Abstract
Background
Surgery for spinal metastasis is rapidly increasing in frequency with procedures ranging from laminectomy to spondylectomy combined with stabilization. This study investigated the effect of various surgical procedures for spinal metastasis of non-small cell lung cancer (NSCLC).
Methods
A single-center consecutive series of patients who underwent surgery for spinal metastasis of NSCLC were retrospectively reviewed. Patients' characteristics, radiographic parameters, operative data, clinical outcomes, and complications were analyzed. Surgical outcomes were assessed according to pain and performance status before and after surgery. Overall survival (OS) rate was estimated using the Kaplan-Meier method. Multivariate analysis was performed to detect factors independently associated with OS using a Cox proportional hazards model.
Results
Twenty-one patients were treated with laminectomy, 24 with corpectomy, 13 with spondylectomy (piecemeal or total en bloc fashion), and all procedures were combined with stabilization. Back pain and performance status improved significantly after surgical treatment among the three groups. Revision surgery due to tumor progression at the index level or spinal metastasis at another level were four patients (19.0%) in the laminectomy group, six patients (25.0%) in the corpectomy group, and one patient (7.7%) in the spondylectomy group. A Charlson comorbidity index and the number of spinal metastasis negatively affected OS (hazard ratio [HR], 19.613 and 2.244). Postoperative chemotherapy, time to metastasis, spondylectomy, and corpectomy had favorable associations with OS (HR, 0.455, 0.487, 0.619, and 0.715, respectively).
Conclusion
Postoperative chemotherapy was the most critical factor in OS of patients with metastatic NSCLC to the spine. An extensive surgical procedure (corpectomy/spondylectomy) with stabilization also could be beneficial for limited patients with spinal metastasis of NSCLC.
Keywords: Non-small Cell Lung Cancer, Spinal Metastasis, Laminectomy, Corpectomy, Spondylectomy, Overall Survival Rate
Graphical Abstract
INTRODUCTION
Lung cancer is one of the most common cancers worldwide and is the leading cause of cancer-related death.1 Non-small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer cases, and its prognosis is better than that of small cell lung cancer. Bone metastasis occurs in 26%–36% of NSCLC patients, and the prognosis of these patients is poor, with a 2-year survival rate of 3%.2 Bony compromise and tumor invasion into the epidural space can occur, leading to axial pain and neurological deficits. The incidence of spinal metastasis is expected to increase as long-term survival becomes more likely due to medical advances in controlling the primary disease. Furthermore, recent advances in surgical techniques and instruments have enabled safe and effective tumor removal and stabilization in patients with spinal metastasis.3,4,5,6 Therefore, it is of the utmost importance to identify the best treatment for patients with metastatic spinal tumors.
To date, the most important and effective treatment in spinal metastasis may be radiotherapy.7 However, surgical stabilization is necessary for some patients with spinal instability.7,8,9 Pain relief and recovery of neurological function also can be achieved by partial or total excision of the tumor from the vertebra anteriorly or posteriorly with sufficient surgical decompression.10 Despite disadvantages such as contamination of tumor cells and residual tumor, curettage or piecemeal excision of vertebral tumors has been commonly practiced.11 However, the local recurrence rate after intralesional resection is high.12 In contrast, spondylectomy is a surgical technique that enables the complete removal of metastatic lesions of the spine.13 Although metastasis generally indicates systemic cancer, it was found that spondylectomy could completely remove tumors locally and prolong the survival of patients with spinal metastases.11,14,15 However, spondylectomy is a technically demanding procedure accompanied by many risks such as excessive bleeding, injury of the major vessels, and spinal cord injury.
This study aimed to compare the outcomes, including back pain, performance status, and survival, of various surgical treatments for patients with NSCLC spinal metastasis and analyze factors associated with OS.
METHODS
Patient population
From October 2004 to December 2019, 421 patients underwent a surgical procedure for spinal metastasis at our institution, where surgery for spinal metastasis was performed on patients who met the following three conditions: 1) spinal metastasis with vertebral body involvement, 2) progressive neurological symptoms or intractable pain, and 3) more than 6 months of expected survival as confirmed by an oncologist. Of these patients, 106 patients had metastasis of NSCLC. We selected patients with spinal metastasis to the thoracic or lumbar spine. Patients who underwent surgery under local anesthesia (biopsy and vertebroplasty) and only decompression surgery were excluded. Ultimately, 58 patients were included in the present study. The patients were divided into three groups according to the surgical method used: laminectomy with fusion, corpectomy with fusion, and spondylectomy with fusion. Laminectomy was performed in cases of spinal cord compression. The surgical indications of corpectomy for spinal metastasis included more than 50% of vertebral body involvement or severe kyphosis (> 20°) due to vertebral body collapse. Spondylectomy was performed for spinal metastasis with radically resectable skip lesions in patients with a good general condition (Eastern Cooperative Oncology Group [ECOG] performance status grade ≤ 3).16
The baseline demographic characteristics, including age, sex, pathology subtype, the presence/absence of epidermal growth factor receptor (EGFR) mutation, time to spinal metastasis from diagnosis of NSCLC, preoperative treatment, preoperative ECOG grade, Charlson comorbidity index (CCI), Tomita score, and Spinal Instability Neoplastic Score (SINS), were reviewed. The surgical details, postoperative treatment, complications, and survival time were also collected from electronic medical records. The clinical outcomes were analyzed using a numeric rating scale (NRS) for back pain and ECOG grade for performance status. All patients in each group visited the spine center at 1 month and 3, 6, 9, and 12 months postoperatively. Since then, if possible, patients have visited the spine center every 3 months. Plain radiographs at preoperative, postoperative, and follow-up time points were analyzed. Any changes on radiographs suggesting local recurrence were investigated using magnetic resonance imaging (MRI).
Surgical procedures
In a laminectomy, a posterior midline approach was used. Subperiosteal dissection was performed, and the paravertebral muscles were retracted. A total laminectomy was performed with a burr to decompress the spinal cord.
In a corpectomy group, an anterior approach was used in two patients, and a posterior approach was used in 22 patients. The anterior approach exposed the metastatic tumor of the lumbar spine through the retroperitoneal approach. Then, the metastatic vertebral body was removed by piecemeal fashion. To reconstruct the bony defect, a titanium mesh cage filled with allograft was used. In the posterior approach, a transpedicular approach was performed in 18 patients, costotransversectomy in three patients, and lateral extracavitary approach in one patient. Metastatic tumor removal and circumferential decompression of the thecal sac were performed.
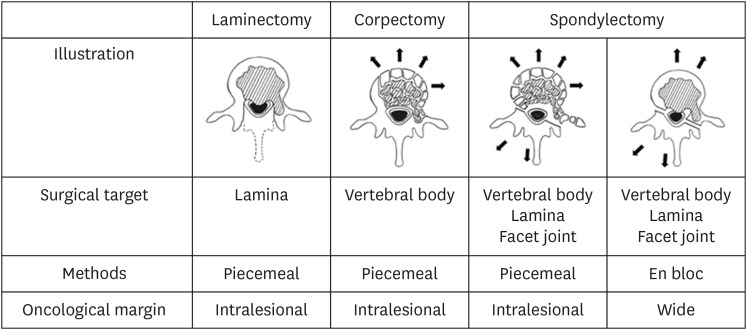
In a spondylectomy group, a combined anterior and posterior approach was used in two patients, and a single posterior approach was used in 11 patients.13,17 In the anterior approach, normal cranial and caudal vertebrae were widely exposed, using a 2- to 3-cm tumor margin to secure it. Discectomy within the tumor-free adjacent disc was performed. In the posterior approach, the ribs and paraspinal muscles with a 2- to 3-cm margin were resected. Laminectomy at the tumor-free site of the affected vertebrae was performed, decompressing the spinal cord. Finally, en bloc extirpectomy was accomplished. Due to the technically demanding procedure, piecemeal total vertebrectomy was performed in some patients (Fig. 1).18
Fig. 1. Schematic representation for describing the different possible techniques to remove spinal metastasis.
A pedicle screw system was used for posterior reconstruction at least two vertebral levels above and below the lesion in all three groups. Pedicle screws were inserted using a freehand technique.19,20
Statistical analysis
One-way analysis of variance was used to analyze the distribution of continuous variables among the three groups. Fisher's exact test was used to compare differences in proportions for categorical variables. Due to missing data, the NRS and ECOG scores of the three groups at the preoperative evaluation and each subsequent visit were compared using generalized estimating equations instead of repeated-measures analysis of variance. The Kaplan-Meier method was used to estimate survival time, and the log-rank method was used to evaluate between-group differences. To detect factors associated with OS, multivariate analysis was performed using a Cox proportional hazards model. All clinical characteristics were evaluated for this regression analysis. The results are reported as P values, hazard ratios (HR), and 95% confidence intervals (CI). All tests were two-sided. A P value < 0.05 was considered to indicate statistical significance. SPSS version 21.0 (IBM Corp., Armonk, NY, USA) was used to perform all statistical analyses.
Ethics statement
The requirement for informed consent was waived due to the retrospective design and minimal risk of the study. The Institutional Review Board of Seoul National University Bundang Hospital approved this study (approval No. B-1912-166-1023).
RESULTS
Preoperative clinical characteristics
Among the patients included in this study, 21 patients underwent decompressive laminectomy, 24 underwent corpectomy, and 13 underwent total spondylectomy. The baseline characteristics of all patients are shown in Table 1. There was no significant difference in mean age among the three groups (62.5 ± 9.5 vs. 61.6 ± 10.2 vs. 62.0 ± 9.2, respectively; P = 0.954). Adenocarcinoma was the most common subtype in all three groups (80.1% vs. 75.0% vs. 61.6%, respectively). The incidence and type of EGFR mutations did not differ significantly among the three groups (P = 0.792). There was no significant difference in time to spinal metastasis from the diagnosis of NSCLC among the three groups (14.5 ± 5.0 vs. 17.9 ± 3.6 vs. 21.4 ± 4.1, respectively; P = 0.147). Also, there were no significant differences in chemotherapy history, history of radiotherapy to spinal metastasis, preoperative ECOG grade, CCI, Tomita score, and SINS among the three groups. The patient in each group had similar follow-up periods without significant differences.
Table 1. Baseline demographic characteristics.
| Parameters | Laminectomy (n = 21) | Corpectomy (n = 24) | Spondylectomy (n = 13) | P value | |
|---|---|---|---|---|---|
| Age, yr | 62.5 ± 9.5 | 61.6 ± 10.2 | 62.0 ± 9.2 | 0.954a | |
| Sex, male:female | 14:7 | 16:8 | 9:4 | 0.999 | |
| Subtype | 0.647b | ||||
| Adenocarcinoma | 17 | 18 | 8 | ||
| Squamous cell carcinoma | 2 | 3 | 4 | ||
| Large-cell carcinoma | 0 | 1 | 0 | ||
| Unclassified carcinoma | 2 | 2 | 1 | ||
| EGFR mutation (+) | 0.792b | ||||
| 19del | 2 | 5 | 1 | ||
| 21L858R | 2 | 3 | 1 | ||
| Time to metastasis, monc | 14.5 ± 5.0 | 17.9 ± 3.6 | 21.4 ± 4.1 | 0.147a | |
| Preop. chemotherapy | 11 (52.4) | 15 (62.5) | 6 (46.2) | 0.625 | |
| Preop. radiotherapy (to spinal metastasis) | 3 (14.3) | 7 (29.2) | 5 (38.5) | 0.257 | |
| Preop. ECOG grade | 2.6 ± 0.5 | 2.5 ± 0.5 | 2.3 ± 0.5 | 0.632a | |
| CCI | 8.3 ± 1.7 | 8.5 ± 1.4 | 8.5 ± 1.7 | 0.897a | |
| Tomita score | 7.8 ± 1.7 | 7.1 ± 1.8 | 7.5 ± 1.9 | 0.354a | |
| SINS | 9.4 ± 1.8 | 10.8 ± 1.5 | 10.5 ± 1.3 | 0.138a | |
| Follow-up months | 10.1 ± 12.8 | 14.3 ± 15.0 | 15.5 ± 15.2 | 0.539a | |
The values are given as means ± standard deviation or number (%) unless otherwise indicated. A P value of < 0.05 was considered to indicate statistical significance.
EGFR = epidermal growth factor receptor, Preop = preoperative, ECOG = Eastern Cooperative Oncology Group, CCI = Charlson comorbidity index, SINS = Spinal Instability Neoplastic Score.
aanalysis of variance; bFisher's exact test; cTime to metastasis from diagnosis of non-small cell lung cancer.
Surgical details and postoperative treatment
Spinal metastases were more frequent in the thoracic spine in all three groups (Table 2). The number of spinal metastasis was significantly different among the three groups (2.5 ± 0.7 vs. 1.3 ± 0.5 vs. 1.1 ± 0.3, respectively; P < 0.001). The operation time was significantly different among the three groups (240.7 ± 57.6 vs. 268.3 ± 102.8 vs. 373.5 ± 84.7 minutes, respectively; P < 0.001). However, there was no significant difference in estimated blood loss among the three groups (P = 0.481).
Table 2. Surgical details and postoperative treatment.
| Parameters | Laminectomy (n = 21) | Corpectomy (n = 24) | Spondylectomy (n = 13) | P value | |
|---|---|---|---|---|---|
| Location of metastasis | 0.695 | ||||
| Thoracic | 14 | 19 | 9 | ||
| Lumbar | 7 | 5 | 4 | ||
| Spinal metastasis | 2.5 ± 0.7 | 1.3 ± 0.5 | 1.1 ± 0.3 | < 0.001a | |
| Instrumented segments | 5.1 ± 1.6 | 4.7 ± 1.1 | 4.9 ± 0.9 | 0.673a | |
| Operation time (min) | 240.7 ± 57.6 | 268.3 ± 102.8 | 373.5 ± 84.7 | < 0.001a | |
| EBL, mL | 811.9 ± 1,164.2 | 983.3 ± 1,451.5 | 1,351.5 ± 993.5 | 0.481a | |
| Postop. ICU transfer | 8 (38.1) | 2 (8.3) | 3 (23.1) | 0.059b | |
| Postop. chemotherapy | 14 (66.7) | 19 (79.2) | 11 (84.6) | 0.531 | |
| Postop. radiotherapy (to spinal metastasis) | 9 (42.9) | 16 (66.7) | 2 (15.4) | 0.011 | |
The values are given as means ± standard deviation or number (%) unless otherwise indicated. A Pvalue of < 0.05 was considered to indicate statistical significance. The boldface type indicates statistical significance.
EBL = estimated blood loss, Postop = postoperative, ICU = intensive care unit.
aanalysis of variance; bFisher's exact test.
The oncologist decided whether or not to start chemotherapy and which chemotherapy would be applied according to the patients' performance status, subtype, and absence/presence of gene mutation. There was no significant difference in the percentage of patients who received chemotherapy after surgery (P = 0.531). However, the percentage of patients who received radiotherapy to spinal metastasis after surgery was significantly different among the three groups (42.9% vs. 66.7% vs. 15.4%, respectively; P = 0.011).
Clinical outcomes
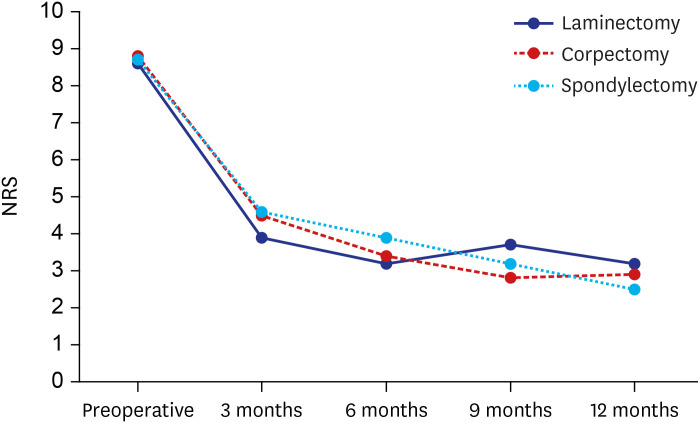
There were no significant differences in the initial NRS for back pain among the three groups (P = 0.890) (Fig. 2). In the laminectomy group, the NRS for back pain was significantly reduced after the operation (from 8.6 ± 1.0 to 3.9 ± 1.9; P = 0.034), and this improvement was maintained well until 1 year postoperatively. The NRS for back pain in the corpectomy and spondylectomy groups was also significantly reduced and maintained well (from 8.8 ± 1.2 to 4.5 ± 0.8; P = 0.017 and from 8.7 ± 1.1 to 4.6 ± 0.8; P = 0.016, respectively). Overall, changes in the NRS for back pain were not significantly different at every measurement.
Fig. 2. Assessments of back pain in the laminectomy group, corpectomy group, and spondylectomy group preoperatively and at postoperative 3, 6, 9, and 12 months.
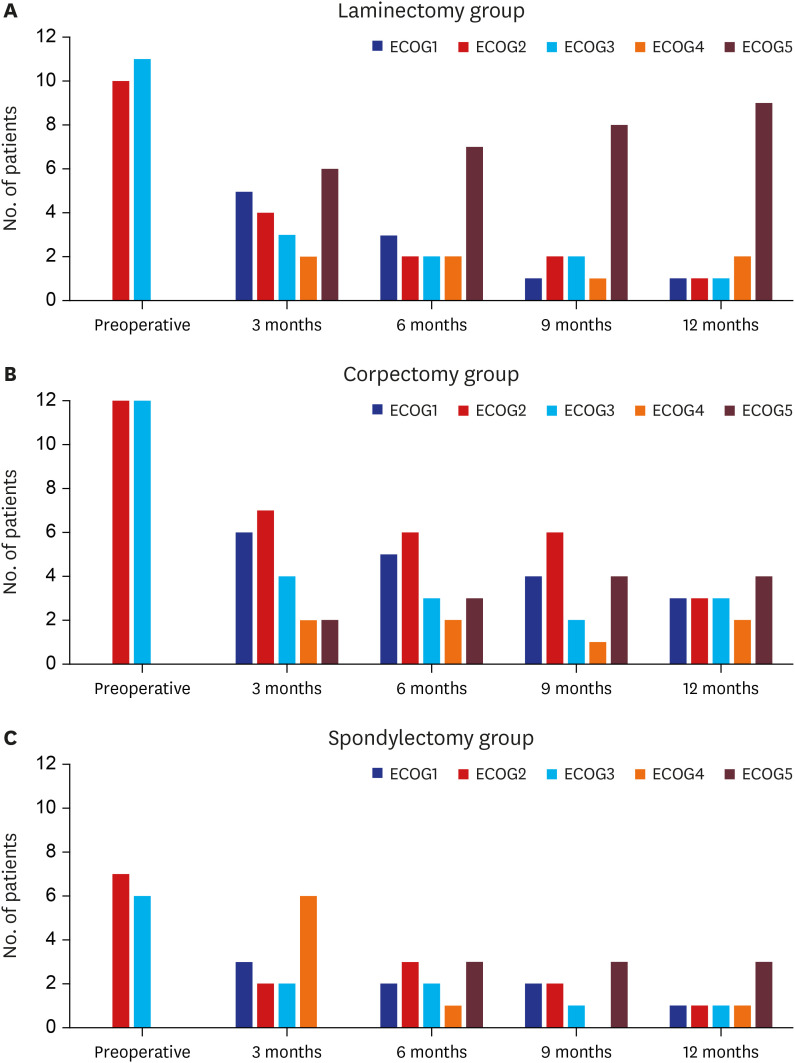
Changes in functional status (ECOG performance) after surgery in the three groups are shown in Fig. 3. The preoperative functional state of all patients in this study was ECOG grade 2 or 3. Three months after the operation, improvement or maintenance of functional status was noted in 34 (58.6%) of the 58 patients, including 11 (52.4%) of the 21 patients after laminectomy, 17 (70.8%) of the 24 patients after corpectomy, and six (46.2%) of the 13 patients after spondylectomy (P = 0.303). Changes in functional status at each visit were not significantly different among the three groups.
Fig. 3. Changes in functional status (ECOG performance) after surgery in the three groups. (A) In the laminectomy group, the ECOG improved or remained unchanged in 3/21 (14.3%) of the patients at 12 months postoperative. (B) In the corpectomy group, the ECOG improved or remained unchanged in 9/24 (37.5%) of the patients at 12 months postoperative. (C) In the spondylectomy group, the ECOG improved or remained unchanged in 3/13 (23.1%) of the patients at 12 months postoperative.
ECOG = Eastern Cooperative Oncology Group.
Complications and tumor progression
There was no significant difference in the complication rate among the three groups (Table 3). In the laminectomy group, there was one patient with wound dehiscence. Another patient underwent revision surgery due to the progression of metastasis at the index level. In the corpectomy group, one patient underwent revision surgery due to postoperative hematoma, and another patient underwent revision surgery due to screw loosening. The other two patients underwent revision surgery due to the progression of metastasis at the index level. In the spondylectomy group, there was one intraoperative vessel injury. With the help of a cardiovascular surgeon, the vessel injury was repaired without further problems. Another patient underwent revision surgery due to rod fracture at 3 years after index surgery.
Table 3. Surgery-related complications and revision surgery.
| Parameters | Laminectomy (n = 21) | Corpectomy (n = 24) | Spondylectomy (n = 13) | P value | |
|---|---|---|---|---|---|
| Any complications | 2 | 4 | 2 | 0.795a | |
| Intraoperative vessel injury | - | - | 1 | ||
| Postoperative hematoma | - | 1 | - | ||
| Wound dehiscence | 1 | - | - | ||
| Screw loosening | - | 1 | - | ||
| Rod fracture | - | - | 1 | ||
| Tumor progression (at the index level) | 1 | 2 | - | ||
A P value of < 0.05 was considered to indicate statistical significance.
aFisher's exact test.
Spinal metastasis at another level occurred in three patients (14.3%) in the laminectomy group, four patients (16.7%) in the corpectomy group, and one patient (7.7%) in the spondylectomy group.
Overall survival (OS)
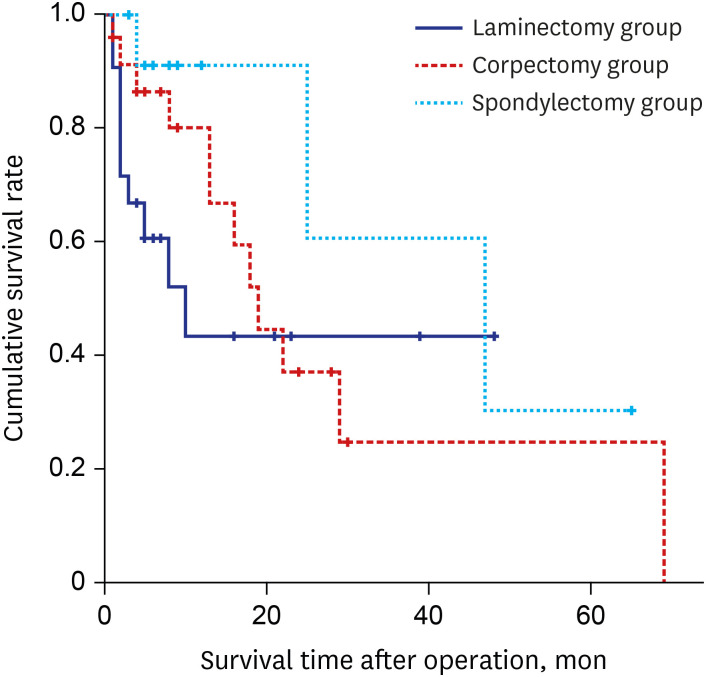
The Kaplan-Meier curve for OS time is shown in Fig. 4. The average survival time of the laminectomy group was 23.3 ± 5.5 months, that of the corpectomy group was 34.8 ± 7.1 months, and that of the spondylectomy group was 47.9 ± 9.3 months. The log-rank test showed a statistically significant difference in survival time among the three groups (P = 0.043).
Fig. 4. Kaplan-Meier graph of postoperative survival status in the three groups. The difference in postoperative survival time in patients treated with laminectomy, corpectomy, and spondylectomy was statistically significant (P = 0.043).
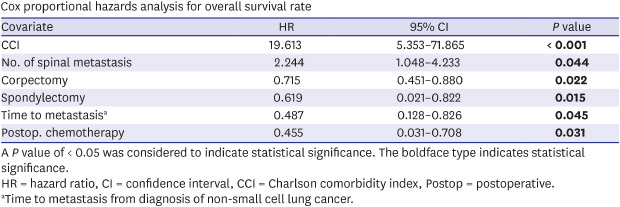
Cox proportional hazards analysis showed that the CCI was the main factor that affected the OS (HR, 19.613; P < 0.001) (Table 4). The number of spinal metastasis (HR, 2.244; P = 0.044) also had significant negative effects on the OS. On the other hand, postoperative chemotherapy (HR, 0.455; P = 0.031), time to spinal metastasis (HR, 2.055; P = 0.045), spondylectomy (HR, 0.619; P = 0.015), and corpectomy (HR, 0.715; P = 0.022) had significant positive effects on the OS.
Table 4. Cox proportional hazards analysis for overall survival rate.
| Covariate | HR | 95% CI | P value |
|---|---|---|---|
| CCI | 19.613 | 5.353–71.865 | < 0.001 |
| No. of spinal metastasis | 2.244 | 1.048–4.233 | 0.044 |
| Corpectomy | 0.715 | 0.451–0.880 | 0.022 |
| Spondylectomy | 0.619 | 0.021–0.822 | 0.015 |
| Time to metastasisa | 0.487 | 0.128–0.826 | 0.045 |
| Postop. chemotherapy | 0.455 | 0.031–0.708 | 0.031 |
A P value of < 0.05 was considered to indicate statistical significance. The boldface type indicates statistical significance.
HR = hazard ratio, CI = confidence interval, CCI = Charlson comorbidity index, Postop = postoperative.
aTime to metastasis from diagnosis of non-small cell lung cancer.
DISCUSSION
Tomita et al.21 proposed a surgical strategy for spinal metastases. Patients with good prognostic scores (scores 2–3) were recommended to undergo wide or marginal excision. Patients with intermediate scores were recommended to undergo marginal or intralesional excision (scores 4–5) or palliative surgery (scores 6–7). Nonsurgical supportive care was recommended for those with a poor prognosis (scores 8–10). In the present study, no significant differences were found in the Tomita score among the three groups (6.8 ± 1.7 vs. 7.1 ± 1.8 vs. 7.5 ± 1.9, respectively; P = 0.537). Nevertheless, all three types of surgery were performed because several spine surgeons performed metastasis surgery, and the study period encompassed numerous developments in surgical instruments and techniques.
Cancer pain can have a significant impact on the quality of life. Intractable pain can even cause a patient to become bed-ridden. Immobilization in bed can lead to complications, including pneumonia, pressure sores, urinary tract infections, thromboembolism, and joint contracture.22,23 In the present study, the NRS for back pain improved significantly at 3 months postoperatively, and the improvements were well maintained until postoperative one year in all three groups. The primary treatment goal of the surgical treatment of spinal metastasis is to achieve functional gain with a modest complication rate and without compromising the remaining survival time. A previous study reported that postoperative ECOG status was an independent prognostic factor for survival time in patients undergoing surgery for metastatic tumors from NSCLC.24 In the present study, all surgical methods resulted in similar outcomes, with no significant differences at any visit.
Tumor progression at the index level or spinal metastasis at another level differed across the groups. In the spondylectomy group, only one patient (7.7%) underwent revision surgery due to spinal metastasis at another level. The rate of local recurrence after spondylectomy in the present study was similar to previous reports (5%–10%).14,15 However, six patients (25.0%) in the corpectomy group and four patients (19.0%) in the laminectomy group underwent revision surgery due to progression at the index level or spinal metastasis at another level. Limited debulking may increase the chance of progression or metastasis. Previous studies have reported that intralesional resections with contaminated margins have a negative effect on local recurrence.25
In the Cox proportional hazards analysis, the CCI was the most significant factor influencing survival (HR, 19.613; 95% CI, 5.353–71.865; P < 0.001). The CCI is known to be a robust predictor of 30-day complications and survival following spinal metastasis surgery.26,27 Moreover, the CCI score is a good indicator of survival in patients with various types of cancer, including NSCLC, colorectal cancer, and prostate cancer.28,29,30 There are several possible reasons for the highly adverse impact of comorbidities on survival. These include the diminished effectiveness of adjuvant therapies, an inability to tolerate adjuvant therapies, and morbidity and mortality attributable to the comorbidities themselves. As expected, the number of spinal metastasis was also associated with a negative impact on survival. A notable finding of the present study was that the time to spinal metastasis (HR, 0.487; P = 0.045) had significant positive effects on the OS. The early-onset type might cause early spinal metastasis as a sign of early spread and aggressiveness and, therefore, is associated with a very poor prognosis. On the other hand, in the late-onset type, spinal metastasis might be an expression of the relatively low malignant potential, with cancer cells only nesting in the beneficial environment of the bone matrix that promotes cancer cell attachment and growth.31,32 The surgical method (corpectomy and spondylectomy) also significantly influenced survival (HR, 0.715 and 0.619, respectively). A retrospective review of corpectomy for metastatic NSCLC to the spine reported satisfactory outcomes.33 Neurologic improvement by at least one Frankel grade was noted in 25 of 31 cases (80%). There was no case of intraoperative mortality. The median survival time was 8.8 months. However, this surgical procedure involves intralesional resection and carries a high risk of incomplete tumor removal and tumor cell contamination. In a study of spondylectomy for lung cancer metastasis to the spine, 66.7% (4 of 6) of patients were still alive after surgery with a mean follow-up of 46.3 months (range, 36–62 months).34 Spondylectomy allows for complete removal of the diseased vertebrae, followed by a circumferential spinal reconstruction that can achieve excellent local control. Considering these results, spine surgeons should not hesitate to perform surgery in patients with spinal metastasis of NSCLC actively. Nevertheless, this study demonstrated that postoperative chemotherapy was the most critical in OS in patients with metastatic NSCLC to the spine (HR, 0.455; P = 0.031).
There were several limitations to our study. First, this report represents a retrospective study that is inherently subject to selection bias. Aggressive surgery, such as corpectomy and spondylectomy, may be conducted for patients with higher performance or good oncologic disease status. As shown in Table 1, there was no difference in baseline demographic characteristics of the three groups, including preoperative chemotherapy and radiotherapy. However, prospective comparative studies with a large series of patients for NSCLC spinal metastasis are needed. Second, relatively few patients were analyzed in this study, which resulted in low statistical power. However, by examining only a single histological type (NSCLC), we could minimize the shortcomings of other studies that analyzed multiple histological types together. Third, in some patients in the spondylectomy group, we performed piecemeal total vertebrectomy rather than total en bloc spondylectomy. We included those patients in the spondylectomy group because the posterior spinal elements (spinous process, lamina, and pedicles) were also removed in addition to the vertebral body. Fourth, the local control rate after surgery was not investigated thoroughly. MRI was not performed in all patients. Only patients with any changes on follow-up X-rays or whose lower extremity weakness progressed were investigated using MRI to confirm local recurrence. Fifth, this study included only patients who underwent surgery. This may represent a selection bias that may underestimate the role of radiotherapy in patients with spinal metastasis. Despite these limitations, our study contains valuable and clinically meaningful information and provides a basis for future research.
Postoperative chemotherapy was the most critical factor in OS in patients with metastatic NSCLC to the spine. However, considering the clinical outcome and OS, an extensive surgical procedure (corpectomy/spondylectomy) with stabilization is beneficial for limited patients with spinal metastasis of NSCLC.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Hyun SJ.
- Data curation: Jung JM.
- Formal analysis: Jung JM.
- Investigation: Jung JM.
- Methodology: Hyun SJ.
- Software: Jung JM.
- Validation: Hyun SJ, Kim KJ.
- Visualization: Jung JM.
- Writing - original draft: Jung JM.
- Writing - review & editing: Hyun SJ, Kim KJ.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Utzschneider S, Wicherek E, Weber P, Schmidt G, Jansson V, Dürr HR. Surgical treatment of bone metastases in patients with lung cancer. Int Orthop. 2011;35(5):731–736. doi: 10.1007/s00264-010-1074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung JM, Chung CK, Kim CH, Yang SH. Minimally invasive surgery without decompression for hepatocellular carcinoma spinal metastasis with epidural spinal cord compression grade 2. J Korean Neurosurg Soc. 2019;62(4):467–475. doi: 10.3340/jkns.2018.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bae J, Lee SH. Minimally invasive spinal surgery for adult spinal deformity. Neurospine. 2018;15(1):18–24. doi: 10.14245/ns.1836022.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wui SH, Hyun SJ, Kang B, Kim KJ, Jahng TA, Kim HJ. Bicortical screw purchase at upper instrumented vertebra (UIV) can cause UIV fracture after adult spinal deformity surgery: a finite element analysis study. Neurospine. 2020;17(2):377–383. doi: 10.14245/ns.1938100.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohrt-Nissen S, Dahl B, Gehrchen M. Choice of rods in surgical treatment of adolescent idiopathic scoliosis: what are the clinical implications of biomechanical properties? - a review of the literature. Neurospine. 2018;15(2):123–130. doi: 10.14245/ns.1836050.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartels RH, van der Linden YM, van der Graaf WT. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58(4):245–259. doi: 10.3322/CA.2007.0016. [DOI] [PubMed] [Google Scholar]
- 8.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 9.Dalbayrak S, Onen MR, Yilmaz M, Naderi S. Clinical and radiographic results of balloon kyphoplasty for treatment of vertebral body metastases and multiple myelomas. J Clin Neurosci. 2010;17(2):219–224. doi: 10.1016/j.jocn.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Weigel B, Maghsudi M, Neumann C, Kretschmer R, Müller FJ, Nerlich M. Surgical management of symptomatic spinal metastases. Postoperative outcome and quality of life. Spine (Phila Pa 1976) 1999;24(21):2240–2246. doi: 10.1097/00007632-199911010-00012. [DOI] [PubMed] [Google Scholar]
- 11.Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. J Orthop Sci. 2006;11(1):3–12. doi: 10.1007/s00776-005-0964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laufer I, Hanover A, Lis E, Yamada Y, Bilsky M. Repeat decompression surgery for recurrent spinal metastases. J Neurosurg Spine. 2010;13(1):109–115. doi: 10.3171/2010.3.SPINE08670. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K, Homma T, Hirano T, Ogose A, Hotta T, Yajiri Y, et al. Margin-free spondylectomy for extended malignant spine tumors: surgical technique and outcome of 13 cases. Spine (Phila Pa 1976) 2007;32(1):142–148. doi: 10.1097/01.brs.0000251045.79708.7a. [DOI] [PubMed] [Google Scholar]
- 14.Demura S, Kawahara N, Murakami H, Abdel-Wanis ME, Kato S, Yoshioka K, et al. Total en bloc spondylectomy for spinal metastases in thyroid carcinoma. J Neurosurg Spine. 2011;14(2):172–176. doi: 10.3171/2010.9.SPINE09878. [DOI] [PubMed] [Google Scholar]
- 15.Cloyd JM, Acosta FL, Jr, Polley MY, Ames CP. En bloc resection for primary and metastatic tumors of the spine: a systematic review of the literature. Neurosurgery. 2010;67(2):435–444. doi: 10.1227/01.NEU.0000371987.85090.FF. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 17.Park JH, Hyun SJ, Kim KJ, Jahng TA. Total en bloc thoracic and lumbar spondylectomy for non-small cell lung cancer with favorable prognostic indicators: is it merely indicated for solitary spinal metastasis? J Korean Neurosurg Soc. 2014;56(5):431–435. doi: 10.3340/jkns.2014.56.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ibrahim A, Crockard A, Antonietti P, Boriani S, Bünger C, Gasbarrini A, et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8(3):271–278. doi: 10.3171/SPI/2008/8/3/271. [DOI] [PubMed] [Google Scholar]
- 19.Hyun SJ, Kim YJ, Cheh G, Yoon SH, Rhim SC. Free hand pedicle screw placement in the thoracic spine without any radiographic guidance : technical note, a cadaveric study. J Korean Neurosurg Soc. 2012;51(1):66–70. doi: 10.3340/jkns.2012.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi HY, Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Freehand S2 alar-iliac screw placement using K-wire and cannulated screw : technical case series. J Korean Neurosurg Soc. 2018;61(1):75–80. doi: 10.3340/jkns.2016.1212.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 22.Dittmer DK, Teasell R. Complications of immobilization and bed rest. Part 1: Musculoskeletal and cardiovascular complications. Can Fam Physician. 1993;39:1428–1432. 1435–1427. [PMC free article] [PubMed] [Google Scholar]
- 23.Teasell R, Dittmer DK. Complications of immobilization and bed rest. Part 2: other complications. Can Fam Physician. 1993;39:1440–1442. 1445–1446. [PMC free article] [PubMed] [Google Scholar]
- 24.Park SJ, Lee CS, Chung SS. Surgical results of metastatic spinal cord compression (MSCC) from non-small cell lung cancer (NSCLC): analysis of functional outcome, survival time, and complication. Spine J. 2016;16(3):322–328. doi: 10.1016/j.spinee.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Sundaresan N, Rothman A, Manhart K, Kelliher K. Surgery for solitary metastases of the spine: rationale and results of treatment. Spine (Phila Pa 1976) 2002;27(16):1802–1806. doi: 10.1097/00007632-200208150-00021. [DOI] [PubMed] [Google Scholar]
- 26.Arrigo RT, Kalanithi P, Cheng I, Alamin T, Carragee EJ, Mindea SA, et al. Charlson score is a robust predictor of 30-day complications following spinal metastasis surgery. Spine (Phila Pa 1976) 2011;36(19):E1274–80. doi: 10.1097/BRS.0b013e318206cda3. [DOI] [PubMed] [Google Scholar]
- 27.Arrigo RT, Kalanithi P, Cheng I, Alamin T, Carragee EJ, Mindea SA, et al. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery. 2011;68(3):674–681. doi: 10.1227/NEU.0b013e318207780c. [DOI] [PubMed] [Google Scholar]
- 28.Birim O, Kappetein AP, Bogers AJ. Charlson comorbidity index as a predictor of long-term outcome after surgery for nonsmall cell lung cancer. Eur J Cardiothorac Surg. 2005;28(5):759–762. doi: 10.1016/j.ejcts.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 29.Shack LG, Rachet B, Williams EM, Northover JM, Coleman MP. Does the timing of comorbidity affect colorectal cancer survival? A population based study. Postgrad Med J. 2010;86(1012):73–78. doi: 10.1136/pgmj.2009.084566. [DOI] [PubMed] [Google Scholar]
- 30.Froehner M, Koch R, Litz RJ, Oehlschlaeger S, Twelker L, Hakenberg OW, et al. Detailed analysis of Charlson comorbidity score as predictor of mortality after radical prostatectomy. Urology. 2008;72(6):1252–1257. doi: 10.1016/j.urology.2008.05.037. [DOI] [PubMed] [Google Scholar]
- 31.Ribatti D, Mangialardi G, Vacca A. Stephen Paget and the ‘seed and soil’ theory of metastatic dissemination. Clin Exp Med. 2006;6(4):145–149. doi: 10.1007/s10238-006-0117-4. [DOI] [PubMed] [Google Scholar]
- 32.Virk MS, Lieberman JR. Tumor metastasis to bone. Arthritis Res Ther. 2007;9(Suppl 1):S5. doi: 10.1186/ar2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YJ, Chang GC, Chen HT, Yang TY, Kuo BI, Hsu HC, et al. Surgical results of metastatic spinal cord compression secondary to non-small cell lung cancer. Spine (Phila Pa 1976) 2007;32(15):E413–8. doi: 10.1097/BRS.0b013e318074d6c7. [DOI] [PubMed] [Google Scholar]
- 34.Murakami H, Kawahara N, Demura S, Kato S, Yoshioka K, Tomita K. Total en bloc spondylectomy for lung cancer metastasis to the spine. J Neurosurg Spine. 2010;13(4):414–417. doi: 10.3171/2010.4.SPINE09365. [DOI] [PubMed] [Google Scholar]