Abstract
Niches are specialized tissue microenvironments that control stem cells functioning. The bone marrow mesenchymal stem cell niche defines a location within the marrow in which mesenchymal stem cells are retained and produce new cells throughout life. Deciphering the signaling mechanisms by which the niche regulates stem cell fate will facilitate the use of these cells for therapy. Recent studies, by using state‐of‐the‐art methodologies, including sophisticated in vivo inducible genetic techniques, such as lineage‐tracing Cre/loxP mediated systems, in combination with pharmacological inhibition, provide evidence that sensory neuron is an important component of the bone marrow mesenchymal stem cell niche. Strikingly, knockout of a specific receptor in sensory neurons blocked stem cell function in the bone marrow. The knowledge arising from these discoveries will be crucial for stem cell manipulation in the future. Here, we review recent progress in our understanding of sensory nerves biology in the stem cell niche.
Keywords: genetic depletion, mesenchymal stem cells, microenvironment, niche, sensory nerves
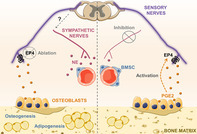
Schematic illustration summarizing the findings from genetic deletion of EP4 specifically from sensory nerve fibers in the bone marrow mesenchymal stem cell niche.
Significance statement.
The understanding of the neural regulation in the stem cell niche in the bone marrow and in other organs still remains limited, and the complexity of these interactions in different microenvironments should be elucidated. This article reviews recent progress in the understanding of sensory nerves biology in stem cell niches. Recent studies provide evidence that sensory neurons are important components of the mesenchymal stem cell niche. The emerging knowledge from this research will be important for the treatment of several disorders.
1. INTRODUCTION
1.1. Bone marrow mesenchymal stem cells (BMSCs)
Adult endogenous stem cells are fundamental for maintaining tissue homeostasis due to their extraordinary capacity to form specialized cell populations in a coordinated way according to the needs of the organism. 1 Mesenchymal stem cells were first discovered within the bone marrow. 2 Subsequent studies have identified mesenchymal stem cells in various other adult tissues. 3 BMSCs are characterized as postnatal self‐renewing multipotent stem cells, forming all skeletal tissues. 2 , 4 In culture, these cells can form a clonal progeny of transplantable cells, equal to the one that generated them. 5 After transplanted in vivo, BMSCs can form bone organoids. 6 A single BMSC is a bona fide stem cell as it can initiate a clonal population in vitro, which then may create a full organoid in vivo with transplantable BMSCs, being self‐renewing and multipotent. 2 , 7 BMSCs are also defined as skeletal stem cells as they can be located within the skeleton, able to give rise to various skeletal tissues, and have an innate capacity to start a recapitulation of bone organogenesis in vivo. 8 , 9 , 10 , 11 BMSCs are essential for the development, lifelong turnover, and regenerative ability of bones in our organism. 12 , 13 The ability of mesenchymal stem cells derived from variable sources to repair tissues placed them in the center of attention of numerous groups due to their promising potential in regenerative medicine for multiple disorders. 14 , 15 Therefore, in the last two decades, it became clear that understanding the biology of these cells may lead to the treatment of several diseases.
1.2. Stem cells and their niches
Accurate regulation over stem cell differentiation is crucial for appropriate tissue homeostasis and organogenesis. 16 Stem cells occupy particular microenvironments, also termed niches, 17 which keep them in an undifferentiated and self‐renewing state. Defining and understanding the mechanisms that restrict niche signaling exclusively to stem cells is crucial to determine how stem cells undergo self‐renewal while their progeny differentiate. Extensive studies in a variety of tissues have highlighted the importance of the microenvironment in modulating stem cell behavior, including skin, 18 intestine, 19 stomach, 20 skeletal muscle, 21 bone marrow, 22 liver, 23 brain, 24 , 25 and others. 26 , 27 Despite significant progress made in our knowledge of which signals foster stem cell quiescence or activation, some constituents of stem cells niches remain unrevealed to date. This is due to the complexity of tissue microenvironment content and its dynamics. Understanding the role of niche components in stem cell behavior is vital for our knowledge of organ homeostasis and disease, and to fully exploit stem cell therapeutic potential.
1.3. BMSC niche
Although the precise location of the BMSC niche has not been determined so far, several studies suggest that MSCs reside in perivascular sites, associated with blood vessels. 28 Therefore, MSCs have been compared to pericytes. 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 Nevertheless, whether these two cell types correspond to the same cell is not clear yet. 11 , 46
BMSCs maintenance within the adult bone is essential for skeletal homeostasis and reconstruction after damage. 47 BMSCs are often in a quiescent state, and extrinsic factors from their medullar niche can activate their self‐renewal, proliferation, or differentiation. Examination of the BMSC microenvironment in the bone marrow revealed that BMSC behavior is markedly affected by interactions with cellular components of this local niche, both directly, by physical contact, or indirectly, by ligation of secreted soluble molecules. 48 Multiple local signaling cues can influence BMSCs fate, such as interleukins, 49 , 50 chemokines, 51 Wnt ligands, 52 , 53 FGF2, 54 and others. 48 , 55 Oxygen also may be a key regulatory factor in the BMSC niche. 56 Perturbations in the architecture of extracellular matrix constituents in the bone marrow influence BMSC behavior. 57 , 58 , 59 , 60 , 61 Moreover, mechanical stimuli from the microenvironment can direct BMSC lineage specification. 57 , 58 , 62 , 63 , 64 Several groups are trying to identify crucial components of the BMSC niche, and how these key components regulate BMSC behavior and fate. The BMSC niche composition is complex and heterogeneous. Besides BMSCs which can regulate themselves, 49 , 65 an array of distinct cell types support and communicate with BMSCs. These include endothelial cells, 59 , 60 , 66 , 67 osteoblasts, 68 osteocytes, 50 , 69 chondrocytes, 70 hematopoietic progenitors, 51 fibroblasts, 71 immune cells, 72 , 73 and others. 74 , 75 As innervations are also present within the bone marrow, they became strong candidates for a role in the BMSC niche. 76 , 77 , 78 , 79
1.4. Nerves in the bone marrow
Nerves penetrating the bone marrow were described for the first time more than 50 years ago. 80 As the bone marrow occupies spaces deep within our body and is encased by an outer hard compact bone, experimental assessments were initially difficult. Later studies confirmed these initial discoveries demonstrating that medullar innervations can signal and communicate with cells in the bone marrow. 81 Innervations in the bone marrow are mostly associated with blood vessels. 80 , 82 The pattern of similar wiring of nerves and blood vessels is well established in other organs, and suggests that they support each other. 83 Accordingly, it is possible that vascular and neuronal networks also have a functional connection within the bone marrow. Because of the perivascular location of BMSCs, the interaction of BMSCs with nerve projections is also likely.
So far, the sympathetic nerves were the most explored nerve fibers in the bone marrow. Innervations expressing tyrosine‐hydroxylase 79 and Neuropeptide Y, 84 an abundant sympathetic co‐transmitter, were detected. Examination of the bone marrow from Neuropeptide Y knockout mouse model revealed a decrease in the number of BMSCs, indicating that Neuropeptide Y signaling may be important for BMSC maintenance. 85 Genetic or pharmacological depletion of sympathetic signaling in the bone marrow triggers the expansion of BMSCs with reduced CFU‐F capacity, also impairing BMSCs' differentiation capacity. 7 , 77 The loss of sympathetic nerves also results in reduction of BMSC ability for hematopoietic stem cell maintenance. 7 , 76 , 77 , 86 Moreover, sympathetic denervation culminates in BMSCs mobilization from their niche to bone‐forming sites. 87 Together, these findings identify that sympathetic innervations can regulate BMSC behavior in the bone marrow, and suggest that BMSCs' maintenance, proliferation, and differentiation are controlled by these nerve fibers.
1.5. Sensory nerves in the bone marrow
Sensory neuronal projections also penetrate the bone marrow microenvironment as abundant single or bundled fibers usually coupled with medullar blood vessels. 79 , 88 These fibers express calcitonin gene‐related peptide, substance P, and/or vasoactive intestinal peptide. 84 , 88 , 89 , 90 , 91 In contrast to sympathetic innervations in the bone marrow, 77 sensory nerves do not diminish with aging. 79 Although the sensory nerves innervate the bone marrow and are located in close proximity to BMSCs, little is known about their role in the bone marrow microenvironment.
Dissecting the complex pathways leading to stem cells activation may be very challenging. Understanding whether sensory nerves are part of the BMSC niche, the signaling mechanisms by which they control the mesenchymal stem cell fate may be crucial for the success of clinical applications. In a recent article in The Journal of Clinical Investigation, Hu and colleagues demonstrated that sensory nerves regulate adult mesenchymal stem cells behavior in vivo via control over sympathetic nerves activity. 92 Using state‐of‐the‐art techniques including sophisticated in vivo inducible genetic approaches, such as lineage‐tracing Cre/loxP mediated technologies, in combination with pharmacological approaches, microtomography, and confocal microscopy, the authors selectively eliminated sensory neurons to analyze their role in bone homeostasis. The authors specifically ablated sensory innervations genetically, by using Advillin‐Cre/TrkA‐floxed and Advillin‐Cre/iDTR mice, and pharmacologically, by capsaicin treatment. These experiments revealed that the absence of sensory neuronal projections promotes fat formation at the expense of osteogenesis in the adult bone marrow. 92 Importantly, the number of BMSCs diminished in vivo, as well as their ability to differentiate into osteoblasts in vitro, 92 suggesting that sensory innervations are essential for BMSCs maintenance in their niche.
It is well established that prostaglandins play essential roles in bone metabolism. 93 Amid prostaglandins, prostaglandin E2 (PGE2) induces bone formation. 94 Strikingly, genetic deletion of prostaglandin E2 receptor (EP4) specifically from sensory nerve fibers, by using Advillin‐Cre/EP4‐floxed mice, inhibited osteogenesis and induced adipogenesis. 92 Moreover, similarly to what was observed after sensory nerves depletion, BMSCs number declined in the bone marrow, along with their capacity to self‐renew and to differentiate into the osteogenic lineage 92 (Figure 1). Genetic ablation of the prostaglandin receptor from other cells present in the bone marrow microenvironment (osteoblasts or BMSCs) did not present alterations in the bone or in BMSC behavior. 92 Overall, these findings imply that EP4 receptor in sensory nerves is crucial for BMSC behavior control in the bone marrow.
FIGURE 1.

Schematic illustration summarizing the findings from genetic deletion of EP4 specifically from sensory nerve fibers in the bone marrow mesenchymal stem cell (BMSC) niche. Sensory fibers innervate the bone marrow BMSC niche. (Right) Sensory nerves maintain MSC number in the bone marrow, which differentiate normally into osteoblasts. (Left) In contrast, genetic ablation of EP4 from sensory innervations reduces the number of BMSCs, likewise their capacity to self‐renew and to differentiate into the osteogenic lineage. This leads to inhibited osteogenesis and induced adipogenesis 92
Hu and colleagues also showed, by using Ocn‐Cre/COX2‐floxed mice and pharmacological inhibition, that osteoblasts are the source of PGE2 which acts on sensory nerves that consequently affect BMSC behavior. 92 Remarkably, Hu and colleagues demonstrated that mechanical loading induces osteoblasts to produce PGE2, linking a physiological state to stem cell regulation. Furthermore, the authors demonstrated that blockage of β2 adrenergic signaling abolishes bone and BMSC changes visualized in Advillin‐Cre/EP4‐floxed mice. These data suggest that sensory innervations affect BMSCs by deactivating sympathetic signaling in the bone marrow. Additionally, as BMSCs are pivotal in bone regeneration, Hu and colleagues examined a mouse model of femur fracture in which EP4 was ablated from sensory nerves. This examination unveiled that EP4 in sensory nerve fibers is vital for osteoinduction of BMSCs for bone fracture healing. Taken together, these results indicate that sensory nerves are fundamental components of the BMSC niche in the bone marrow which regulate BMSC functions. This study reveals a new component of the BMSC niche: sensory nerves; linking physiological states to BMSCs activation. Here, we discuss the findings from this work, and evaluate recent advances in our understanding of the bone marrow microenvironment and sensory nerves biology.
2. PERSPECTIVES/FUTURE DIRECTIONS
2.1. Specificity of transgenic mouse models
Hu and colleagues explored tissue‐specific null mutant mouse models (Advillin‐Cre/EP4‐floxed, Ocn‐Cre/COX2‐floxed, Ocn‐Cre/EP4‐floxed, and Lepr‐Cre/EP4‐floxed mice). 92 Some caveats need to be given due consideration when using such systems, including insufficient gene knockdown and compensatory pathway upregulation. Thus, the analysis of the expression level of the gene being deleted as well as of other genes that may be affected will clarify this point. Moreover, gene ablation that occurs in the germline can culminate in developmental compensatory mechanisms. 95 , 96 , 97 Models with inducible time and tissue‐specific gene ablation would overcome potential physiological compensatory processes that can alter the true function of a specific gene. 98 Such strategies are being used to study the role of individual proteins in distinct pathophysiologic conditions. 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 Thus, this may be addressed by analyzing bone marrow from Advillin‐CreER/EP4‐floxed mice in which EP4 deletion in sensory nerve fibers can be temporally controlled.
The main findings from this work are based on the data obtained from Advillin‐Cre mice. 92 Note, however, that expression of advillin is not restricted to peripheral sensory neurons that innervate the bone marrow. Thus, in Advillin‐Cre/EP4‐floxed mice, EP4 is also eliminated from sensory nerves in several other tissues, besides the bone. Therefore, it remains to be explored whether BMSC phenotype in this mouse model is due to EP4 deletion that happens specifically in the bone. Moreover, it was recently discovered that advillin is also expressed in the peripheral neuronal projections innervating the vasculature coming from sympathetic, parasympathetic, and enteric neurons. 107 Interestingly, even some non‐neuronal cells may be targeted in these mice, that is, Merkel 107 , 108 and Tuft 109 cells. Hence, it is possible that some of the effects observed in BMSCs in Advillin‐Cre/EP4‐floxed mice are not due to sensory neurons exclusively. To clarify whether EP4 is eliminated from any other medullar components, the bone marrow from Advillin‐Cre/EP4‐floxed/TdTomato mice should be examined in which all components from which EP4 is being deleted will be labeled. Furthermore, mouse models more specific to sensory nerves can be used for comparison, such as Nav1.8‐Cre mice.
2.2. Mechanism by which sensory nerves affect BMSCs
Cao's group data revealed that sensory nerves regulate BMSC behavior in the bone marrow. 92 Yet it remains uncertain whether this happens by an indirect mechanism via BMSC niche components or by directly acting on BMSCs. The authors suggest that this regulation is via sympathetic nervous system, based on pharmacological inhibition with β adrenergic antagonists. 92 As beta‐blockers may have off‐target side effects, 110 a direct evidence that sympathetic neurons are the main/only functionally important path for the effect of sensory nerves on BMSCs is still needed. To verify the role of sympathetic input on sensory nerves’ function within the BMSC niche, genetic sympathectomy may be performed in combination with sensory nerve ablation. This may be achieved by using TH‐Cre/iDTR mice, in which diphtheria toxin injection causes apoptosis of peripheral sympathetic nerves. 111
Interestingly, in another recent work from the same group, Chen and colleagues suggest that sensory nerves' effect on bone formation is through the hypothalamus. 112 The authors show that, in mice stimulated by PGE2, CREB is phosphorylated in the hypothalamus, and this is inhibited by knockout of EP4 receptors in sensory nerves. Albeit CREB phosphorylation in the hypothalamus may affect sympathetic nerves, 113 no direct evidence that sensory nerves act on MSCs via hypothalamus is available yet. Therefore, it remains an exciting open question whether/how sensory nerves affect sympathetic nerves function in the BMSC niche. In addition to experiments in transgenic mouse models, single cell transcriptomic analysis will help us understand the central and peripheral nervous system involvement on the role of sensory nerves within the BMSC niche.
Importantly, sensory nerves may be stimulated to release a variety of neuropeptides, such as calcitonin gene related peptide, vasoactive intestinal peptide, tachykinins (Substance P, neurokinin A, neurokinin B), and others. 114 , 115 , 116 , 117 Whether sensory nerves act directly on bone marrow BMSCs through any of these mediators remains unknown, and should be addressed in future studies.
2.3. Sensory nerves role in other stem cell niches
After Schofield Raymond proposed the notion of stem cell niche for hematopoietic stem cells, 17 this concept was adopted for several other stem cells. Stem cell specialized microenvironments have been described for BMSCs, 118 neural stem cells, 119 , 120 satellite cells, 21 hair follicles stem cells, 121 intestinal stem cells, 122 gonadal stem cells, 123 liver stem cells, 23 and others. Cao's group indicates sensory nerves for the first‐time as a component of a stem cell niche. 92 As bone marrow BMSCs, key components of hematopoietic stem cell niche, are affected by sensory nerves, hematopoietic stem cells will be as well. Nonetheless, it will be important to examine whether sensory nerves can regulate hematopoietic stem cells also independently from their effect on BMSCs. As sensory nerves innervate multiple organs, 124 future studies should explore the role of these nerves in stem cell niches of other organs.
One such organ is the skin, largely innervated by sensory nerve fibers. 125 Interestingly, a recent study showed that hyperactivation of the sympathetic nervous system leads to reduced number of melanocyte stem cells in their dermal niche 126 (Figure 2). These stem cells reside in the hair follicle microenvironment. 127 Zhang and colleagues used Cre/loxP mediated technologies in combination with chemogenetics to evaluate stress effect on melanocyte stem cells in the hair follicle niche. These experiments revealed that hyperactivation of sympathetic nerves leads to melanocyte stem cells activation, proliferation, and consequently elimination from their niche. 126 Interestingly, resinoferatoxin, which causes sensory nerve denervation as well, 128 was used to induce stress. This suggests that possibly sensory nerves also may have a role in the hair follicle melanocyte stem cell niche.
FIGURE 2.
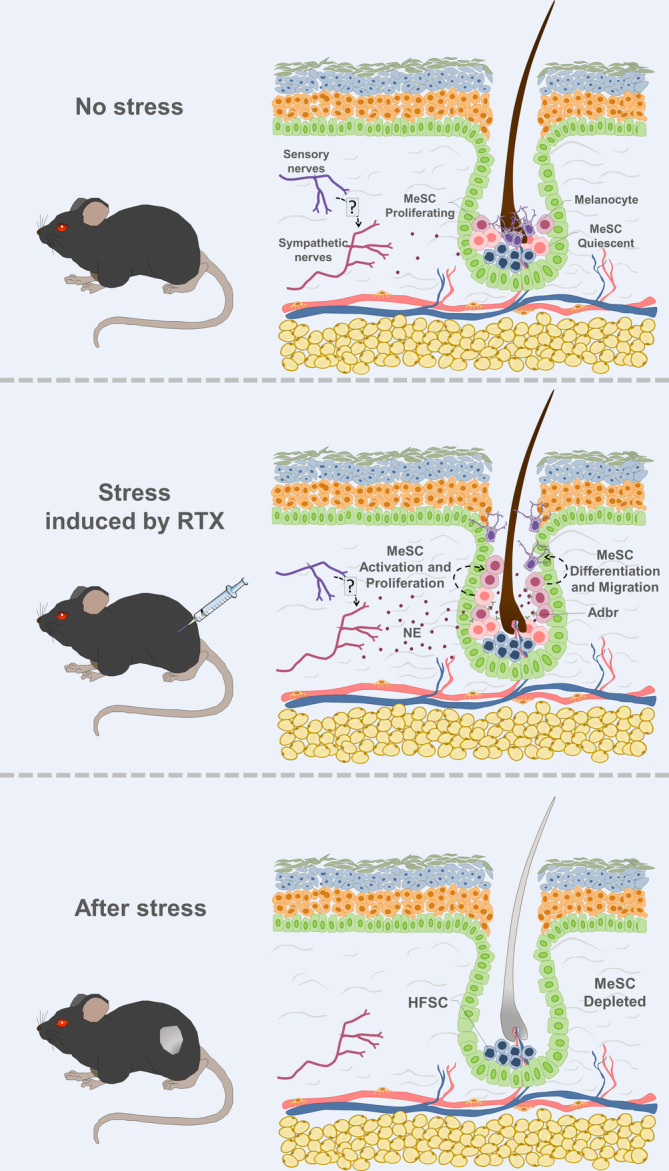
Sensory nerves role in the hair follicle melanocyte stem cell (MeSC) niche. Two main stem cell populations are present in the hair follicle bulge: MeSCs and hair follicle stem cells (HFSCs). MeSCs generate new pigmented hair. Zhang and colleagues revealed that hyperactivation of sympathetic nerves leads to MeSCs activation, proliferation, and consequently elimination from their niche, leading to hair discoloration. 126 Interestingly, resinoferatoxin (RTX), which causes sensory nerve denervation as well, was used to induce stress. Adrb2, β2‐adrenergic receptor; NE, norepinephrine
Curiously, a recent study using elegant techniques including sophisticated lung transplants in combination with in vivo lineage‐tracing technologies, identified surprisingly a niche for hematopoietic stem cells in the lungs. 129 , 130 As these organs are densely innervated by sensory nerves, 131 the role of sensory innervations in the pulmonary hematopoietic stem cell niche should be explored.
Interestingly, Cao's group showed that mice with sensory nerves genetic depletion present also impaired hepatic regeneration after hepatectomy, suggesting that sensory nerves compose the liver stem cell niche as well. 112 Notably, the mechanism by which sensory nerves are activated in the liver is different from what happens in the bone marrow, as deletion of EP4 receptor in sensory nerves did not alter hepatic regeneration. 112 During fetal development, hematopoietic stem cells are located in the liver from where they later migrate through the bloodstream to the bone marrow preceding birth, where they remain throughout the adult life. 132 , 133 , 134 , 135 Their expansion in the fetal liver is dependent of portal vessel‐associated hepatic mesenchymal stem cells. 132 It will be interesting to explore whether sensory nerves also regulate hepatic mesenchymal stem cells in the fetal liver, and consequently hematopoietic stem cells.
2.4. Sensory nerve involvement in BMSCs' other functions
The most well‐established functions of BMSCs, based on which they were named, are related to their capacity to differentiate in multiple cell types, replacing damaged cells. 136 Notably, in addition to their regenerative activities, BMSCs have also been described to present immunomodulatory, immunosuppressive, and anti‐inflammatory characteristics. 137 , 138 , 139 These capabilities are the basis for the medical exploration by numerous clinical trials of BMSCs in the therapy for inflammatory and immune disorders. 140 , 141 , 142 BMSCs can affect the behavior of various immune cells, such as T cells, macrophages, and others. 143 , 144 , 145 These interactions may grant homeostasis within the tissue. 146 , 147
In the last decade, several groups have focused their attention on exploring the immune‐modulating capacity of the peripheral nervous system. Recent studies have shown that sensory neurons are critical mediators of inflammatory processes in diverse tissues. 128 , 148 , 149 Nevertheless, what are the cellular and molecular mechanisms by which sensory nerves regulate immune responses remain uncertain. The regulation of BMSCs behavior by sensory innervations brings the question whether stem cells are involved in immune regulatory effects of sensory neurons. Also, it will be important to investigate which cues derived from sensory nerves may affect BMSCs and how.
2.5. Sensory nerves heterogeneity
Hu and colleagues assume sensory nerves to be a homogeneous population in their study. 92 However, sensory nerves have been shown to be heterogeneous, comprising various subtypes with distinct functions. 150 Sensory neurons even within the same ganglia may differ in their embryonic origins. 151
Classically, different sensory neurons have been divided based on specific molecular markers and cell‐body diameters. For instance, sensory neurons expressing neurotrophic receptor tyrosine kinase 2 and neurotrophic receptor tyrosine kinase 3 with large cell‐body diameter, sensory neurons expressing calcitonin gene‐related peptide with medium‐sized cell‐body diameter, and sensory neurons expressing purinergic receptor P2X ligand gated ion channel 3 with small cell‐body diameter. 152 More recent elegant studies categorized them, by single‐sensory neuron RNA sequencing, into at least 11 distinct neuronal subtypes based on their transcriptomic patterns. 153 , 154 Due to the crucial role played by sensory nerves discovered by Hu et al, the question arises as to whether the sensory nerves subpopulations differ in their capacity to regulate BMSC niche.
Importantly, BMSCs are also heterogeneous in their morphology, distribution, anatomical location, origin, molecular markers, and function. 155 , 156 , 157 , 158 , 159 At least two subpopulations have been described in the bone marrow. 157 Thus, whether only a fraction of BMSCs respond to sensory nerves in response to mechanical loading still needs to be elucidated. It would be important to evaluate whether distinct BMSCs' subsets behave differently during sensory nerves activation.
2.6. Sensory nerves in the cancer microenvironment
Nerves have been reported to promote tumor growth and spread. 160 , 161 , 162 Classically, sensory nerves have been associated with tumor‐associated pain. 163 , 164 Interestingly, a new study shows that sensory nerve fibers are involved in tumor progression in vivo. 124 The authors show, by using Nav1.8‐Cre/TdTomato mice, the presence of sensory nerves within the tumor microenvironment. Prazeres and colleagues specifically depleted sensory innervations within the tumor microenvironment, in a model of genetic depletion of Nav1.8+ sensory neuronal projections or by chemical depletion using resiniferatoxin. These experiments revealed that sensory nerve ablation induces changes that lead to worse outcomes in tumor‐bearing mice. 124 Moreover, low expression of genes related to sensory nerves correlate with worse outcomes in human melanoma biopsies. 124 Taken together, these findings suggest that sensory innervations participate in cancer progression, by inhibiting tumor growth. However, the cellular and molecular mechanisms by which sensory nerves influence cancer development remain to be studied.
Lately, roles in cancer progression have been assigned to tumor‐associated MSCs, which arise as an important component of the tumor microenvironment. 165 MSCs within the tumors contribute to tumor‐associated immunosuppression, inflammation, angiogenesis, tumor growth, metastasis, and therapeutic resistance in various cancer types. 166 , 167 Understanding whether/how tumoral sensory nerves‐derived cues influence MSCs will facilitate our profound comprehension of the functions of sensory nerves and MSCs within the tumor microenvironment. This may lead to the discovery of new cancer therapies that target sensory nerves and MSCs. Not less important will be the exploration of sensory nerves' roles in cancer initiation by cancer stem cells, 168 or on cancer reactivation by dormant disseminated tumor cells 169 (Figure 3).
FIGURE 3.
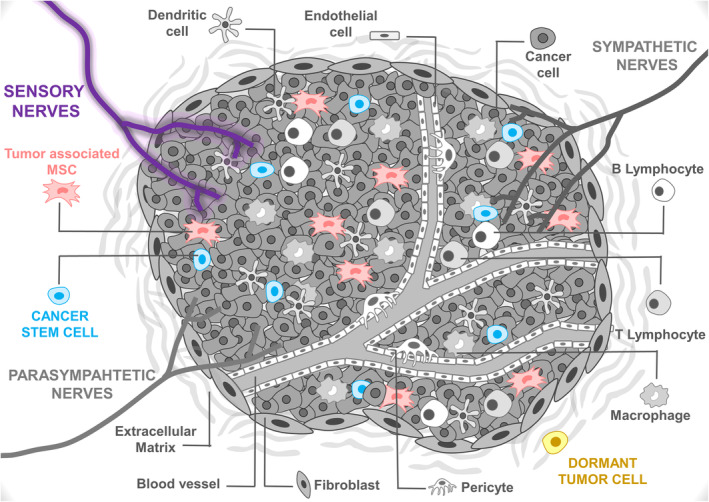
Sensory nerves' possible roles in the tumor microenvironment. Sensory neuronal projections infiltrate the tumor microenvironment. The study of Prazeres and colleagues indicates that sensory nerves block cancer progression. 124 Cellular and molecular mechanisms by which sensory nerves influence cancer development remain uncertain. Future works should examine whether sensory nerves regulate tumor‐associated mesenchymal stem cells (MSCs), cancer stem cells, or/and dormant tumor cells
3. CONCLUSION
In conclusion, the study by Hu and colleagues reveals a novel important role in BMSCs regulation of sensory neurons which innervate the bone marrow. 92 However, our understanding of the neural regulation in the stem cell niche in the bone marrow and other organs remains limited, and the complexity of these interactions in different microenvironments should be elucidated in future studies. A big challenge for the future will be to translate these findings to human patients. Whether sensory nerves are essential components in the human bone marrow stem cell niche remains to be determined. Improving the availability of human bone marrow samples will be crucial to reach this goal. Future developments in this research are promising.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors wrote and commented on the manuscript.
ACKNOWLEDGMENTS
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra‐1708‐15285, a grant from Pró‐reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016), a grant from CNPQ (Universal, Process No. 405977/2018‐2), a grant from National Institute of Science and Technology in Theranostics and Nanobiotechnology (CNPq/CAPES/FAPEMIG, Process No. 465669/2014‐0), a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED‐00570‐16)], a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED‐00313‐16)], and a productivity fellowship from the National Council for Scientific and Technological Development (CNPq); Akiva Mintz is supported by the National Institute of Health (1R01CA179072‐01A1) and by the American Cancer Society Mentored Research Scholar grant (124443‐MRSG‐13‐121‐01‐CDD). Caroline C. Picoli, Alinne C. Costa, and Pedro H. D. M. Prazeres are supported by doctoral fellowships from CAPES. Pedro A. C. Costa is supported by a postdoctoral fellowship (PNPD) from CAPES. Gabryella S. P. Santos is supported by a doctoral fellowship from CNPq. Walison N. Silva and Beatriz G. S. Rocha are supported by master fellowships from CAPES.
Picoli CC, Costa AC, Rocha BGS, et al. Sensory nerves in the spotlight of the stem cell niche. STEM CELLS Transl Med. 2021;10:346–356. 10.1002/sctm.20-0284
Caroline C. Picoli and Alinne C. Costa are co‐first authors.
Funding information American Cancer Society Mentored Research Scholar, Grant/Award Number: 124443‐MRSG‐13‐121‐01‐CDD; National Institute of Health, Grant/Award Number: 1R01CA179072‐01A1; National Council for Scientific and Technological Development; FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED‐00313‐16)]; FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED‐00570‐16)]; National Institute of Science and Technology in Theranostics and Nanobiotechnology; CNPQ; Pró‐reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); Instituto Serrapilheira/Serra‐1708‐15285
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106(4):619‐633. [DOI] [PubMed] [Google Scholar]
- 2. Sacchetti B, Funari A, Michienzi S, et al. Self‐renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324‐336. [DOI] [PubMed] [Google Scholar]
- 3. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301‐313. [DOI] [PubMed] [Google Scholar]
- 4. Ylostalo J, Bazhanov N, Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single‐cell‐derived colonies. Exp Hematol. 2008;36(10):1390‐1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serafini M, Sacchetti B, Pievani A, et al. Establishment of bone marrow and hematopoietic niches in vivo by reversion of chondrocyte differentiation of human bone marrow stromal cells. Stem Cell Res. 2014;12(3):659‐672. [DOI] [PubMed] [Google Scholar]
- 7. Mendez‐Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bianco P, Kuznetsov SA, Riminucci M, et al. Postnatal skeletal stem cells. Methods Enzymol. 2006;419:117‐148. [DOI] [PubMed] [Google Scholar]
- 9. Kramann R, Schneider RK, DiRocco DP, et al. Perivascular Gli1+ progenitors are key contributors to injury‐induced organ fibrosis. Cell Stem Cell. 2015;16(1):51‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guimaraes‐Camboa N, Cattaneo P, Sun Y, et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell. 2017;20:345‐359.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Birbrair A, Borges IDT, Gilson Sena IF, et al. How plastic are pericytes? Stem Cells Dev. 2017;26(14):1013‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frenette PS, Pinho S, Lucas D, Scheiermann C. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping‐stone for regenerative medicine. Annu Rev Immunol. 2013;31:285‐316. [DOI] [PubMed] [Google Scholar]
- 13. Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370(1):82‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samsonraj RM, Raghunath M, Nurcombe V, Hui JH, van Wijnen AJ, Cool SM. Concise review: multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Translational Medicine. 2017;6(12):2173‐2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miranda VHS, Gomes TR, Eller DE, et al. Liver damage in schistosomiasis is reduced by adipose tissue‐derived stem cell therapy after praziquantel treatment. PLoS Negl Trop Dis. 2020;14(8):e0008635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287(5457):1427‐1430. [DOI] [PubMed] [Google Scholar]
- 17. Schofield R. The relationship between the spleen colony‐forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1‐2):7‐25. [PubMed] [Google Scholar]
- 18. Fuchs E. Finding one's niche in the skin. Cell Stem Cell. 2009;4(6):499‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tan DW, Barker N. Intestinal stem cells and their defining niche. Curr Top Dev Biol. 2014;107:77‐107. [DOI] [PubMed] [Google Scholar]
- 20. Bartfeld S, Koo BK. Adult gastric stem cells and their niches. Wiley Interdiscip Rev Dev Biol. 2017;6(2):1–12. [DOI] [PubMed] [Google Scholar]
- 21. Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche [in Eng]. Physiol Rev. 2013;93(1):23‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370(1):82‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haussinger D, Kordes C. Space of Disse: a stem cell niche in the liver. Biol Chem. 2019;401(1):81‐95. [DOI] [PubMed] [Google Scholar]
- 24. Azevedo PO, Lousado L, Paiva AE, et al. Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience. 2017;363:62‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andreotti JP, Prazeres P, Magno LAV, et al. Neurogenesis in the postnatal cerebellum after injury. Int J Dev Neurosci. 2018;67:33‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157(1):41‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lousado L, Prazeres P, Andreotti JP, et al. Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis. 2017;8(10):e3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Birbrair A, Zhang T, Wang ZM, et al. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 2014;6:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci. 2015;128(2):81‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Costa MA, Paiva AE, Andreotti JP, et al. Pericytes constrict blood vessels after myocardial ischemia. J Mol Cell Cardiol. 2018;116:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Birbrair A, Zhang T, Files DC, et al. Type‐1 pericytes accumulate after tissue injury and produce collagen in an organ‐dependent manner. Stem Cell Res Ther. 2014;5(6):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Birbrair A, Delbono O. Pericytes are essential for skeletal muscle formation. Stem Cell Rev. 2015;11(4):547‐548. [DOI] [PubMed] [Google Scholar]
- 33. Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Type‐1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol. 2013;305(11):C1098‐C1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Birbrair A, Zhang T, Wang ZM, et al. Role of pericytes in skeletal muscle regeneration and fat accumulation [in Eng]. Stem Cells Dev. 2013;22(16):2298‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Birbrair A, Zhang T, Wang ZM, et al. Type‐2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307(1):C25‐C38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prazeres P, Turquetti AOM, Azevedo PO, et al. Perivascular cell alphav integrins as a target to treat skeletal muscle fibrosis. Int J Biochem Cell Biol. 2018;99:109‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prazeres P, Almeida VM, Lousado L, et al. Macrophages generate pericytes in the developing brain. Cell Mol Neurobiol. 2018;38(4):777‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Birbrair A, Sattiraju A, Zhu D, et al. Novel peripherally derived neural‐like stem cells as therapeutic carriers for treating glioblastomas. Stem Cells Translational Medicine. 2017;6(2):471‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Birbrair A, Zhang T, Wang ZM, et al. Skeletal muscle pericyte subtypes differ in their differentiation potential [in Eng]. Stem Cell Res. 2013;10(1):67‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Birbrair A, Zhang T, Wang ZM, et al. Skeletal muscle neural progenitor cells exhibit properties of NG2‐glia [in Eng]. Exp Cell Res. 2013;319(1):45‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Guerra DAP, Paiva AE, Sena IFG, et al. Targeting glioblastoma‐derived pericytes improves chemotherapeutic outcome. Angiogenesis. 2018;21(4):667‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sena IFG, Paiva AE, Prazeres P, et al. Glioblastoma‐activated pericytes support tumor growth via immunosuppression. Cancer Med. 2018;7(4):1232‐1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Coatti GC, Frangini M, Valadares MC, et al. Pericytes extend survival of ALS SOD1 mice and induce the expression of antioxidant enzymes in the murine model and in IPSCs derived neuronal cells from an ALS patient. Stem Cell Rev Rep. 2017;13(5):686‐698. [DOI] [PubMed] [Google Scholar]
- 44. Valle IB, Schuch LF, da Silva JM, et al. Pericyte in oral squamous cell carcinoma: a systematic review. Head Neck Pathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Picoli CC, Coimbra‐Campos LMC, Guerra DAP, et al. Pericytes act as key players in spinal cord injury. Am J Pathol. 2019;189(7):1327‐1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernardes SS, Pinto MCX, Amorim JH, et al. Glioma pericytes promote angiogenesis by producing periostin. Cell Mol Neurobiol. 2020; 10.1007/s10571-020-00975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Knight MN, Hankenson KD. Mesenchymal stem cells in bone regeneration. Adv Wound Care (New Rochelle). 2013;2(6):306‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9(1):11‐21. [DOI] [PubMed] [Google Scholar]
- 49. Pricola KL, Kuhn NZ, Haleem‐Smith H, Song Y, Tuan RS. Interleukin‐6 maintains bone marrow‐derived mesenchymal stem cell stemness by an ERK1/2‐dependent mechanism. J Cell Biochem. 2009;108(3):577‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liao C, Zhang C, Jin L, Yang Y. IL‐17 alters the mesenchymal stem cell niche towards osteogenesis in cooperation with osteocytes. J Cell Physiol. 2020;235(5):4466‐4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Overstraeten‐Schlogel N, Beguin Y, Gothot A. Role of stromal‐derived factor‐1 in the hematopoietic‐supporting activity of human mesenchymal stem cells. Eur J Haematol. 2006;76(6):488‐493. [DOI] [PubMed] [Google Scholar]
- 52. Boland GM, Perkins G, Hall DJ, Tuan RS. Wnt 3a promotes proliferation and suppresses osteogenic differentiation of adult human mesenchymal stem cells. J Cell Biochem. 2004;93(6):1210‐1230. [DOI] [PubMed] [Google Scholar]
- 53. Baksh D, Tuan RS. Canonical and non‐canonical Wnts differentially affect the development potential of primary isolate of human bone marrow mesenchymal stem cells. J Cell Physiol. 2007;212(3):817‐826. [DOI] [PubMed] [Google Scholar]
- 54. Tsutsumi S, Shimazu A, Miyazaki K, et al. Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun. 2001;288(2):413‐419. [DOI] [PubMed] [Google Scholar]
- 55. de Alvarenga EC, Silva WN, Vasconcellos R, Paredes‐Gamero EJ, Mintz A, Birbrair A. Promyelocytic leukemia protein in mesenchymal stem cells is essential for leukemia progression. Ann Hematol. 2018;97(10):1749‐1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buravkova LB, Andreeva ER, Gogvadze V, et al. Mesenchymal stem cells and hypoxia: where are we? Mitochondrion. 2014;19(Pt A):105‐112. [DOI] [PubMed] [Google Scholar]
- 57. Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677‐689. [DOI] [PubMed] [Google Scholar]
- 58. Ward DF Jr, Salasznyk RM, Klees RF, et al. Mechanical strain enhances extracellular matrix‐induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular‐related kinase‐dependent pathway. Stem Cells Dev. 2007;16(3):467‐480. [DOI] [PubMed] [Google Scholar]
- 59. Lozito TP, Kuo CK, Taboas JM, Tuan RS. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107(4):714‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lozito TP, Taboas JM, Kuo CK, Tuan RS. Mesenchymal stem cell modification of endothelial matrix regulates their vascular differentiation. J Cell Biochem. 2009;107(4):706‐713. [DOI] [PubMed] [Google Scholar]
- 61. Klees RF, Salasznyk RM, Vandenberg S, Bennett K, Plopper GE. Laminin‐5 activates extracellular matrix production and osteogenic gene focusing in human mesenchymal stem cells. Matrix Biol. 2007;26(2):106‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143‐147. [DOI] [PubMed] [Google Scholar]
- 63. McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6(4):483‐495. [DOI] [PubMed] [Google Scholar]
- 64. Winer JP, Janmey PA, McCormick ME, et al. Bone marrow‐derived human mesenchymal stem cells become quiescent on soft substrates but remain responsive to chemical or mechanical stimuli. Tissue Eng Part A. 2009;15(1):147‐154. [DOI] [PubMed] [Google Scholar]
- 65. Zaragosi LE, Ailhaud G, Dani C. Autocrine fibroblast growth factor 2 signaling is critical for self‐renewal of human multipotent adipose‐derived stem cells. Stem Cells. 2006;24(11):2412‐2419. [DOI] [PubMed] [Google Scholar]
- 66. Tao J, Sun Y, Wang QG, Liu CW. Induced endothelial cells enhance osteogenesis and vascularization of mesenchymal stem cells. Cells Tissues Organs. 2009;190(4):185‐193. [DOI] [PubMed] [Google Scholar]
- 67. Paiva AE, Lousado L, Almeida VM, et al. Endothelial cells as precursors for osteoblasts in the metastatic prostate cancer bone. Neoplasia. 2017;19(11):928‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Csaki C, Matis U, Mobasheri A, Shakibaei M. Co‐culture of canine mesenchymal stem cells with primary bone‐derived osteoblasts promotes osteogenic differentiation. Histochem Cell Biol. 2009;131(2):251‐266. [DOI] [PubMed] [Google Scholar]
- 69. Heino TJ, Hentunen TA, Vaananen HK. Conditioned medium from osteocytes stimulates the proliferation of bone marrow mesenchymal stem cells and their differentiation into osteoblasts. Exp Cell Res. 2004;294(2):458‐468. [DOI] [PubMed] [Google Scholar]
- 70. Gerstenfeld LC, Barnes GL, Shea CM, Einhorn TA. Osteogenic differentiation is selectively promoted by morphogenetic signals from chondrocytes and synergized by a nutrient rich growth environment. Connect Tissue Res. 2003;44(Suppl 1):85‐91. [PubMed] [Google Scholar]
- 71. Ejaz A, Hatzmann FM, Hammerle S, et al. Fibroblast feeder layer supports adipogenic differentiation of human adipose stromal/progenitor cells. Adipocyte. 2019;8(1):178‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Albiero M, Poncina N, Ciciliot S, et al. Bone marrow macrophages contribute to diabetic stem cell mobilopathy by producing oncostatin M. Diabetes. 2015;64(8):2957‐2968. [DOI] [PubMed] [Google Scholar]
- 73. Kanashiro A, Hiroki CH, da Fonseca DM, et al. The role of neutrophils in neuro‐immune modulation. Pharmacol Res. 2020;151:104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sena IFG, Borges IT, Lousado L, et al. LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell Cycle. 2017;16(21):2018‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sena IFG, Prazeres P, Santos GSP, et al. Identity of Gli1(+) cells in the bone marrow. Exp Hematol. 2017;54:12‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Katayama Y, Battista M, Kao WM, et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124(2):407‐421. [DOI] [PubMed] [Google Scholar]
- 77. Maryanovich M, Zahalka AH, Pierce H, et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med. 2018;24(6):782‐791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Recalde A, Richart A, Guerin C, et al. Sympathetic nervous system regulates bone marrow‐derived cell egress through endothelial nitric oxide synthase activation: role in postischemic tissue remodeling. Arterioscler Thromb Vasc Biol. 2012;32(3):643‐653. [DOI] [PubMed] [Google Scholar]
- 79. Chartier SR, Mitchell SAT, Majuta LA, Mantyh PW. The changing sensory and sympathetic innervation of the young, adult and aging mouse femur. Neuroscience. 2018;387:178‐190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Calvo W. The innervation of the bone marrow in laboratory animals. Am J Anat. 1968;123(2):315‐328. [DOI] [PubMed] [Google Scholar]
- 81. Jung WC, Levesque JP, Ruitenberg MJ. It takes nerve to fight back: the significance of neural innervation of the bone marrow and spleen for immune function. Semin Cell Dev Biol. 2017;61:60‐70. [DOI] [PubMed] [Google Scholar]
- 82. Tabarowski Z, Gibson‐Berry K, Felten SY. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 1996;98(4):453‐457. [DOI] [PubMed] [Google Scholar]
- 83. Carmeliet P, Tessier‐Lavigne M. Common mechanisms of nerve and blood vessel wiring. Nature. 2005;436(7048):193‐200. [DOI] [PubMed] [Google Scholar]
- 84. Bjurholm A, Kreicbergs A, Terenius L, Goldstein M, Schultzberg M. Neuropeptide Y‐, tyrosine hydroxylase‐ and vasoactive intestinal polypeptide‐immunoreactive nerves in bone and surrounding tissues. J Auton Nerv Syst. 1988;25(2–3):119‐125. [DOI] [PubMed] [Google Scholar]
- 85. Park MH, Jin HK, Min WK, et al. Neuropeptide Y regulates the hematopoietic stem cell microenvironment and prevents nerve injury in the bone marrow. EMBO J. 2015;34(12):1648‐1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mendez‐Ferrer S, Battista M, Frenette PS. Cooperation of beta(2)‐ and beta(3)‐adrenergic receptors in hematopoietic progenitor cell mobilization. Ann N Y Acad Sci. 2010;1192:139‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Du Z, Wang L, Zhao Y, et al. Sympathetic denervation‐induced MSC mobilization in distraction osteogenesis associates with inhibition of MSC migration and osteogenesis by norepinephrine/adrb3. PLoS One. 2014;9(8):e105976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Castaneda‐Corral G, Jimenez‐Andrade JM, Bloom AP, et al. The majority of myelinated and unmyelinated sensory nerve fibers that innervate bone express the tropomyosin receptor kinase A. Neuroscience. 2011;178:196‐207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Weihe E, Nohr D, Michel S, et al. Molecular anatomy of the neuro‐immune connection. Int J Neurosci. 1991;59(1‐3):1‐23. [DOI] [PubMed] [Google Scholar]
- 90. Hara‐Irie F, Amizuka N, Ozawa H. Immunohistochemical and ultrastructural localization of CGRP‐positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone. 1996;18(1):29‐39. [DOI] [PubMed] [Google Scholar]
- 91. Jimenez‐Andrade JM, Bloom AP, Stake JI, et al. Pathological sprouting of adult nociceptors in chronic prostate cancer‐induced bone pain. J Neurosci. 2010;30(44):14649‐14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hu B, Lv X, Chen H, et al. Sensory nerves regulate mesenchymal stromal cell lineage commitment by tuning sympathetic tones. J Clin Invest. 2020;130:3483‐3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Blackwell KA, Raisz LG, Pilbeam CC. Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab. 2010;21(5):294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yoshida K, Oida H, Kobayashi T, et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci USA. 2002;99(7):4580‐4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kastner P, Mark M, Chambon P. Nonsteroid nuclear receptors: what are genetic studies telling us about their role in real life? Cell. 1995;83(6):859‐869. [DOI] [PubMed] [Google Scholar]
- 96. Nassar MA, Stirling LC, Forlani G, et al. Nociceptor‐specific gene deletion reveals a major role for Nav1.7 (PN1) in acute and inflammatory pain. Proc Natl Acad Sci USA. 2004;101(34):12706‐12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Henriques F, Lopes MA, Franco FO, et al. Toll‐like receptor‐4 disruption suppresses adipose tissue remodeling and increases survival in cancer cachexia syndrome. Sci Rep. 2018;8(1):18024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Silva WN, Prazeres P, Paiva AE, et al. Macrophage‐derived GPNMB accelerates skin healing. Exp Dermatol. 2018;27(6):630‐635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lau J, Minett MS, Zhao J, et al. Temporal control of gene deletion in sensory ganglia using a tamoxifen‐inducible Advillin‐Cre‐ERT2 recombinase mouse. Mol Pain. 2011;7:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Almeida VM, Paiva AE, Sena IFG, et al. Pericytes make spinal cord breathless after injury. Neuroscientist. 2017;24(5):440‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Santos GSP, Magno LAV, Romano‐Silva MA, Mintz A, Birbrair A. Pericyte plasticity in the brain. Neurosci Bull. 2019;35(3):551‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Santos GSP, Prazeres P, Mintz A, et al. Role of pericytes in the retina. Eye. 2017;32(3):483‐486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Azevedo PO, Paiva AE, Santos GSP, et al. Cross‐talk between lung cancer and bones results in neutrophils that promote tumor progression. Cancer Metastasis Rev. 2018;37(4):779‐790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Azevedo PO, Sena IFG, Andreotti JP, et al. Pericytes modulate myelination in the central nervous system. J Cell Physiol. 2017;233(8):5523‐5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Silva WN, Leonel C, Prazeres P, et al. Role of Schwann cells in cutaneous wound healing. Wound Repair Regen. 2018;26(5):392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Guerra DAP, Paiva AE, Sena IFG, et al. Adipocytes role in the bone marrow niche. Cytometry A. 2018;93(2):167‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hunter DV, Smaila BD, Lopes DM, et al. Advillin is expressed in all adult neural crest‐derived neurons. eNeuro. 2018;5(5):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ranade SS, Woo SH, Dubin AE, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516(7529):121‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Esmaeilniakooshkghazi A, George SP, Biswas R, Khurana S. Mouse intestinal tuft cells express advillin but not villin. Sci Rep. 2020;10(1):8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pasznik P, Rutkowska E, Niewieczerzal S, Cielecka‐Piontek J, Latek D. Potential off‐target effects of beta‐blockers on gut hormone receptors: in silico study including GUT‐DOCK‐A web service for small‐molecule docking. PLoS One. 2019;14(1):e0210705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lucas D, Scheiermann C, Chow A, et al. Chemotherapy‐induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19(6):695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chen H, Hu B, Lv X, et al. Prostaglandin E2 mediates sensory nerve regulation of bone homeostasis. Nat Commun. 2019;10(1):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Oury F, Yadav VK, Wang Y, et al. CREB mediates brain serotonin regulation of bone mass through its expression in ventromedial hypothalamic neurons. Genes Dev. 2010;24(20):2330‐2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Helyes Z, Than M, Oroszi G, et al. Anti‐nociceptive effect induced by somatostatin released from sensory nerve terminals and by synthetic somatostatin analogues in the rat. Neurosci Lett. 2000;278(3):185‐188. [DOI] [PubMed] [Google Scholar]
- 115. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Akiba Y, Kato S, Katsube K, et al. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004;321(1):219‐225. [DOI] [PubMed] [Google Scholar]
- 117. Goncalves WA, Rezende BM, de Oliveira MPE, et al. Sensory ganglia‐specific TNF expression is associated with persistent nociception after resolution of inflammation. Front Immunol. 2019;10:3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kuhn NZ, Tuan RS. Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol. 2010;222(2):268‐277. [DOI] [PubMed] [Google Scholar]
- 119. Andreotti JP, Lousado L, Magno LAV, Birbrair A. Hypothalamic neurons take center stage in the neural stem cell niche. Cell Stem Cell. 2017;21(3):293‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Andreotti JP, Silva WN, Costa AC, et al. Neural stem cell niche heterogeneity. Semin Cell Dev Biol. 2019;95:42‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jahoda CA, Christiano AM. Niche crosstalk: intercellular signals at the hair follicle. Cell. 2011;146(5):678‐681. [DOI] [PubMed] [Google Scholar]
- 122. Spit M, Koo BK, Maurice MM. Tales from the crypt: intestinal niche signals in tissue renewal, plasticity and cancer. Open Biol. 2018;8(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Greenspan LJ, de Cuevas M, Matunis E. Genetics of gonadal stem cell renewal. Annu Rev Cell Dev Biol. 2015;31:291‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Prazeres PHDM, Leonel C, Silva WN, et al. Ablation of sensory nerves favors melanoma progression. J Cell Mol Med. 2020;24(17):9574‐9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Leonel C, Sena IFG, Silva WN, et al. Staphylococcus epidermidis role in the skin microenvironment. J Cell Mol Med. 2019;23(9):5949‐5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Zhang B, Ma S, Rachmin I, et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020;577(7792):676‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Hsu YC, Li L, Fuchs E. Emerging interactions between skin stem cells and their niches. Nat Med. 2014;20(8):847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Riol‐Blanco L, Ordovas‐Montanes J, Perro M, et al. Nociceptive sensory neurons drive interleukin‐23‐mediated psoriasiform skin inflammation. Nature. 2014;510(7503):157‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lefrancais E, Ortiz‐Munoz G, Caudrillier A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544(7648):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Borges IDT, Sena IFG, de Azevedo PO, et al. Lung as a niche for hematopoietic progenitors. Stem Cell Rev Rep. 2017;13(5):567‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Talbot S, Abdulnour RE, Burkett PR, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87(2):341‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Khan JA, Mendelson A, Kunisaki Y, et al. Fetal liver hematopoietic stem cell niches associate with portal vessels. Science. 2016;351(6269):176‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Al‐Drees MA, Yeo JH, Boumelhem BB, et al. Making blood: the Haematopoietic niche throughout ontogeny. Stem Cells Int. 2015;2015:571893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Palis J, Robertson S, Kennedy M, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073‐5084. [DOI] [PubMed] [Google Scholar]
- 135. Tavian M, Peault B. Embryonic development of the human hematopoietic system. Int J Dev Biol. 2005;49(2–3):243‐250. [DOI] [PubMed] [Google Scholar]
- 136. Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow‐derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16(4):557‐564. [PubMed] [Google Scholar]
- 137. Sala E, Genua M, Petti L, et al. Mesenchymal stem cells reduce colitis in mice via release of TSG6, independently of their localization to the intestine. Gastroenterology. 2015;149(1):163‐176.e120. [DOI] [PubMed] [Google Scholar]
- 138. Soler R, Orozco L, Munar A, et al. Final results of a phase I‐II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23(4):647‐654. [DOI] [PubMed] [Google Scholar]
- 139. Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells for the treatment of multiple sclerosis and other neurological diseases. Lancet Neurol. 2011;10(7):649‐656. [DOI] [PubMed] [Google Scholar]
- 140. Anderson JD, Johansson HJ, Graham CS, et al. Comprehensive proteomic analysis of mesenchymal stem cell exosomes reveals modulation of angiogenesis via nuclear factor‐KappaB signaling. Stem Cells. 2016;34(3):601‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yuan O, Lin C, Wagner J, et al. Exosomes derived from human primed mesenchymal stem cells induce mitosis and potentiate growth factor secretion. Stem Cells Dev. 2019;28(6):398‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Showalter MR, Wancewicz B, Fiehn O, et al. Primed mesenchymal stem cells package exosomes with metabolites associated with immunomodulation. Biochem Biophys Res Commun. 2019;512(4):729‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499‐3506. [DOI] [PubMed] [Google Scholar]
- 144. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726‐736. [DOI] [PubMed] [Google Scholar]
- 145. Kim J, Hematti P. Mesenchymal stem cell‐educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37(12):1445‐1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow‐derived mesenchymal stromal cells turn activated macrophages into a regulatory‐like profile. PLoS One. 2010;5(2):e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang QZ, Su WR, Shi SH, et al. Human gingiva‐derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28(10):1856‐1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Talbot S, Abdulnour RE, Burkett PR, et al. Silencing nociceptor neurons reduces allergic airway inflammation. Neuron. 2015;87(2):341‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Chiu IM, Heesters BA, Ghasemlou N, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Kruger L, Light AR, Schweizer FE. Axonal terminals of sensory neurons and their morphological diversity. J Neurocytol. 2003;32(3):205‐216. [DOI] [PubMed] [Google Scholar]
- 151. Harlow DE, Barlow LA. Embryonic origin of gustatory cranial sensory neurons. Dev Biol. 2007;310(2):317‐328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Woolf CJ, Ma Q. Nociceptors—noxious stimulus detectors. Neuron. 2007;55(3):353‐364. [DOI] [PubMed] [Google Scholar]
- 153. Usoskin D, Furlan A, Islam S, et al. Unbiased classification of sensory neuron types by large‐scale single‐cell RNA sequencing. Nat Neurosci. 2015;18(1):145‐153. [DOI] [PubMed] [Google Scholar]
- 154. Li CL, Li KC, Wu D, et al. Somatosensory neuron types identified by high‐coverage single‐cell RNA‐sequencing and functional heterogeneity. Cell Res. 2016;26(8):967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Dias Moura Prazeres PH, Sena IFG, Borges IDT, et al. Pericytes are heterogeneous in their origin within the same tissue. Dev Biol. 2017;427(1):6‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Asada N, Kunisaki Y, Pierce H, et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol. 2017;19(3):214‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Morikawa S, Baluk P, Kaidoh T, Haskell A, Jain RK, McDonald DM. Abnormalities in pericytes on blood vessels and endothelial sprouts in tumors. Am J Pathol. 2002;160(3):985‐1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Nehls V, Denzer K, Drenckhahn D. Pericyte involvement in capillary sprouting during angiogenesis in situ. Cell Tissue Res. 1992;270(3):469‐474. [DOI] [PubMed] [Google Scholar]
- 160. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression [in Eng]. Science. 2013;341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 161. Zahalka AH, Arnal‐Estape A, Maryanovich M, et al. Adrenergic nerves activate an angio‐metabolic switch in prostate cancer. Science. 2017;358(6361):321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Bapat AA, Hostetter G, Von Hoff DD, et al. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695‐707. [DOI] [PubMed] [Google Scholar]
- 164. Jimenez‐Andrade JM, Ghilardi JR, Castaneda‐Corral G, et al. Preventive or late administration of anti‐NGF therapy attenuates tumor‐induced nerve sprouting, neuroma formation, and cancer pain. Pain. 2011;152(11):2564‐2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Shi Y, Du L, Lin L, et al. Tumour‐associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16(1):35‐52. [DOI] [PubMed] [Google Scholar]
- 167. Paiva AE, Lousado L, Guerra DAP, et al. Pericytes in the premetastatic niche. Cancer Res. 2018;78(11):2779‐2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133‐143. [DOI] [PubMed] [Google Scholar]
- 169. Aguirre‐Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7(11):834‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


