Abstract
Brain‐derived neurotropic factor (BDNF), which is secreted by mesenchymal stem cells (MSCs), protects against severe intraventricular hemorrhage (IVH)‐induced brain injuries. Although the paracrine protective effects of MSCs are mediated primarily by extracellular vesicles (EVs), the therapeutic efficacy of MSC‐derived EVs and the role of the BDNF in the EVs have not been studied. This study aimed to determine whether MSC‐derived EVs attenuate severe IVH‐induced brain injuries, and if so, whether this protection is mediated by BDNF transfer. We compared the therapeutic efficacy of MSCs, MSC‐derived EVs with or without BDNF knockdown, and fibroblast‐derived EVs in vitro in rat cortical neuronal cells challenged with thrombin and in vivo in newborn rats by injecting 200 μL of blood at postnatal day (P) 4 and transplanting 1 × 105 MSCs or 20 μg of EVs at P6. The MSCs and MSC‐derived EVs, but not the EVs derived from BDNF‐knockdown MSCs or fibroblasts, significantly attenuated in vitro thrombin‐induced neuronal cell death and in vivo severe IVH‐induced brain injuries such as increased neuronal cell death, astrogliosis, and inflammatory responses; reduced myelin basic protein and neurogenesis; led to progression of posthemorrhagic hydrocephalus; and impaired behavioral test performance. Our data indicate that MSC‐derived EVs are as effective as parental MSCs in attenuating severe IVH‐induced brain injuries, and this neuroprotection is primarily mediated by BDNF transfer via EVs.
Keywords: brain‐derived neurotropic factor, extracellular vesicles, intraventricular hemorrhage, mesenchymal stem cells
Recent reports showed that stem cells transplantation was effective on improving brain injury from severe intraventricular hemorrhage (IVH) in newborn rats. As a next step, because stem cells secrete bioactive extracellular vesicles (EVs), the authors tried to prove the therapeutic effects of EVs as next‐generation therapeutic candidates against newborn IVH. Intracerebroventricular transplantation of EVs secreted from stem cells effectively attenuated brain injury from severe IVH via transferring brain‐derived neurotrophic factor in newborn rats.
Significance statement.
Intraventricular hemorrhage in premature infants remains a major cause of mortality and long‐term morbidities, including seizure, cerebral palsy, and developmental delay, because there are few effective treatments. Recently, stem cells have emerged as a new therapeutic strategy due to recent reports showing that stem cells transplantation improved brain injury from severe intraventricular hemorrhage in newborn rats. Transplantation of extracellular vesicles secreted from stem cells effectively attenuated brain injury from severe intraventricular hemorrhage via transferring brain‐derived neurotrophic factor in newborn rats.
1. INTRODUCTION
Intraventricular hemorrhage (IVH), which results from the rupture of the germinal matrix through the ependymal into the lateral ventricle, is a common and serious disorder, especially in the most immature preterm infants. 1 , 2 , 3 Brain injuries induced by severe IVH and the ensuing posthemorrhagic hydrocephalus (PHH) result in increased mortality and neurologic morbidities including seizure, cerebral palsy, and developmental retardation in premature infants. 4 , 5 , 6 Since no effective therapy is currently available to ameliorate brain injury or prevent the progress of PHH after severe IVH in premature infants, developing a new and effective treatment to improve the outcome of this devastating and intractable disorder is an urgent issue.
Recently, we have shown that xenotransplantation of human mesenchymal stem cells (MSCs) significantly attenuates the progress of PHH and brain damage after severe IVH in immunocompetent newborn rats. 7 Furthermore, a phase I clinical trial has shown that intraventricular transplantation of allogenic human MSCs into extremely preterm infants with severe IVH is safe and feasible. 8 These data above may suggest that MSC treatment might be a promising option for treating IVH of premature infants. However, despite their valuable therapeutic effects, transplanting viable MSCs has several disadvantages, such as concerns regarding potential tumorigenicity and other side effects such as pulmonary embolism and cellular rejection by the host. 9 , 10 , 11
The benefits of MSC transplantation are primarily mediated by secretion of various paracrine factors through extracellular vesicles (EVs), a nuclear membrane vesicle measuring 40‐100 nm in diameter containing numerous proteins, lipids, and RNAs similar to those present in parent MSCs, rather than by direct regenerative mechanism. 9 , 12 , 13 , 14 , 15 Recent studies have shown that MSC‐derived EVs could recapitulate the therapeutic efficacy of parent MSCs in various neonatal disorders, including bronchopulmonary dysplasia, 16 fetal hypoxic ischemic brain damage, 17 and hypoxia induced pulmonary hypertension, 17 , 18 via the delivery of messenger RNA, microRNA, and proteins. 13 , 16 , 17 , 18 Moreover, as this cell‐free therapy could bypass the concerns associated with viable MSC transplantation, the use of MSC‐derived EVs might be a potential surrogate for stem cell therapy in cases of severe IVH. Nonetheless, the therapeutic efficacy of MSC‐derived EVs for the treatment of severe IVH has not been tested.
In the present study, we assessed whether the intraventricular injection of MSC‐derived EVs is as effective as MSCs alone in a newborn rat pup model of severe IVH, and if so, whether this neuroprotection is related to protein transfer from EVs to the damaged host brain tissue. The transfer of brain‐derived neurotropic factor (BDNF) was specifically inspected, as we have previously proved the essential role of MSC‐secreted BDNF in improving the progress of PHH and brain injuries after severe IVH in newborn rats. 1 , 19
2. MATERIALS AND METHODS
2.1. Mesenchymal stem cells
Human umbilical cord blood (UCB)‐derived MSCs obtained from a single donor and manufactured in strict compliance with good manufacturing process at passage 6 (Medipost Co, Ltd, Seoul, Korea) were used in this study. 8 , 20 , 21 , 22 The characterization, 23 differentiation potential, 12 , 21 immunophenotypic results, 12 and karyotypic stability up to passage 11 of MSCs 24 have been reported in our previous studies. Human fibroblasts (MRC‐5; no. 10171) were purchased from the Korean Cell Line Bank (Seoul, Korea).
2.2. BDNF‐knockdown EVs
BDNF and scrambled small interfering RNAs (siRNAs; sc‐42 121 and sc‐37 007) were purchased from Santa Cruz Biotechnology (Santa Cruz, California). For BDNF knockdown, MSCs were transfected with siRNA oligonucleotides targeting BDNF using Oligofectamine (Invitrogen, Carlsbad, California), as described previously. 25 Using the identical method above, scrambled siRNA was transfected into MSCs to make a negative control. From the conditioned media of the BDNF siRNA‐ or scrambled siRNA‐transfected MSCs, EVs were isolated.
2.3. Isolation of EVs
Details of EV isolation from the MSC culture supernatant have been described in our previous studies. 13 , 16 , 20 Briefly, after culturing 5 × 106 MSCs per 100‐mm plates, the cells were serum starved and EVs were obtained by ultracentrifugation of the cell culture media and stored at −80°C until further use.
2.4. Quantification of EV
Details of EV quantification also have been described in our previous studies. 13 , 20 The total EV protein content was quantified by measuring the protein concentration using the Bradford assay. 13 , 20 The distribution of EVs was analyzed by measuring the rate of Brownian motion using a NanoSight (NanoSight NS300; Malvern, Worcestershire, UK) that is equipped with fast video capture and a particle tracking software. 13 , 16 , 20 The obtained EVs were resuspended in phosphate‐buffered saline (PBS; 500 μL, 1 mg/mL total protein) and characterized for size and polydispersity. 13 , 20 Dynamic light scattering and zeta potential determinations were performed with a Zetasizer nanoseries instrument (532‐nm laser wavelength; Malvern Instruments Ltd, Worcestershire, UK) to determine the size of the EVs. 13 , 20 The EV size date refers to the scattering intensity distribution (z‐average).
2.5. Scanning electron microscopy
After fixation in 2.5% glutaraldehyde, isolated EVs were loaded on a polycarbonate membrane. 13 , 20 The membrane was washed with PBS and distilled water and then dehydrated with acetone. 13 , 20 The acetone was removed by critical point drying using liquid carbon dioxide. 13 , 20 Samples were mounted on aluminum stubs with carbon tape and on an scanning electron microscopy (SEM) stub. 13 , 20 After sputter coating with 3‐5 nm of platinum, the samples were examined using an SEM (Zeiss Auriga Workstation, Oberkochen, Germany). 13 , 20
2.6. Transmission electron microscopy
EVs (5 μL) were fixed with 2% glutaraldehyde, loaded on 200‐mesh formvar/carbon‐coated electron microscopic grids (Electron Microscopy Sciences, Washington, Pennsylvania), and incubated for 10 minutes. After washing with distilled water, they were stained with 2% uranyl acetate in water for 1 minute. 13 , 20 Transmission electron micrographs were obtained using a Tecnai Spirit G2 transmission electron microscope (FEI, Hillsboro, Oregon) operating at 120 kV. 13 , 20
2.7. Thrombin exposure in vitro cell culture
To induce in vitro neuronal damage introduced from the hemorrhagic condition, 25 , 26 primarily cultured rat cortical neuronal cells were exposed to 40 U of thrombin (Reyon Pharmaceutical Co, Ltd, Seoul, South Korea) and then incubated in neurobasal medium (GIBCO, Gaithersburg, Maryland) alone or with naïve MSCs or EVs derived from fibroblasts, nontransfected MSCs, scrambled siRNA‐transfected MSCs, or BDNF siRNA‐transfected MSCs. 25 Cell viability was evaluated with a colorimetric 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyl tetrazolium (MTT) assay (Dojindo Molecular Technologies Inc., Rockville, Maryland). 25 Relative viabilities were determined by normalizing to 0% (no cells) and 100% (untreated cells) controls. 25 Cytotoxicity was determined by lactate dehydrogenase (LDH) releases, according to the supplier's instructions (Roche, Mannheim, Germany). 20 The level of malondialdehyde (MDA), as a marker of oxidative stress, was measured in cell lysates in duplicate, using the Oxiselect TBARS assay kit containing thiobarbituric acid‐reactive substances (Cell Biolabs, San Diego, California), according to the manufacturer's instructions. 20
2.8. Animal model
All experimental protocols described in this study were approved by the Institutional Animal Care and Use Committee of the Samsung Biomedical Research Institute, and the study followed the institutional and National Institutes of Health guidelines for laboratory animal care. All animal procedures were performed in an Association for the Assessment and Accreditation of Laboratory Animal Care International‐accredited specific pathogen‐free facility. Newborn Sprague‐Dawley rats (Orient Co., Seoul, Korea) reared with their dams in the standard cage were used in this study. Rats were reared under an alternating 12‐hours light/dark cycle with constant room humidity and temperature. 25 At postnatal day (P) 4, IVH was induced by intracerebroventricular injection of a 200 μL of fresh maternal whole blood (100 μL each into the right and left ventricles), as described previously. 1 , 7 , 25 , 27 , 28 , 29 , 30 At P5, brain magnetic resonance imaging (MRI) was performed to confirm baseline severe IVH, and only severe IVH‐induced rats replicating grade III/IV IVH in human infants 2 were included. At P6, IVH‐induced rats were randomly divided into seven experimental groups: normal control (NC, n = 13), IVH control (IC, n = 14), IVH with MSC (IM, n = 13), IVH with nontransfected MSC‐derived EV (IMEV, n = 11), IVH with fibroblast‐derived EV (IFEV, n = 13), IVH with scrambled siRNA‐transfected MSC‐derived EV (IMscrEV, n = 13), and IVH with BDNF siRNA‐transfected MSC‐derived EVs groups (IMBDNFsiREV, n = 14). At P6, 5 × 105 naïve MSCs in 50 μL of saline, 20 μg of EVs derived from naïve MSCs, scrambled siRNA‐ or BDNF siRNA‐transfected MSCs, fibroblasts in 50 μL of saline, or an equal volume of saline (for the control group) were administered intraventricularly. Follow‐up brain MRI was performed at P11 and P32. The general conditions of the rat pups were monitored on a daily basis, especially for the initial 7 days after IVH modeling, and then on a weekly basis until sacrifice. All procedures were performed under inhaled anesthesia using a mixture of isoflurane (Ifran; Hana Pharm. Co, Ltd, Seoul, Korea) and 2:1 nitrous oxide/oxygen. 25 Animals were sacrificed with CO2 inhalation on P32, and brain tissue samples were prepared. 25
2.9. In vivo MRI and assessment of the ventricle‐to‐whole‐brain volume ratio
Brain MRI was performed at P5, P11, and P32, and the ventricle: whole‐brain volume ratio was assessed to monitor the progress of ventricular dilatation and volume ratio of ventricles, as described previously. 7 , 25 , 28 , 29 , 30
2.10. Functional behavioral test
To evaluate sensorimotor function, the negative geotaxis and rotarod tests were performed at P32, as described previously. 7 , 19 , 25 , 28 , 29 , 30
2.11. Terminal deoxynucleotidyl transferase dUTP nick‐end labeling assay and immunohistochemistry
Cell death was assessed using the immunofluorescent terminal deoxynucleotidyl transferase dUTP nick‐end labeling (TUNEL) technique, and the extent of reactive gliosis, reactive microglia, myelination, and neurogenesis, as evidenced by neuronal specific glial fibrillary acidic protein (GFAP), ED1, myelin basic protein (MBP), and doublecortin, respectively, were immunohistochemically evaluated, as described in our previous studies. 7 , 19 , 25 , 28 , 29 , 30
2.12. Enzyme‐linked immunosorbent assay
The levels of inflammatory cytokines, including interleukin (IL) 1α, IL‐1β, IL‐6, and tumor necrosis factor (TNF)‐α, in the periventricular brain tissue homogenates were measured using the Millipex MAP ELISA Kit (Millipore, Billerica, Massachusetts). 25 Human‐ and rat‐specific BDNFs were measured using an enzyme‐linked immunosorbent assay (ELISA) kit (Quantikine ELISA Kit; R&D Systems, Minneapolis, Minnesota), as described previously. 25
2.13. Statistical analyses
Data are expressed as the mean ± SE of the mean. For continuous variables, statistical comparison between groups was performed using one‐way analysis of variance and Tukey's post hoc analysis. For the time‐course variables, a univariate general linear model for repeated measures with Tukey's post hoc comparison was performed. All data were analyzed using SPSS version 18.0 (IBM, Chicago, Illinois). A P value of <.05 was considered statistically significant.
3. RESULTS
3.1. Characterization of MSC‐derived EVs
In representative SEM and transmission electron microscopy images, the isolated EVs were spheroidal, with a peak size distribution of 100 nm in diameter, as measured by nanoparticle tracking analysis (Figure 1A,B). The isolated EVs were positive for the exosome‐specific markers CD9, CD63, and CD81, but they were negative for mitochondrial cytochrome C, GM130, and fibrillarin (Figure 1C).
FIGURE 1.
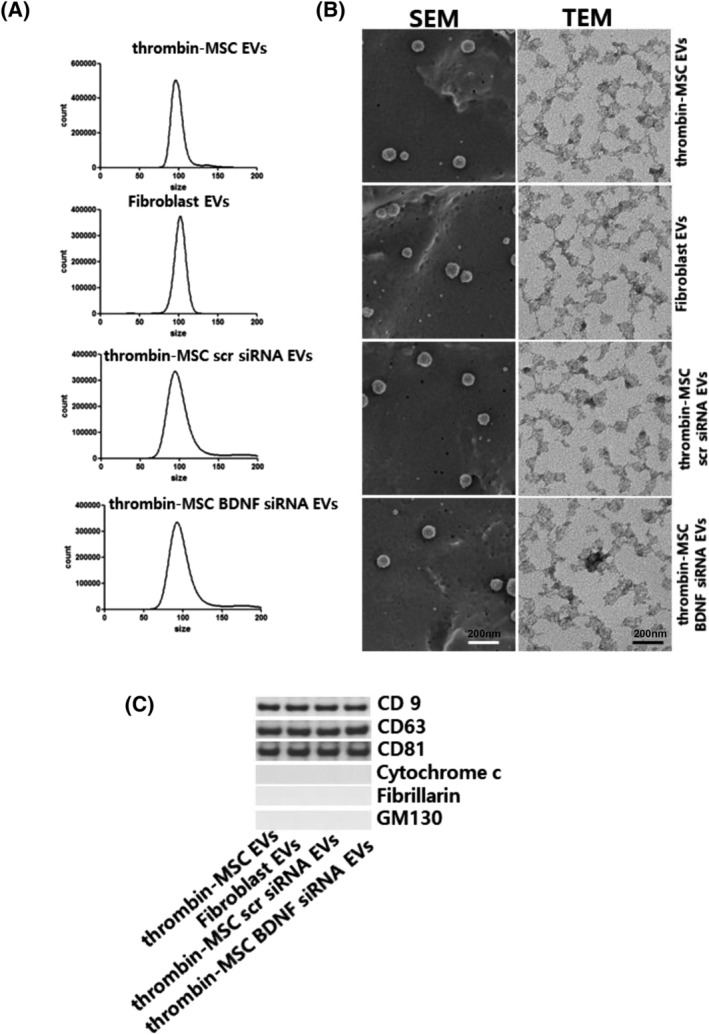
Confirmation of extracellular vesicles (EVs). EVs were isolated from the cell culture media using ultracentrifugation. A, The size and number of EVs as measured using NanoSightNS300 and Nanoparticle Tracking Analysis software. B, Scanning electron micrograph (SEM) of EVs loaded on a polycarbonate membrane. Transmission electron micrograph (TEM) of EVs. EVs on copper grids and stained with uranyl acetate. Representative SEM (left) and TEM (right). C, Representative immunoblot for the organelle marker proteins in mesenchymal stem cell‐derived EVs, CD9, CD63, CD81, cytochrome C for mitochondria, fibrillarin for the nucleus, and GM130 for the Golgi
3.2. BDNF‐knockdown EVs and human‐ and rat‐specific BDNF levels
Human‐ and rat‐specific BDNF protein levels were assessed with ELISA in cultured rat cortical neuronal cells after thrombin exposure with or without human MSCs or MSC‐derived EVs (Supporting Information Figure S1).
No human BDNF‐specific protein was observed in the supernatant of the normal or thrombin‐exposed rat cortical neuronal cells cultured alone or cocultured with fibroblast‐derived EVs. The human BDNF protein level in the thrombin‐exposed rat cortical neuronal cells supernatant was significantly higher when cocultured with MSCs, naïve MSC‐derived EVs or scrambled siRNA‐transfected MSC‐derived EVs than when cocultured with BDNF siRNA‐transfected MSC‐derived EVs (Supporting Information Figure S1A).
The significant decrease in the rat BDNF protein level in the thrombin‐exposed control group, compared with the NC group, was significantly improved by coculture with MSCs, nontransected, or scrambled siRNA‐transfected MSC‐derived EVs than by coculture with BDNF siRNA‐transfected MSC‐ or fibroblast‐derived EVs (Supporting Information Figure S1B).
3.3. Protection of MSC‐derived EVs in vitro
Thrombin exposure significantly increased neuronal cell death which evidenced by increase in TUNEL‐positive neuronal cells in the thrombin‐exposed groups, compared with the NC group. This was substantially attenuated by coculture with MSCs, naïve MSC‐derived EVs, or scrambled siRNA‐transfected MSC‐derived EVs, but not with BDNF siRNA‐transfected MSC‐ or fibroblast‐derived EVs (Figure 2A,B).
FIGURE 2.
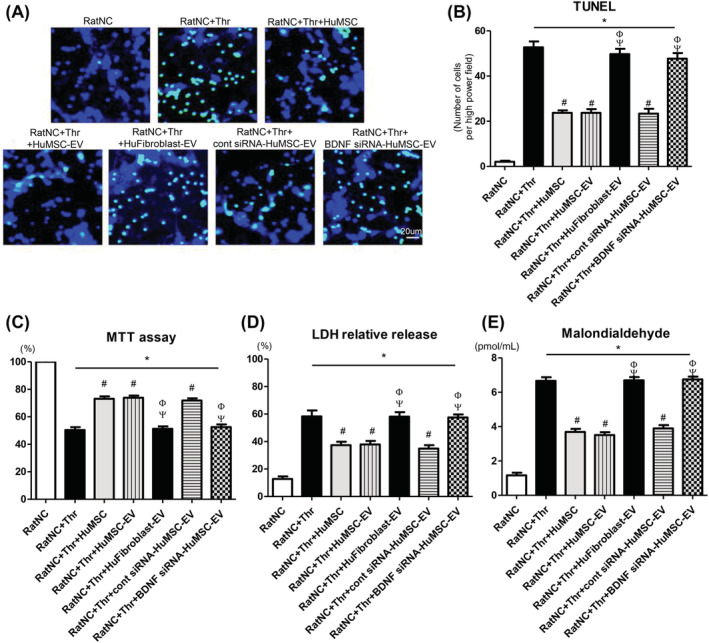
Neuroprotective effect of extracellular vesicles on in vitro thrombin‐induced primary cultured rat cortical neurons. A, Representative fluorescence micrographs of the penumbra area with staining for terminal deoxynucleotidyl transferase dUTP nick‐end labeling (TUNEL) (green, TUNEL‐positive); B, The number of TUNEL‐positive cells captured using fluorescent microscopy; C, Cell viability, expressed as relative proliferation rate (%) to the normal control group; D, Cytotoxicity, expressed as relative lactate dehydrogenase (LDH) release (%) to positive control (100% fully killed cells); E, Malondialdehyde (MDA) level. RatNC, rat neuronal cell normal control; Thr, thrombin treatment; HuMSC, Human MSCs treatment; HuMSC‐EV, extracellular vesicles from naïve human MSCs; HuFibroblast‐EV, extracellular vesicles from human fibroblasts; cont siRNA HuMSC‐EV, extracellular vesicles from human MSCs transfected with scrambled siRNA; BDNF siRNA HuMSC‐EV, extracellular vesicles from human MSCs transfected with BDNF siRNA. Data are presented as mean ± SE of the mean. *P < .05 compared with the rat neuronal cells normal control, #P < .05 compared with the rat neuronal cells treated with thrombin injury control, Φ P < .05 compared with rat neuronal cells treated with thrombin and human MSCs, Ψ P < .05 compared with rat neuronal cells treated with thrombin and human MSCs‐EVs
Thrombin exposure significantly reduced neuronal cell survival, increased LDH release, and increased MDA level in cultured rat neuronal cell groups than in the NC group (Figure 2C‐E). The thrombin‐induced increase in cell death, LDH release, and MDA level were obviously attenuated by coculture with MSCs, naïve MSC‐derived EVs, or scrambled siRNA‐transfected MSC‐derived EVs, but not with BDNF siRNA‐transfected MSC‐ or fibroblast‐derived EVs.
3.4. Serial brain MRI
Figure 3A shows representative serial brain MRIs from each animal group performed at 1, 7, and 28 days after induction of severe IVH (ie, at P5, P11, and P32, respectively). Although the ventricular dilatation severity at P5 was not different among the study groups, the progressive ventriculomegaly noticed in the nontreated IVH control (IC) group on follow‐up brain MRI performed at P11 and P32 was clearly attenuated in the IM, IMEV, and IMscrEV groups, but not in the IFEV or IMBDNFsiREV group (Figure 3B).
FIGURE 3.
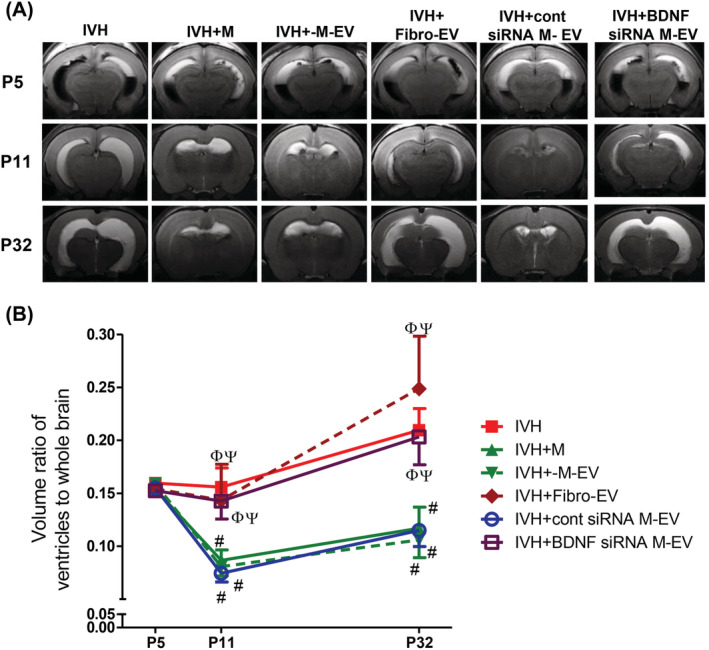
BDNF knockdown in the extracellular vesicles abolished the therapeutic effects of MSCs in attenuating ventricular dilatation and its progression after severe intraventricular hemorrhage (IVH). A, Representative serial brain magnetic resonance imaging (MRIs) from each group 1, 7, and 28 days after inducing IVH (at P5, P11, and P32, respectively). B, Ventricle: whole brain volume ratio as measured by MRI. Data are expressed as mean ± SE of the mean. Data are presented as mean ± SE of the mean. *P < .05 compared with the normal control, #P < .05 compared with the IVH injury control, Φ P < .05 compared with IVH + MSCs, Ψ P < .05 compared with IVH + MSCs‐EVs. NC, normal control; IVH, intraventricular hemorrhage control; M, MSCs transplantation; M‐EV, extracellular vesicles from naïve MSCs; Fibro‐EV, extracellular vesicles from fibroblasts; cont siRNA M‐EV, extracellular vesicles from MSCs transfected with scrambled siRNA; BDNF siRNA M‐EV, extracellular vesicles from MSCs transfected with BDNF siRNA
3.5. Protection of MSC‐derived EVs in vivo
The severe IVH‐induced periventricular brain injuries assessed at P32, including increased TUNEL‐positive cell death, GFAP‐positive astrocytosis, ED1‐positive gliosis, and impaired myelination and neurogenesis (evidenced by reduced MBP and doublecortin levels, respectively), in the IC group were significantly improved in the IM, IMEV, and IMscrEV groups, but not in the IFEV or IMBDNFsiREV group (Figure 4A,B).
FIGURE 4.
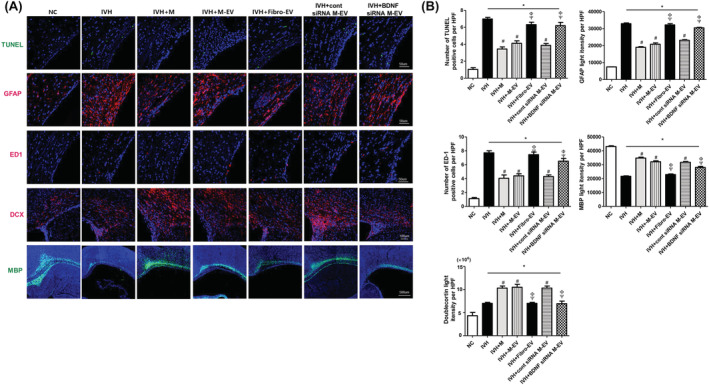
BDNF knockdown in the extracellular vesicles abolished the therapeutic effects of MSCs in improving brain myelination and in attenuating cell death and reactive gliosis after severe IVH. A, Representative immunofluorescence photomicrographs of the periventricular area with staining for TUNEL (green), glial fibrillary acidic protein (GFAP) (red), ED1 (red), doublecortin (DCX) (red), myelin basic protein (MBP) (green), and 4′,6‐diamidino‐2‐pheylindole (DAPI) (blue). B, The average number of TUNEL and ED1‐positive cells and mean light intensity of GFAP, DCX, and MBP immunofluorescence per high‐power field (HPF) in each group. Data are expressed as mean ± SE of the mean. Data are presented as mean ± SE of the mean. *P < .05 compared with the normal control, #P < .05 compared with the IVH injury control, Φ P < .05 compared with IVH + MSCs, Ψ P < .05 compared with IVH + MSCs‐EVs. NC, normal control; IVH, intraventricular hemorrhage control; M, MSCs transplantation; M‐EV, extracellular vesicles from naïve MSCs; Fibro‐EV, extracellular vesicles from fibroblasts; cont siRNA M‐EV, extracellular vesicles from MSCs transfected with scrambled siRNA; BDNF siRNA M‐EV, extracellular vesicles from MSCs transfected with BDNF siRNA
The increased inflammatory cytokines such as IL‐1α, IL‐1β, IL‐6, and TNF‐α in the cerebrospinal fluid and periventricular brain tissue at P32 observed in the IC group were significantly improved in the IM, IMEV, and IMscrEV groups, but not in the IFEV or IMBDNFsiREV group (Figure 5A,B).
FIGURE 5.
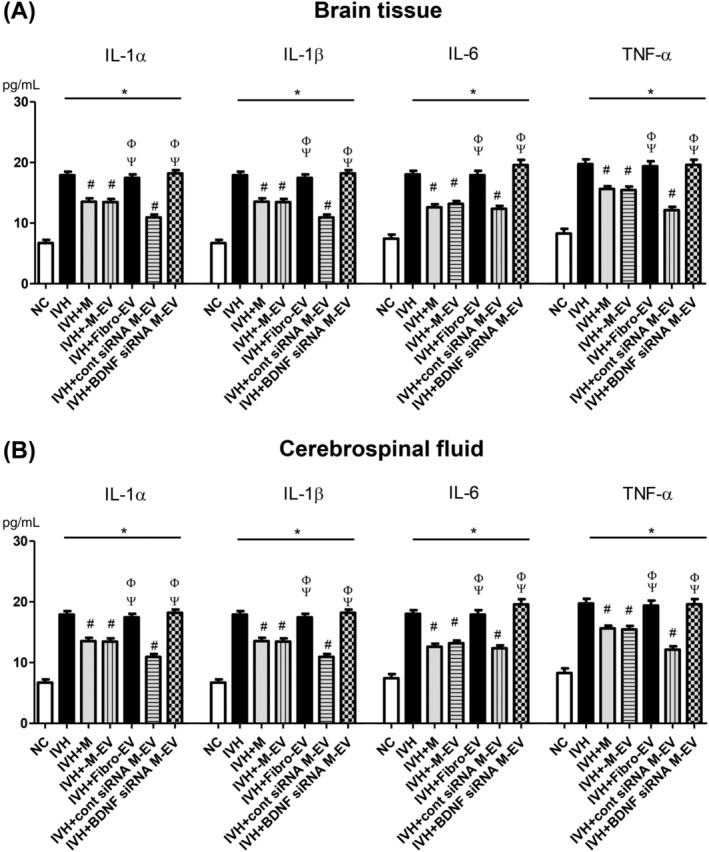
BDNF knockdown in the extracellular vesicles abolished the therapeutic effects of MSCs in downregulating brain inflammation after severe IVH. Levels of inflammatory cytokines including interleukin (IL)‐1α, IL‐β, and IL‐6 and tumor necrosis factor (TNF)‐α in brain tissue homogenates, A, and cerebrospinal fluid, B, on P32. Data are expressed as mean ± SE of the mean. Data are presented as mean ± SE of the mean. *P < .05 compared with the normal control, #P < .05 compared with the IVH injury control, Φ P < .05 compared with IVH + MSCs, Ψ P < .05 compared with IVH + MSCs‐EVs. NC, normal control; IVH, intraventricular hemorrhage control; M, MSCs transplantation; M‐EV, extracellular vesicles from naïve MSCs; Fibro‐EV, extracellular vesicles from fibroblasts; cont siRNA M‐EV, extracellular vesicles from MSCs transfected with scrambled siRNA; BDNF siRNA M‐EV, extracellular vesicles from MSCs transfected with BDNF siRNA
3.6. Functional behavior tests
In the negative geotaxis test, a significantly longer time was required to rotate from the head‐down position to the head‐up position on a slanted slope in the IC group than in NC group (Figure 6A). This functional impairment observed in the IC group was significantly improved in the IM, IMEV, and IMscrEV groups, but not in the IFEV or IMBDNFsiREV group.
FIGURE 6.
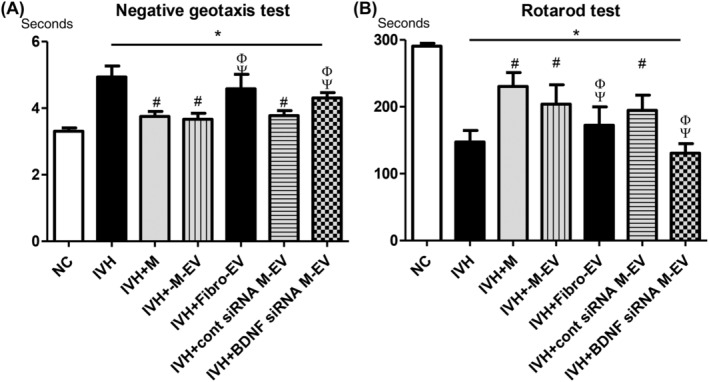
BDNF knockdown in the extracellular vesicles abolished the therapeutic effects of MSCs in improving behavioral function after severe IVH. Sensorimotor functional outcomes on the negative geotaxis test, A, and rotarod test, B, on P32. Data are expressed as mean ± SE of the mean. Data are presented as mean ± SE of the mean. *P < .05 compared with the normal control, #P < .05 compared with the IVH injury control, Φ P < .05 compared with IVH + MSCs, Ψ P < .05 compared with IVH + MSCs‐EVs. NC, normal control; IVH, intraventricular hemorrhage control; M, MSCs transplantation; M‐EV, extracellular vesicles from naïve MSCs; Fibro‐EV, extracellular vesicles from fibroblasts; cont siRNA M‐EV, extracellular vesicles from MSCs transfected with scrambled siRNA; BDNF siRNA M‐EV, extracellular vesicles from MSCs transfected with BDNF siRNA
In the rotarod test, the latency to fall was significantly shorter in the IC group than in the NC group (Figure 6B). This impaired performance was significantly improved in the IM, IMEV, and IMscrEV groups, but not in the IFEV or IMBDNFsiREV groups.
4. DISCUSSION
In the present study, MSC‐derived, but not fibroblast‐derived, EVs displayed similar therapeutic effects as parental MSCs in attenuating both thrombin‐induced neuronal cell death in vitro and severe IVH‐induced brain damages in vivo. These findings above may suggest that the neuroprotective potency of EVs are specific to parental MSCs from which EVs are originated. To our best knowledge, this is the first study presenting that MSC‐derived EVs could recapitulate the therapeutic efficacy of parent MSCs in attenuating severe IVH‐induced brain damages. As this cell‐free therapy can overcome the potential concerns and side effects associated with viable MSC transplantation, 9 , 10 , 11 the use of MSC‐derived EVs might be a potential alternative for live stem cell therapy in cases of severe IVH.
The therapeutic benefits of MSC‐derived EV transplantation are mediated by delivering cargo containing various proteins, lipids, and RNAs similar to those present in the parent MSCs. 13 , 16 , 18 , 31 Previously, we have reported on the critical role of BDNF secreted by transplanted MSCs in mediating its protective effects including improving inflammatory response, cell death, astrogliosis, and PHH advance as well as improving neurogenesis and myelination after severe IVH in rat pup model. 19 , 25 In the present study, the neuroprotective effects of MSC‐derived EVs, which are as effective as their parental MSCs, such as significantly attenuating the severe IVH‐induced increase in TUNEL‐positive apoptotic cell death, inflammatory responses, oxidative stress, and astrogliosis as well as improving the reduced brain myelination and neurogenesis, were abolished with BDNF siRNA‐transfection. These findings may implicate that the BDNF enclosed within the donor MSC‐EVs might be one of the essential secreted factors that play a critical role in protecting against severe IVH‐induced brain injuries in newborn rats.
Since BDNF is a critical factor for neuronal survival in various disorders such as focal ischemia, hypoglycemia, meningitis, and IVH, 19 , 25 , 32 , 33 , 34 our data showing thrombin‐induced increase in oxidative stress and the ensuing cell death in rat neuronal cell culture in vitro observed in the IC group might be related to the obvious decrease in the rat BDNF level below the threshold level for survival. Furthermore, along with the detectable human BDNF level checked in the naïve MSC‐derived EVs coculture group, the rat BDNF level was significantly increased, compared with that of the IC group, which corresponding well with the concurrent improvement of thrombin‐induced increase in oxidative stress and the resultant cell death in the rat neuronal cell culture. After coculture with BDNF siRNA‐transfected MSC‐derived EVs but not with scrambled siRNA‐transfected EVs, the human and rat BDNF level was significantly diminished compared with coculture with the naïve MSC‐derived EVs. Furthermore, the beneficial effects of the MSC‐EVs against thrombin‐induced upregulated oxidative stress and the ensuing apoptosis were abolished. Taken together, these findings suggest that the human BDNF transferred through MSC‐derived EVs devote to the recovery of host rat BDNF level above the threshold level essential for neuronal survival, ultimately leading to protection against severe IVH‐induced brain injuries.
Although the BDNF expression was significantly restored in the group cocultured with naïve MSC‐EVs compared with that in the nontreated control group, the absolute BDNF level was much lower than that of the NC group. Recently, we have reported that preconditioning, especially with thrombin, significantly boosts MSC‐derived EVs production and enriches their cargo contents with various paracrine factors. 13 , 20 Furthermore, thrombin‐preconditioned EVs promote proangiogenic activity in vitro and enhance cutaneous wound healing in vivo. 13 Considering the concerns for use of genetically engineered BDNF‐overexpressing MSC‐derived EVs, this simple, safe, and effective thrombin preconditioning regimen for better EV therapy could be easily translated into clinical use.
Besides histopathologic improvements, improvements in learning, memory, and cognition may be the primary concerns of parents of the infants with IVH. In the present study, the negative geotaxis and rotarod tests were done to examine sensorimotor learning after severe IVH in rats. The IVH‐induced impaired performance of these tests were significantly improved with MSCs, naïve MSCs, and scrambled siRNA‐transfected MSC‐derived EV transplantation, but not with BDNF siRNA‐transfected EV transplantation. In the present and previous studies, 19 , 25 MSC‐derived EVs provided neuroprotection by attenuating IVH‐induced neuronal cell death and enhancing neurogenesis both in the hippocampus and periventricular brain tissue. Collectively, these findings suggest that the BDNF in the EVs might be one of the critical factors in neuroprotection, which improves sensorimotor function after severe IVH.
In this study, we arbitrarily determined the therapeutic method of intracerebroventricular injection of 20 μg of EVs at P6 based on the literature review, 18 , 35 , 36 preclinical data of MSC‐derived EV transplantation for bronchopulmonary dysplasia, 16 and our previous experiences from MSC transplantation in the IVH rat pup model, which indicate that therapeutic effects was enhanced with local injection than systemic infusion 30 at an early time‐point after injury. 28 Further study are warranted to establish the optimal timing, route, and dosage of MSC‐derived EV.
In our previous study, 25 we observed that additionally supplemented recombinant human BDNF dose dependently recovered abolished neuroprotective action of BDNF knockdown MSCs, from which the EVs for the present study were derived. In this context, BDNF knockdown MSC‐EV group in the present study might be rescued by adding back BDNF supplement and this should be confirmed by further study.
5. CONCLUSION
EVs derived from MSCs are as therapeutically effective as the parental MSCs in improving IVH‐induced brain injuries, and the BDNF transferred through EVs might act as a key secretory factor mediating beneficial neuroprotective action, including attenuation of inflammatory response, apoptosis, astrogliosis, and PHH advance as well as improve brain myelination and neurogenesis after severe IVH in newborn rats.
CONFLICT OF INTEREST
W.S.P., Y.S.C., D.K.S., and S.Y.A. declared potential conflicts of interest arising from a filed or issued patent titled “Pharmaceutical composition for treating cerebrovascular diseases, containing stem cell‐derived exosome as active ingredient” as coinventors, not as patentees. The other authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
S.Y.A., D.K.S., W.S.P.: conception and design, provision of study material or patients, analyzed and interpreted the results, manuscript writing and revision, final approval of manuscript; Y.E.K., S.S., Y.S.C.: administrative support, collection and/or assembly of data, analyzed and interpreted the results, manuscript writing, approval of manuscript.
Supporting information
Figure S1: The human‐specific BDNF (A) and rat‐specific BDNF (B) levels were measured in the cell culture media in each group. Data are presented as mean ± SE of the mean. Abbreviations: RatNC, rat neuronal cell normal control; Thr, thrombin treatment; HuMSC, Human MSCs treatment, HuMSC‐EV; extracellular vesicles from naïve human MSCs, HuFibroblast‐EV; extracellular vesicles from human fibroblasts, cont siRNA HuMSC‐EV; extracellular vesicles from human MSCs transfected with scrambled siRNA, BDNF siRNA HuMSC‐EV; extracellular vesicles from human MSCs transfected with BDNF siRNA. Data are presented as mean ± SE of the mean. *P < .05 compared with the rat neuronal cells normal control, #P < .05 compared with the rat neuronal cells treated with thrombin injury control, Φ P < .05 compared with rat neuronal cells treated with thrombin and human MSCs, Ψ P < .05 compared with rat neuronal cells treated with thrombin and human MSCs‐EVs.
ACKNOWLEDGMENT
This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI14C3484), grants from the National Research Foundation of Korea (NRF) funded by the Korea Ministry of Science, ICT & Future Planning/the Ministry of Education, Science and Technology (2017R1A2B2011383, 2020R1A2C2010645), and SMC‐Ottogi Research Fund (SMX1200831).
Ahn SY, Sung DK, Kim YE, Sung S, Chang YS, Park WS. Brain‐derived neurotropic factor mediates neuroprotection of mesenchymal stem cell‐derived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. STEM CELLS Transl Med. 2021;10:374–384. 10.1002/sctm.20-0301
So Yoon Ahn and Dong Kyung Sung contributed equally to this work as co‐first authors.
Funding information SMC‐Ottogi Research Fund, Grant/Award Number: SMX1200831; ICT & Future Planning/the Ministry of Education, Science and Technology, Grant/Award Numbers: 2020R1A2C2010645, 2017R1A2B2011383; National Research Foundation of Korea; Ministry of Health & Welfare, Republic of Korea, Grant/Award Number: HI14C3484; Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI)
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr. 2014;57:251‐256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529‐534. [DOI] [PubMed] [Google Scholar]
- 3. Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993‐1994. Pediatrics. 2000;105:1216‐1226. [DOI] [PubMed] [Google Scholar]
- 4. International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. International PHVD Drug Trial Group. Lancet. 1998;352:433‐440. [PubMed] [Google Scholar]
- 5. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Ventriculomegaly Trial Group. Arch Dis Child. 1990;65:3‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kennedy CR, Ayers S, Campbell MJ, et al. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: follow‐up at 1 year. Pediatrics. 2001;108:597‐607. [DOI] [PubMed] [Google Scholar]
- 7. Ahn SY, Chang YS, Sung DK, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497‐504. [DOI] [PubMed] [Google Scholar]
- 8. Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I dose‐escalation clinical trial. Stem Cells Translational Medicine. 2018;7:847‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendt M, Rezvani K, Shpall E. Mesenchymal stem cell‐derived exosomes for clinical use. Bone Marrow Transplant. 2019;54:789‐792. [DOI] [PubMed] [Google Scholar]
- 10. Jeong JO, Han JW, Kim JM, et al. Malignant tumor formation after transplantation of short‐term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang S, Guo L, Ge J, et al. Excess integrins cause lung entrapment of mesenchymal stem cells. Stem Cells. 2015;33:3315‐3326. [DOI] [PubMed] [Google Scholar]
- 12. Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood‐derived mesenchymal stem cells attenuate hyperoxia‐induced lung injury in neonatal rats. Cell Transplant. 2009;18:869‐886. [DOI] [PubMed] [Google Scholar]
- 13. Sung DK, Chang YS, Sung SI, Ahn SY, Park WS. Thrombin preconditioning of extracellular vesicles derived from mesenchymal stem cells accelerates cutaneous wound healing by boosting their biogenesis and enriching cargo content. J Clin Med. 2019;8(4):533–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25:73‐78. [DOI] [PubMed] [Google Scholar]
- 15. Chang YS, Ahn SY, Jeon HB, et al. Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury. Am J Respir Cell Mol Biol. 2014;51:391‐399. [DOI] [PubMed] [Google Scholar]
- 16. Ahn SY, Park WS, Kim YE, et al. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell‐derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ophelders DR, Wolfs TG, Jellema RK, et al. Mesenchymal stromal cell‐derived extracellular vesicles protect the fetal brain after hypoxia‐ischemia. Stem Cells Translational Medicine. 2016;5:754‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhu YG, Feng XM, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice. Stem Cells. 2014;32:116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ko HR, Ahn SY, Chang YS, et al. Human UCB‐MSCs treatment upon intraventricular hemorrhage contributes to attenuate hippocampal neuron loss and circuit damage through BDNF‐CREB signaling. Stem Cell Res Ther. 2018;9:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sung DK, Sung SI, Ahn SY, Chang YS, Park WS. Thrombin preconditioning boosts biogenesis of extracellular vesicles from mesenchymal stem cells and enriches their cargo contents via protease‐activated receptor‐mediated signaling pathways. Int J Mol Sci. 2019;20(12):2899‐2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jang YK, Jung DH, Jung MH, et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85:212‐225. [DOI] [PubMed] [Google Scholar]
- 22. Kim JY, Kim DH, Kim DS, et al. Galectin‐3 secreted by human umbilical cord blood‐derived mesenchymal stem cells reduces amyloid‐beta42 neurotoxicity in vitro. FEBS Lett. 2010;584:3601‐3608. [DOI] [PubMed] [Google Scholar]
- 23. Lee JK, Lee MK, Jin HJ, et al. Efficient intracytoplasmic labeling of human umbilical cord blood mesenchymal stromal cells with ferumoxides. Cell Transplant. 2007;16:849‐857. [DOI] [PubMed] [Google Scholar]
- 24. Ahn SY, Chang YS, Sung DK, et al. Cell type‐dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy. 2015;17:1025‐1035. [DOI] [PubMed] [Google Scholar]
- 25. Ahn SY, Chang YS, Sung DK, et al. Pivotal role of brain derived neurotrophic factor secreted by mesenchymal stem cells in severe intraventricular hemorrhage in the newborn rats. Cell Transplant. 2016;26(1):145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hua Y, Wu J, Keep RF, et al. Tumor necrosis factor‐alpha increases in the brain after intracerebral hemorrhage and thrombin stimulation. Neurosurgery. 2006;58:542‐550. discussion 542–550. [DOI] [PubMed] [Google Scholar]
- 27. Huo J, Sun S, Geng Z, et al. Bone marrow‐derived mesenchymal stem cells promoted cutaneous wound healing by regulating keratinocyte migration via beta2‐adrenergic receptor signaling. Mol Pharm. 2018;15:2513‐2527. [DOI] [PubMed] [Google Scholar]
- 28. Park WS, Sung SI, Ahn SY, et al. Optimal timing of mesenchymal stem cell therapy for neonatal intraventricular hemorrhage. Cell Transplant. 2016;25:1131‐1144. [DOI] [PubMed] [Google Scholar]
- 29. Kim S, Kim YE, Hong S, et al. Reactive microglia and astrocytes in neonatal intraventricular hemorrhage model are blocked by mesenchymal stem cells. Glia. 2020;68:178‐192. [DOI] [PubMed] [Google Scholar]
- 30. Ahn SY, Chang YS, Sung DK, et al. Optimal route for mesenchymal stem cells transplantation after severe intraventricular hemorrhage in newborn rats. PLoS One. 2015;10:e0132919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia‐induced pulmonary hypertension. Circulation. 2012;126:2601‐2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kokaia Z, Andsberg G, Yan Q, Lindvall O. Rapid alterations of BDNF protein levels in the rat brain after focal ischemia: evidence for increased synthesis and anterograde axonal transport. Exp Neurol. 1998;154:289‐301. [DOI] [PubMed] [Google Scholar]
- 33. Li L, Shui QX, Zhao ZY. Regulation of brain‐derived neurotrophic factor (BDNF) expression following antibiotic treatment of experimental bacterial meningitis. J Child Neurol. 2003;18:828‐834. [DOI] [PubMed] [Google Scholar]
- 34. Lindvall O, Ernfors P, Bengzon J, et al. Differential regulation of mRNAs for nerve growth factor, brain‐derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc Natl Acad Sci U S A. 1992;89:648‐652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Monsel A, Zhu YG, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell‐derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Rocco G, Baldari S, Toietta G. Towards therapeutic delivery of extracellular vesicles: strategies for in vivo tracking and biodistribution analysis. Stem Cells Int. 2016;2016:5029619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: The human‐specific BDNF (A) and rat‐specific BDNF (B) levels were measured in the cell culture media in each group. Data are presented as mean ± SE of the mean. Abbreviations: RatNC, rat neuronal cell normal control; Thr, thrombin treatment; HuMSC, Human MSCs treatment, HuMSC‐EV; extracellular vesicles from naïve human MSCs, HuFibroblast‐EV; extracellular vesicles from human fibroblasts, cont siRNA HuMSC‐EV; extracellular vesicles from human MSCs transfected with scrambled siRNA, BDNF siRNA HuMSC‐EV; extracellular vesicles from human MSCs transfected with BDNF siRNA. Data are presented as mean ± SE of the mean. *P < .05 compared with the rat neuronal cells normal control, #P < .05 compared with the rat neuronal cells treated with thrombin injury control, Φ P < .05 compared with rat neuronal cells treated with thrombin and human MSCs, Ψ P < .05 compared with rat neuronal cells treated with thrombin and human MSCs‐EVs.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


