Abstract
The present study was conducted to investigate effects and mechanism of quercetin on lipids metabolism in broilers. 480 AA broilers were randomly allotted to four treatments (0, 0.2, 0.4, and 0.6 g/kg quercetin) for 42 days. Compared with the control, 0.6 g/kg quercetin significantly decreased percentage of abdominal fat (P < 0.05); 0.2, 0.4, and 0.6 g/kg quercetin significantly decreased relative abundance of Lachnospiraceae and Desulfovibrionaceae (P < 0.05, P < 0.05, P < 0.01; P < 0.01, P < 0.01, P < 0.01); 0.2 g/kg quercetin significantly increased mRNA expression of PI3K, AMPKα1, AMPKα2, AMPKβ2, LKB1 (P < 0.01, P < 0.01, P < 0.05, P < 0.01, P < 0.05), and significantly reduced mRNA expression of SREBP1 and PPARγ (P < 0.01, P < 0.05); 0.4 g/kg quercetin significantly increased mRNA expression of LKB1 and PKB (P < 0.05, P < 0.01) and significantly reduced mRNA expression of ACC, HMGR, PPARγ, and SREBP1 (P < 0.05, P < 0.01, P < 0.01, P < 0.01); 0.6 g/kg quercetin significantly increased mRNA expression of AMPKγ, LKB1, CPT1, PPARα, PKB (P < 0.01, P < 0.01, P < 0.01, P < 0.05, P < 0.05), and significantly reduced the mRNA expression of PI3K, ACC, HMGR, PPARγ, SREBP1 (P < 0.05, P < 0.05, P < 0.01, P < 0.01, P < 0.01); 0.2 g/kg quercetin significantly increased protein expression of AMPK (P < 0.01); 0.6 g/kg quercetin significantly increased protein expression of LKB1 (P < 0.01), 0.2 and 0.6 g/kg quercetin significantly increased protein expression of PI3K, PKB, CPT1 (P < 0.05, P < 0.01, P < 0.05, P < 0.01, P < 0.01, P < 0.01), and significantly reduced protein expression of ACC and SREBP1 (P < 0.01, P < 0.01, P < 0.01, P < 0.01). In conclusion, quercetin improved lipid metabolism by modulating gut microbial and AMPK/PPAR signaling pathway in broilers.
Keywords: quercetin, lipids metabolism, microbial, AMPK, PPAR
Introduction
Abdominal fat is essentially excessive accumulation of lipid. Therefore, the research of lipid metabolism becomes the focus at present. Lipid metabolism has also been linked with differences in the composition of the gut microbiota (Huazano-Garcia et al., 2017). In high-fat diet (HFD) fed Wistar rats, an increase in abundance of the families Coriobacteriaceae and Enterobacteriaceae was reported that may directly alter host physiology (Lecomte et al., 2015). The previous results of transcriptome sequencing showed that adenosine monophosphate activated protein kinase (AMPK) signaling pathway was the main signal pathway of lipid metabolism (Wang et al., 2020). Activated AMPK pathway reduced lipid synthesis by suppressing the expression of downstream targets (Zhao et al., 2019). Moreover, activation of PPAR pathway significantly alleviated lipid metabolic disorders (Cai et al., 2020). PPAR is an AMPK downstream target (Diniz et al., 2021), AMPK/PPAR signaling pathway was the main signal pathway of lipid metabolism.
Plant polyphenol, especially flavonoids, is a kind of safe additives with multiple biological activities. It drew public attention because of anti-bacterial action, anti-inflammation, anti-cancer and immune enhancement, etc. (George et al., 2016; Goya et al., 2017). Quercetin (International Union of Pure and Applied Chemistry nomenclature for quercetin is 3,3′,4′,5,7-pentahydroxyflvanone), a flavonoid found in fruits and vegetables, is categorized as a flavonol, which is one of the six subclasses of flavonoid compounds (Li et al., 2016a, b). Quercetin supplementation also improved antibacterial capacity, antioxidation and lipid metabolism in broilers (Sohaib et al., 2015). The previous studies in our laboratory showed that quercetin improved immune function and antibacterial activities in broilers (Wang et al., 2018; Yang et al., 2020). However, the percentage of abdominal fat was the most important indicator of carcass characteristics in broilers, the objective of this study was to investigate the mechanism of quercetin on lipid metabolism in broilers.
Materials and Methods
Birds, Diets, and Experimental Design
All procedures were performed in accordance with the guidelines set forth by the Animal Welfare Committee of Northeast Agricultural University (Harbin, China). Housing, management and care of the birds confirmed to the guidelines of Agricultural Animal in Agricultural Research and Teaching of Heilongjiang Province (HEI Animal Management Certificate No. 11928).
Four hundred and eighty AA broilers (1 day old) were obtained from a commercial facility (Yinong Poultry, Harbin, China). Birds were randomly allotted to four experimental treatments comprising six replicates of 20 birds in each replicate. All birds were raised in stainless steel cages (316 mm × 400 mm × 400 mm) under continuous light in a controlled room for 42 days. The room temperature was maintained at 33°C for the first 3 days. Then the temperature was reduced to 24°C until the end of the experiment. Water and experimental diets were provided ad libitum.
The experimental diets were based on corn and soybean meal, and quercetin was added at four concentrations: 0, 0.2, 0.4, and 0.6 g/kg of diet. Feeding was divided into two phases: the starter from 1 to 21 days and the grower from 22 to 42 days. The basal diet was formulated to meet the nutritional requirements suggested according to Chinese Broiler Feeding Standards (NY/T33-2004) (Table 1). Quercetin (purity of quercetin dihydrate powder ≥97%, Sigma-Aldrich, St. Louis, MO, United States) was mixed in basal diet.
TABLE 1.
Calculated composition of basal diets and nutrient level.
| % (Air-dry basis) | ||
| Composition | 1–21 days | 21–42 days |
| Ingredients | ||
| Corn | 57.50 | 62.30 |
| Soybean meal | 34.50 | 30.00 |
| Fish meal | 1.00 | 1.00 |
| Soybean oil | 3.00 | 3.00 |
| Sodium chloride | 0.30 | 0.30 |
| Dicalcium phosphate | 1.65 | 1.70 |
| Limestone | 1.52 | 1.17 |
| Methionine | 0.20 | 0.20 |
| Choline | 0.10 | 0.10 |
| Multivitamin premixa | 0.03 | 0.03 |
| Mineral premixb | 0.20 | 0.20 |
| Nutrient level | ||
| Metabolizable energy (MJ/kg) | 12.33 | 12.50 |
| Crude protein | 21.75 | 19.72 |
| Lysine | 1.18 | 1.04 |
| Methionine + Cysteine | 0.91 | 0.86 |
| Ca | 1.07 | 0.60 |
| Total P | 0.70 | 0.68 |
| Available P | 0.46 | 0.45 |
aContent per kilogram of diet: 1,500 IU of vitamin A; 3,200 IU of vitamin D3; 10 IU of vitamin E, 0.5 mg of vitamin K, 1.8 mg of vitamin B1, 3.6 mg of vitamin B2, 3.5 mg of vitamin B6, 0.01 mg of vitamin B12, 0.15 mg of biotin, 0.55 mg of folic acid, 30 mg of niacin, and 10 mg of pantothenic acid. bContent: 8 mg of Cu (CuSO4⋅5H2O), 0.35 mg of I (KI), 80 mg of Fe (FeSO4⋅7H2O), 60 mg of Mn (MnSO4⋅H2O), 0.15 mg of Se (NaSeO3), and 40 mg of Zn (ZnO).
Methods
Carcass Characteristics
At the age of 42 days, 12 chickens per treatment (6 per replicate pen) of randomly chosen were slaughtered for carcass analyses. Each of these birds was deprived of feed for 12 h and individually weighed just prior to slaughter. Percentage of carcass, eviscerated and semi-eviscerated, breast muscle, thigh muscle and abdominal fat was calculated according to the weight of the carcass, eviscerated, semi-eviscerated, breast muscle, thigh muscle, and abdominal fat.
Metagenome Sequencing
The whole ileal contents were collected and frozen in liquid nitrogen and sent to Geneis (Beijing) Co. Ltd. for metagenome sequencing using Illumina HiSeq 2500 platform. Microbial DNA was extracted from 12 ileum samples using the improved metagenomic DNA extraction method. The quality of the extracted metagenomic DNA was checked on 0.8% agarose gel visualized on a gel documentation system (Alphaimager HP, United States). The quantity and purity of the DNA was assessed using Nanodrop LITE spectrophotometer (Thermo Scientific, United States). ABI Steponeplus Real-Time PCR System and Agilent 2100 Bioanalyze were used to detect the output and quality of the constructed library.
AMPK Signaling Pathway
Real-time quantitative PCR (RT-qPCR)
Liver tissue was individually homogenized, and total RNA was extracted using the TRIZOL reagent. The Superscript First-Strand Synthesis System (Life Technologies, Grand Island, NY, United States) was adopted to synthesize first-strand cDNA from the total RNA. The quantity of purified cDNAs was determined by RT-qPCR (Life Technologies, Grand Island, NY, United States). β-actin was used as the internal control in this study (Table 2).
TABLE 2.
Parameters of primer pairs for the genes.
| Gene | Primer sequence | Product size | GenBank accession |
| PPARα | F: 5′-TAACGGAGTTCCAATCGC-3′ | 222 bp | NM 001001464 |
| R: 5′-AACCCTTACAACCTTCACAA-3′ | |||
| PPARγ | F: 5′-CACTGCAGGAACAGAACAAAGAA-3′ | 67 bp | NM 001001460 |
| R: 5′-TCCACAGAGCGAAACTGACATC-3′ | |||
| PKB | F: 5′-CTGATGATGCCAAGGAGATT-3′ | 175 bp | NM 205055.1 |
| R: 5′-TGGTCAGGAGGAGTGATTGT-3′ | |||
| AMPKα1 | F: 5′-AAGGTTGGCAAGCATGAGTT-3′ | 492 bp | NM 001039603 |
| R: 5′-TTCTGGGCCTGCATATAACC-3′ | |||
| AMPKα2 | F: 5′-AGCACGCCAACAGAGACTTCTT-3′ | 399 bp | NM 001039605 |
| R: 5′-ATCATCAAAGGGCAAAGTGC-3′ | |||
| AMPKβ1 | F: 5′-CCAGGAGCCCTATGTCTGTAAG-3′ | 113 bp | NM 001039912 |
| R: 5′-CAGGATCGCAAGAAATGCC-3′ | |||
| AMPKβ2 | F: 5′-GCACTGCCCATCCCTCTAA-3′ | 125 bp | NM 001044662 |
| R: 5′-CGGCTGCCACGAAACAA-3′ | |||
| AMPKγ | F: 5′-AGAGGTCCCAAAGCCTGAGTT-3′ | 118 bp | NM 001034827 |
| R: 5′-GAAGATGCCCAGAGCCACA-3′ | |||
| PI3K | F: 5′-CGGATGTTGCCTTACGGTTGT-3′ | 162 bp | NM 001004410.1 |
| R: 5′-GTTCTTGTCCTTGAGCCACTGAT-3′ | |||
| ACC | F: 5′-CACTTCGAGGCGAAAAACTC-3′ | 447 bp | NM 205505 |
| R: 5′-GGAGCAAATCCATGACCACT-3′ | |||
| CPT1 | F: 5′-CAATGAGGTACTCCCTGAAA-3′ | 337 bp | NM oo102898 |
| R: 5′-CATTATTGGTCCACGCCCTC-3′ | |||
| HMGCR | F: 5′-AGCTGCAACCCTGAGGAAACT-3′ | 1268 bp | AB109635 |
| R: 5′-AGCCATCACTGTAGCACACAC-3′ | |||
| SREBP1 | F: 5′-GAGGAAGGCCATCGAGTCA-3′ | 392 bp | AY 029224 |
| R: 5′-GGAAGACAAAGGCACAGAGG-3′ | |||
| LKB1 | F: 5′-GGGGAGACAGAAGGGAACAGA-3′ | 158 bp | NM 001045833 |
| R:5′-TGAGAGGGATGCTTGAATACGA-3′ | |||
| β-actin | F: 5′-TGCGTGACATCAAGGAGAAG-3′ | 300 bp | L08165 |
| R: 5′-TGCCAGGGTACATTGTGGTA-3′ | |||
| 18sRNA | F:5′-TAGATAACCTCGAGCCGATCGCA-3′ | 312 bp | AF 173612 |
| R:5′-GACTTGCCCTCCAATGGATCC TC-3′ |
Western blot
Briefly, equal amounts of protein samples (30 μg) were loaded into SDS-PAGE apparatus and transferred to PVDF membranes. PVDF membranes were then probed with primary antibodies against targeted proteins. Images were detected by a ChemiDoc XRS + imaging system (Bio-Rad, Hercules, CA, United States), and bands of the target proteins were quantified with the ImageJ software. GAPDH was used as internal control in this study.
Statistical Analysis
The data was treated using a one-way analysis of variance as a completely randomized design with four treatments and six replicates for each treatment using SPSS 20.0 statistical software, the results were expressed as means ± standard error of the mean (SEM), P < 0.05 was considered as statistically significant criteria. Calculated ΔCt (corrected sample) = mean value of target gene–mean value of internal reference gene, ΔΔCt = ΔCt − mean value of control group. The results of western blot were analyzed by Gel-Pro analyzer 4 software.
Results and Discussion
Effect of Quercetin on Carcass Characteristics in Broilers
There are few reports on the effects of dietary quercetin supplementation on the carcass characteristics in broilers. 0.2% sea buckthorn flavonoid supplementation significantly increased the dressing percentage, improved eviscerated and semi-eviscerated percentage in AA broilers (Li et al., 2008). However, our study showed that no significant differences in percentage of dressing, eviscerated weight, semi-eviscerated of AA broilers were observed (P > 0.05), compared with control (Table 3). The difference of carcass characteristics probably resulted from complicated constituent of flavonoids from sea buckthorn, and/or diverse bioavailability and synergism of various flavonoids (Ross and Kasum, 2002; Guo and Bruno, 2015).
TABLE 3.
Effects of dietary quercetin on carcass characteristics in AA broilers (%).
| Diet (quercetin, g/kg) | ||||
| Items | 0 | 0.2 | 0.4 | 0.6 |
| Dressing percentage | 94.41 ± 1.15 | 94.20 ± 1.42 | 93.10 ± 3.99 | 90.89 ± 6.63 |
| Eviscerated percentage | 69.86 ± 3.17 | 71.51 ± 2.03 | 69.49 ± 4.69 | 69.32 ± 3.61 |
| Semi-eviscerated percentage | 85.48 ± 2.51 | 86.09 ± 1.55 | 84.72 ± 4.37 | 84.97 ± 3.91 |
| Percentage of abdominal fat | 1.53 ± 0.23a | 1.48 ± 0.20a | 1.33 ± 0.15ab | 1.19 ± 0.26b |
In the same row, values with different small letter superscripts mean significant difference (P < 0.05); Values with different capital letter superscripts mean significant difference (P < 0.01); Values with no letter or the same letter superscripts mean no significant difference (P > 0.05). Values are expressed as means ± SEM, and n = 6 for all groups. The same as the follows.
Some studies had shown that quercetin promoted fat metabolism in rats (Peng et al., 2017; Rocca et al., 2018). Abdominal fat deposition was reduced by Hawthorn extract in the drinking water of chickens (Ahmadipour et al., 2014). Kim reported that high intake of dietary flavonoids may be associated with a decreased prevalence of abdominal obesity in broilers (Cao et al., 2012). In the present study, the percentage of abdominal fat was significantly decreased by 0.6 g/kg quercetin supplementation (P < 0.05) (Table 3). The result was supported by the findings which fermented Ginkgo biloba leaves (including abundant flavonoid) in the diet of broilers decreased abdominal fat deposition in adults (Seong-Ah et al., 2020). Lipid accumulation may contribute to abdominal fat, therefor, accumulation of lipids could be attributed to the downregulation of fatty-acid oxidation and adipogenic and lipogenic pathways upregulation and increased delivery of fatty acid to abdominal (Foulds et al., 2017).
Effect of Quercetin on Relative Abundance of Ileal Microflora in AA Broilers at the Family
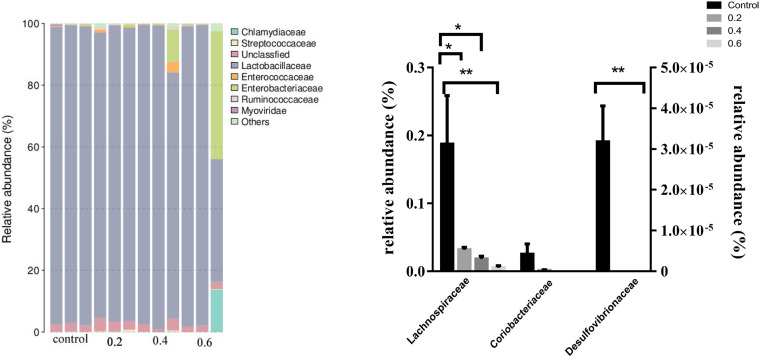
Accumulating evidence indicates that microorganisms improved host physiology and lipid metabolism. Metagenomic sequencing technology is widely used in microflora detection. The Desulfovibrionaceae family (Proteobacteria phyla) is gram-negative sulfate-reducing bacteria involved in the production of lipopolysaccharides and endotoxins and well-known inflammation-inducing capacity (Cani et al., 2008; Zhao L. et al., 2017). Desulfovibrionaceae was thought to be positively associated with obesity (Delzenne and Cani, 2011), the metagenomic analyses showed that the tea extracts changed the overall composition of gut microbiota and decreased the relative abundance of family Rikenellaceae and Desulfovibrionaceae in mice (Liu J. et al., 2019). Green tea polyphenol (epigallocatechin-3-gallate-EGCG) significantly decreased the relative abundance of Desulfovibrionaceae in HFD fed mice (Ushiroda et al., 2019). The abundance of bacterial genera Desulfovibrionaceae was significantly decreased in Wasabi-treated rats (Thomaz et al., 2020). Moreover, the relative abundance of Desulfovibrionaceae in the HFD group was significantly higher than that in the ND group, however, after chlorogenic acid treatment, the relative abundance of this bacteria was decreased in mice (Wang et al., 2019). Lachnospiraceae, a family of clostridia, a kind of digestive tract-associated bacteria, correlates with increased fat mass and lipid level (Kameyama and Itoh, 2014; Murugesan et al., 2016; Pataky et al., 2016). Lachnospiraceae family was accompanied with the increasing body weight in germ-free ob/ob mice (Zhao L. et al., 2017). Previous studies have shown that Lachnospiraceae may protect against obesity and colon cancer in humans by producing butyric acid (Meehan and Beiko, 2014). Additionally, Lachnospiraceae was largely decreased when HFD-induced mice were simultaneously administrated chlorogenic acid (Wang et al., 2019). Furthermore, high dose of Fuzhuan brick tea (FBT) reduced the levels of Lachnospiraceae and Desulfovibrionaceae, compared with the baseline level, the beneficial effects on HFD-induced obese mice were associated with regulating the relative abundance of Lachnospiraceae and Desulfovibrionaceae (Liu D. et al., 2019). Coriobacteriaceae family in the gut was associated with the development of metabolic syndrome (Luccia et al., 2015). Coriobacteriaceae belonging to the phylum Actinobacteria were involved in bile acid metabolism, had a negative effect on cholesterol homeostasis through increasing cholesterol absorption (Zhao N. Q. et al., 2017). Specific species within Coriobacteriaceae were known for metabolizing compounds such as the isoflavones daidzein and genistein to equol (Bangsgaard Bendtsen et al., 2012; Flórez et al., 2019). Equol may significantly affect blood lipids in vitro (Zhang T. et al., 2013). In the current study, at the family level, 0.2, 0.4 and 0.6 g/kg quercetin supplementation significantly reduced the relative abundance of Lachnospiraceae and Desulfovibrionaceae (P < 0.05, P < 0.05, P < 0.01; P < 0.01, P < 0.01, P < 0.01); However, 0.2, 0.4, and 0.6 g/kg quercetin supplementation did not influence the relative abundance of Coriobacteriaceae (P > 0.05) (Figure 1). Together with the above results, we inferred that quercetin reduced percentage of abdominal fat through beneficial modulation of the gut microbiota.
FIGURE 1.
Effect of quercetin on relative abundance of ileal microflora in AA broilers at the family level. Note: The results of relative quantification were expressed as 2– ΔΔCT. The quantification of control was 1, namely 2– ΔΔCT = 1. The value 2– ΔΔCT of treatment group was a multiple of control. N = 3. ∗P < 0.05, ∗∗P < 0.01. Values are mean ± SEM (n = 3).
Quercetin Improved Lipids Metabolism Through AMPK/PPAR Signal Pathway in Broilers
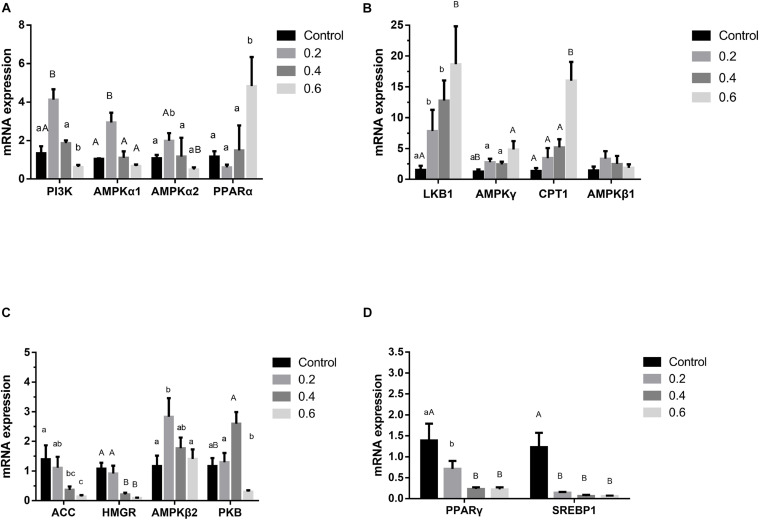
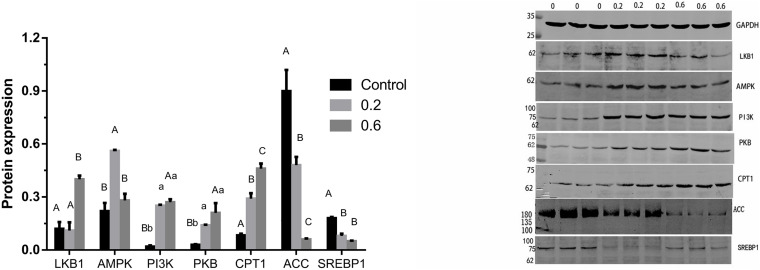
The previous study in our lab found 505 differentially expressed genes of AMPK signal pathway in the quercetin treatment, compared with the control, and the liver was the main metabolic site of lipid in broilers (Wang et al., 2020); 0.04% quercetin supplementation decreased fat content of liver in laying hens (Zhang L. et al., 2013). Therefore, these findings together with the present study confirmed that quercetin regulated fat metabolism, thus reduced abdominal fat deposition in broilers. However, the mechanism of action remains to be unclear. AMPK plays a key role in regulating lipid and glucose metabolism, acts as an energy sensor, regularly responding to cellular energy demands by sensing the balance in AMP to ATP ratio (Zhao N. Q. et al., 2017). AMPK, a heterotrimer kinase composed of catalytic and regulatory subunit, is classified into three different receptor subtypes, AMPKα, AMPKβ, and AMPKγ. AMPK signaling pathway coordinates glucose metabolism by regulating glycolysis and gluconeogenesis, and controls lipid metabolism by acting on fatty acid synthesis and fatty acid oxidation (Hardie, 2011; Do et al., 2012). Some studies suggested that the enhanced AMPK signaling may attenuate liver lipid accumulation and hepatic fibrosis in mice (Wang et al., 2013; Woods et al., 2017). Phosphorylated AMPK level was down-regulated in the diabetic liver, and Sonchus oleraceus Linn increased the expression of AMPK in diabetes mice (Chen et al., 2020). Ginsenoside Rk3 (G-Rk3) mediated hepatic lipid accumulation via activating the AMPK/Akt signaling pathway in mice (Liu Y. et al., 2019). Licochalcone A activates AMPK to increase lipolysis in liver (Liou et al., 2019). Quercetin exerted anti-adipogenic effects in 3T3-L1 cells by activating the AMPK signaling pathway (Yang et al., 2008). A previous study reported that quercetin increased the phosphorylation of AMPK in cultured smooth muscle cells and aortic arteries, which also exhibited increased levels of acetyl CoA carboxylase, a downstream protein of AMPK, implicating the increased activity of AMPK following quercetin administration (Ahn et al., 2008). In addition, flaxseed polysaccharide interacts with intestinal flora, upregulates AMPK, and inhibited lipid accumulation in obese mice (Luo et al., 2019). In the current study, 0.6 g/kg quercetin supplementation significantly increased AMPKγ mRNA expression (P < 0.01). Simultaneously, 0.2 g/kg quercetin supplementation significantly increased mRNA expression of AMPKα1, AMPKα2, AMPKβ2 (P < 0.01, P < 0.05, P < 0.05) (Figure 2) and protein expression of AMPK (P < 0.01) (Figure 3). These findings suggested that quercetin regulated lipid metabolism through increasing the expression of AMPK.
FIGURE 2.
Effects of quercetin on genes relating to the AMPK/PPARα signaling pathway in liver of AA broilers. Note: The results of relative quantification were expressed as 2– ΔΔCT. The quantification of control was 1, namely 2– ΔΔCT = 1. The value 2– ΔΔCT of treatment group was a multiple of control. N = 6. Mean values without a common letter are significantly different, P < 0.05. Values are mean ± SEM (n = 6).
FIGURE 3.
Effects of quercetin on protein relating to the AMPK/PPARα signaling pathway in liver of AA broilers. Note: Data are presented as mean ± SEM (n = 3–5). Bars with different lowercase letters are significantly different (P < 0.05) Bars with different capital letters are significantly different (P < 0.01).
The AMPK Upstream Pathway
Phosphatidylinositol 3-kinase (PI3K) and serine-threonine protein kinase (PKB/AKT), regarded as signal transduction molecules in cells, are associated with varieties of biological processes, including apoptosis, insulin resistance, and adipogenesis (Maingrette and Renier, 2003; Jiménez-Castro et al., 2013; Pang et al., 2013). Previous studies elucidated that the PI3K-PKB/AKT mediated signaling pathway was participated in lipid accumulation process via phosphorylating or activating substrates (Peng et al., 2015; Zhu et al., 2015; Manning and Toker, 2017). The activated insulin receptor activates PI3K and PKB/AKT as well as downstream glucose and lipid metabolism. In the current study, 0.2 g/kg quercetin supplementation significantly increased mRNA and protein expression of PI3K and protein expression of PKB (P < 0.01, P < 0.05); 0.6 g/kg quercetin supplementation significantly increased protein expression of PI3K and PKB (P < 0.01, P < 0.01) (Figures 2, 3). Taken together, in line with the previous research that quercetin improved lipid metabolism and reduced abdominal fat deposition by activating PI3K/PKB signal pathway (Ying et al., 2020). And together with the results of AMPK in this experiment, our findings indicated that quercetin increased the expression of AMPK via stimulating PI3K-PKB/AKT kinase activity.
Liver kinase B1 (LKB1) is a serine/threonine protein kinase which was first discovered in studying Peutz-Jeghers syndrome. AMPK activity is mainly regulated by LKB1 in mice (Sakamoto et al., 2005) and chickens (Proszkowiec-Weglarz et al., 2006). LKB1 regulation of the AMPK family plays well-established roles in increasing fat oxidation (Thomson et al., 2007), while mediating part of the response to oxidative stress (Chen et al., 2016). LKB1 is upstream of AMPK and a family of 12 other Ser/Thr kinases closely related to AMPK, which would potentially be regulated of fat acid oxidation by LKB1 in mice (Kim et al., 2018). LKB1 is considered the major route of AMPK activation because an LKB1 deficiency results in an almost complete loss of AMPK activity (Jeppesen et al., 2013). Our study results showed that 0.2, 0.4 and 0.6 g/kg quercetin supplementation significantly increased LKB1 mRNA expression (P < 0.05, P < 0.05, P < 0.01), and 0.6 g/kg quercetin supplementation significantly increased protein expression of LKB1 (P < 0.01) (Figure 2). Together with the results of AMPK in this experiment, our findings indicated that quercetin-activated LKB1 up-regulated AMPK expression.
The AMPK Downstream Pathway
Acetyl-CoA carboxylase (ACC), a rate-limiting enzyme involved in the production of malonyl-CoA, is used for fatty acyl-CoA biosynthesis, stimulates CPT1 and reduces the flux of substrates in the fatty acid anabolic pathway (Carling et al., 2008; Lage et al., 2008). ACC inactivation is related to the predominance of β-oxidation, which provides energy to the body (Zhou et al., 2001). AMPK may regulate the transcription and expression of ACC in hypothalamus of mammals and avian species (Xue and Kahn, 2006; Xu et al., 2011, 2012). Curcumin significantly decreased levels of ACC1 to inhibit lipid metabolism (Qiu et al., 2016). Our study showed that 0.4 and 0.6 g/kg quercetin supplementation significantly reduced ACC mRNA expression (P < 0.05, P < 0.05); 0.2 and 0.6 g/kg quercetin supplementation significantly reduced protein expression of ACC (P < 0.01, P < 0.01). Together with the results of AMPK in this experiment, the current results were consistent with Watt MJ’s study which AMPK activation down-regulated ACC expression in liver of broilers (Watt et al., 2006).
Carnitine palmitoyl transterase-1 (CPT1) is considered as a mitochondrial gateway for fatty acid entering into the matrix, is also the main modulator of hepatic mitochondrial β-oxidation flux. CPT1 adjusts the β-oxidation of fatty acids by catalyzing the conversion of fatty acyl-CoA into fatty acylcarnitine in mitochondria (Zheng et al., 2013). Joubert et al. (2010) demonstrated that AMPK may regulate fatty acid metabolism by the CPT in muscle of broilers. Nobiletin increased hepatic CPT1 mRNA, thus promoted fatty acid oxidation (Mulvihill et al., 2011). Instant fermented teas heighten energy expenditure by increasing the expression of the CPT-1 gene (Sun et al., 2019). In the current study, 0.6 g/kg quercetin supplementation significantly increased CPT1 mRNA expression (P < 0.01); 0.2 and 0.6 g/kg quercetin supplementation significantly increased protein expression of CPT1 (P < 0.01, P < 0.01) (Figure 2). Together with the results of AMPK in this experiment, our findings showed that quercetin down-regulated the expression of ACC in liver, indicating that inhibition of ACC by AMPK activation contributed to increased CPT 1 activity.
Peroxisome proliferator activated receptors (PPARs) are nuclear transcription factors, which are classified into three different receptor subtypes, PPARα, PPARβ, and PPARγ. PPARs are particularly expressed in tissues with high lipid catabolic capacities, such as liver, skeletal muscle and brown adipose tissue, play a crucial role in lipid metabolism by regulating oxidation and disintegration of fatty acids, lipid transportation, assembly of lipoproteins through modulating transcription of their downstream genes (Daigo et al., 2007; Park et al., 2014; Beatriz et al., 2017). Berbamine treatment increased the PPARα expression, a vital transcription factor to fatty acid oxidation (Ankita et al., 2020). Meanwhile, previous studies have revealed that AMPK activation is accompanied by increased PPARα expression (Baar, 2004; Lee et al., 2006). Wogonin exhibited beneficial effects in lipid metabolism through activating AMPK and PPARα (Bak et al., 2014). The transcription factor PPARγ plays a key role in regulating adipogenesis and is expressed in the late stages of differentiation. Red yeast buckwheat (RYB) treatment significantly suppressed the mRNA and protein expression of PPARγ in 3T3-L1 cells (Hong et al., 2017). Rosehip extract inhibited lipid accumulation in white adipose tissue by suppressing the expression of PPARγ (Akifumi et al., 2013). Flavonol kaempferol decreased the AMPK activation-mediated PPARγ expression (Zhang and Liu, 2011). Our study results showed that 0.6 g/kg quercetin supplementation significantly increased PPARα mRNA expression (P < 0.05), and 0.2, 0.4, and 0.6 g/kg reduced PPARγ mRNA expression (P < 0.05, P < 0.01, P < 0.01) (Figure 2). It suggested that AMPK activation was accompanied by increased PPARα and reduced PPARγ expression. AMPK activation by quercetin in the present study regulated ACC, CPT1, and PPAR expression, thus increased lipid β-oxidation, therefore, decreased fat deposition.
Sterol regulatory element binding proteins (SREBPs) play pivotal roles in both lipogenesis and cholesterol homeostasis (Zhu et al., 2019). SREBP1 is particularly involved in activation of the genes controlling fatty acid metabolism and de novo lipogenesis (Hua et al., 2016). SREBP1c manages adipogenesis by activating some genes connected to the synthesis of fatty acids and triglyceride (Li et al., 2011; Hu et al., 2020). Berberine may prevent lipid metabolism disorders by down-regulating SREBP and up-regulating AMPKα (Li et al., 2011). Several studies demonstrated that AMPKα reduced lipid synthesis by restraining SREBP activity and promoted fatty acid oxidation to control hepatic energy metabolism in liver (Park et al., 2008; Li et al., 2011). Our study has shown that 0.2, 0.4, and 0.6 g/kg quercetin supplementation significantly reduced mRNA and protein expression of SREBP1 (P < 0.01, P < 0.01, P < 0.01, P < 0.01, P < 0.01) (Figures 2, 3). Together with the results of AMPK in this experiment, our findings indicated that quercetin decreased SREBP1 expression thought AMPK activation in liver of broilers.
3-Hydroxy-3-Methylglutaryl-CoA reductase (HMGR) is a rate-limiting enzyme for cholesterol synthesis. Transcriptional and pathway analysis results showed that the overexpression of HMGR was correlated with the down-regulation of AMPK gene expression (Lin et al., 2020). Schisandra chinensisfruit (SF) extract may decrease lipid accumulation by up-regulating lipolytic factors (AMPK) and decreasing the expression or activity of lipogenic modulators (HMGR) (Liu et al., 2015). Artemisia species treatment significantly reduced HMGR and PPARα activation in comparison with high fat diet mice (Wang et al., 2013). In the current study, 0.4 and 0.6 g/kg quercetin supplementation significantly reduced HMGR mRNA expression (P < 0.01, P < 0.01) (Figure 2). Our findings were supported by Haitao Liu’s study (Liu et al., 2015) that AMPK activation down-regulated HMGR expression in liver of broilers. The fat of meat is an important component in meat quality and impacts animal productivity. Therefore, it suggested that quercetin-activated AMPK in the present study down-regulated HMGR and SREBP1 expression, thus decreased lipid deposition.
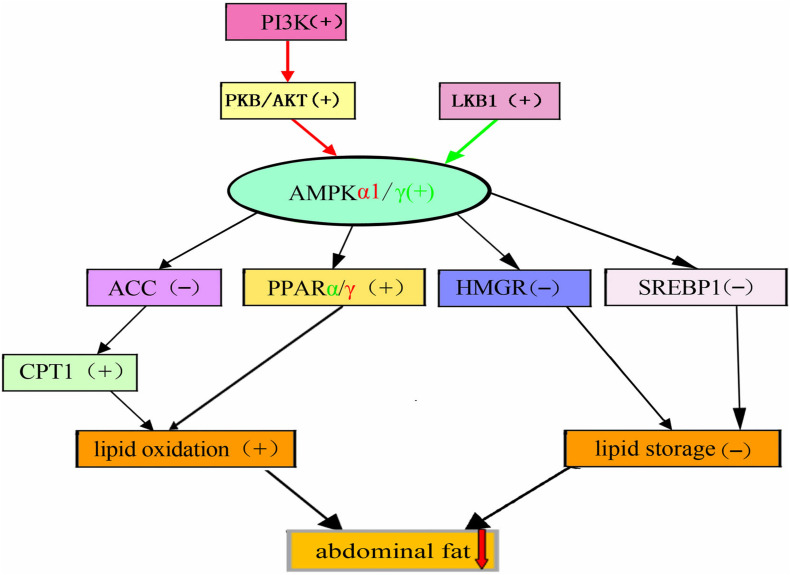
Quercetin reduced the expression levels of SREBP1, PPARγ, and HMGR in liver by activating the AMPK signaling pathway. Consequently, adipogenesis was restricted, thereby reduced lipid synthesis. The present study also found that quercetin decreased ACC expression and increased the expression of CPT1 and PPARα by activating AMPK, thus prevented fatty acid intake and promoted lipolysis and fatty acid oxidation. However, quercetin activated the AMPK signaling pathway through increasing the expression of PI3K, PKB/ATK and LKB1. Therefore, the current results demonstrated that dietary quercetin supplementation improved lipid metabolism, which promoted lipid oxidation and reduced lipid deposition by regulating AMPK/PPAR signaling pathway in liver of broilers (Figure 4).
FIGURE 4.
Proposed model of AMPK actions on gene expressions in liver of chickens fed with quercetin [(–), Down, (+), Up] change.
Conclusion
The present results showed that dietary quercetin supplementation might change the abdominal fat deposition by regulating AMPK/PPAR signaling pathway and gut microbial in broilers. Activation of the AMPK/PPAR signaling pathway and modulation of the gut microbiota attenuated abdominal fat accumulation by accelerating lipolysis and fatty-acid oxidation and inhibiting fatty acid uptake and lipid synthesis, accumulation of lipids could be repression, quercetin may be used as functional additive to improve lipid metabolism.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by the HEI Animal Management Certificate No. 11928.
Author Contributions
MW participated in the design of the study and critically revised the first manuscript. SW, HL, HW, and LY provided some technical support for the experiment. BW, MD, and YM performed the experiments and participated in the statistical analysis. YL modified the manuscript and have given final approval of the version to be submitted. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (32072749).
References
- Ahmadipour B., Hassanpour H., Asadi E., Khajali F., Khajali F. (2014). Kelussia odoratissima mozzaf -a promising medicinal herb to prevent pulmonary hypertension in broiler chickens reared at high altitude. J. Ethnopharmacol. 159 49–54. 10.1016/j.jep.2014.10.043 [DOI] [PubMed] [Google Scholar]
- Ahn J., Lee H., Kim S., Park J., Ha T. (2008). The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophl. Res. Commun. 373 545–549. 10.1016/j.bbrc.2008.06.077 [DOI] [PubMed] [Google Scholar]
- Akifumi N., Norihisa N., Yoichi M., Nobuhito S. (2013). Rosehip extract inhibits lipid accumulation in white adipose tissue by suppressing the expression of peroxisome proliferator- activated receptor gamma. Prev. Nutr. Food Sci. 18 85–91. 10.3746/pnf.2013.18.2.085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankita S., Sumit K. A., Neha S., Upendra N. D., Poonam K. (2020). Berbamine induced AMPK activation regulates mTOR/SREBP-1c axis and Nrf2/ARE pathway to allay lipid accumulation and oxidative stress in steatotic HepG2 cells. Euro. J. Pharmacol. 62 1490–1499. 10.2337/db12-1160 [DOI] [PubMed] [Google Scholar]
- Baar K. (2004). Involvement of PPARγ co-activator-1, nuclear respiratory factors 1 and 2, and PPARα in the adaptive response to endurance exercise. Proc. Nutr. Soc. 63 269–273. 10.1079/PNS2004334 [DOI] [PubMed] [Google Scholar]
- Bak E. J., Kim J., Choi Y. H., Kim J. H., Lee D. E., Woo G. H., et al. (2014). Wogonin ameliorates hyperglycemia and dyslipidemia via pparα activation in db/db mice. Clin. Nutr. 33 156–163. 10.1016/j.clnu.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Bangsgaard Bendtsen K. M., Krych L., Sørensen D. B., Pang W., Nielsen D. S., Josefsen K., et al. (2012). Gut microbiota composition is correlated to grid floor induced stress and behavior in the BALB/c mouse. PLoS One 7:e46231. 10.1371/journal.pone.0046231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatriz B., Tuva B. D., Indira M., Mathijs G., Sverre H., Sergio M. L. P., et al. (2017). Leukocyte overexpression of intracellular nampt attenuates atherosclerosis by regulating pparγ-dependent monocyte differentiation and function. Arterioscl. Throm. Vas. 37 1157–1167. 10.1161/ATVBAHA.116.308187 [DOI] [PubMed] [Google Scholar]
- Cai H., Wen Z., Li X. M., Meng K., Yang P. L. (2020). Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microb. Biotechnol. 104 5959–5972. 10.1007/s00253-020-10620-0 [DOI] [PubMed] [Google Scholar]
- Cani P. D., Bibiloni R., Knauf C., Waget A., Neyrinck A. M., Delzenne N. M., et al. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57 1470–1481. 10.2337/db07-1403 [DOI] [PubMed] [Google Scholar]
- Cao F. L., Zhang X. H., Yu W. W., Zhao L. G., Wang T. (2012). Effect of feeding fermented ginkgo biloba leaves on growth performance, meat quality, and lipid metabolism in broilers. Poul. Sci. 91 1210–1221. 10.3382/ps.2011-01886 [DOI] [PubMed] [Google Scholar]
- Carling D., Sanders M., Woods A. (2008). The regulation of AMP-activated protein kinase by upstream kinases. Inter. J. Obesity. 32 S55–S59. [DOI] [PubMed] [Google Scholar]
- Chen L., Lin X., Fan X., Qian Y., Lu Q., Teng H. (2020). Sonchus oleraceus Linn extract enhanced glucose homeostasis through the AMPK/Akt/GSK-3beta signaling pathway in diabetic liver and HepG2 cell culture. Food Chem. Toxicol. 136:111072. 10.1016/j.fct.2019.111072 [DOI] [PubMed] [Google Scholar]
- Chen T., Moore T. M., Ebbert M. T., McVey N. L., Madsen S. R., Hallowell D. M. (2016). Liver kinase B1 inhibits the expression of inflammation-relatedgenes postcontraction in skeletal muscle. J. Appl. Physiol. 120 876–888. 10.1152/japplphysiol.00727.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigo Y. S., Naoichi S. T., Takehiro T. S., Hiroyuki M. R., Ryoko Y. S., Seiji N. M., et al. (2007). Adaptor protein sh2-b linking receptor-tyrosine kinase and akt promotes adipocyte differentiation by regulating peroxisome proliferator-activated receptor gamma messenger ribonucleic acid levels. Mol. Endocrinol. 21 1120–1131. 10.1210/me.2006-0413 [DOI] [PubMed] [Google Scholar]
- Delzenne N. M., Cani P. D. (2011). Interaction between obesity and the gut microbiota: relevance in nutrition. Annu. Rev. Nutr 31 15–31. [DOI] [PubMed] [Google Scholar]
- Diniz T. A., Junior E. A. D. L., Alexandre A. T., Biondo L. A., Jose C. R. N. (2021). Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 266:118868 10.1016/j.lfs.2020.118868 [DOI] [PubMed] [Google Scholar]
- Do G. M., Jung U. J., Park H. J., Kwon E. Y., Jeon S. M., McGregor R. A., et al. (2012). Resveratrol ameliorates diabetes-related metabolic changes via activation of AMP-activated protein kinase and its downstream targets in db/db mice. Mol. Nutr. Food Res. 56 1282–1291. 10.1002/mnfr.201200067 [DOI] [PubMed] [Google Scholar]
- Flórez A. B., Vázquez L., Rodríguez J., Redruello B., Mayo B. (2019). Transcriptional regulation of the equol biosynthesis gene cluster in Adlercreutzia equolifaciens DSM19450T. Nutrients 11 993–1005. 10.3390/nu11050993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds C. E., Trevio L. S., York B., Walker C. L. (2017). Endocrine-disrupting chemicals and fatty liver disease. Nat. Rev. Endocrinol. 13 445–457. 10.1038/nrendo.2017.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George V. C., Dellaire G., Rupasinghe H. P. V. (2016). Plant flavonoids in cancer chemoprevention: role in genome stability. J. Nutr. Biochem. 45 1–14. [DOI] [PubMed] [Google Scholar]
- Goya L., Martin M. A., Sarria B., Ramos S., Mateos R., Bravo L. (2017). Effect of cocoa and its flavonoids on biomarkers of inflammation: studies of cell culture. Anim. Hum. Nutr. 8 212–234. 10.3390/nu8040212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y., Bruno R. S. (2015). Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 26 201–210. 10.1016/j.jnutbio.2014.10.008 [DOI] [PubMed] [Google Scholar]
- Hardie D. G. (2011). Sensing of energy and nutrients by AMP-activated protein kinase. Am. J. Clin. Nutr 93 891S–896S. 10.3945/ajcn.110.001925 [DOI] [PubMed] [Google Scholar]
- Hong H., Park J., Lumbera W. L., Hwang S. G. (2017). Monascus ruber\r, -Fermented buckwheat (Red Yeast Buckwheat) suppresses Adipogenesis in 3T3-L1 Cells. J. Med. Food. 20 352–359. 10.1089/jmf.2016.3761 [DOI] [PubMed] [Google Scholar]
- Hu Y. Y., Yin F. W., Liu Z. Y., Xie H. K., Xu Y. H., Zhou D. Y., et al. (2020). Acerola polysaccharides ameliorate high-fat diet-induced non-alcoholic fatty liver disease through reduction of lipogenesis and improvement of mitochondrial functions in mice. Food Funct. 11 1037–1048. 10.1039/C9FO01611B [DOI] [PubMed] [Google Scholar]
- Hua S., Li Y., Su L., Liu X. (2016). Diosgenin ameliorates gestational diabetes through inhibition of sterol regulatory element-binding protein-1. Biomed. Pharmacother. 84 1460–1465. 10.1016/j.biopha.2016.10.049 [DOI] [PubMed] [Google Scholar]
- Huazano-Garcia A., Shin H., Lopez M. G. (2017). Modulation of gut microbiota of overweight mice by agavins and their association with body weight loss. Nutrients 9:821. 10.3390/nu9090821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen J., Maarbjerg S. J., Jordy A. B., Fritzen A. M., Pehmoller C., Sylow L., et al. (2013). LKB1 regulates lipid oxidation during exercise independently of AMPK. Diabetes. 62 1490–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Castro M. B., Casillas-Ramírez A., Mendes-Braz M., Massip-Salcedo M., Gracia-Sancho J., Elias-Miró M., et al. (2013). Adiponectin and resistin protect steatotic livers undergoing transplantation. J. Hepatol. 59 1208–1214. 10.1016/j.jhep.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Joubert R., Métayer Coustard S., Swennen Q., Sibut V., Crochet S., Cailleau-Audouin E., et al. (2010). The beta-adrenergic system is involved in the regulation of the expression of the expression of avian uncoupling protein in the chicken. Domest. Anim. Endocrin. 38 115–125. 10.1016/j.domaniend.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Kameyama K., Itoh K. (2014). Intestinal colonization by a Lachnospiraceae bacterium contributes to the development of diabetes in obese mice. Microbes. Environ. 29 427–430. 10.1264/jsme2.ME14054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. G., Kim J. R., Choi H. C. (2018). Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. J. Med. Food 21 146–153. 10.1089/jmf.2017.4052 [DOI] [PubMed] [Google Scholar]
- Lage R., Diéguez C., Vidal-Puig A., López M. (2008). Ampk: a metabolic gauge regulating whole-body energy homeostasis. Trends. in Mole. Med. 14 539–549. 10.1016/j.molmed.2008.09.007 [DOI] [PubMed] [Google Scholar]
- Lecomte V., Kaakoush N. O., Maloney C. A., Raipuria M., Huinao K. D., Mitchell H. M., et al. (2015). Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One 10:e0126931. 10.1371/journal.pone.0126931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. J., Kim M., Park H. S., Kim H. S., Jeon M. J., Oh K. S., et al. (2006). AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Bioph. Res. Commun. 340 291–295. 10.1016/j.bbrc.2005.12.011 [DOI] [PubMed] [Google Scholar]
- Li Y., Jing F. U., Wang B. D., Wang Y. B., Shan A. S. (2008). Effect of flavones of sea buckthorn on carcass characteristics and meat quality of arbor acres broilers. C J. Anim. Vet. Sci. 39 1217–1223. 10.1007/s10499-007-9164-4 [DOI] [Google Scholar]
- Li Y., Xu S., Mihaylova M. M., Zheng B., Hou X., Jiang B., et al. (2011). AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13 376–388. 10.1016/j.cmet.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Yao J., Han C., Yang J., Chaudhry M. T., Wang S., et al. (2016a). Quercetin, inflammation and immunity. Nutrients 8:167. 10.3390/nu8030167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao X. M., Feng X. Y., Liu X. M., Deng C., Hu C. H. (2016b). Berberine alleviates olanzapine-induced adipogenesis via the ampkα–srebp pathway in 3t3-l1 cells. Inter. J. Mole. Sci. 17 1865–1877. 10.3390/ijms17111865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. C., Wei C., Zhang X. G., You W., Li G. F. (2020). HMGR overexpression and interference affects the expression of steroidogenic genes and cholesterol content in bovine intramuscular adipocytes. Sci. Rep. 10:16606 10.1038/s41598-020-73626-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou C. J., Lee Y. K., Ting N. C., Chen Y. L., Shen S., Wu S. C. (2019). Protective effects of licochalcone A ameliorates obesity and non-fatty liver disease via promotion of the Sirt-1/AMPK pathway in mice fed a high-fat diet. Cells 8 447–467. 10.3390/cells8050447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Huang J., Luo Y., Wen B., Wu W., Zeng H., et al. (2019). Fuzhuan brick tea attenuates high-fat diet-induced obesity and associated metabolic disorders by shaping gut microbiota. J. Agri. Food Chem. 67 13589–13604. 10.1021/acs.jafc.9b05833 [DOI] [PubMed] [Google Scholar]
- Liu H. T., Wu C. G., Wang S., Gao S. M., Liu J. S., Dong Z. Q., et al. (2015). Extracts and lignans of Schisandra chinensis fruit alter lipid and glucose metabolism in vivo and in vitro. J. Fun. Foods. 19 296–307. 10.1016/j.jff.2015.09.049 [DOI] [Google Scholar]
- Liu J., Hao W., He Z., Kwek E., Zhao Y., Zhu H., et al. (2019). Beneficial effects of tea water extracts on the body weight and gut microbiota in C57BL/6J mice fed with a high-fat diet. Food Funct. 10 2847–2860. 10.1039/C8FO02051E [DOI] [PubMed] [Google Scholar]
- Liu Y., Deng J., Fan D. (2019). Ginsenoside Rk3 ameliorates high-fat-diet/streptozocin induced type 2 diabetes mellitus in mice via the AMPK/Akt signaling pathway. Food Funct. 10 2538–2551. 10.1039/c9fo00095j [DOI] [PubMed] [Google Scholar]
- Luccia B., Crescenzo R., Mazzoli A., Cigliano L., Venditti P., Walser J. C. (2015). Iossa, S. Rescue of fructose-induced metabolic syndrome by antibiotics or faecal transplantation in a rat model of obesity. PLoS One 10:e0134893. 10.1371/journal.pone.0134893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. M., Qi J. M., Wang W. J., Luo Z. H., Liu L., Zhang G. G., et al. (2019). Antiobesity effect of flaxseed polysaccharide via inducing satiety due to leptin resistance removal and promoting lipid metabolism through the AMP-activated protein kinase (AMPK) signaling pathway. J. Agri. Food Chem. 67 7040–7049. 10.1021/acs.jafc.9b02434 [DOI] [PubMed] [Google Scholar]
- Maingrette F., Renier G. (2003). Leptin increases lipoprotein lipase secretion by macrophages: involvement of oxidative stress and protein kinase C. Diabetes. 52 2121–2128. 10.2337/diabetes.52.8.2121 [DOI] [PubMed] [Google Scholar]
- Manning B. D., Toker A. (2017). Akt/pkb signaling: navigating the network. Cell 169 381–405. 10.3390/ijms14059751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan C. J., Beiko R. G. (2014). A phylogenomic view of ecological specialization in the lachnospiraceae, a family of digestive tract-associated bacteria. Genom. Biol. Evol. 6 703–713. 10.1093/gbe/evu050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. E., Assini J. M., Lee J. K., Allister E. M., Sutherland B. G., Koppes J. B., et al. (2011). Nobiletin attenuates vldl overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 60 1446–1457. 10.2337/db10-0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan S., García-Mena J., Pizano-Zárate M. L., Maya O., Galván- Rodríguez F. M., Miranda-Brito C., et al. (2016). The role of the colon microbiota in the Mexican children obesity. World Congress on Targeting Microbiota 3 1–4. 10.18143/JISM_v3i1_1625 [DOI] [Google Scholar]
- Pang L., Zhang Y., Yu Y., Zhang S. (2013). Resistin promotes the expression of vascular endothelial growth factor in ovary carcinoma cells. Int. J. Mol. Sci. 14 9751–9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. J., Yun J., Jang S. H., Kang S. N., Jeon B. S., Ko Y. G., et al. (2014). Coprinus comatus cap inhibits adipocyte differentiation via regulation of PPARγ and Akt signaling pathway. PLoS One 9:e105809. 10.1371/journal.pone.0105809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K. G., Min A. K., Koh E. H., Kim H. S., Kim M. O., Park H. S., et al. (2008). Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatol. 48 1477–1486. 10.1042/bst0310220 [DOI] [PubMed] [Google Scholar]
- Pataky Z., Genton L., Spahr L., Lazarevic V., Terraz S., Gaïa N., et al. (2016). Impact of hypocaloric hyperproteic diet on gut microbiota in overweight or obese patients with nonalcoholic fatty liver disease: a pilot study. Dig. Dis. Sci. 61 2721–2731. 10.1007/s10620-016-4179-1 [DOI] [PubMed] [Google Scholar]
- Peng J., Li Q., Li K., Zhu L., Lin X., Lin X., et al. (2017). Quercetin improves glucose and lipid metabolism of diabetic rats: involvement of akt signaling and SIRT1. J. Diabetes Res. 2017 1–10. 10.1155/2017/3417306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X. F., Chen R. C., Wu Y., Huang B., Tang C. P., Chen J. F., et al. (2015). Pparγ–pi3k/akt–no signal pathway is involved in cardiomyocyte hypertrophy induced by high glucose and insulin. J. Dia. Compl. 29 755–760. 10.1016/j.jdiacomp.2015.04.012 [DOI] [PubMed] [Google Scholar]
- Proszkowiec-Weglarz M., Richards M. P., Ramachandran R., Mcmurtry J. P. (2006). Characterization of the amp-activated protein kinase pathway in chickens[J]. Comp. Biochem. Phys. B Biochem. Mol. Biol. 143 92–106. 10.1016/j.cbpb.2005.10.009 [DOI] [PubMed] [Google Scholar]
- Qiu P. Y., Man S. L., Li J., Liu J., Zhang L. M., Yu P., et al. (2016). Overdose intake of curcumin initiates the unbalanced state of bodies. J. Agri. Food Chem. 64 2765–2771. 10.1021/acs.jafc.6b00053 [DOI] [PubMed] [Google Scholar]
- Rocca C., Albano L., Granieri M. C., Amelio D., Nettore I. C., Macchia P. E., et al. (2018). Novel anti-obesity quercetin-derived Q2 prevents metabolic disorders in rats fed with high-fat diet. Vasc. Pharmacol. 103–105:58 10.1016/j.vph.2017.12.030 [DOI] [Google Scholar]
- Ross J. A., Kasum C. M. (2002). Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 22 19–34. 10.1146/annurev.nutr.22.111401.144957 [DOI] [PubMed] [Google Scholar]
- Sakamoto K., McCarthy A., Smith D., Gree K. A., Grahame Hardie D., Ashworth A., et al. (2005). Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO 24 1810–1820. 10.1038/sj.emboj.7600667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong-Ah K., Jiyoon K., Shinyoung J., Gyung-Ah W., Sangah S., Hyojee J. (2020). Association between dietary flavonoid intake and obesity among adults in Korea. Appl. Physiol. Nutr. Metab. 45 203–212. 10.1139/apnm-2019-0211 [DOI] [PubMed] [Google Scholar]
- Sohaib M., Butt M. S., Shabbir M. A., Shahid M. (2015). Lipid stability, antioxidant potential and fatty acid composition of broilers breast meat as influenced by quercetin in combination with alpha-tocopherol enriched diets. Lipids Health. Dis. 14 1–15. 10.1186/s12944-015-0058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Wang Y. W., Song P. P., Wang H. S., Xu N., Wang Y. J., et al. (2019). Antiobesity effects of instant fermented teas in vitro and in mice with high-fat-diet-induced obesity. Food Funct. 10 3502–3513. 10.1039/c9fo00162j [DOI] [PubMed] [Google Scholar]
- Thomaz F. S., Tomsett K. I., Panchal S. K., Worrall S., Nitert M. D. (2020). Wasabi supplementation alters the composition of the gut microbiota of diet-induced obese rats. J. Funct. Foods 67 103868–103876. 10.1016/j.jff.2020.103868 [DOI] [Google Scholar]
- Thomson D. M., Brown J. D., Fillmore N., Condon B. M., Kim H. J., Barrow J. R. (2007). LKB1 and the regulation of malonyl-CoA and fatty acid oxidation in muscle. Am. J. Physiol. Endocrinol. Metab. 293 1572–1579. 10.1152/ajpendo.00371.2007 [DOI] [PubMed] [Google Scholar]
- Ushiroda C., Naito Y., Takagi T., Uchiyama K., Mizushima K., Higashimura Y., et al. (2019). Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J. Clini. Biochemis. Nutr. 65 34–46. 10.3164/jcbn.18-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Mao Y. J., Wang B., Wang S. S., Lu H., Ying L. L., et al. (2020). Quercetin improving lipid metabolism by regulating lipid metabolism pathway of ileum mucosa in broilers. Oxid. Med. Cell. Longev. 2020 1–11. 10.1155/2020/8686248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yao J., Zhou B., Yang J., Chaudry M. T., Wang M., et al. (2018). Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Protect. 81 68–78. 10.4315/0362-028X.JFP-17-214 [DOI] [PubMed] [Google Scholar]
- Wang Z., Lam K. L., Hu J., Ge S. H., Zhou A., Zheng B. D., et al. (2019). Chlorogenic acid alleviates obesity and modulates gut microbiota in high−fat−fed mice. Food Sci. Nutr. 7 579–588. 10.1002/fsn3.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. Q., Zhang X. H., Yu Y., Tipton R. C., Raskin I., Ribnicky D., et al. (2013). Artemisia scoparia extract attenuates non-alcoholic fatty liver disease in diet-induced obesity mice by enhancing hepatic insulin and AMPK signaling independently of FGF21 pathway. Metabolism 62 1239–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt M. J., Steinberg G. R., Chen Z. P., Kemp B. E., Febbraio M. A. (2006). Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J. Physiol.. 574 139–147. 10.1113/jphysiol.2006.107318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Williams J. R., Muckett P. J., Mayer F. V., Liljevald M., Bohlooly Y. M., et al. (2017). Liver-specific activation of AMPK prevents steatosis on a high-fructose diet. Cell Rep. 18 3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Denbow C. J., Meiri N., Denbow D. M. (2012). Fasting of 3-day-old chicks leads to changes in histone h3 methylation status. Physiol. Behav. 105 276–282. 10.1016/j.physbeh.2011.06.023 [DOI] [PubMed] [Google Scholar]
- Xu P., Siegel P. B., Denbow D. M. (2011). Genetic selection for body weight in chickens has altered responses of the brain\”s ampk system to food intake regulation effect of ghrelin, but not obestatin. Behav. Brain Res. 221 216–226. 10.1016/j.bbr.2011.02.034 [DOI] [PubMed] [Google Scholar]
- Xue B., Kahn B. B. (2006). Ampk integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J. Physiol. 574 73–83. 10.1113/jphysiol.2006.113217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. X., Maria T. C., Zhou B., Xiao F. L., Wang M., Mao Y. J., et al. (2020). Quercetin improves immune function in Arbor Acre broilers through activation of NF-κB signaling pathway. Poul. Sci. 99 906–913. 10.1016/j.psj.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Y., Della-Fera M. A., Rayalam S., Ambati S., Hartzell D. L., Park H. J., et al. (2008). Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 82 1032–1039. 10.1016/j.lfs.2008.03.003 [DOI] [PubMed] [Google Scholar]
- Ying L., Chaudhry M., Xiao F., Mao Y., Wang M., Wang B., et al. (2020). The effects and mechanism of quercetin dietary supplementation in streptozotocin-induced hyperglycemic Arbor Acre broilers. Oxid. Med. Cell. Longev. 2020 1–11. 10.1155/2020/9585047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yao L. I., Feng X. A., Liu Y., Jin F., Hu L. L., et al. (2013). Effect of quercetin on production performance and lipids metabolism of laying hens. C. Anim. Hus. Vet. Med 40 89–93. [Google Scholar]
- Zhang T., Liang X., Shi L., Wang L., Chen J., Kang C., et al. (2013). Estrogen receptor and PI3K/Akt signaling pathway involvement in S-(-)equol-induced activation of Nrf2/ARE in endothelial cells. PLoS One 8:e79075. 10.1371/journal.pone.0079075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu D. (2011). Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur. J. Pharmacol. 670 325–332. 10.1016/j.ejphar.2011.08.011 [DOI] [PubMed] [Google Scholar]
- Zhao L., Zhang Q., Ma W., Tian F., Shen H., Zhou M. (2017). Combination of quercetin and resveratrol reduces obesity in high fat diet-fed rats by modulation of gut microbiota. Food Funct. 8 4644–4656. [DOI] [PubMed] [Google Scholar]
- Zhao N. Q., Li X. Y., Wang L., Feng Z. L., Li X. F., Wen Y. F., et al. (2017). Palmitate induces fat accumulation by activating C/EBPBβ-mediated G0S2 expression in hepg2 cells. World J. Gastroentero 2017 7705–7715. 10.3748//wjg.v23.i43.7705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z., Wang C., Zhang L. (2019). Lactobacillus plantarum NA136 improves the non-alcoholic fatty liver disease by modulating the AMPK/Nrf2 pathway. Appl. Microbiol. Biotechnol. 103 5843–5850. 10.1007/s00253-019-09703-4 [DOI] [PubMed] [Google Scholar]
- Zheng J. L., Luo Z., Liu C. X., Chen Q. L., Tan X. Y., Zhu Q. L. (2013). Differential effects of acute and chronic zinc (Zn) exposure on hepatic lipid deposition and metabolism in yellow catfish Pelteobagrus fulvidraco. Aquat. Toxicol. 132 173–181. 10.1016/j.aquatox.2013.02.002 [DOI] [PubMed] [Google Scholar]
- Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., et al. (2001). Role of amp-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108 1167–1174. 10.1172/JCI13505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D., Wang J., Sun X., Chen J., Duan Y., Pan J., et al. (2015). Septin4_i1 regulates apoptosis in hepatic stellate cells through peroxisome proliferator-activated receptor-?/akt/b-cell lymphoma 2 pathway. J. Histochem. Cytochem. 63 163–169. 10.1369/0022155414567230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Du W., Liu Y., Cheng M., Wang X., Zhang C., et al. (2019). Prolonged high-glucose exposure decreased SREBP-1/FASN/ACC in Schwann cells of diabetic mice via blocking PI3K/Akt pathway. J. Cell. Biochem. 120 5777–5789. 10.1002/jcb.27864 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.