 71.Jamrozik K, Broadhurst RJ, Anderson CS, Stewart-Wynne EG. The role of lifestyle factors in the etiology of stroke: a population-based case-control study in Perth, Western Australia. Stroke. 1994;25:51–59. doi: 10.1161/01.str.25.1.51. [DOI] [PubMed] [Google Scholar]
- 72.Yamagishi K, Iso H, Kitamura A, Sankai T, Tanigawa T, Naito Y, et al. Smoking raises the risk of total and ischemic strokes in hypertensive men. Hypertens Res. 2003;26:209–217. doi: 10.1291/hypres.26.209. [DOI] [PubMed] [Google Scholar]
- 73.Kelly TN, Gu D, Chen J, Huang JF, Chen JC, Duan X, et al. Cigarette smoking and risk of stroke in the Chinese adult population. Stroke. 2008;39:1688–1693. doi: 10.1161/STROKEAHA.107.505305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269:232–236. [PubMed] [Google Scholar]
- 75.United States, Public Health Service, Office of the Surgeon General. United States, Department of Health and Human Services . The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 76.Bateman BT, Schumacher HC, Bushnell CD, Pile-Spellman J, Simpson LL, Sacco RL, et al. Intracerebral hemorrhage in pregnancy: frequency, risk factors, and outcome. Neurology. 2006;67:424–429. doi: 10.1212/01.wnl.0000228277.84760.a2. [DOI] [PubMed] [Google Scholar]
- 77.Nakamura K, Barzi F, Lam TH, Huxley R, Feigin VL, Ueshima H, et al. Cigarette smoking, systolic blood pressure, and cardiovascular diseases in the Asia-Pacific region. Stroke. 2008;39:1694–1702. doi: 10.1161/STROKEAHA.107.496752. [DOI] [PubMed] [Google Scholar]
- 78.Tian DZ, Wei W, Dong YJ. Influence of COL1A2 gene variants on the incidence of hypertensive intracerebral hemorrhage in a Chinese population. Genet Mol Res. 2016;15:gmr7369. doi: 10.4238/gmr.15017369. [DOI] [PubMed] [Google Scholar]
- 79.Thrift AG, McNeil JJ, Forbes A, Donnan GA. Three important subgroups of hypertensive persons at greater risk of intracerebral hemorrhage. Melbourne Risk Factor Study Group. Hypertension. 1998;31:1223–1229. doi: 10.1161/01.hyp.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 80.Leppälä JM, Paunio M, Virtamo J, Fogelholm R, Albanes D, Taylor PR, et al. Alcohol consumption and stroke incidence in male smokers. Circulation. 1999;100:1209–1214. doi: 10.1161/01.cir.100.11.1209. [DOI] [PubMed] [Google Scholar]
- 81.Thrift AG, McNeil JJ, Forbes A, Donnan GA. Risk of primary intracerebral haemorrhage associated with aspirin and non-steroidal anti-inflammatory drugs: case-control study. BMJ. 1999;318:759–764. doi: 10.1136/bmj.318.7186.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958–967. doi: 10.1016/S2213-8587(15)00316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Emerging Risk Factors Collaboration. Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zodpey SP, Tiwari RR, Kulkarni HR. Risk factors for haemorrhagic stroke: a case-control study. Public Health. 2000;114:177–182. [PubMed] [Google Scholar]
- 85.Dinesh Shah A, Langenberg C, Rapsomaniki E, Denaxas S, Pujades-Rodriguez M, Gale CP, et al. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1·9 million people. Lancet. 2015;385 Suppl 1:S86. doi: 10.1016/S0140-6736(15)60401-9. [DOI] [PubMed] [Google Scholar]
- 86.Faigle R, Marsh EB, Llinas RH, Urrutia VC, Gottesman RF. Race-specific predictors of mortality in intracerebral hemorrhage: differential impacts of intraventricular hemorrhage and age among blacks and whites. J Am Heart Assoc. 2016;5:e003540. doi: 10.1161/JAHA.116.003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saloheimo P, Lapp TM, Juvela S, Hillbom M. The impact of functional status at three months on long-term survival after spontaneous intracerebral hemorrhage. Stroke. 2006;37:487–491. doi: 10.1161/01.STR.0000198868.78546.fc. [DOI] [PubMed] [Google Scholar]
- 88.Yao X, Xu Y, Siwila-Sackman E, Wu B, Selim M. The HEP score: a nomogram-derived hematoma expansion prediction scale. Neurocrit Care. 2015;23:179–187. doi: 10.1007/s12028-015-0147-4. [DOI] [PubMed] [Google Scholar]
- 89.Zhou JF, Wang JY, Luo YE, Chen HH. Influence of hypertension, lipometabolism disorders, obesity and other lifestyles on spontaneous intracerebral hemorrhage. Biomed Environ Sci. 2003;16:295–303. [PubMed] [Google Scholar]
- 90.Neaton JD, Wentworth DN, Cutler J, Stamler J, Kuller L. Risk factors for death from different types of stroke. Multiple Risk Factor Intervention Trial Research Group. Ann Epidemiol. 1993;3:493–499. doi: 10.1016/1047-2797(93)90103-b. [DOI] [PubMed] [Google Scholar]
- 91.Sturgeon JD, Folsom AR, Longstreth WT, Jr, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38:2718–2725. doi: 10.1161/STROKEAHA.107.487090. [DOI] [PubMed] [Google Scholar]
- 92.Song YM, Cho HJ. Risk of stroke and myocardial infarction after reduction or cessation of cigarette smoking: a cohort study in Korean men. Stroke. 2008;39:2432–2438. doi: 10.1161/STROKEAHA.107.512632. [DOI] [PubMed] [Google Scholar]
- 93.Tse LA, Fang XH, Wang WZ, Qiu H, Yu IT. Incidence of ischaemic and haemorrhagic stroke and the association with smoking and smoking cessation: a 10-year multicentre prospective study in China. Public Health. 2012;126:960–966. doi: 10.1016/j.puhe.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2684–2698. doi: 10.1161/CIRCULATIONAHA.104.492215. [DOI] [PubMed] [Google Scholar]
- 95.Carreras G, Lugo A, Gallus S, Cortini B, Fernández E, López MJ, et al. Burden of disease attributable to second-hand smoke exposure: a systematic review. Prev Med. 2019;129:105833. doi: 10.1016/j.ypmed.2019.105833. [DOI] [PubMed] [Google Scholar]
- 96.Horning EC, Horning MG, Carroll DI, Stillwell RN, Dzidic I. Nicotine in smokers, non-smokers and room air. Life Sci. 1973;13:1331–1346. doi: 10.1016/0024-3205(73)90154-9. [DOI] [PubMed] [Google Scholar]
- 97.Zhang LF, Yang J, Hong Z, Yuan GG, Zhou BF, Zhao LC, et al. Proportion of different subtypes of stroke in China. Stroke. 2003;34:2091–2096. doi: 10.1161/01.STR.0000087149.42294.8C. [DOI] [PubMed] [Google Scholar]
- 98.World Health Organization; Monitor tobacco use and prevention policies (tobacco control) https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/gho-tobacco-control-monitor. Accessed January 21, 2020. [Google Scholar]
- 99.He Y, Jiang B, Li LS, Li LS, Ko L, Wu L, et al. Secondhand smoke exposure predicted COPD and other tobacco-related mortality in a 17-year cohort study in China. Chest. 2012;142:909–918. doi: 10.1378/chest.11-2884. [DOI] [PubMed] [Google Scholar]
- 100.Nishino Y, Tsuji I, Tanaka H, Nakayama T, Nakatsuka H, Ito H, et al. Stroke mortality associated with environmental tobacco smoke among never-smoking Japanese women: a prospective cohort study. Prev Med. 2014;67:41–45. doi: 10.1016/j.ypmed.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 101.Hou L, Han W, Jiang J, Liu B, Wu Y, Zou X, et al. Passive smoking and stroke in men and women: a national population-based case-control study in China. Sci Rep. 2017;7:45542. doi: 10.1038/srep45542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.He Y, Lam TH, Jiang B, Wang J, Sai X, Fan L, et al. Passive smoking and risk of peripheral arterial disease and ischemic stroke in Chinese women who never smoked. Circulation. 2008;118:1535–1540. doi: 10.1161/CIRCULATIONAHA.108.784801. [DOI] [PubMed] [Google Scholar]
- 103.Malek AM, Cushman M, Lackland DT, Howard G, McClure LA. Secondhand smoke exposure and stroke: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Prev Med. 2015;49:e89–e97. doi: 10.1016/j.amepre.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, et al. Smoking and mortality: beyond established causes. N Engl J Med. 2015;372:631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 105.Levine PH. An acute effect of cigarette smoking on platelet function: a possible link between smoking and arterial thrombosis. Circulation. 1973;48:619–623. doi: 10.1161/01.cir.48.3.619. [DOI] [PubMed] [Google Scholar]
- 106.Hawkins RI. Smoking, platelets and thrombosis. Nature. 1972;236:450–452. doi: 10.1038/236450a0. [DOI] [PubMed] [Google Scholar]
- 107.Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. 2013;10:219–230. doi: 10.1038/nrcardio.2013.8. [DOI] [PubMed] [Google Scholar]
- 108.Renaud S, Blache D, Dumont E, Thevenon C, Wissendanger T. Platelet function after cigarette smoking in relation to nicotine and carbon monoxide. Clin Pharmacol Ther. 1984;36:389–395. doi: 10.1038/clpt.1984.193. [DOI] [PubMed] [Google Scholar]
- 109.Hijioka M, Matsushita H, Hisatsune A, Isohama Y, Katsuki H. Therapeutic effect of nicotine in a mouse model of intracerebral hemorrhage. J Pharmacol Exp Ther. 2011;338:741–749. doi: 10.1124/jpet.111.182519. [DOI] [PubMed] [Google Scholar]
- 110.Anan J, Hijioka M, Kurauchi Y, Hisatsune A, Seki T, Katsuki H. Cortical hemorrhage-associated neurological deficits and tissue damage in mice are ameliorated by therapeutic treatment with nicotine. J Neurosci Res. 2017;95:1838–1849. doi: 10.1002/jnr.24016. [DOI] [PubMed] [Google Scholar]
- 111.Go GO, Park H, Lee CH, Hwang SH, Han JW, Park IS. The outcomes of spontaneous intracerebral hemorrhage in young adults: a clinical study. J Cerebrovasc Endovasc Neurosurg. 2013;15:214–220. doi: 10.7461/jcen.2013.15.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen CJ, Ding D, Ironside N, Buell TJ, Southerland AM, Koch S, et al. Cigarette smoking history and functional outcomes after spontaneous intracerebral hemorrhage. Stroke. 2019;50:588–594. doi: 10.1161/STROKEAHA.118.023580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ironside N, Chen CJ, Pucci J, Connolly ES. Effect of cigarette smoking on functional outcomes in patients with spontaneous intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2019;28:2496–2505. doi: 10.1016/j.jstrokecerebrovasdis.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 114.Wang L, Kittaka M, Sun N, Schreiber SS, Zlokovic BV. Chronic nicotine treatment enhances focal ischemic brain injury and depletes free pool of brain microvascular tissue plasminogen activator in rats. J Cereb Blood Flow Metab. 1997;17:136–146. doi: 10.1097/00004647-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 115.Davis JW, Shelton L, Eigenberg DA, Hignite CE, Watanabe IS. Effects of tobacco and non-tobacco cigarette smoking on endothelium and platelets. Clin Pharmacol Ther. 1985;37:529–533. doi: 10.1038/clpt.1985.83. [DOI] [PubMed] [Google Scholar]
- 116.Blache D, Bouthillier D, Davignon J. Acute influence of smoking on platelet behaviour, endothelium and plasma lipids and normalization by aspirin. Atherosclerosis. 1992;93:179–188. doi: 10.1016/0021-9150(92)90254-e. [DOI] [PubMed] [Google Scholar]
- 117.Hladovec J. Endothelial injury by nicotine and its prevention. Experientia. 1978;34:1585–1586. doi: 10.1007/BF02034689. [DOI] [PubMed] [Google Scholar]
- 118.Goos JD, Henneman WJ, Sluimer JD, Vrenken H, Sluimer IC, Barkhof F, et al. Incidence of cerebral microbleeds: a longitudinal study in a memory clinic population. Neurology. 2010;74:1954–1960. doi: 10.1212/WNL.0b013e3181e396ea. [DOI] [PubMed] [Google Scholar]
- 119.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke. 2013;44:995–1001. doi: 10.1161/STROKEAHA.111.000038. [DOI] [PubMed] [Google Scholar]
- 120.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 121.Qureshi AI, Suarez JI, Parekh PD, Sung G, Geocadin R, Bhardwaj A, et al. Risk factors for multiple intracranial aneurysms. Neurosurgery. 1998;43:22–26. doi: 10.1097/00006123-199807000-00013. [DOI] [PubMed] [Google Scholar]
- 122.Qureshi AI, Sung GY, Suri MF, Straw RN, Guterman LR, Hopkins LN. Factors associated with aneurysm size in patients with subarachnoid hemorrhage: effect of smoking and aneurysm location. Neurosurgery. 2000;46:44–50. [PubMed] [Google Scholar]
- 123.Juvela S. Prevalence of risk factors in spontaneous intracerebral hemorrhage and aneurysmal subarachnoid hemorrhage. Arch Neurol. 1996;53:734–740. doi: 10.1001/archneur.1996.00550080048012. [DOI] [PubMed] [Google Scholar]
- 124.Malovichko MV, Zeller I, Krivokhizhina TV, Xie Z, Lorkiewicz P, Agarwal A, et al. Systemic toxicity of smokeless tobacco products in mice. Nicotine Tob Res. 2019;21:101–110. doi: 10.1093/ntr/ntx230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol. 2011;300:H1518–H1529. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marcos-Ramiro B, García-Weber D, Millán J. TNF-induced endothelial barrier disruption: beyond actin and Rho. Thromb Haemost. 2014;112:1088–1102. doi: 10.1160/TH14-04-0299. [DOI] [PubMed] [Google Scholar]
- 127.Ferrero E, Villa A, Ferrero ME, Toninelli E, Bender JR, Pardi R, et al. Tumor necrosis factor alpha-induced vascular leakage involves PECAM1 phosphorylation. Cancer Res. 1996;56:3211–3215. [PubMed] [Google Scholar]
- 128.Romer LH, McLean NV, Yan HC, Daise M, Sun J, DeLisser HM. IFN-gamma and TNF-alpha induce redistribution of PECAM-1 (CD31) on human endothelial cells. J Immunol. 1995;154:6582–6592. [PubMed] [Google Scholar]
- 129.Abbruscato TJ, Lopez SP, Mark KS, Hawkins BT, Davis TP. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J Pharm Sci. 2002;91:2525–2538. doi: 10.1002/jps.10256. [DOI] [PubMed] [Google Scholar]
- 130.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, et al. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 131.Kastrup A, Gröschel K, Ringer TM, Redecker C, Cordesmeyer R, Witte OW, et al. Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke. 2008;39:2385–2387. doi: 10.1161/STROKEAHA.107.505420. [DOI] [PubMed] [Google Scholar]
- 132.Hossain M, Sathe T, Fazio V, Mazzone P, Weksler B, Janigro D, et al. Tobacco smoke: a critical etiological factor for vascular impairment at the blood-brain barrier. Brain Res. 2009;1287:192–205. doi: 10.1016/j.brainres.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazzone P, Tierney W, Hossain M, Puvenna V, Janigro D, Cucullo L. Pathophysiological impact of cigarette smoke exposure on the cerebrovascular system with a focus on the blood-brain barrier: expanding the awareness of smoking toxicity in an underappreciated area. Int J Environ Res Public Health. 2010;7:4111–4126. doi: 10.3390/ijerph7124111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yamauchi A, Dohgu S, Nishioku T, Shuto H, Naito M, Tsuruo T, et al. An inhibitory role of nitric oxide in the dynamic regulation of the blood-brain barrier function. Cell Mol Neurobiol. 2007;27:263–270. doi: 10.1007/s10571-007-9139-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hossain M, Mazzone P, Tierney W, Cucullo L. In vitro assessment of tobacco smoke toxicity at the BBB: do antioxidant supplements have a protective role? BMC Neurosci. 2011;12:92. doi: 10.1186/1471-2202-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ghosh D, Mishra MK, Das S, Kaushik DK, Basu A. Tobacco carcinogen induces microglial activation and subsequent neuronal damage. J Neurochem. 2009;110:1070–1081. doi: 10.1111/j.1471-4159.2009.06203.x. [DOI] [PubMed] [Google Scholar]
- 137.Naik P, Fofaria N, Prasad S, Sajja RK, Weksler B, Couraud PO, et al. Oxidative and pro-inflammatory impact of regular and denicotinized cigarettes on blood brain barrier endothelial cells: is smoking reduced or nicotine-free products really safe? BMC Neurosci. 2014;15:51. doi: 10.1186/1471-2202-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Alonso JR, Cardellach F, Casademont J, Miró O. Reversible inhibition of mitochondrial complex IV activity in PBMC following acute smoking. Eur Respir J. 2004;23:214–218. doi: 10.1183/09031936.03.00038203. [DOI] [PubMed] [Google Scholar]
- 139.Bhagwat SV, Vijayasarathy C, Raza H, Mullick J, Avadhani NG. Preferential effects of nicotine and 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-1-butanone on mitochondrial glutathione S-transferase A4-4 induction and increased oxidative stress in the rat brain. Biochem Pharmacol. 1998;56:831–839. doi: 10.1016/s0006-2952(98)00228-7. [DOI] [PubMed] [Google Scholar]
- 140.Bobinger T, Burkardt P, Huttner HB, Manaenko A. Programmed cell death after intracerebral hemorrhage. Curr Neuropharmacol. 2018;16:1267–1281. doi: 10.2174/1570159X15666170602112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 142.Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J. NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol. 2014;75:209–219. doi: 10.1002/ana.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu Z, Wang Z, Yu L, Ding Y, Xu Y, Xu N, et al. GCN2 reduces inflammation by p-eIF2α/ATF4 pathway after intracerebral hemorrhage in mice. Exp Neurol. 2019;313:16–25. doi: 10.1016/j.expneurol.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 144.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- 145.Jung JE, Sun G, Bautista Garrido J, Obertas L, Mobley AS, Ting SM, et al. The mitochondria-derived peptide humanin improves recovery from intracerebral hemorrhage: implication of mitochondria transfer and microglia phenotype change. J Neurosci. 2020;40:2154–2165. doi: 10.1523/JNEUROSCI.2212-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Al-Senani FM, Zhao X, Grotta JC, Shirzadi A, Strong R, Aronowski J. Proteasome inhibitor reduces astrocytic iNOS expression and functional deficit after experimental intracerebral hemorrhage in rats. Transl Stroke Res. 2012;3:146–153. doi: 10.1007/s12975-011-0108-y. [DOI] [PubMed] [Google Scholar]
- 147.Tang J, Liu J, Zhou C, Ostanin D, Grisham MB, Neil Granger D, et al. Role of NADPH oxidase in the brain injury of intracerebral hemorrhage. J Neurochem. 2005;94:1342–1350. doi: 10.1111/j.1471-4159.2005.03292.x. [DOI] [PubMed] [Google Scholar]
- 148.Chen YC, Chen CM, Liu JL, Chen ST, Cheng ML, Chiu DT. Oxidative markers in spontaneous intracerebral hemorrhage: leukocyte 8-hydroxy-2’-deoxyguanosine as an independent predictor of the 30-day outcome. J Neurosurg. 2011;115:1184–1190. doi: 10.3171/2011.7.JNS11718. [DOI] [PubMed] [Google Scholar]
- 149.Hayashi K, Hasegawa Y, Takemoto Y, Cao C, Mukasa A, Kim-Mitsuyama S. Enhanced oxidative stress contributes to worse prognosis and delayed neurofunctional recovery after striatal intracerebral hemorrhage in 5XFAD mice. Eur J Neurosci. 2020;51:1806–1814. doi: 10.1111/ejn.14596. [DOI] [PubMed] [Google Scholar]


