Abstract
Salmonella Enteritidis (SE) are important zoonotic pathogens, and can be easily transferred to humans by contaminated animal products. Epidemic surveys of SE are necessary in current modern large-scale chicken farms. In this study, Salmonella strains were isolated from possibly infected samples collected at 3 independent farms, and their serotype, drug resistances, virulence genes, and genetic similarity were analyzed by molecular genetic analysis technologies including multilocus sequence typing (MLST), clustered regularly interspaced short palindromic repeats (CRISPR), pulsed-field gel electrophoresis (PFGE), and whole-genome sequencing (WGS). A total of 346 Salmonella strains were isolated from 3,598 samples (9.61%); 329 isolates were identified as SE (95.09%) and 308 isolates were multidrug resistant (93.62%). Virulotyping based on 6 virulence genes showed high similarity in SE isolates of each farm, with the exception of 2 isolates. All SE isolates were found to be the same ST11 type by MLST, and 22 strains of 150 SE isolates selected at random were found to belong to 1 cluster by PFGE and the same SET1 type by CRISPR. WGS results further revealed that these isolates belonged to the same clonal cluster, with high genetic similarity of 99.80 to 100.00%. All these results indicated that these SE isolates were overwhelmingly dominant and demonstrated high genetic similarity, which revealed that the same SE clone might be transmitted in these farms.
Key words: Salmonella Enteritidis, antimicrobial susceptibility, virulotype, genetic similarity, molecular genetic analysis technology
Introduction
Food safety is of high concern worldwide, with a major focus on pathogenic microbes. Salmonella spp. are among the most frequently isolated foodborne pathogens (Bounar-Kechih et al., 2012), ranking second among the 31 major pathogens in the United States of America (Scallan et al., 2011). To date, over 2,600 Salmonella serotypes have been identified, more than half of which belong to Salmonella enterica subsp. enterica (Li et al., 2018), accounting for the majority of Salmonella infections in humans (Ren et al., 2016). Moreover, Salmonella spp. are important zoonotic pathogens worldwide and are responsible for 93.8 million foodborne illnesses and 155,000 deaths per year globally (Taylor et al., 2018). In 2013, 82,694 confirmed salmonellosis cases were reported by 27 European Union member states, resulting in a notification rate of 20.4 cases per 100,000 people (Kwambana-Adams et al., 2015). As in previous years, the 2 most commonly reported Salmonella serovars in 2013 were Salmonella Enteritidis (SE) and Salmonella Typhimurium, which represented 39.5 and 20.2%, respectively, of all reported serovars associated with confirmed human cases.
Salmonella spp. can be transmitted to humans through the food chain (Angulo et al., 2004; Kilonzo-Nthenge et al., 2008; Marshall and Levy, 2011), and these species are predominantly found in poultry, eggs, and dairy products (Silva and Aguiar, 2011; Uche et al., 2017; Velasquez et al., 2018). Specifically, chicken and by-products are a major source of Salmonella infection in clinical and nonclinical settings. There are serious consequences when chickens on a farm are infected by Salmonella. In August 2010, 0.5 billion eggs from the California-based poultry producer Foster Farms, 0.17 billion eggs from the Iowa-based poultry producer Hillandale Farms, and 0.38 billion eggs from Wright County Egg Farms were recalled. These recalls were ordered because the eggs might have been contaminated with Salmonella, and 1,000 cases of human salmonellosis had been caused by eggs that had been sold. In 2014, the Centers for Disease Control and Prevention reported a multistate outbreak of human Salmonella infection linked to live poultry in the backyard flocks in Atlanta, GA. In 2012, 300 cases of human salmonellosis were reported, and 80% of the reported ill people had contact with live poultry 1 wk before the illness began (CDC, USA). These Salmonella incidents caused social panic in addition to severe economic losses and human deaths.
In China, an increasing number of chickens are raised on closed large-scale modern farms, as opposed to on a small scale in backyards. These chickens and their products are currently the main sources of food supplies and will continue to be in the future, as in the United States. The safety of chickens with regard to Salmonella is also of high concern to the Chinese government. Thus, it is necessary to examine Salmonella infections on modern large-scale chicken farms. Such investigations will offer information regarding control strategies and Salmonella clearance for different serotypes to reduce the incidence of salmonellosis in humans, which will meet government requirements for food safety.
In this study, we investigated Salmonella infections on 3 modern large-scale chicken farms in different provinces of China. Based on the drug resistance of SE isolates, certain virulence genes, and their molecular characteristics, including multilocus sequence typing (MLST), clustered regularly interspaced short palindromic repeats (CRISPR), pulsed-field gel electrophoresis (PFGE), and whole-genome sequencing (WGS) assessments, we found SE to be a predominant serovar, with a very close relationship with regard to genetic evolution of SE among the 3 farms.
Materials and methods
Farms Selection and Samples Collection
This Salmonella survey was carried out on 3 modern large-scale chicken farms A, B, and C, in Shandong, Jiangsu, and Hebei provinces, respectively, with more than 10 million chickens. Suspected Salmonella-infected samples were continuously collected between September 2016 and July 2018 from 3 different breeder farms, once every 2 mo; samples included eggs, dead embryos, and sick and dead chickens, as well as drag samples from hatcheries according to the method described by Bailey et al. (2001). The chicken carcasses and samples were stored at 4°C and transported to the laboratory within 48 h in an insulated ice chest containing ice packs. Dead embryos were transferred to the laboratory within 24 h. Microbial analysis was performed immediately upon arrival of samples in the laboratory.
Salmonella Isolation and Serotype Identification
Liver and yolk sac samples were aseptically collected from the sick and dead chickens and dead embryos, respectively. Approximately 10 g liver tissue or yolk sac was suspended in 100 mL buffered peptone water and incubated at 37°C for 16 to 20 h; 1-mL aliquots of these pre-enriched cultures were inoculated into 10 mL Rappaport-Vassiliadis enrichment broth (BD Difco, Sparks, MD) and continuously cultured at 42°C for 24 h. The broth cultures were streaked onto xylose lysine Tergitol 4 (BD Difco) agar plates, which were incubated at 37°C for 24 h. Isolated strains exhibiting a typical Salmonella phenotype were selected, and their biochemical characteristics were confirmed using an API 20E test kit (bioMerieux, Marcy I'Etoile, France). Salmonella isolates were serotyped by slide agglutination for O and H antigens using commercially available antisera (Tianrun Bio-Pharmaceutical, Ningbo, China) according to the manufacturer's instructions.
Antimicrobial Susceptibility Testing
The antimicrobial susceptibility test was performed according to the guidelines of the Clinical and Laboratory Standards Institute (Liljebjelke et al., 2017). Agar diffusion assays were performed using Mueller-Hinton agar with disks containing 7 classes of antimicrobial agents (Brain Heart Infusion, Oxoid, Basingstoke, UK): ampicillin, 10 μg; amoxicillin/clavulanic acid, 20/10 μg; cefazolin, 30 μg; meropenem, 10 μg; aztreonam, 30 μg; kanamycin, 30 μg; gentamicin, 10 μg; streptomycin, 10 μg; amikacin, 30 μg; ciprofloxacin, 5 μg; enrofloxacin, 5 μg; nalidixic acid, 30 μg; trimethoprim/sulfamethoxazole, 1.25/23 μg; chloramphenicol, 30 μg; and nitrofurantoin, 300 μg. All Salmonella isolates were tested for susceptibility to each of these antibiotics.
Virulotyping Based on Selected Virulence Genes
Salmonella genomic DNA was extracted according to the QIAamp DNA Mini kit protocol (Qiagen GmbH, Hilden, Germany). The extracted genomic DNA was stored at 70°C for later use.
PCR was conducted in individual reactions using primers targeting the following genes: prgH, sopB, spiC, orfL, pefA, and spvC (Manning et al., 2015; Gole et al., 2017). The specific primer sequences are shown in Table 1. Fifty SE isolates randomly selected from each farm were tested.
Table 1.
Primers for virulence gene.
| Gene | Primer sequence (5′–3′) | Annealing temperature (°C) | Amplicon (bp) | Reference |
|---|---|---|---|---|
| prgH | F: GCCCGAGCAGCCTGAGAAGTTAGAAA | 55 | 755 | Manning et al., (2015) |
| R: TGAAATGAGCGCCCCTTGAGCCAGTC | ||||
| sopB | F: GAAGACTACCAGGCGCACTT | 55 | 804 | Gole et al., (2017) |
| R: TTGTGGATGTCCACGGTGAG | ||||
| spiC | F: CCTGGATAATGACTATTGAT | 56 | 300 | Manning et al., (2015) |
| R: AGTTTATGGTGATTGCGTAT | ||||
| orfL | F: GGAGTATCGATAAAGATGTT | 56 | 331 | Manning et al., (2015) |
| R: GCGCGTAACGTCAGAATCAA | ||||
| pefA | F: GCGCCGCTCAGCCGAACCAG | 58 | 154 | Manning et al., (2015) |
| R: CAGCAGAAGCCCAGGAAACAGTG | ||||
| spvC | F: TCTCTGCATTTCGCCACCAT | 58 | 563 | Manning et al., (2015) |
| R: TGCACAACCAAATGCGGAAG |
Molecular Genetic Analysis by Multi-Technologies
MLST
Seven housekeeping genes, aroC, dnaN, hemD, hisD, purE, sucA, and thrA (primers are shown in Table 2), recommended by the University College Cork (http://mst.ucc.ie) were assessed in the MLST assay (Sukhnanand et al., 2005). The amplified PCR products were sent to GenScript (Nanjing) Co., Ltd. for sequencing. The sequencing results were evaluated using MEGA v7.0 (Kumar et al., 2016), and the corresponding minimum spanning tree diagram was generated using BioNumerics v7.5 (Applied Maths NV, Sint-Martens-Latem, Belgium).
Table 2.
Amplification and sequencing primers of Salmonella for MLST.
| Locus | Sequence of primers (5′–3′) |
Size of products (bp) | |
|---|---|---|---|
| Amplification primers | Sequencing primers | ||
| aroC | F: CCTGGCACCTCGCGCTATAC | F: GGCACCAGTATTGGCCTGCT | 826 |
| R: CCACACACGGATCGTGGCG | R: CATATGCGCCACAATGTGTTG | ||
| dnaN | F: ATGAAATTTACCGTTGAACGTGA | F: CCGATTCTCGGTAACCTGCT | 833 |
| R: AATTTCTCATTCGAGAGGATTGC | R: CCATCCACCAGCTTCGAGGT | ||
| hemD | F: ATGAGTATTCTGATCACCCG | F: GTGGCCTGGAGTTTTCCACT | 666 |
| R: ATCAGCGACCTTAATATCTTGCCA | R: GACCAATAGCCGACAGCGTAG | ||
| hisD | F: GAAACGTTCCATTCCGCGCAGAC | F: GTCGGTCTGTATATTCCCGG | 894 |
| R: CTGAACGGTCATCCGTTTCTG | R: GGTAATCGCATCCACCAAATC | ||
| purE | F: ATGTCTTCCCGCAATAATCC | F: CGCATTATTCCGGCGCGTGT | 510 |
| R: TCATAGCGTCCCCCGCGGATC | R: CGCGGATCGGGATTTTCCAG | ||
| sucA | F: AGCACCGAAGAGAAACGCTG | F: AGCACCGAAGAGAAACGCTG | 643 |
| R: GGTTGTTGATAACGATACGTAC | R: GGTTGTTGATAACGATACGTAC | ||
| thrA | F: GTCACGGTGATCGATCCGGT | F: ATCCCGGCCGATCACATGAT | 852 |
| R: CACGATATTGATATTAGCCCG | R: CTCCAGCAGCCCCTCTTTCAG | ||
Abbreviation: MLST, multilocus sequence typing.
CRISPR
Primers for CRISPR are listed in Table 3 (Fei et al., 2017). The PCR products were sent to GenScript (Nanjing) Co., Ltd. for sequencing. The sequencing results were uploaded to a website (http://crispr.i2bc.paris-saclay.fr/Server/) for processing to determine the spacer arrangement of SE and to export the files in binary form. A corresponding color map was completed.
Table 3.
Amplification and sequencing primers of Salmonella for CRISPR.
| Locus | Primer sequence (5′–3′) | Size of products (bp) |
|---|---|---|
| CRISPR1 | F: GCTGGTGAAACGTGTTTATCC | 1,000–2,000 |
| R: ATTCCGGTAGATYTKGATGGAC | ||
| CRISPR2 | F: AACGCCATGGCCTCCTCCTG | 1,000–2,000 |
| R: CAAAATCAGYAAATTAGCTGCTGTTC |
Abbreviation: CRISPR, clustered regularly interspaced short palindromic repeats.
PFGE
PFGE was conducted as a part of routine surveillance. PFGE for Salmonella strains was performed according to the PulseNet protocol using Xba I as the restriction enzyme (New England Biolabs, Leusden, The Netherlands). Cluster analysis was performed with BioNumerics 5.1 using the Dice similarity coefficient and the unweighted pair group method using average linkages dendrogram type (optimization 0.5%, position tolerance 1.5%).
WGS
Genomic DNA of the selected Salmonella isolates was extracted using the QIAamp DNA Mini kit (Qiagen GmbH), and WGS was performed by Novogene Co. Ltd., Beijing, China. The genomic DNA sequencing results were analyzed using the Illumina HiSeq platform to generate 150-bp parallel (Almeida et al., 2018) reads with a data volume of approximately 150 Gb/strain clean reads (Thermo Fisher Scientific, Waltham, MA). Gene fragments were assembled using SPAdes v3.11 (Illumina, Inc., San Diego, CA) with the parameter para: –careful–cov cutoff auto. Genomic similarity analysis was performed on the assembly results using the Mash assay (Lindsay et al., 2018).
Results
High Prevalence of SE as a Predominant Serovar
The prevalence of Salmonella on the breeder farms is summarized in Table 4. Overall, the Salmonella isolation rate of farm A (Shandong) was the highest (14.02%), followed by farm C (Hebei) (9.84%), and farm B (Jiangsu) scored the lowest (5.66%). A total of 346 Salmonella isolates were recovered from 3,598 samples (9.61%). These Salmonella isolates showed low diversity, with 95.09% being identified as SE (Table 5).
Table 4.
Salmonella isolation from different samples in 3 farms.
| Samples |
Salmonella positive/samples (% ratio) |
Total |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A (Shandong) | |||||||||||||
| Eggs | 0/17 | 2/26 (7.7) | 1/23 (4.3) | 3/32 (9.4) | 1/25 (4) | 1/29 (3.4) | 2/24 (8.3) | 0/19 | 2/21 (9.5) | 0/25 | 4/25 (16) | 0/21 | 16/287 (5.6) |
| Dead embryos | 11/34 (32.4) | 9/30 (30) | 11/37 (29.7) | 7/27 (25.9) | 7/29 (24.1) | 7/25 (28) | 10/33 (30.3) | 11/35 (31.4) | 9/32 (28.1) | 8/29 (27.6) | 7/23 (30.4) | 11/36 (30.6) | 108/370 (29.2) |
| Sick/dead chickens | - | 0/1 | 1/1 (100) | - | 1/3 (33.3) | 0/2 | - | 1/3 (33.3) | 1/2 (50) | - | 1/2 (50) | 0/1 | 5/15 (33.3) |
| Drag samples | 2/28 (7.1) | 2/37 (5.4) | 1/35 (2.9) | 0/35 | 5/39 (12.8) | 2/36 (5.6) | 2/33 (6.1) | 0/29 | 3/35 (8.6) | 2/32 (6.3) | 2/34 (5.9) | 1/32 (3.1) | 22/405 (5.4) |
| Total | 151/1,077 (14.02) | ||||||||||||
| B (Jiangsu) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggs | 2/40 (5) | 0/43 | 3/52 (5.8) | 0/36 | 1/39 (2.6) | 2/41 (4.9) | 1/43 (2.3) | 0/33 | 2/40 (5) | 0/39 | 2/42 (4.8) | 1/36 (2.8) | 14/484 (2.9) |
| Dead embryos | 5/28 (17.9) | 6/31 (19.4) | 4/23 (17.4) | 4/25 (16) | 3/22 (13.6) | 4/27 (14.8) | 6/28 (21.4) | 4/25 (16) | 3/23 (13.0) | 3/25 (12) | 4/22 (18.2) | 3/23 (13.0) | 49/302 (16.2) |
| Sick/dead chickens | 0/2 | - | - | 0/2 | 1/1 (100) | 0/1 | - | 0/1 | - | 1/2 (50) | 0/2 | 0/1 | 2/12 (16.7) |
| Drag samples | 0/37 | 0/41 | 0/40 | 1/35 (2.9) | 1/42 (2.4) | 0/41 | 2/43 (4.7) | 1/36 (2.8) | 0/42 | 1/40 (2.5) | 1/37 (2.7) | 0/39 | 7/473 (1.5) |
| Total | 72/1,271 (5.66) | ||||||||||||
| C (Hebei) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eggs | 2/41 (4.9) | 0/37 | 3/30 (10) | 3/43 (7.0) | 4/40 (10) | 2/38 (5.3) | 3/42 (7.1) | 1/40 (2.5) | 4/39 (10.3) | 2/43 (4.7) | 1/36 (2.8) | 3/39 (7.7) | 28/468 (6.0) |
| Dead embryos | 6/21 (28.6) | 8/24 (33.3) | 5/22 (22.7) | 8/26 (30.8) | 6/26 (23.1) | 6/20 (30) | 6/23 (26.1) | 5/21 (23.8) | 7/25 (28) | 6/23 (26.1) | 5/22 (22.7) | 5/24 (20.1) | 73/277 (26.4) |
| Sick/dead chickens | - | - | 0/1 | - | 0/2 | - | 1/2 (50) | 1/1 (100) | 0/3 | 2/2 (100) | 0/2 | 0/3 | 4/16 (25) |
| Drag samples | 2/42 (4.8) | 0/43 | 0/39 | 4/36 (11.1) | 2/42 (4.8) | 3/46 (6.5) | 1/41 (2.4) | 0/35 | 0/45 | 4/40 (10) | 0/41 | 2/39 (5.1) | 18/489 (3.7) |
| Total | 123/1,250 (9.84) | ||||||||||||
Table 5.
Classification of Salmonella serovars in 3 farms.
| Farm | No. of samples | No. of Salmonella isolates (%) | No. of Salmonella isolates (%) |
||||
|---|---|---|---|---|---|---|---|
| SE | ST | SIND | SINF | SD | |||
| A (Shandong) | 1,077 | 151 (14.02) | 144 (95.36) | 6 (3.97) | 1 (0.67) | ||
| B (Jiangsu) | 1,271 | 72 (5.66) | 70 (97.22) | 2 (2.78) | |||
| C (Hebei) | 1,250 | 123 (9.84) | 115 (93.50) | 4 (3.25) | 2 (1.62) | 2 (1.62) | |
| Total | 3,598 | 346 (9.61) | 329 (95.09) | 12 (3.46) | 1 (0.29) | 2 (0.58) | 2 (0.58) |
Abbreviations: SD, Salmonella Derby; SE, Salmonella Enteritidis; SIND, Salmonella Indiana; SINF, Salmonella Infantis; ST, Salmonella Typhimurium.
Little Difference in Antimicrobial Susceptibility
The antimicrobial susceptibilities or drug resistances observed are summarized in Table 6. A total of 329 SE isolates were resistant to at least 1 antimicrobial agent, and 308 SE isolates exhibited multidrug resistance (93.62%). The highest resistance rates recorded corresponded to nalidixic acid (96.35%), ampicillin (57.75%), and streptomycin (53.19%). There was little difference in the drug resistance of SE isolates among the 3 breeder farms.
Table 6.
Antibiotic susceptibility among 329 SE isolates in 3 breeder farms.
| Drugs | No. of drug-resistant strains (%) |
||||
|---|---|---|---|---|---|
| A n=144 | B n=70 | C n=115 | Total n=329 | ||
| β-Lactams | AMP | 93 (64.58) | 52 (74.29) | 45 (39.13) | 190 (57.75) |
| AMC | 68 (47.22) | 14 (20.00) | 1 (0.87) | 83 (25.23) | |
| CZO | 15 (10.42) | 4 (5.71) | 8 (6.96) | 27 (8.21) | |
| MEM | 3 (2.08) | 3 (4.29) | 0 (0) | 6 (1.82) | |
| ATM | 14 (9.72) | 3 (4.29) | 7 (6.09) | 24 (7.29) | |
| Aminoglycosides | KAN | 0 (0) | 4 (5.71) | 6 (5.22) | 10 (3.04) |
| GEN | 0 (0) | 4 (5.71) | 1 (0.87) | 5 (1.52) | |
| STR | 92 (63.89) | 50 (71.43) | 33 (28.70) | 175 (53.19) | |
| AMK | 0 (0) | 4 (5.71) | 0 (0) | 4 (1.22) | |
| Quinolones | CIP | 15 (10.42) | 57 (81.43) | 3 (2.61) | 75 (22.80) |
| ENR | 66 (45.83) | 33 (47.14) | 3 (2.61) | 102 (31.00) | |
| NAL | 143 (99.31) | 69 (98.57) | 105 (91.30) | 317 (96.35) | |
| Sulfonamides | SXT | 3 (2.08) | 4 (5.71) | 3 (2.61) | 10 (3.04) |
| Chloramphenicols | CHL | 1 (0.69) | 5 (7.14) | 13 (11.30) | 19 (5.78) |
| Nitrofurantoin | NIT | 134 (93.06) | 12 (17.14) | 2 (1.74) | 148 (44.98) |
Abbreviations: AMC, amoxicillin/clavulanic acid; AMK, amikacin; AMP, ampicillin; ATM, aztreonam; CHL, chloramphenicol; CIP, ciprofloxacin; CZO, cefazolin; ENR, enrofloxacin; GEN, gentamicin; KAN, kanamycin; MEM, meropenem; NAL, nalidixic acid; NIT, nitrofurantoin; SE, Salmonella Enteritidis; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole.
High Similarity in Virulotype
Salmonella virulence was assessed by PCR using 50 SE isolates randomly selected from each farm. All SE isolates tested positive for the pefA and spvC genes (Table 7). Only 2 isolates differed: 1 SE isolate from the Shandong A farm carried only orfL, spiC, pefA, and spvC genes without prgH and sopB genes; and 1 SE isolate from the Jiangsu B farm harbored only pefA and spvC genes without prgH, sopB, orfL, and spiC genes. The rate of virulence gene positivity among the remaining SE isolates tested was 100%.
Table 7.
PCR results for the presence of virulence genes in SE isolates in 3 breeder farms.
| Farm | PCR results | No. of resistant strains |
|---|---|---|
| A (Shandong) | prgH-sopB-orfL-spiC-pefA-spvC | 49 |
| orfL-spiC-pefA-spvC | 1 | |
| B (Jiangsu) | prgH-sopB-orfL-spiC-pefA-spvC | 49 |
| pefA-spvC | 1 | |
| C (Hebei) | prgH-sopB-orfL-spiC-pefA-spvC | 50 |
Abbreviation: SE, Salmonella Enteritidis.
High Genetic Similarity by Multi-Technologies
MLST
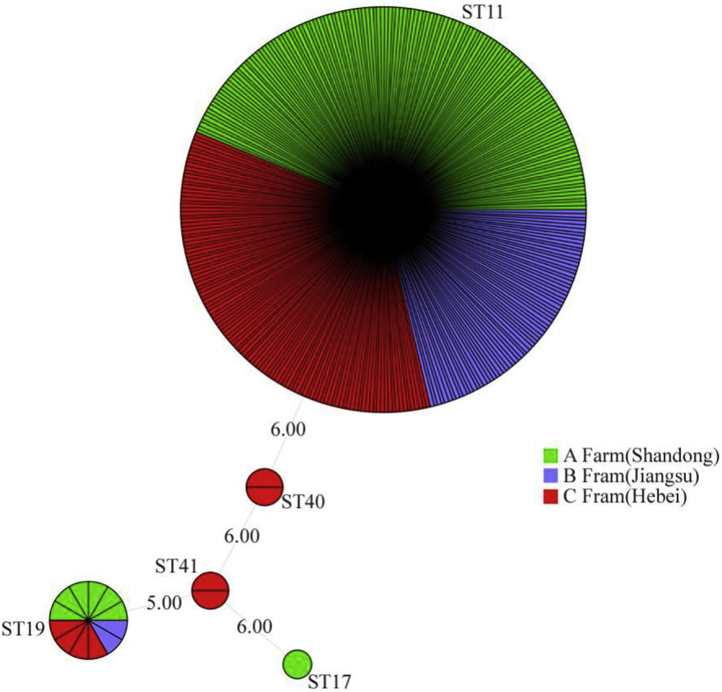
MLST was performed on the Salmonella isolates, and the sequencing results showed 95.09% (329 out of 346) of these Salmonella isolates to be ST11, indicating that they are SE. MLST results for the SE isolates showed that the nucleotide sequence ranges of the aroC, dnaN, hemD, hisD, purE, sucA, and thrA sites were completely identical: 5, 2, 3, 7, 6, 6, and 11, respectively (Figure 1).
Figure 1.
Three hundred and twenty nine of 346 isolates from 3 breeder farms were ST11-type (SE) based on MLST. Abbreviations: MLST, multilocus sequence typing; SE, Salmonella Enteritidis.
CRISPR
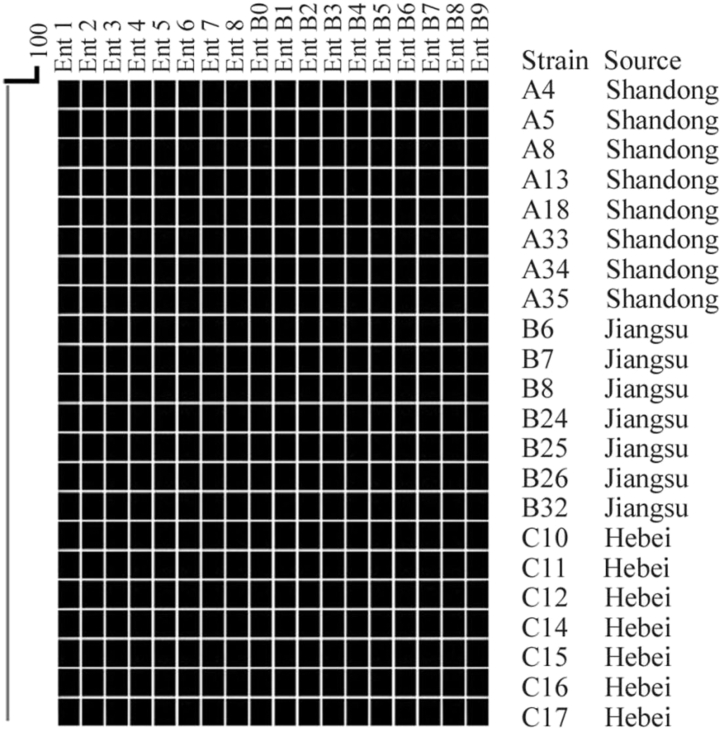
All SE isolates from the 3 breeder farms were subjected to CRISPR typing (Fei et al., 2017). Specific results with the same SET1 CRISPR type are presented in Figure 2. CRISPR1-Spacer had 8 spacers, including Ent1, Ent2, Ent3, Ent4, Ent5, Ent6, Ent7, and Ent8. The CRISPR2 segments had 10 spacers, including EntB0 and EntB1, EntB2, EntB3, EntB4, EntB5, EntB6, EntB7, EntB8, and EntB9. The spacer composition and arrangement of CRISPR types revealed that all the tested SE isolates from the 3 breeder farms were of the SET1 type (Figure 3), which was the same as the CRISPR type of SE P125109 (submission number: AM933172) in the National Center for Biotechnology Information library.
Figure 2.
CRISPR typing dendrogram of SE isolates in 3 breeder farms.
Figure 3.
Twenty two representative SE isolates were SET1-type based on CRISPR typing. Abbreviations: CRISPR, clustered regularly interspaced short palindromic repeats; SE, Salmonella Enteritidis.
PFGE
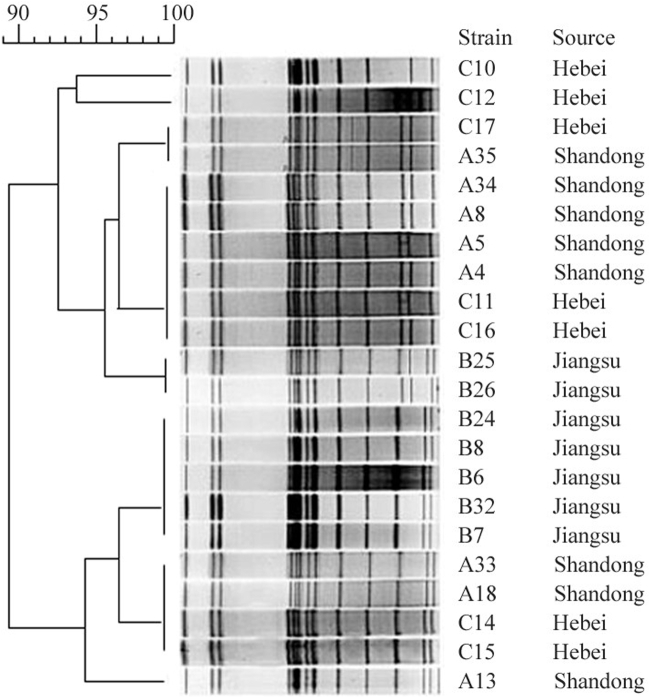
The same 22 SE isolates from the 3 breeder farms were subjected to PFGE subtyping. The Dice coefficient was greater than 0.85 for the same cluster, and Salmonella in the same cluster was considered homologous. A homology of 100% was considered to represent the same PFGE subtype, and the 22 SE strains were divided into 1 cluster and 8 PFGE subtypes (Figure 4). The similarity of the bands was high, indicating that these SE isolates on the 3 chicken farms had very similar genetic relationships, though a few bands differed significantly.
Figure 4.
Twenty two representative SE isolates belonged to 1 same cluster based on PFGE. Abbreviations: PFGE, pulsed-field gel electrophoresis; SE, Salmonella Enteritidis.
WGS
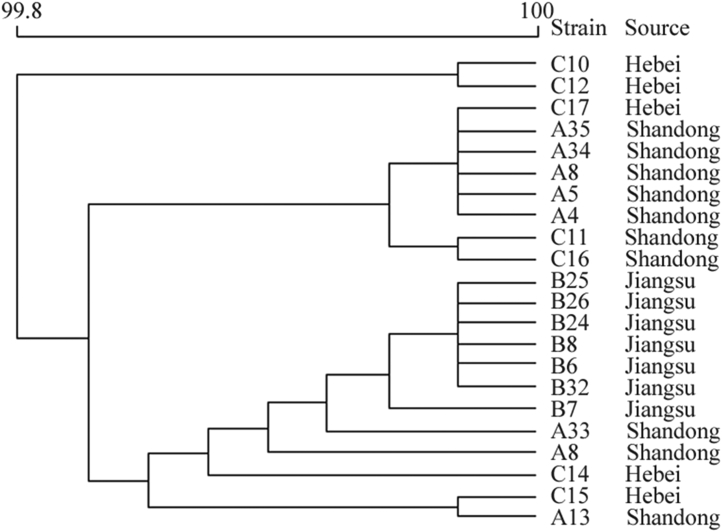
Genomic DNA sequencing results showed that the similarity among the 22 representative SE isolates from the 3 breeder farms was 99.80 to 100.00% (Figure 5); these isolates also belonged to the same clonal cluster. WGS analysis further indicated the possibility that the same SE strain was pandemic on the 3 breeder farms.
Figure 5.
Twenty two representative SE isolates belonged to 1 same clonal cluster based on WGS. Abbreviations: SE, Salmonella Enteritidis; WGS, whole-genome sequencing.
Discussion
Salmonella infection remains a major public health concern worldwide (Flockhart et al., 2017), contributing to the economic burden of both industrialized and underdeveloped countries due to the costs associated with surveillance, prevention, and treatment of salmonellosis (Uche et al., 2017). In China, an increasing number of chickens are raised on closed large-scale modern farms and not on a small scale in backyards. These chickens and their by-products are major sources of food and will continue to be a major source in the future, as in the western developed countries (Besser, 2018).
In the past, chickens were raised on a small scale in the backyard, and many different serotypes of Salmonella were isolated, with rich diversity, including Salmonella Pullorum, Salmonella Gallinarum, SE, and Salmonella Typhimurium (Ford et al., 2018). Although SE can also infect chickens, the birds show no clinical symptoms, and few die under normal feeding conditions. Thus, SE has been easily neglected. In addition, Salmonella has a placeholder effect: once SE becomes established on a farm, it diffuses via horizontal and vertical transmission. Vertical transmission of SE can lead to infection of eggs and offspring to cause generational magnification, persisting on a farm for long time (Taylor et al., 2018). In this survey, Salmonella strains were isolated from many eggs, dead embryos, and sick and dead chickens (Bailey et al., 2001; Fei et al., 2017), though we cannot conclude which Salmonella strain was the main factor causing death among embryos and chickens. As SE in chickens can exclude other Salmonella serotypes, SE can readily become the predominant serotype.
In this survey, Salmonella isolates exhibited low diversity, and SE was the predominant serotype on modern large-scale chicken farms in China, consistent with reports from other countries (Silva et al., 2011; Uche et al., 2017). Additionally, a very close genetic relationship among the SE isolates from the 3 breeder farms in China was observed, and the same pandemic SE strain might be present on these farms. We sought to address why SE is prevalent on different remote poultry farms and has a very close genetic relationship, though it might be the same pandemic strain. This transition may be due to the introduction by breeders, which had been confirmed that SE was able to be transmitted following the chick supply chain (Fei et al., 2017). Some modern large-scale chicken farms have no self-cultivated ancestral chickens, with their chickens obtained from different domestic ancestral chicken farms. Conversely, some modern large-scale chicken farms have their own ancestral chickens, which were imported directly from farms abroad; nonetheless, it cannot be guaranteed in a commercial contract that these ancestral chickens were not infected with SE. According to many reports (Eriksson et al., 2018), Salmonella Pullorum has been purged from chicken farms in developed western countries, whereas SE has not been completely purged. Many of the breeder farms involved in this experiment were recorded as importing chickens from abroad. It is possible that Salmonella was introduced when chickens were imported, which may account for this type of SE. Regardless, there is a lack of sufficient evidence for this hypothesis that this large cluster of SE derives from the same source as the common SE in exporting countries, and further investigation is needed.
Four technologies, including MLST, CRISPR, PFGE, and WGS, were employed for genetic analysis, with the results of MLST and CRISPR being similar and those of PFGE and WGS showing high similarity. PFGE indicated that the representative SE isolates assessed belonged to 1 cluster, with a Dice coefficient greater than 0.85. WGS also showed that the isolates belonged to the same clonal cluster, with similarity of 99.80 to 100.00%. These 4 methods indicated that genetic differences among these SE isolates were very small, and the fact that the isolates have a close relationship suggests the same pandemic strain in these farms. If this is true, SE will become a main target during Salmonella clearance. Many targeted measures, such as a novel vaccine, can be used to control this type of SE, which is the main serotype in these farms.
However, antimicrobial susceptibility testing revealed little differences in drug resistance among these Salmonella isolates in different breeder farms. It is possible that the same bacterium can undergo changes in drug resistance to adapt to different environments and antibiotic pressures.
The severe problem of SE in modern large-scale chicken farms is not restricted to China; it is in fact a worldwide issue, and it is currently difficult to control this type of SE in these farms. However, researchers in China and other countries continue to study novel methods to protect poultry and livestock against SE. The high genetic similarity of SE as a predominant serotype in large-scale chicken farms in China will offer a new targeted strain to design vaccines.
Acknowledgments
This work was supported by the National Key Research and Development Program Special Project (2016YFD0501607), the Special Fund for Agro-scientific Research in the Public Interest (201403054), the National Natural Science Foundation of China (31320103907), and Open Project of State Key Laboratory of Genetically Engineered Veterinary Vaccines (AGVSKL-ZY-201803).
Disclosures
No conflict of interest exists among authors during manuscript submission and publishing.
Footnotes
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.psj.2020.12.038.
Supplementary data
References
- Angulo J.A., Angulo N., Yu J. Antagonists of the neurokinin-1 or dopamine D1 receptors confer protection from methamphetamine on dopamine terminals of the mouse striatum. Ann. Ny. Acad. Sci. 2004;1025:171–180. doi: 10.1196/annals.1316.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida F., Seribelli A.A., Medeiros M.I.C., Rodrigues D.D.P., MelloVarani A.D., Luo Y., Allard M.W., Falcão J.P. Phylogenetic and antimicrobial resistance gene analysis of Salmonella Typhimurium strains isolated in Brazil by whole genome sequencing. PLoS One. 2018;13:e0201882. doi: 10.1371/journal.pone.0201882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bounar-Kechih S., Hamdi T.M., Mezali L., Assaous F., Rahal K. Antimicrobial resistance of 100 Salmonella strains isolated from Gallus gallus in 4 wilayas of Algeria. Poult. Sci. 2012;91:1179–1185. doi: 10.3382/ps.2011-01620. [DOI] [PubMed] [Google Scholar]
- Bailey J.S., Stern N.J., Fedorka-Cray P., Craven S.E., Cox N.A., Cosby D.E., Ladely S., Musgrove M.T. Sources and movement of Salmonella through integrated poultry operations: a multistate epidemiological investigation. J. Food Prot. 2001;64:1690–1697. doi: 10.4315/0362-028x-64.11.1690. [DOI] [PubMed] [Google Scholar]
- Besser J.M. Salmonella epidemiology: a whirlwind of change. Food Microbiol. 2018;71:55–59. doi: 10.1016/j.fm.2017.08.018. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Soderlund R., Ernholm L., Melin L., Jansson D.S. Diagnostics, epidemiological observations and genomic subtyping in an outbreak of pullorum disease in non-commercial chickens. Vet. Microbiol. 2018;217:47–52. doi: 10.1016/j.vetmic.2018.02.025. [DOI] [PubMed] [Google Scholar]
- Fei X., He X., Guo R., Yin C., Geng H., Wu K., Yin K., Geng S., Pan Z., Li Q., Jiao X. Analysis of prevalence and CRISPR typing reveals persistent antimicrobial-resistant Salmonella infection across chicken breeder farm production stages. Food Control. 2017;77:102–109. [Google Scholar]
- Flockhart L., Pintar K., Cook A., McEwen S., Friendship R., Kelton D., Pollari F. Distribution of Salmonella in humans, production animal operations and a watershed in a foodnet Canada sentinel site. Zoonoses. Public Hlth. 2017;64:41–52. doi: 10.1111/zph.12281. [DOI] [PubMed] [Google Scholar]
- Ford L., Moffatt C.R.M., Fearnley E., Miller M., Gregory J., Sloan-Gardner T.S., Polkinghorne B.G., Bell R., Franklin N., Williamson D.A., Glass K., Kirk M.D. The epidemiology of Salmonella enterica outbreaks in Australia, 2001–2016. Front. Sustain. Food Syst. 2018;2:86. [Google Scholar]
- Gole V.C., Woodhouse R., Caraguel C., Moyle T., Rault J.L., Sexton M., Chousalkar K. Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 2017;83:3313–3316. doi: 10.1128/AEM.03313-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwambana-Adams B., Darboe S., Nabwera H., Foster-Nyarko E., Ikumapayi U.N., Secka O., Betts M., Bradbury R., Wegmüller R., Lawal B. Salmonella infections in the Gambia, 2005-2015. Clin. Infect. Dis. 2015;61:S354–S362. doi: 10.1093/cid/civ781. [DOI] [PubMed] [Google Scholar]
- Kilonzo-Nthenge A., Nahashon S.N., Chen F., Adefope N. Prevalence and antimicrobial resistance of pathogenic bacteria in chicken and Guinea fowl. Poult. Sci. 2008;87:1841–1848. doi: 10.3382/ps.2007-00156. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang X., Yin K., Hu Y., Xu H., Xie X., Xu L., Fei X., Chen X., Jiao X. Genetic analysis and CRISPR typing of Salmonella enterica serovar Enteritidis from different sources revealed potential transmission from poultry and pig to human. Int. J. Food Microbiol. 2018;266:119–125. doi: 10.1016/j.ijfoodmicro.2017.11.025. [DOI] [PubMed] [Google Scholar]
- Liljebjelke K.A., Hofacre C.L., White D.G., Ayers S., Lee M.D., Maurer J.J. Diversity of antimicrobial resistance phenotypes in Salmonella isolated from commercial poultry farms. Front. Vet. Sci. 2017;4:96. doi: 10.3389/fvets.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay C., Flint J., Lilly K., Hope K., Durrheim D.N. Retrospective use of whole genome sequencing to better understand an outbreak of Salmonella enterica serovar Mbandaka in New South Wales, Australia. Western. Pac. Surveill. Response J. 2018;9:20–25. doi: 10.5365/wpsar.2017.8.4.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24:718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning J., Gole V., Chousalkar K. Screening for Salmonella in backyard chickens. Prev. Vet. Med. 2015;120:241–245. doi: 10.1016/j.prevetmed.2015.03.019. [DOI] [PubMed] [Google Scholar]
- Ren X., Li M., Xu C., Cui K., Feng Z., Fu Y., Zhang J., Liao M. Prevalence and molecular characterization of Salmonella enterica isolates throughout an integrated broiler supply chain in China. Epidemiol. Infect. 2016;144:2989–2999. doi: 10.1017/S0950268816001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan E., Hoekstra R.M., Angulo F.J., Tauxe R.V., Widdowson M.-A., Roy S.L., Jones J.J., Griffin P.M. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.I., Aguiar H.G. Diversification in the first year of food life. Acta Med. Port. 2011;24:1035–1040. [PubMed] [Google Scholar]
- Sukhnanand S., Alcaine S., Warnick L.D., Su W., Hof J., Craver M.P.J., McDonough P., Boor K.J., Wiedmann M. DNA sequence-based subtyping and evolutionary analysis of selected Salmonella enterica serotypes. J. Clin. Microbiol. 2005;43:3688–3698. doi: 10.1128/JCM.43.8.3688-3698.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J., Leite D., Fernandes M., Mena C., Gibbs P.A., Teixeira P. Campylobacter spp. as a foodborne pathogen: a review. Front. Microbiol. 2011;2:200. doi: 10.3389/fmicb.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M., Cox W., Otterstatter M., With N. de., Galanis E. Evaluation of agricultural interventions on human and poultry-related Salmonella Enteritidis in British columbia. Foodborne. Pathog. Dis. 2018;15:39–43. doi: 10.1089/fpd.2017.2302. [DOI] [PubMed] [Google Scholar]
- Uche I.V., MacLennan C.A., Saul A. A systematic review of the incidence, risk factors and case fatality rates of invasive nontyphoidal Salmonella (iNTS) disease in Africa (1966 to 2014) PLoS Neglect. Trop. D. 2017;11:e0005118. doi: 10.1371/journal.pntd.0005118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez C.G., Macklin K.S., Kumar S., Bailey M., Ebner P.E., Oliver H.F., Martin-Gonzalez F.S., Singh M. Prevalence and antimicrobial resistance patterns of Salmonella isolated from poultry farms in southeastern United States. Poult. Sci. 2018;97:2144–2152. doi: 10.3382/ps/pex449. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.