Dear Sir:
Previous clinical trials to prevent post-stroke cognitive impairment, such as Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) and Secondary Prevention of Small Subcortical Strokes (SPS3), failed to show clinically meaningful results [1,2]. There is an evidence that cilostazol, a phosphodiesterase-3 inhibitor, could suppress cognitive decline in patients with dementia [3], and decrease amyloid beta accumulation [4]. Probucol, a cholesteryl ester transfer protein activator with lipid-lowering and anti-oxidative effects, has a beneficial effect on cognition by inhibiting amyloid beta-induced hippocampal synaptic impairment [5]. Thus, we aimed to determine the efficacy of cilostazol and probucol for preventing poststroke cognitive decline in patients with multiple cerebral microbleeds (CMBs) or a history of prior intracerebral hemorrhage (ICH); a population that is expected to have a high risk for future cognitive decline.
PreventIon of CArdiovascular events in iSchemic Stroke patients with high risk of cerebral hemOrrhage for reducing COGnitive decline (PICASSO-COG) is a predetermined substudy of the PICASSO trial, which is a randomized double-blinded placebo-controlled trial with a 2×2 factorial design: cilostazol versus aspirin, and probucol versus no probucol [6]. The design and analysis plan have been previously reported [7]. The key inclusion criteria were non-cardioembolic ischemic stroke or transient ischemia attack and previous ICH or multiple CMBs on gradient echo imaging. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) and the Montreal Cognitive Assessment (MoCA) at randomization and at 4, 13, 25, 37, and 49 months after randomization. The cognitive function at the second visit (4 months after randomization) in patients who were randomized within 3 months after stroke was set as the baseline function, while the cognitive function at the first visit (1 month after enrollment) was set as the baseline for those randomized beyond 90 days after stroke. The baseline cognitive assessment was therefore conducted between 4 and 7 months after stroke onset in all participants eligible for the PICASSO-COG substudy. The primary outcome was a change in MMSE score, and a restricted maximum likelihoodbased mixed effects model with repeated measurements was used. The efficacy of each treatment was analyzed separately because the interaction effect between the antiplatelets and lipid-lowering treatment was not significant. Detailed information on the analyses, including sensitivity and subgroup analyses, are presented in the Supplementary methods.
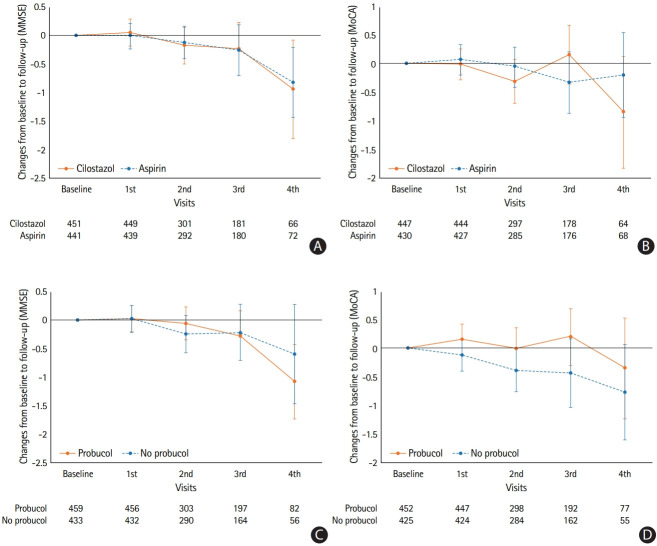
As shown in Supplementary Figure 1, among 1,382 subjects, 1,240 completed cognitive evaluations at randomization and 892 subjects (877 for the MoCA) were finally included (Supplementary Table 1) [7]. The baseline characteristics were not significantly different between the treatment groups, except the proportion of those with baseline MMSE ≤24 (Supplementary Table 2) [1]. Cilostazol did not show any significant differences in preventing cognitive decline in comparison with aspirin (Figure 1 and Supplementary Table 3). In the subgroup analysis according to the baseline MMSE score, the decrease in the MMSE score in the aspirin group of those with baseline MMSE ≤24 was more pronounced than that in the cilostazol group although the treatment effect was not significant (Supplementary Table 4). In the propensity score-matched subsets considering the baseline differences in the proportions of those with baseline MMSE ≤24, the cilostazol group showed a favorable outcome in those with mild to moderate white matter hyperintensities (WMHs) (Supplementary Table 5). Otherwise, no significant results were found in the subgroups and sensitivity analysis. Probucol treatment did not show any beneficial effect in the primary outcome using the MMSE. When analyzed according to the MoCA scores, probucol showed a favorable effect in preventing cognitive decline compared with the no probucol group (Supplementary Table 3). This effect was also observed in the subgroups without diabetes mellitus, with concomitant lipid-lowering agents, with baseline MMSE >24, and without severe WMH (Supplementary Table 4).
Figure 1.
Mean changes in cognitive scores from baseline to each follow-up in (A, B) cilostazol vs. aspirin and (C, D) probucol vs. no probucol groups. (A, C) Mini-Mental State Examination (MMSE) and (B, D) Montreal Cognitive Assessment (MoCA).
Longitudinal cognitive profiles of the study population might explain why this trial failed to prove the hypothesis. The demographics of the study subjects were comparable to those of the SPS3 trial [2]. However, 69.3% of the PICASSO-COG subjects had moderate or severe WMH, while half of the subjects in the SPS3 had none or mild WMH. In this distinctive population, the magnitude of observed cognitive change was smaller than what we had expected [7]. There are several reasons to consider. It has been reported that cognitive decline in patients with moderate to severe WMH was mainly observed in processing speed and executive function [8]. In the SPS3 trial conducted in patients with lacunar infarction, verbal fluency was mainly impaired in addition to episodic memory [9]. Memory dysfunction has also been reported to be affected by actually mediating executive dysfunction [8]. Therefore, the MMSE was not sensitive enough to capture these long-term cognitive changes. The MoCA has been reported to be more sensitive to the stroke population than the MMSE; however, the MoCA total score seems inadequate to quantify changes over a 2-year study period. For subsequent clinical trials, neuropsychological tests that more sensitively assess changes over time in the target population, such as fluency, trail-making, and the Stroop test should be adopted. In another aspect, the active risk factor control in the trial setting might prevent the cognitive deterioration of study subjects, including the control group, and made it difficult to verify the effectiveness of the trial drug. This can be conceived from the findings from the previous trials for vascular cognitive impairment, which showed stable cognitive trajectories in placebo arms [10]. Lastly, it is possible that the heterogeneity of WMH might have been affected [11]. The theoretically hypothesized cognitive decline might not be actually observed in patients with WMH of causes other than ischemic origin. However, since the subjects of this trial had ischemic stroke based on the inclusion criteria and had preceding ICH/multiple CMBs, the proportion of these patients is not expected to be high.
We predetermined the time window of baseline cognitive evaluation between 4 and 7 months after entry event to minimize the effects of acute stroke on cognitive function [12]. The intervals between index-stroke and baseline evaluations were 1 month in the PRoFESS trial and 74 to 76 days in the SPS3 trial [1,2]. If we were to include the spontaneous cognitive recovery after stroke in our analysis, the effects of the study medication could be exaggerated or underestimated.
As a limitation, the current study population did not seem to fulfill the criteria of reliable cognitive decline, and the trial needed much longer follow-up to show a significant change in the MMSE score [7]. In addition, a treatment effect could have occurred between the index-stroke and the baseline assessment. Since we limited our analysis to those who underwent baseline evaluations for 4 to 7 months after index-stroke, we could not address this possibility in our analysis.
To the best of our knowledge, this is the first clinical trial comparing the efficacy of aspirin, cilostazol, and probucol in preventing poststroke cognitive decline. Cilostazol and probucol did not show any significant differences compared to aspirin and no probucol. However, when patients were assessed by the MoCA, probucol reduced cognitive decline after stroke.
Acknowledgments
This work was supported by Korea Otsuka Pharmaceutical Company.
Sun U. Kwon declares grants from Korea Otsuka Pharmaceutical Company. All other authors declare no competing interests.
Supplementary materials
Supplementary materials related to this article can be found online at https://doi.org/10.5853/jos.2020.03650.
References
- 1.Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7:875–884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearce LA, McClure LA, Anderson DC, Jacova C, Sharma M, Hart RG, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol. 2014;13:1177–1185. doi: 10.1016/S1474-4422(14)70224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ihara M, Nishino M, Taguchi A, Yamamoto Y, Hattori Y, Saito S, et al. Cilostazol add-on therapy in patients with mild dementia receiving donepezil: a retrospective study. PLoS One. 2014;9:e89516. doi: 10.1371/journal.pone.0089516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SH, Kim JH, Bae SS, Hong KW, Lee DS, Leem JY, et al. Protective effect of the phosphodiesterase III inhibitor cilostazol on amyloid β-induced cognitive deficits associated with decreased amyloid β accumulation. Biochem Biophys Res Commun. 2011;408:602–608. doi: 10.1016/j.bbrc.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 5.Santos DB, Peres KC, Ribeiro RP, Colle D, dos Santos AA, Moreira EL, et al. Probucol, a lipid-lowering drug, prevents cognitive and hippocampal synaptic impairments induced by amyloid β peptide in mice. Exp Neurol. 2012;233:767–775. doi: 10.1016/j.expneurol.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Kim BJ, Lee EJ, Kwon SU, Park JH, Kim YJ, Hong KS, et al. Prevention of cardiovascular events in Asian patients with ischaemic stroke at high risk of cerebral haemorrhage (PICASSO): a multicentre, randomised controlled trial. Lancet Neurol. 2018;17:509–518. doi: 10.1016/S1474-4422(18)30128-5. [DOI] [PubMed] [Google Scholar]
- 7.Yu KH, Hong KS, Oh MS, Lee J, Lee JS, Kwon SU, et al. Design and rationale for a cognitive outcome substudy in ischemic stroke patients with high risk of cerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:2061–2066. doi: 10.1016/j.jstrokecerebrovasdis.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Jokinen H, Kalska H, Mäntylä R, Ylikoski R, Hietanen M, Pohjasvaara T, et al. White matter hyperintensities as a predictor of neuropsychological deficits post-stroke. J Neurol Neurosurg Psychiatry. 2005;76:1229–1233. doi: 10.1136/jnnp.2004.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacova C, Pearce LA, Costello R, McClure LA, Holliday SL, Hart RG, et al. Cognitive impairment in lacunar strokes: the SPS3 trial. Ann Neurol. 2012;72:351–362. doi: 10.1002/ana.23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black S, Román GC, Geldmacher DS, Salloway S, Hecker J, Burns A, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke. 2003;34:2323–2330. doi: 10.1161/01.STR.0000091396.95360.E1. [DOI] [PubMed] [Google Scholar]
- 11.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 12.Sachdev PS, Lo JW, Crawford JD, Mellon L, Hickey A, Williams D, et al. STROKOG (stroke and cognition consortium): an international consortium to examine the epidemiology, diagnosis, and treatment of neurocognitive disorders in relation to cerebrovascular disease. Alzheimers Dement (Amst) 2016;7:11–23. doi: 10.1016/j.dadm.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.