Abstract
Background & Aims
cAMP responsive element-binding protein 3 like 3 (CREB3L3) is a membrane-bound transcription factor involved in the maintenance of lipid metabolism in the liver and small intestine. CREB3L3 controls hepatic triglyceride and glucose metabolism by activating plasma fibroblast growth factor 21 (FGF21) and lipoprotein lipase. In this study, we intended to clarify its effect on atherosclerosis.
Methods
CREB3L3-deficifient, liver-specific CREB3L3 knockout, intestine-specific CREB3L3 knockout, both liver- and intestine-specific CREB3L3 knockout, and liver CREB3L3 transgenic mice were crossed with LDLR−/− mice. These mice were fed with a Western diet to develop atherosclerosis.
Results
CREB3L3 ablation in LDLR−/− mice exacerbated hyperlipidemia with accumulation of remnant APOB-containing lipoprotein. This led to the development of enhanced aortic atheroma formation, the extent of which was additive between liver- and intestine-specific deletion. Conversely, hepatic nuclear CREB3L3 overexpression markedly suppressed atherosclerosis with amelioration of hyperlipidemia. CREB3L3 directly up-regulates anti-atherogenic FGF21 and APOA4. In contrast, it antagonizes hepatic sterol regulatory element-binding protein (SREBP)–mediated lipogenic and cholesterogenic genes and regulates intestinal liver X receptor–regulated genes involved in the transport of cholesterol. CREB3L3 deficiency results in the accumulation of nuclear SREBP proteins. Because both transcriptional factors share the cleavage system for nuclear transactivation, full-length CREB3L3 and SREBPs in the endoplasmic reticulum (ER) functionally inhibit each other. CREB3L3 promotes the formation of the SREBP-insulin induced gene 1 complex to suppress SREBPs for ER-Golgi transport, resulting in ER retention and inhibition of proteolytic activation at the Golgi and vice versa.
Conclusions
CREB3L3 has multi-potent protective effects against atherosclerosis owing to new mechanistic interaction between CREB3L3 and SREBPs under atherogenic conditions.
Keywords: CREB3L3, SREBP, Hyperlipidemia, Enterohepatic Circulation
Abbreviations used in this paper: Apo, apolipoprotein; CREB3L3, cAMP responsive element-binding protein 3 like 3; ER, endoplasmic reticulum; FFA, free fatty acid; FGF21, fibroblast growth factor 21; GFP, green fluorescent protein; HDL, high-density lipoprotein; HPLC, high-performance liquid chromatography; HSV, herpes simplex virus; Insig, insulin-induced gene; KO, knockout; LPL, lipoprotein lipase; LXR, liver X receptor; PLA, proximity ligation assay; PPARα, peroxisome proliferator-activated receptor alpha; S1P, site-1 protease; SREBF, sterol regulatory element-binding factor; SREBP, sterol regulatory element-binding protein; TBA, total bile acid; TC, total cholesterol; Tg, transgenic; TG, triglyceride; VLDL, very-low-density lipoprotein; WD, Western diet
Graphical abstract
Summary.
Deficiency of CREB3L3 accelerates atherosclerosis, whereas liver CREB3L3 overexpression mice improve atherosclerosis. Hepatic and intestinal CREB3L3 cooperate to regulate whole-body lipid metabolism. Premature CREB3L3 suppresses the intracellular transport from ER to nuclear and the cleavage processes of SREBP.
cAMP responsive element-binding protein 3 like 3 (CREB3L3) is expressed only in the liver and intestinal cells,1 where the CREB3L3 protein localizes in the endoplasmic reticulum (ER) and is transported to the Golgi apparatus and nucleus.1, 2, 3 Nuclear expression of the active form of CREB3L3 in the nucleus is increased under fasting. This is consistent with the finding that CREB3L3 mRNA expression is higher during fasting than refeeding.4 CREB3L3 reduces plasma triglyceride (TG) levels by increasing the hepatic expression of apolipoprotein (Apo)-encoding genes such as apolipoprotein A4 (Apoa4), Apoa5, and Apoc2,1 which activate blood lipoprotein lipase (LPL) activity. CREB3L3−/− mice exhibit massive hepatic lipid metabolite accumulation and significantly increased plasma TG levels or nonalcoholic steatohepatitis when fed an atherogenic high-fat diet.5 APOA4 regulates high-density lipoprotein (HDL) metabolism by activating lecithin-cholesterol acyltransferase, a key enzyme involved in cholesterol transfer to newly synthesized HDL particles.6,7 This leads to stimulation of cholesterol efflux from macrophages8 and activation of receptor-mediated uptake of HDL by hepatocytes.9 Overexpression of APOA4 in mice prevents the development of atherosclerosis.10, 11, 12
CREB3L3 and peroxisome proliferator-activated receptor alpha (PPARα) synergistically activate hepatic fibroblast growth factor 21 (Fgf21) expression.13,14 Synthesized FGF21 proteins are secreted into the circulation and transported to peripheral tissues. This includes brain and skeletal muscle, as well as white adipose tissue and brown adipose tissue, in which FGF21 activates lipolysis and thermogenesis, respectively.15 These effects improve diabetes and hyperlipidemia by reducing plasma glucose, insulin, TG, and cholesterol. FGF21 suppresses atherosclerotic development by reducing hypercholesterolemia, oxidative stress, and vascular smooth muscle cell proliferation via adiponectin-dependent and -independent mechanisms.16,17 FGF21 regulates monocyte and macrophage recruitment, proliferation, and inflammatory functions in bloods and myocardial tissues, preventing macrophage accumulation, inflammation, and fibrosis.16,18,19
Recently, it has been shown that CREB3L3 plays a crucial role in lipoprotein metabolism, and LDLR−/−CREB3L3−/− mice develop significantly more atherosclerotic lesions in the aortas than LDLR−/− mice.20 However, the contribution of hepatic and intestinal CREB3L3 to atherosclerosis remains unclear. In this study, we revealed that LDLR−/−CREB3L3−/− mice exhibited severe atherosclerosis by inducing sterol regulatory element-binding protein (SREBP) activation. Liver- and intestine-specific CREB3L3-knockout (KO) in LDLR−/− mice (LDLR−/− LKO and LDLR−/− IKO, respectively) also showed accelerated atherosclerosis formation compared with LDLR−/− mice. Conversely, hepatic CREB3L3 overexpression (TgCREB3L3) in LDLR−/− (LDLR−/−TgCREB3L3) mice suppressed atherosclerosis. Collectively, we propose that CREB3L3 in enterohepatic circulation plays a crucial role in atherosclerosis development, and the mechanism involved in this process warrants further investigation.
Results
CREB3L3 Deletion Promotes Atherosclerosis With Severe Hyperlipidemia at an Early Stage of Western Diet and Irrespective of Sex
To evaluate the early stage of atherosclerosis in LDLR−/−CREB3L3−/− mice, female and male LDLR−/−CREB3L3−/− mice were fed a Western diet (WD) for 5 weeks. The LDLR−/−CREB3L3−/− mice showed a significant increase in atherosclerotic lesion formation in both the entire aorta and aortic root compared with control LDLR−/− mice, which showed barely detectable lesions at this stage (Figures 1A and B, 2A and B). Plasma TG, total cholesterol (TC), total bile acid (TBA), and free fatty acid (FFA) levels were markedly higher in female and male LDLR−/−CREB3L3−/− mice than in LDLR−/− mice (Figures 1C and D, 2C and D). High-performance liquid chromatography (HPLC) analysis revealed marked accumulation of TG and cholesterol and significant enrichment of APOB-containing lipoprotein fractions in female and male LDLR−/−CREB3L3−/− mice (Figures 1C and 2C). Significant increases in very-low-density lipoprotein (VLDL)-APOB proteins (APOB100 and APOB40) were observed in female and male LDLR−/−CREB3L3−/− mice relative to LDLR−/− mice (Figures 1C and 2C). Overexpression of APOA4, a target of CREB3L3,21 has been reported to possess anti-atherogenic properties.10, 11, 12 Plasma APOA4 levels in LDLR−/−CREB3L3−/− mice were significantly lower than those measured in LDLR−/−mice (Figures 1C and 2C). Plasma levels of FGF21, an anti-atherogenic hormone, were significantly reduced in female and male LDLR−/−CREB3L3−/− mice (Figures 1D and 2D). Collectively, we hypothesized that the absence of CREB3L3 induced severe combined hyperlipidemia with pro-atherogenic plasma lipoprotein and hormonal profiles. Of note, the atherosclerosis lesions were fewer but clearly visible in both female and male LDLR−/−CREB3L3−/− mice even when they consumed a normal diet, which gave rise to almost negligible atheroma in LDLR−/−mice (Figure 3A, B, D, and E). Plasma TG, TC, and FFA levels were markedly higher in female and male LDLR−/−CREB3L3−/− mice than in LDLR−/− mice (Figure 3C and D). Plasma TBA levels were higher in female LDLR−/−CREB3L3−/− mice than in LDLR−/− mice but reversed in male LDLR−/−CREB3L3−/− mice (Figure 3C and D).
Figure 1.
A WD for 5 weeks promotes atherosclerosis in LDLR−/−CREB3L3−/−mice. Ten- to 11-week-old female LDLR−/− and LDLR−/−CREB3L3−/− mice were fed a WD for 5 weeks. Samples were collected in a fed state. (A) Representative images of entire Sudan IV-stained aortas from LDLR−/− (n = 14) and LDLR−/−CREB3L3−/− (n = 17) mice. Surface area occupied by lesions was quantified. ∗∗P < .01 vs LDLR−/− mice. (B) Representative aortic root sections from LDLR−/− (n = 10) and LDLR−/−CREB3L3−/− (n = 16) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic root lesion areas were quantified. ∗∗P < .01 vs LDLR−/− mice. (C) Plasma TG and TC levels (n = 7, respectively). HPLC analysis of plasma lipoprotein profiles specific for plasma TG and cholesterol. APOB100 and APOB48 in VLDL fractions and its quantification (n = 7, respectively). ∗∗P < .01 vs LDLR−/− mice. Plasma APOA4 levels. (D) Plasma levels of TBA (n = 7 each), FFA (n = 7 each), and FGF21 (n = 5–6 per group). ∗P < .05 vs LDLR−/− mice. (E) Plasma aspartate and alanine aminotransferase (AST and ALT) levels. n = 9–10 per group. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. (F) Histology of liver sections and liver TG, TC, and TBA levels (n = 5–8 per group). ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. (G) Hematoxylin-eosin staining, Oil Red O staining, and periodic acid–Schiff (PAS) staining of small intestines from these mice. (H) Intestinal TG and TC levels of these mice (n = 6–8 per group). (I) Quantification of campesterol and β-sitosterol levels of female mice (n = 7–8 per group). ∗∗P < .01 vs LDLR−/− mice.
Figure 2.
A WD for 5 weeks promotes atherosclerosis in male LDLR−/−CREB3L3−/−mice. Ten- to 11-week-old male LDLR−/− and LDLR−/−CREB3L3−/− mice were fed a WD for 5 weeks. Samples were collected in a fed state. (A) Representative images of entire Sudan IV-stained aortas from LDLR−/− (n = 11) and LDLR−/−CREB3L3−/− (n = 11) mice. Surface area occupied by lesions was quantified. ∗∗P < .01 vs LDLR−/− mice. (B) Representative aortic root sections from LDLR−/− (n = 11) and LDLR−/−CREB3L3−/− (n = 11) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic root lesion areas were quantified. ∗P < .05 vs LDLR−/− mice. (C) Plasma TG and TC levels (n = 11 each). HPLC analysis of plasma lipoprotein profiles specific for plasma TG and cholesterol. APOB100 and APOB48 were isolated from VLDL fractions via ultracentrifugation, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, stained with Coomassie Brilliant Blue, and quantified. n = 6–7 per group. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. Plasma APOA4 levels were determined by Western blotting. (D) Plasma TBA, FFA, and FGF21 levels. n = 5–11 per group. ∗P < .05 vs LDLR−/− mice. (E) Histology of liver sections, and liver TG, TC, and TBA levels. n = 9–15 per group. ∗P < .05 vs LDLR−/− mice.
Figure 3.
Even feeding with normal diet promotes atherosclerosis in LDLR−/−CREB3L3−/−mice. Sixteen-week-old female (A–C) and male (D–F) LDLR−/− and LDLR−/−CREB3L3−/− mice were fed a normal diet. Samples were collected in a fed state. (A) Representative aortic root sections from female LDLR−/− (n = 10) and LDLR−/−CREB3L3−/− (n = 11) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic lesion areas were quantified. ∗∗P < .01 vs LDLR−/− mice. (B) Representative images of entire Sudan IV-stained aortas from LDLR−/− (n = 7) and LDLR−/−CREB3L3−/− (n = 6) mice. Surface area occupied by lesions was quantified. ∗∗P < .01 vs LDLR−/− mice. (C) Plasma TG, TC, TBA, and FFA levels of female LDLR−/− and LDLR−/−CREB3L3−/− mice (n = 5–8 per group). ∗∗P < .01 vs LDLR−/− mice. (D) Representative aortic root sections from male LDLR−/− (n = 15) and LDLR−/−CREB3L3−/− (n = 17) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic lesion areas were quantified. ∗∗P < .01 vs LDLR−/− mice. (E) Representative images of entire Sudan IV-stained aortas from male LDLR−/− (n = 7) and LDLR−/−CREB3L3−/− (n = 10) mice. Surface area occupied by lesions was quantified. ∗P < .05 vs LDLR−/− mice. (F) Plasma TG, TC, TBA, and FFA levels of male LDLR−/− and LDLR−/−CREB3L3−/− mice (n = 11–16 per group). ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice.
Deficiency of CREB3L3 Dysregulates Hepatic Lipid Metabolism and Subsequently Exacerbates Steatohepatitis
Plasma aspartate aminotransferase and alanine aminotransferase levels were also increased (Figure 1E), suggesting more severe liver injury in LDLR−/−CREB3L3−/− mice versus LDLR−/− mice. Histologic liver sections from female and male LDLR−/−CREB3L3−/− mice exhibited severe lipid accumulation (Figures 1F and 2E). Liver TG and TC levels in female and male LDLR−/−CREB3L3−/− mice were higher than those of LDLR−/− mice (Figures 1F and 2E). Liver TBA levels in female LDLR−/−CREB3L3−/− mice tended to decrease but not significantly, and there was no change between male genotypes (Figures 1F and 2E). These findings support that LDLR−/−CREB3L3−/− mice increase de novo lipogenesis and hepatosteatosis. Taken together, we found that LDLR−/−CREB3L3−/− mice develop both atherosclerosis and hepatosteatosis, regardless of sex differences. Therefore, the disruption of CREB3L3 is a very strong risk factor for the onset and progression of arteriosclerosis. Subsequently, we mainly used female mice for the study of KO mice.
Deletion of CREB3L3 in the Small Intestine Promotes Lipid Absorption From Diet, Contributing to Hyperlipidemia
CREB3L3 is also expressed in the intestines. Histologic analysis with periodic acid–Schiff staining did not reveal differences in small intestinal mucin-producing goblet cells (Figure 1G). However, enhanced lipid accumulation in the villi of female LDLR−/−CREB3L3−/− mice fed a WD for 5 weeks were detected and quantitatively confirmed (Figure 1G and H). This evidence suggested that there is a dysregulation of lipid metabolism in the small intestines of LDLR−/−CREB3L3−/− mice. The levels of cholesterol absorption markers, such as campesterol and β-sitosterol,22 in the plasma of LDLR−/−CREB3L3−/− mice were significantly higher than those observed in LDLR−/− mice (Figure 1I). Hence, CREB3L3 deletion in the small intestine may cause an increase in intestinal cholesterol absorption, thus exacerbating hyperlipidemia. LDLR−/−CREB3L3−/− mice showed an apparent increase in chylomicron production (Figure 4), supporting the notion that deficiency of CREB3L3 increases the activity of TG absorption in the intestine and subsequent chylomicron-TG production. Taken together, CREB3L3 deletion in the small intestine contributes to hyperlipidemia. Conversely, as we previously reported, intestinal CREB3L3-overexpressing mice exhibited suppression of plasma TC levels when fed the same diet via the suppression of cholesterol absorption in the intestine.23 These findings indicate that hepatic CREB3L3 regulates TG metabolism, and that intestinal CREB3L3 regulates cholesterol and TG absorption in the small intestine, further suggesting that CREB3L3 regulates systemic lipid metabolism in enterohepatic circulation.
Figure 4.
LDLR−/−CREB3L3−/−mice show increased intestinal TG absorption. Eight-week-old female LDLR−/− and LDLR−/−CREB3L3−/− mice were fasted for 3 hours and intravenously injected with Triton WR-1339. After injection (2 hours), mice received 100 μL olive oil orally. Plasma was collected at 0, 1, 2, and 3 hours after administration. n = 7 each. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice.
Arteriosclerosis Is Exacerbated in LDLR−/−CREB3L3−/− Mice After WD Feeding for 3 Months, a Standard Condition of the Evaluation
LDLR−/−CREB3L3−/− mice exhibited early severe atherosclerosis and remained in these phenotypes even after a WD feeding for 3 months. Similar to the observation after the 5-week diet, female LDLR−/−CREB3L3−/− mice revealed a significant increase in atherosclerotic lesion formation (Figure 5A and B). Plasma lipid levels were markedly higher in LDLR−/−CREB3L3−/− mice than in LDLR−/− mice (Figure 5C and D), accompanied by a marked accumulation of both TG in the chylomicron, VLDL, intermediate density lipoprotein, and low-density lipoprotein fractions, and the entire APOB-containing lipoproteins of LDLR−/−CREB3L3−/− mice (Figure 5C). Plasma FGF21 levels of LDLR−/−CREB3L3−/− mice were significantly lower than those of LDLR−/− mice (Figure 5D). These findings indicate that even after feeding with a WD for 3 months, deficiency of CREB3L3 leads to the development of severe atherosclerosis with severe combined hyperlipidemia.
Figure 5.
A WD for 3 months promotes atherosclerosis in LDLR−/−CREB3L3−/−mice. Ten- to 11-week-old female LDLR−/− and LDLR−/−CREB3L3−/− mice were fed a WD for 3 months. Samples were collected in a fed state. (A) Representative images of entire Sudan IV-stained aortas from LDLR−/− (n = 10) and LDLR−/−CREB3L3−/− (n = 11) mice. Surface area occupied by lesions was quantified. (B) Representative aortic root sections from LDLR−/− (n = 9) and LDLR−/−CREB3L3−/− (n = 10) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic root lesion areas were quantified. ∗∗P < .01 vs LDLR−/− mice. (C) Plasma TG (n = 6–7) and TC (n = 11 each) levels. HPLC analysis of plasma lipoprotein profiles specific for plasma TG and cholesterol. ∗∗P < .01 vs LDLR−/− mice. (D) Plasma TBA, FFA, and FGF21 levels (n = 10–11 per group). ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice.
Liver and Intestine CREB3L3 Deficiencies Additively Develop Atherosclerosis
To define the tissue-specific contribution of CREB3L3 in the suppression of atherosclerosis, CREB3L3 LKO and IKO mice24 were crossed with LDLR−/− mice, generating LDLR−/−CREB3L3 LKO and LDLR−/−CREB3L3 IKO mice, respectively. By further crossing of these mice, LDLR−/− mice specifically deficient in both liver and intestine CREB3L3 (LDLR−/−CREB3L3 DKO) were generated. The general plasma biochemical phenotypes of these mice on a normal diet were evaluated at 8 weeks; both LDLR−/−CREB3L3 LKO and LDLR−/−CREB3L3 IKO mice showed higher plasma TG levels than LDLR−/−flox mice (Figure 6A). Plasma TC levels of LDLR−/−CREB3L3 LKO were significantly higher; however, the levels of LDLR−/−CREB3L3 IKO mice were not changed compared with those of LDLR−/−flox mice. LDLR−/−CREB3L3 DKO mice showed increases in both plasma TG and TC levels additively with liver and small intestine defects (Figure 6B). HPLC analysis exhibited higher plasma TG and cholesterol levels, which were distributed over APOB-containing lipoproteins in the following order: DKO, LKO, IKO, and flox mice (Figure 6A and B). LDLR−/−CREB3L3 DKO particularly showed a peak in the chylomicron fraction and a decrease in HDL cholesterol. The pattern of plasma FFA levels was similar to that of plasma TG levels (Figure 6C). The pattern of plasma TBA levels was similar to that of plasma TC levels (Figure 6C), suggesting that liver CREB3L3 deletion leads to bile acid metabolism disorders. Plasma FGF21 levels of LDLR−/−CREB3L3 LKO and LDLR−/−CREB3L3 DKO mice were significantly lower than those of LDLR−/−flox mice (Figure 6C), indicating that these levels were dependent on hepatic CREB3L3 in contrast to the contribution of CREB3L3 of both organs to plasma lipids. Collectively, the data indicate that both liver and intestine CREB3L3 additively contribute to lipid metabolism.
Figure 6.
Plasma lipoprotein profiles in female LDLR−/−mice after deletion of tissue-specific CREB3L3. Samples were collected from 8-week-old female LDLR−/− CREB3L3 flox, LDLR−/− CREB3L3 LKO, LDLR−/− CREB3L3 IKO, and LDLR−/− CREB3L3 DKO mice in a fed state. (A and B) Plasma TG (A) and TC (B) levels (n = 17–29 per group). ∗P < .05 and ∗∗P < .01 among genotypes. HPLC analysis of plasma lipoprotein profiles of TG and cholesterol. (C) Plasma levels of TBA (n = 6–7 each), FFA (n = 18–25 per group), and FGF21 (n = 16–17 per group). ∗∗P < .01 among genotypes.
After feeding the mice with a WD for 3 months, atherosclerotic lesion areas in all groups of KO mice were greater than those recorded in control flox mice. Increases in the estimations by both the entire area and cross section at the sinus were as follows (ascending order): IKO, LKO, and DKO mice (Figure 7A and B). Because of the absence of both liver and intestine CREB3L3, the development of atherosclerosis was further exacerbated in LDLR−/−CREB3L3 DKO mice. This effect was presumably induced by the absence of both collaboratively disturbing lipid metabolism and atherogenic risks (Figure 7A and B).
Figure 7.
A WD for 3 months exacerbates development of atherosclerosis after deletion of tissue-specific CREB3L3 in LDLR−/−mice fed. Ten- to 11-week-old female LDLR−/−flox (flox), LDLR−/−liver-specific CREB3L3 knockout (LKO), LDLR−/−intestine-specific CREB3L3 knockout (IKO), and LDLR−/−liver- and intestine-specific CREB3L3 knockout (DKO) mice were fed a WD for 3 months; samples were collected in a fed state. (A) Representative images of entire Sudan IV-stained aortas from flox (n = 10), LKO (n = 8), IKO (n = 13), and DKO (n = 10) mice. Surface area occupied by the lesions was quantified. ∗P < .05 and ∗∗P < .01 among genotypes. (B) Representative aortic root sections from flox (n = 9), LKO (n = 8), IKO (n = 11), and DKO (n = 10) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic root lesion areas were quantified. ∗P < .05 and ∗∗P < .01 among genotypes.
Hepatic CREB3L3 Activation Suppresses the Formation of Atherosclerotic Lesions in LDLR−/− Mice That Were Fed a WD
Mice with hepatic overexpression of active CREB3L3 (TgCREB3L3) (Figure 8)14 were crossed with LDLR−/− mice. LDLR−/− and LDLR−/−TgCREB3L3 mice were fed a WD for 3 months and subsequently subjected to an atherosclerosis analysis. Lesions were markedly suppressed in both the entire aorta and aortic root of LDLR−/−TgCREB3L3 mice (Figure 9A and B), indicating that hepatic CREB3L3 overexpression attenuates the WD-induced development of atherosclerosis. In addition, because FGF21 (a main CREB3L3 target) exerts a protective effect against atherosclerosis, the contribution of FGF21 in these phenotypes was estimated by crossing LDLR−/−TgCREB3L3 mice with FGF21−/− mice to generate LDLR−/−TgCREB3L3FGF21−/− mice, followed by being fed on a WD diet for 3 months. Deletion of FGF21 was confirmed by showing that plasma FGF21 levels were significantly increased in LDLR−/−TgCREB3L3 mice and not detected in FGF21−/− background mice (Figure 9C). Consistent with a previous report,16 LDLR−/−FGF21−/− mice showed a trend of more severe development of atherosclerosis. However, LDLR−/−FGF21−/− TgCREB3L3 mice maintained a significant suppression of atherosclerosis to a similar extent in the presence of FGF21. Notably, the inhibition rate by CREB3L3 overexpression was estimated to be approximately 50% and 40% in LDLR−/− and LDLR−/−FGF21−/− mice, respectively (Figure 9A and B). These data suggested that the anti-atherogenic effect of CREB3L3 is not mediated primarily through FGF21. CREB3L3 overexpression significantly reduced the plasma TG, TBA, and FFA levels of LDLR−/− mice and LDLR−/−FGF21−/− mice. There were no differences in plasma TC levels among all genotypes (Figure 9C). As a causative factor for hyperlipidemia and atherosclerosis in a previous report,1 plasma levels of APOA4, an LPL modulator, and a CREB3L3 target gene,1 were similarly increased in mice overexpressing CREB3L3 among both LDLR−/−TgCREB3L3 and LDLR−/−FGF21−/−TgCREB3L3 mice (Figure 9D). In gain of function, CREB3L3 target APOA4, but not FGF21, contributed to the suppressive effects of hepatic CREB3L3 on the development of atherosclerosis. Taken together with the observations in KO mice, it can be concluded that CREB3L3 prevents atherosclerosis.
Figure 8.
Ectopic active form of CREB3L3 protein in the liver and intestine of LDLR−/−TgCREB3L3 mice. Levels of the ectopic active form of CREB3L3 protein in the liver and small intestine of 8-week-old male LDLR−/− and LDLR−/−TgCREB3L3 mice were determined by Western blotting.
Figure 9.
WD for 3 months suppresses development of atherosclerosis in LDLR−/−TgCREB3L3 mice. Ten- to 11-week-old male LDLR−/−, LDLR−/−TgCREB3L3, LDLR−/−FGF21−/−, and LDLR−/−FGF21−/−TgCREB3L3 mice were fed a WD for 3 months. Samples were collected in a fed state. (A) Representative images of entire Sud IV-stained aortas from LDLR−/− (n = 22), LDLR−/−TgCREB3L3 (n = 8), LDLR−/−FGF21−/− (n = 14), and LDLR−/−FGF21−/−TgCREB3L3 (n = 14) mice. Surface area occupied by lesions was quantified. ∗P < .05 and ∗∗P < 0.01 among genotypes. (B) Representative aortic root sections from LDLR−/− (n = 18), LDLR−/−TgCREB3L3 (n = 11), LDLR−/−FGF21−/− (n = 11), and LDLR−/−FGF21−/−TgCREB3L3 (n = 12) mice. Cross sections were stained with Oil Red O and hematoxylin. Aortic root lesion areas were quantified. ∗∗P < .01 among genotypes. (C) Plasma TG, TC, TBA, FFA, and FGF21 levels. n = 6–21; ∗P < .05 and ∗∗P < .01 among genotypes. (D) Plasma APOA4 levels were detected by Western blotting.
CREB3L3 Regulates TG Metabolism by Controlling Apolipoproteins in the Liver of LDLR−/− Mice
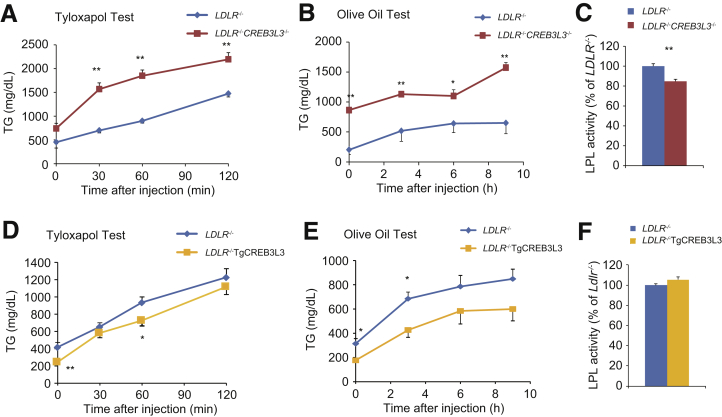
We next investigated the potential risk factors linked to atherosclerosis-prone CREB3L3 deficiency, starting with TG metabolism. VLDL secretions from the liver were significantly increased in LDLR−/−CREB3L3−/− mice (Figure 10A). Consistent with a previous study,1 the expression of LPL activators (eg, Apoc2 and Apoa5) was significantly reduced in LDLR−/−CREB3L3−/− mice, whereas the expression of the LPL inhibitor Apoc3 was increased (Figure 11A). One of the LPL activators, Apoa4, tended to decrease but not significantly (Figure 11A). These changes contribute to hypertriglyceridemia by inhibiting LPL activity and impairing TG clearance. In accordance with the decreased plasma LPL activity, TG clearance was remarkably decreased in LDLR−/−CREB3L3−/− mice (Figure 10B and C).
Figure 10.
VLDL secretion and TG clearance in LDLR−/−CREB3L3−/−and LDLR−/−TgCREB3L3 mice. (A and D) TG production rates (tyloxapol test) in 8-week-old female LDLR−/− (n = 6) and LDLR−/−CREB3L3−/− (n = 11) mice (A) and male LDLR−/− (n = 6) and LDLR−/−TgCREB3L3 (n = 6) mice (D). Mice were starved for 24 hours and intravenously injected with Triton WR-1339. Plasma was collected at 0, 30, 60, and 120 minutes after injection. ∗∗P < .01 vs LDLR−/− mice. (B and E) Postprandial TG responses (olive oil test) in 9-week-old female LDLR−/− (n = 7–8) and LDLR−/−CREB3L3−/− (n = 8) mice (B) and male LDLR−/− (n = 7) and LDLR−/−TgCREB3L3 (n = 5) mice (E). Mice were starved for 16 hours, followed by oral administration of 200 μL olive oil. Plasma was collected at 0, 3, 6, and 9 hours after administration. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. (C and F) Plasma LPL activity in 8-week-old female LDLR−/− (n = 5) and LDLR−/−CREB3L3−/− mice (n = 6) (C) and male LDLR−/− (n = 5) and LDLR−/−TgCREB3L3 (n = 5) mice (F). ∗P < .05 vs LDLR−/− mice.
Figure 11.
Gene expression in LDLR−/−, LDLR−/−CREB3L3−/−, and LDLR−/−TgCREB3L3 mice. Gene expression in livers of 8-week-old female LDLR−/− and LDLR−/−CREB3L3−/− mice (n = 11 per group) (A) and male LDLR−/− and LDLR−/− TgCREB3L3 mice (n = 7 per group) (B) in a fed state with normal diet. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. (C) Gene expression in intestines of 8-week-old male LDLR−/− and LDLR−/− TgCREB3L3 mice in a fed state with normal diet (n = 7 per group). ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice.
In contrast to LDLR−/−CREB3L3−/− mice, hepatic CREB3L3 overexpression significantly increased hepatic Apoa4 and Apoc2 expression (Figure 11B). Consistent with a previous report,25 CREB3L3 overexpression increased bile acid synthesis-related gene expression, including that of cytochrome P450 family 7 subfamily A member 1 (Cyp7a1) and Cyp8b1 (Figure 11B). Cyp7a1 and Cyp8b1 were regulated by FXR/SHP signaling,26 but Fxr and Shp were not changed in both LDLR−/−CREB3L3−/− and LDLR−/−TgCREB3L3 mice compared with LDLR−/− mice (Figure 11A and B). LDLR−/−TgCREB3L3 mice exhibited an apparent increase in TG clearance (Figure 10E) but no difference in VLDL secretions compared with LDLR−/− mice (Figure 10D). There was no difference in plasma LPL activity between LDLR−/− and LDLR−/−TgCREB3L3 mice (Figure 10F). However, changes in apolipoproteins could partially modulate plasma LPL activity and lead to a consequent decrease in plasma TG-rich lipoprotein levels. FGF21 also has the ability to reduce plasma TG levels.27 Our findings suggest that CREB3L3 activates FGF21 and LPL regulatory genes, resulting in a reduction in plasma TG levels.
Deficiency of CREB3L3 in the Small Intestine of LDLR−/− Mice Dysregulates Liver X Receptor Signaling
Next we focused on intestinal lipid metabolism. The expression of LXRα/β and liver X receptor (LXR) signaling molecules, adenosine triphosphate binding cassette subfamily A member 1 (Abca1), Abcg5, and Abcg8, was significantly down-regulated in the intestines of LDLR−/−CREB3L3−/− mice. In contrast, the expression of Abcg1 tended to decrease, but not significantly (Figure 11C). Fxr and Shp were not changed (Figure 11C). On the basis of a previous report demonstrating that intestinal overexpression of active LXRα in LDLR−/− (LDLR−/−TgLXRα) mice improves atherosclerosis,28 we also speculated that the suppression of LXR signaling in intestines of LDLR−/−CREB3L3−/− mice contributes to the acceleration of atherosclerosis. LDLR−/−TgLXRα mice consistently exhibited significantly reduced intestinal cholesterol absorption.28 Therefore, the increase in intestinal cholesterol absorption in LDLR−/−CREB3L3−/− mice may depend on the down-regulation of LXR signaling in the small intestine. Reductions in Abcg5/8, which increased cholesterol excretion into the intestinal lumen, also led to accumulation of cholesterol in the intestines of LDLR−/−CREB3L3−/− mice. Similar to the liver, Apoa4 and Apoc2 expression was decreased in the small intestines of LDLR−/−CREB3L3−/− mice (Figure 11C).
Deficiency of CREB3L3 Activates the Hepatic Expression of SREBP-1 and -2 Target Genes in the Liver of LDLR−/− Mice
To further investigate the integral mechanism, we determined the hepatic gene expression profiles of CREB3L3−/− and hepatic transgenic (Tg) mice. Consistent with the previously described profiles of CREB3L3−/− mice,14 genes downstream of CREB3L3, including fatty acid oxidation-related genes (eg, Ppara), carnitine palmitoyltransferase 1a, liver (Cpt1a), and Fgf21, were decreased in LDLR−/−CREB3L3−/− mice (Figure 11A). Hepatic CREB3L3 overexpression significantly increased hepatic Ppara and the expression of its target genes (eg, Cpt1a and Fgf21) in LDLR−/− mice (Figure 11B). Lipogenic genes regulated by SREBP-1c were entirely up-regulated in LDLR−/−CREB3L3−/− mice including fatty acid synthase (Fasn), stearoyl-coenzyme A desaturase 1 (Scd1), ELOVL family member 6, and elongation of long-chain fatty acids (yeast) (Elovl6) (Figure 11A). Another SREBP-1 target, patatin-like phospholipase domain containing 3 (Pnpla3), which is a central regulator of hepatic TG metabolism and fat accumulation,29 was also remarkably increased in LDLR−/−CREB3L3−/− mice (Figure 11A). Notably, in contrast to the marked induction of target genes, the expression of its encoding gene, sterol regulatory element binding transcription factor 1 (Srebf1), which is the gene name of SREBP1, was only slightly increased. The expression of cholesterol synthesis genes governed by SREBP-2, such as 3-hydroxy-3-methylglutaryl-CoA synthase 1 (Hmgcs1), HMGCoA reductase (Hmgcr), and squalene epoxidase (Sqle), was increased in LDLR−/−CREB3L3−/− mice, although the encoding gene Srebf2 did not exhibit apparent changes (Figure 11A). These changes in SREBP-related genes implicate the functional activation of SREBPs at the posttranslational level. In contrast, the expression of Srebfs and its target genes per se was not altered between LDLR−/− and LDLR−/−TgCREB3L3 mice despite the hepatic overexpression of nuclear, and not full-length, CREB3L3 (Figure 11B).
Interaction Between CREB3L3 and SREBP in Hepatic Lipid Metabolism
To explain that the hepatic expression of lipogenic and cholesterogenic genes was strongly up-regulated in LDLR−/−CREB3L3−/− mice without appreciable induction of Srebf expression, we evaluated the proteolytic cleavage of SREBPs by the amount of precursor and nuclear SREBP proteins. Western blotting revealed that the levels of both the premature (membrane; pSREBP-1) and active (nuclear) forms of SREBP-1 (nSREBP-1), as well as the active form of SREBP-2 (nSREBP-2), were robustly increased in the livers of LDLR−/−CREB3L3−/− mice (Figure 12A). This finding suggests the activated proteocleavage of both proteins and activation of these target genes. SREBPs and CREB3L3 share a set of proteases (S1P and S2P) involved in the cleavage process of transcriptional activation at the Golgi.3 Thus, the cleavage of these proteins may be competitive to each other. Specifically, we hypothesized that the presence of premature CREB3L3 (pCREB3L3) inhibits the cleavage of pSREBPs in a competitive manner. Accordingly, pCREB3L3 expression was restored in LDLR−/−CREB3L3−/− mice via infection with an adenovirus encoding pCREB3L3 (Ad-pCREB3L3) to determine whether pCREB3L3 could suppress pSREBP cleavage, thus reducing nSREBP accumulation. As expected, pCREB3L3 overexpression reduced nSREBP-1 and nSREBP-2 accumulation in LDLR−/−CREB3L3−/− mice, with only small changes noted in the expression of Srebfs (Figure 12B). pCREB3L3 overexpression decreased the plasma TG and TC levels compared with those measured in mice infected with control green fluorescent protein (GFP) (Figure 12B). Genes related to SREBP cleavage were also investigated. An SREBP cleavage activator, Scap, was increased in LDLR−/−CREB3L3−/− mice (Figure 12C); this partly explains the activation of SREBP cleavage in LDLR−/−CREB3L3−/− mice. Consistent with a previous report, insulin-induced gene 2a (Insig2a), a retention factor of SREBP-SREBF chaperone (SREBP-SCAP) complex in the ER, is a target gene of CREB3L3.30 Insig2a was decreased in LDLR−/−CREB3L3−/− mice (Figure 12C). Other retention factors, such as Insig1 and Insig2b, were not changed in LDLR−/−CREB3L3−/− mice (Figure 12C). Meanwhile, overexpression of nuclear CREB3L3 in LDLR−/− mice failed to affect the expression of Insig genes (Figure 12C). This discrepancy between loss and gain of CREB3L3 in LDLR−/− mice indicates that the CREB3L3-INSIG2a pathway is not sufficient to provide an explanation for the strong SREBP activation by CREB3L3 deficiency, supporting the aforementioned new hypothesis. In a cell-based reporter assay using an SREBP response element (SRE)-containing luciferase (SRE-Luc), endogenous SREBP cleavage activity was detected by transfection with pSREBP-1a, an isoform of SREBPs with strong transcriptional activity, as evidenced by SRE-Luc activity (Figure 12D). Further co-transfection with pCREB3L3 significantly suppressed this pSREBP-1a cleavage. However, the active form of CREB3L3 (nCREB3L3) did not (Figure 12D), further supporting the competition in the cleavage between pCREB3L3 and pSREBP.
Figure 12.
Premature CREB3L3 suppresses cleavage of SREBPs. (A) Immunoblotting analysis of SREBPs in hepatic nuclear extracts and total cell lysates from 8-week-old female LDLR−/− and LDLR−/−CREB3L3−/− mice. Each lane was a pooled sample from 3 mouse livers. (B) Immunoblotting analysis of SREBPs in hepatic nuclear extracts and total cell lysates. Each lane was a pooled sample from 3 mouse livers. Values were indicated as fold changes of band intensity versus GFP infection, hepatic expression of Srebfs, and plasma TG and TC levels in 10-week-old female LDLR−/− and LDLR−/−CREB3L3−/− mice infected with adenoviruses encoding GFP and the low and high dose of full-length CREB3L3 (pCREB3L3) after feeding with normal diet for 6 days. n = 4–8. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. (C) Hepatic SREBP cleavage modulator gene expression in 8-week-old female LDLR−/−and LDLR−/−CREB3L3−/− and 8-week-old male LDLR−/− and LDLR−/− TgCREB3L3 mice in a fed state with normal diet. n = 4–6. ∗P < .05 and ∗∗P < .01 vs LDLR−/− mice. (D) pCREB3L3 suppresses SREBP transcriptional activity. pCREB3L3 or the active form of CREB3L3 (mCREB3L3) and pSREBP expression vectors were co-transfected with an SRE-luc vector into HEK293 cells. Luciferase activity was determined after 48 hours. n = 4–8. ∗P < .05 and ∗∗P < .01 vs Control.
Antagonism Between CREB3L3 and SREBP Occurs at Trafficking From the ER to the Golgi
An immunoprecipitation assay showed a direct association between the 2 precursor proteins pCREB3L3 and pSREBP-1c (Figure 13A). Further association analysis exhibited that pCREB3L3 bound to the components related to SREBP-1 translocation from the ER to the Golgi, including INSIG1 and SCAP, respectively (Figure 13B and C). SCAP is an escort protein of pSREBP-1c for its translocation from the ER to the Golgi, and INSIG1 suppresses the translocation of the SREBP-SCAP complex.31 The pSREBP-1c and SCAP association was increased in a CREB3L3 dose-dependent manner (Figure 13D). Similarly, the pSREBP-1 and INSIG1 association was also increased in a CREB3L3 dose-dependent manner (Figure 13E). Taken together, pCREB3L3 supports the INSIG1-pSREBP-1c-SCAP complex formation, supporting that pCREB3L3 induces SREBP-1 retention in the ER. To determine the effects of pCREB3L3 on the cellular localization of SREBPs, mCherry-tagged pSREBP-1c and SCAP with or without GFP-tagged pCREB3L3 vectors were co-transfected into HEK293 cells. Immunohistochemistry analysis revealed that SREBP-1c was localized in the nucleus merging with a nuclear marker, 4´,6-diamidino-2-phenylindole (DAPI), when co-transfected with SCAP. This indicated that SCAP enhanced the transport and cleavage of SREBP and caused its nuclear transfer (Figure 13F). However, when also co-transfected with pCREB3L3, SREBP-1c and CREB3L3 were not colocalized in nucleus. This evidence supports the notion that their direct binding leads to the suppression of SREBP cleavage by pCREB3L3 (Figure 13F). Organelle marker immunostaining indicated that the colocalization of SREBP-1 and CREB3L3 occurs at the ER because both proteins and the ER marker (calnexin) were merged (Figure 13G). The Golgi marker GM130 in SREBP-1c/SCAP transfection showed a partial signal of SREBP-1c merging at the Golgi, presumably a remnant of the uncleaved one and the other partial signal of unmerged one presumably cleaved into the nucleus (Figure 13H). SREBP-1c/SCAP/CREB3L3 co-transfection caused only marginal signaling merging of SREBP-1c-CREB3L3 at the Golgi (Figure 13H). The data indicate that CREB3L3 inhibited the SCAP escort of SREBP-1c to the Golgi by forming the complex. The complex remained in the ER, thus not allowing the entry of SREBP-1c to the Golgi. To further investigate whether pCREB3L3 disrupts the pSREBPs-S1P complex, we used an in situ microscopy approach through a proximity ligation assay (PLA). Herpes simplex virus (HSV)–tagged pSREBP-1c and Halo-tagged S1P were co-transfected into HEK293 cells. Complexes between pSREBP-1c and S1P were observed as red dots around the nucleus (Figure 13I). As CREB3L3 inhibited its complex formation, the red dots were significantly decreased in addition to pCREB3L3 transfection (Figure 13I). This indicates that pCREB3L3 inhibited the formation of the complex between pSREBP-1c and S1P. These findings suggest that the physical association of CREB3L3 with SREBPs inhibits the SCAP-mediated transport of SREBP-1 from the ER to the Golgi, the processing by S1P, and, consequently, SREBP transcriptional activity. To verify this observation vice versa (ie, whether CREB3L3 cleavage is conversely inhibited by SREBP), we evaluated the nCREB3L3 protein levels in SREBF1−/− mice. Hepatic nCREB3L3 protein levels in SREBF1−/− mice were clearly increased compared with those measured in wild-type mice (Figure 13J). These results support that CREB3L3 and SREBP-1 can antagonize each other through direct mutual interaction at the precursor protein level.
Figure 13.
Premature CREB3L3 associates with SREBP and SREBP transport regulatory proteins. (A–E) Physical association among CREB3L3, SREBP, and SREBP transport regulatory proteins. Indicated vectors were co-transfected into HEK293 cells. After 24 hours, cell lysates were collected and immunoprecipitated with an anti-V5 antibody. Immunoprecipitants were detected with the indicated antibodies. (F–H) Localization of SREBP-1c and CREB3L3 in the cellular component. mCherry-pSREBP-1c (mCherry-BP-1c) and SREBF chaperone (SCAP), with/without GFP-pCREB3L3 (GFP-CREB3L3), were co-transfected into HEK293 cells. 4´,6-diamidino-2-phenylindole (DAPI) for the nucleus (F), calnexin for ER (G), and GM130 for the Golgi apparatus (H) were immunostained. (I) CREB3L3 inhibits SREBPs-S1P interaction. Using the DuoLink PLA, red dots showed the pSREBP-1c-S1P association. ∗P < .05. (J) Immunoblot analysis of CREB3L3 in hepatic nuclear extracts and total cell lysates from 16-week-old male wild-type (WT) and SREBF1−/− mice. (K) Schematic representation of atherosclerosis development via a competitive transport interaction of SREBPs and CREB3L3.
Discussion
The present study clearly exhibited that CREB3L3 profoundly impacts the atherosclerotic phenotypes. CREB3L3 deletion caused both hypertriglyceridemia and hypercholesterolemia and accelerated aortic atheroma formation in LDLR−/− mice fed either a WD or a normal diet, irrespective of sex, and from both hepatic and intestinal origins. Conversely, hepatic nuclear CREB3L3 overexpression strikingly attenuated WD-induced hyperlipidemia and atherosclerosis progression in LDLR−/− mice. Numerous studies, as well as the present work, confirmed that the primary role of CREB3L3 is the regulation of TG metabolism.1,14,24,32,33 TG has been proposed as the major atherosclerosis risk factor, highlighting the potential cholesterol-related or more comprehensive mechanisms of the anti-atherogenic effects of CREB3L3.
In the process of clarifying the causative mediators of the anti-atherogenic effect of CREB3L3, FGF21 (a major hepatic CREB3L3 target gene that regulates both lipid and glucose metabolism) had been a strong candidate. It has been reported that FGF21 ameliorates atherosclerosis and leads to the hepatic activation of SREBP-2 in APOE−/−FGF21−/− mice.16,34 Although the present study also showed that some metabolic phenotypes observed in LDLR−/−CREB3L3−/− mice were attributed to decreases in plasma FGF21 levels, deficiency of FGF21 in LDLR−/−TgCREB3L3 mice did not cancel the improvement in atherosclerosis, distracting the hypothesis that FGF21 is the main contributor to anti-atherosclerosis.
The IKO mice also exhibited atheroma formation, which was comparable to that noted in LKO mice, confirming the role of intestinal CREB3L3 in anti-atherogenic action. Consistent with previous report that CREB3L3 controls LXR acitivity,35 decreased LXR signaling and increased lipid contents were observed in the intestines of LDLR−/−CREB3L3−/− mice. Overexpression of the intestinal-specific active form of LXR in LDLR−/− mice on a WD increased fecal neutral sterol excretion and exhibited protection against atherosclerosis.28 These results support the hypothesis that intestinal CREB3L3 contributes to cholesterol metabolism via LXR. LDLR−/−CREB3L3−/− mice had higher plasma plant sterol levels than LDLR−/− mice, explaining that CREB3L3 deficiency in the small intestines increases cholesterol absorption. Collectively, the data suggest that both liver- and intestine-specific CREB3L3 deficiency additively promote atherosclerosis.
We showed that CREB3L3 deficiency increases the levels of hepatic nSREBPs and, consequently, plasma lipids. This led us to speculate and confirm the two-step CREBH-mediated suppression of SREBP-1c activation: (1) CREB3L3 induces the retention of SPREBP-1c in the ER by promoting the formation of the CREB3L3/INSIG1/SCAP/SREBP complex, and (2) CREB3L3 physically competes with SREBPs for cleavage by S1P and S2P in the Golgi. Therefore, CREB3L3 deletion may result in primarily decreased TG catabolism and enhanced lipogenesis as a secondary consequence of SREBP-1c activation, leading to a marked accumulation of TG-rich remnant lipoproteins and severe hypertriglyceridemia. In addition, both increased cholesterol absorption due to the absence of intestinal CREB3L3 and cholesterol synthesis mediated by SREBP-2 activation in the liver significantly enriched these lipoproteins with cholesterol and played a major role in the production of more atherogenic lipoproteins.
Functional competition between SREBPs and CREB3L3 implicates profound physiological consequences for lipid and energy regulation. CREB3L3 and SREBPs use the same activation process of intramembrane proteolysis regulation. Through this process, transmembrane proteins are cleaved to release cytosolic domains that translocate into the nucleus and thereby regulate gene transcription. CREB3L3 is cleaved by the processing enzymes S1P and S2P in the Golgi apparatus in a manner similar to that of SREBP cleavage.3 Therefore, we initially hypothesized that pCREB3L3 would competitively inhibit SREBP processing. We showed that CREB3L3, SREBP-1c, and INSIG1 physically interact (Figure 13E), possibly to inhibit the trafficking of the SREBP-INSIG1-CREB3L3 complex from the ER to the Golgi. Recently, it was reported that CREB3L3 increases Insig2a expression, which in turn suppresses SREBP activation.30 Consistently, its expression was decreased in the livers of LDLR−/−CREB3L3−/− mice but not changed in those of LDLR−/−TgCREB3L3 mice. In addition, LDLR−/−TgCREB3L3 mice (overexpressing nCREB3L3) did not exhibit apparently altered SREBPs and activation of their target genes in the liver. Certainly, adenoviral overexpression of pCREB3L3 in the livers of LDLR−/−CREB3L3−/− mice reduced the expression of nSREBP-1 and nSEEBP-2 proteins (Figure 12B). This result indicated that in LDLR−/− mice, SREBP cleavage was regulated by the existence of pCREB3L3 rather than INSIGs. It was previously reported that CREB3L3 suppresses LXRα-induced Srebf1c expression by inhibiting LXRα binding to the Srebf1c promoter.36 However, our data did not show changes in SREBF1c and its target genes in LDLR−/−TgCREB3L3 mice.
We propose the new concept that CREB3L3, SREBPs, and INSIG1 physically interact at the ER, inhibiting the transportation of SREBPs to the Golgi apparatus, and CREB3L3 competes with SREBPs in access to S1P in the Golgi apparatus. Loss of this interaction because of CREB3L3 deficiency induces SREBP-1 and -2 cleavage and promotes the induction of TG and cholesterol synthesis (Figure 13K). This mechanism also explains why the overexpression of nuclear CREB3L3 did not suppress hepatic SREBP target genes (Figure 11B), because nuclear CREB3L3 does not compete with pSREBPs. In the normal liver, Creb3l3 is up-regulated during fasting and down-regulated under feeding conditions; Srebf1c is regulated in a reciprocal manner. Thus, the encounter of the 2 factors does not actively occur on the ER under normal nutritional states. However, in metabolic disturbances with high atherogenic risks, such as db/db or ob/ob mice, these 2 factors could be expressed collaterally14 and interact with and inhibit each other. Finally, enhancement of nCREB3L3 with decreased pCREB3L3 in the SREBF1−/− liver (Figure 13J) supports this hypothesis. Functional antagonism between CREB3L3 and SREBPs in atherosclerosis is consistent on the basis of the anti-atherogenic action of CREB3L3 from the current data and pro-atherogenic action of SREBP-1 from our previous work.37 CREB3L3 and SREBPs are regulators of the catabolism and anabolism of lipids, respectively. Hence, it is conceivable to configure a mechanism by which mutual interaction and balance of the counterpartners maintain the whole-body energy balance and atherosclerosis risks.
Conclusions
Collectively, our study is the first to identify the crucial role of CREB3L3 enterohepatic interplay in lipid metabolism and prevention of atherosclerosis. Therefore, CREB3L3 may be a new target against atherosclerosis. Protection from atherosclerosis by overexpression of nuclear CREB3L3 in mice was greater than that expected from the amelioration of hyperlipidemia in LDLR−/−TgCREB3L3 mice. CREB3L3 is deeply involved in cellular stress and inflammation, which we have not investigated in the present study. Therefore, further study is warranted to address these aspects.
Methods
Mice
This project was approved by and conducted under the guidelines of the Animal Care Committee of the University of Tsukuba. CREB3L3tm1.1Sad/J (CREB3L3−/−) mice5 and LDLR−/− mice38 were purchased from the Jackson Laboratory (Bar Harbor, ME). CREB3L3−/− mice were crossed onto an LDLR−/− background to generate LDLR−/−CREB3L3−/− mice. Tg mice overexpressing amino acids 1–320 of human CREB3L3 under control of the phosphoenolpyruvate carboxykinase promoter on the C57BL/6J background (hereafter referred to as TgCREB3L3) were generated as previously described.14 FGF21−/− mice were provided by Professors Morichika Konishi and Nobuyuki Ito.39 TgCREB3L3 mice were crossed with LDLR−/− mice to produce LDLR−/−TgCREB3L3 mice and subsequently crossed with FGF21−/− mice to produce LDLR−/−FGF21−/−TgCREB3L3 mice. CREB3L3flox/flox (flox) mice were generated using the CRISPR/Cas 9 system as previously described.24 Flox mice were crossed with B6.Cg-Tg(Alb-Cre)21Mgn/J (albumin Cre Tg; Jackson Laboratory)40 and/or villin Cre Tg mice (Jackson Laboratory)41 to produce LKO, IKO, and DKO mice. These mice were crossed with LDLR−/− mice, generating LDLR−/−flox, LDLR−/−LKO, LDLR−/−IKO, and LDLR−/−DKO mice, respectively. SREBF1−/− mice were generated as previously described.42 Sixteen-week-old male wild-type and SREBF1−/− mice were fasted for 24 hours and fed with high-sucrose diet for 12 hours.43 All mice were maintained on normal diet (Oriental Yeast Company, Tokyo, Japan) and a 14-hour light/10-hour dark cycle. For the atherosclerosis analyses, mice were fed a WD (D12079B [34% sucrose, 21% fat, 0.15% cholesterol]; Research Diets, Inc, New Brunswick, NJ) under the indicated conditions.37 For adenoviral infection, 8- to 10-week-old female LDLR−/−CREB3L3−/− mice were infected with the indicated adenovirus at 1.0 (low) and 5.0 (high dose) × 108 plaque-forming units/g body weight; samples were collected 6 days later while in a fed state. All animal husbandry procedures and animal experiments were consistent with the University of Tsukuba Regulations of Animal Experiment and approved by the Animal Experiment Committee of the University of Tsukuba.
Determination of Metabolic Parameters
Plasma levels of glucose, TGs, TC, TBA, FFA, alanine aminotransferase, and aspartate aminotransferase were measured using Wako enzymatic kits (Wako Pure Chemical Industries, Osaka, Japan). Plasma insulin was measured with a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (Sibayagi, Gunma, Japan). Plasma FGF21 was measured with a mouse/rat FGF21 Quantikine ELISA kit (R&D Systems, Minneapolis, MN). Hepatic TG, TC, and TBA contents were measured as previously described.23,43 Intestinal TG and TC contents were measured using the same protocol. Plasma APOA4 was detected by Western blotting with an anti-APOA4 antibody (sc-19036; Santa Cruz Biotechnology, Santa Cruz, CA).
HPLC Analysis
For the lipoprotein distribution analysis, pooled plasma samples from 4 or 5 mice per group were analyzed via upgraded HPLC analysis, as previously described (Skylight Biotech Inc, Tokyo, Japan).44
Isolation of the VLDL Fraction
VLDL (d < 1.006 g/mL) was isolated via ultracentrifugation with a TLA120.2 rotor (Beckman Coulter, Brea, CA). VLDL fractions were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and subjected to Coomassie brilliant blue staining.
Determination of Plasma Sterol Levels
For the sterol distribution analysis, pooled plasma samples were quantified by using a gas chromatography method (Skylight Biotech Inc).
Immunoblotting
Total cell and nuclear fraction lysates were prepared as previously described45 and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Subsequently, the samples were subjected to Western blotting analysis using antibodies against SREBP-1 (sc-12332; Santa Cruz Biotechnology), SREBP-2 (10007663; Cayman Chemical, Ann Arbor, MI), α-tubulin (05-829; Millipore, Burlington, MA), lamin A/C (#2032; Cell Signaling Technology, Danvers, MA), V5 (R960; Life Technologies, Carlsbad, CA), HSV (69171-3; Novagen, Millipore), MYC (9E10; Santa Cruz Biotechnology), and hemagglutinin (3F10; Roche, Basel, Switzerland) antibodies.
Immunoprecipitation
HEK293 cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and penicillin/streptomycin. Indicated plasmids were transfected with X-tremeGENE 9 (Roche) according to the instructions provided by the manufacturer. V5-tagged full-length mouse CREB3L3 cDNA was inserted into pcDNA3.1 (Invitrogen, Carlsbad, CA); GFP-tagged full-length mouse CREB3L3 cDNA was inserted into pEGFP (GFP-CREB3L3) (Clontech, Mountain View, CA); mCherry-tagged human SREBP-1c was inserted into pmCherry (mCherry-SREBP-1c) (Clontech); and hemagglutinin-tagged hamster SCAP, Myc-tagged mouse INSIG1, HSV-tagged human SREBP-1c, and HSV-tagged human SREBP-2 were inserted into pCMV. Cell lysates were immunoprecipitated with antibodies against V5, and immunoprecipitants were subjected to immunoblotting with the indicated antibodies as previously described.14
Duolink PLA
HEK293 cells were co-transfected with HSV-tagged pSREBP-1c and Halo-tagged S1P with/without pCREB3L3. Cells were fixed with 3.7% formalin in phosphate-buffered saline for 30 minutes before being permeabilized with 0.2% Triton X-100 in phosphate-buffered saline for 10 minutes. Cells were subsequently subjected to the PLA using the Duolink red kit (Sigma–Aldrich, St Louis, MO) according to the instructions provided by the manufacturer.
Immunocytochemistry
HEK293 cells were transfected with mCherry-tagged pSREBP-1c, SCAP, and GFP-tagged pCREB3L3 using X-tremeGENE 9 (Roche). Cells were grown on coverslips, fixed with 4% paraformaldehyde for 15 minutes, and permeabilized with 0.1% Triton X-100 for 5 minutes. After blocking in 1% bovine serum albumin for 30 minutes, the cells were incubated with primary and secondary antibodies for 1 hour each. The ER and Golgi apparatus were stained using anti-calnexin (610523; BD Biosciences, San Jose, CA) and anti-GM130 antibodies (610822; BD Biosciences), respectively. Immunoreactive complexes were visualized with Alexa Fluor 405-conjugated secondary antibody (ab175660; Abcam, Cambridge, UK), and nuclei were visualized by staining with DAPI.
Promoter Analysis
HEK293 cells were transfected with the indicated luciferase reporter, expression plasmids, and a reference pRL-SV40 plasmid (Promega, Madison, WI) using X-tremeGENE 9 (Roche). SRE-luc vector45, 46, 47 and human SREBP-1a45,46 have been previously described. After a 48-hour incubation, firefly luciferase activity in cells was measured and normalized to Renilla luciferase activity.
Atherosclerotic Lesion Analysis
Ten- to 11-week-old male and female LDLR−/− and LDLR−/−CREB3L3−/− mice (age 10–11 weeks) were fed a WD containing 34% sucrose, 21% fat, and 0.15% cholesterol (D12079B; Research Diets, Inc) for 5 weeks or 3 months. Ten- to 11-week-old male LDLR−/− and LDLR−/−TgCREB3L3 mice (age 10–11 weeks) were fed a WD for 3 months. The mice were subsequently euthanized to extract their hearts and aortas. Hearts were fixed in 4% formalin for >48 hours. The basal half of each heart was embedded in Tissue-Tek OCT compound (Sakura Finetek, Torrance, CA). Cross sections were stained with Oil Red O and hematoxylin. Aortas were cut along the midline from the iliac arteries to the aortic root, pinned flat, and treated with Sudan IV for 15 minutes to stain lesions, followed by 70% ethanol destaining and fixation in 4% phosphate-buffered formalin.37 Atherosclerotic lesions were quantified using the Photoshop CS software (Adobe Systems Inc, San Jose, CA).
TG Production
Mice were deprived of food for 24 hours and subsequently injected with Triton WR-1339 (0.5 mg/g body weight; Sigma–Aldrich) via the tail veins to block the clearance of nascent APOB-containing lipoproteins. Blood samples were collected at 0, 30, 60, and 120 minutes after injection.37
Postprandial TG Response
Mice were deprived of food for 16 hours, followed by oral administration of 200 μL olive oil.1 Blood samples were collected at 0, 3, 6, and 9 hours after administration.
Intestinal TG Absorption
Mice were fasted for 3 hours and injected with Triton WR-1339 (1 mg/g body weight; Sigma–Aldrich) via the tail veins. After injection (2 hours), mice received 100 μL olive oil orally.48 Blood samples were collected for up to 3 hours after the injection.
Determination of Plasma LPL Activity
Mice were injected with 100 U/kg body weight of heparin (Novo Heparin; Mochida Pharmaceutical Co, Ltd, Tokyo, Japan) via the tail veins. Blood samples were collected at 20 minutes after administration. Plasma LPL activity was determined by using an LPL activity assay kit (Roar Biochemical, Inc, Huntington, NY) according to the instructions provided by the manufacturer.
Preparation of Recombinant Adenovirus
cDNAs encoding human full-length of CREB3L3 (NM_032607.2) and GFP were cloned into pENTR4 vectors (Life Technologies). In addition, adenovirus vectors were recombined with pAd/CMV/V5-DEST vectors (Life Technologies). Recombinant adenoviruses were produced in 293A cells (Invitrogen) and purified via cesium chloride gradient centrifugation, as previously described.43
Analysis of Gene Expression
Total RNA was isolated from cells and tissues using Trizol reagent (Invitrogen) and Sepasol (Nacalai, Kyoto, Japan). Real-time polymerase chain reaction analysis templates were prepared via cDNA synthesis (Invitrogen) from total RNA. Real-time polymerase chain reaction was performed using the ABI Prism 7300 System (Applied Biosystems, Inc, Foster City, CA) with SYBR Green Master Mix (Roche) and TB Green Premix EX Taq II (TAKARA Bio, Shiga, Japan).49 Primer sequences are described in Table 1.
Table 1.
Primers Used for Real-Time Polymerase Chain Reaction Analysis
| Gene name | Forward | Reverse |
|---|---|---|
| Abca1 | AAAACCGCAGACATCCTTCAG | CATACCGAAACTCGTTCACCC |
| Abcg1 | CCATGAATGCCAGCAGCTACT | CTGTGAAGTTGTTGTCCACCTTCT |
| Abcg5 | AGGGCCTCACATCAACAGAG | GCTGACGCTGTAGGACACAT |
| Abcg8 | AGTGGTCAGTCCAACACTCTG | GAGACCTCCAGGGTATCTTGAA |
| Acox1 | CGATCCAGACTTCCAACATGAG | CCATGGTGGCACTCTTCTTAACA |
| Angptl3 | TCTACTGTGATACCCAATCAGGC | CATGTTTCGTTGAAGTCCTGTGA |
| Angptl4 | GCATCCTGGGACGAGATGAAC | CCCTGACAAGCGTTACCACA |
| Angptl6 | TTGGGCGTCCAGAAGGAGAA | CAGTCCTCTAGGAGTATCAGCAG |
| Apoa1 | TCACCCACACCCTTCAGGAT | CTGGCTCCCTGTCAGGAAGA |
| Apoa4 | TTACCCAGCTAAGCAACAATGC | GAGGGTACTGAGCTGCTGAGTGA |
| Apoa5 | GCGAGTTCTGCCGTAGGAC | CCCAACCCCATCAAATGTGA |
| Apob | TTGGCAAACTGCATAGCATCC | TCAAATTGGGACTCTCCTTTAGC |
| Apoc2 | CCAAGGAGGTTGCCAAAGAC | TGCCTGCGTAAGTGCTCATG |
| Apoc3 | TACAGGGCTACATGGAACAAGC | CAGGGATCTGAAGTGATTGTCC |
| Bip | ACATCAAGCAGTACCAGATCAC | AACCCCGATGAGGCTGTAGC |
| Bsep | CAATGTTCAGTTCCTCCGTTCA | TCTCTTTGGTGTTGTCCCCATA |
| Cpt1a | CCTGGGCATGATTGCAAAG | GGACGCCACTCACGATGTT |
| Creb3l3 | CCTGTTTGATCGGCAGGAC | CGGGGGACCATAATGGAGA |
| Cyclophilin | TGGCTCACAGTTCTTCATAACCA | ATGACATCCTTCAGTGGCTTGTC |
| Cyp7a1 | GCTGAGAGCTTGAAGCACAAGA | TTGAGATGCCCAGAGGATCAC |
| Cyp8b1 | CTAGGGCCTAAAGGTTCGAGT | GTAGCCGAATAAGCTCAGGAAG |
| Cyp27a1 | CCAGGCACAGGAGAGTACG | GGGCAAGTGCAGCACATAG |
| Dhcr7 | CACCGGCCGTGCTAGTCTGG | CAGGCTTGTAGCCCGTTCACCTC |
| Dgat1 | CGTGGGCGACGGCTACT | GAAACCACTGTCTGAGCTGAACA |
| Dgat2 | GCCCGCAGCGAAAACA | GTCTTGGAGGGCTGAGAGGAT |
| Elovl6 | ACAATGGACCTGTCAGCAAA | CTACCAGTGCAGGAAGATCAGT |
| Fasn | ATCCTGGAACGACGAGAACACGATCT | AGAGACGTGTCATCCTGGACTT |
| Fbwx7a | CTCACCAGCTCTCCTCTCCATT | GCTGAACATGGTACAAGGCCA |
| Fgf21 | AGATCAGGGAGGATGGAACA | TCAAAGTGAGGCGATCCATA |
| Fxr | CTCTGCTCACAGCGATCGTC | CACCGCCTCTCTGTCCTTGA |
| Hmgcs1 | AACTGGTGCAGAAATCTCTAGC | GGTTGAATAGCTCAGAACTAGCC |
| Hmgcr | GAGAAGAAGCCTGCTGCATA | CGTCAACCATAGCTTCCGTAGTT |
| Insig1 | TCACAGTGACTGAGCTTCAGCA | TCATCTTCATCACACCCAGGAC |
| Insig2a | CCCTCAATGAATGTACTGAAGGATT | TGTGAAGTGAAGCAGACCAATGT |
| Insig2b | CCGGGCAGAGCTCAGGAT | GAAGCAGACCAATGTTTCAATGG |
| Lxra | AGCAACAGTGTAACAGGCGCT | ACGATGGCCAGCTCAGTAAAGT |
| Lxrb | ATGTCTTCCCCCACAAGTTCT | GACCACGATGTAGGCAGAGC |
| Mttp | AGCTTTGTCACCGCTGTGC | TCCTGCTATGGTTTGTTGGAAGT |
| Npc1l1 | ATCCTCATCCTGGGCTTTGC | GCAAGGTGATCAGGAGGTTGA |
| Pltp | GACGACGAGAGGATGGTGTACG | GTCGGACTCAGGAGAACAATGC |
| Pnpla3 | TCACCTTCGTGTGCAGTCTC | CCTGGAGCCCGTCTCTGAT |
| Ppara | TTGTGGCTGGTCAAGTTCGG | GCTCTCTGTGTCCACCATGT |
| S14 | ATGCAAGTGCTAACGAAACGC | GGAGTACCGATCCATGACTGTC |
| Scap | ATTTGCTCACCGTGGAGATGTT | GAAGTCATCCAGGCCACTACTAATG |
| Scd1 | AGATCTCCAGTTCTTACACGACCAC | CTTTCATTTCAGGACGGATGTCT |
| Shp | CAAGGAGTATGCGTACCTGAAG | CCTGGCACATCTGGGTTGAAG |
| Sqle | AAATCAGAGCCGTGGGCTAC | GGAAGTGACACAGTTCTATG |
| Srebf1c | CGGCGCGGAAGCTGT | TGCAATCCATGGCTCCGT |
| Srebf2 | CTGCAGCCTCAAGTGCAAAG | CAGTGTGCCATTGGCTGTCT |
| Vldlr | TTCCTAGCTCATCCTCTTGCAC | CTGACCCAGTGAATTTATTGGC |
Statistical Analysis
Statistical significance was determined by using unpaired Student t tests and one-way analysis of variance with Tukey’s post hoc using the GraphPad Prism software (San Diego, CA). Differences with P values <.05 were considered significant. Data are expressed as the mean ± standard error of the mean.
Acknowledgments
The authors thank Enago (www.enago.jp) for the English language review.
CRediT Authorship Contributions
Hitoshi Shimano (Conceptualization: Lead; Funding acquisition: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Yoshimi Nakagawa (Conceptualization: Equal; Data curation: Equal; Funding acquisition: Equal; Investigation: Equal; Writing – original draft: Equal; Writing – review & editing: Equal),
Yunong Wang (Investigation: Equal)
Song-iee Han (Investigation: Equal)
Kanako Okuda (Investigation: Equal)
Asayo Oishi (Investigation: Equal)
Yuka Yagishita (Investigation: Equal)
Kae Kumagai (Investigation: Equal)
Hiroshi Ohno (Investigation: Equal)
Yoshinori Osaki (Investigation: Equal)
Yuhei Mizunoe (Investigation: Equal)
Masaya Araki (Investigation: Equal)
Yuki Murayama (Supervision: Equal)
Hitoshi Iwasaki (Supervision: Equal)
Morichika Konishi (Resources: Equal)
Nobuyuki Itoh (Resources: Equal)
Takashi Matsuzaka (Supervision: Equal)
Hirohito Sone (Investigation: Equal)
Nobuhiro Yamada (Investigation: Equal)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by Grants-in-Aid from the Ministry of Science, Education, Culture and Technology of Japan (25282214 and 16H03253 to Y.N., and 17H06395 and 18H04051 to H.Shimano), AMED-CREST (to H.Shimano), Japan Heart Foundation/Novartis Grant for Research Award on Molecular and Cellular Cardiology (to Y.N.), Uehara Memorial Foundation (to Y.N.), Ono Medical Research Foundation (to Y.N.), Mochida Memorial Foundation for Medical and Pharmaceutical Research (to Y.N.), Suzuken Memorial Foundation (to Y.N.), Senshin Medical Research Foundation (to Y.N.), Takeda Science Foundation (to Y.N.), Japan Foundation for Applied Enzymology (to Y.N.), Banyu Life Science Foundation International (to Y.N.), and Yamaguchi Endocrine Research Foundation (to Y.N.).
Contributor Information
Yoshimi Nakagawa, Email: ynaka@inm.u-toyama.ac.jp.
Hitoshi Shimano, Email: hshimano@md.tsukuba.ac.jp.
References
- 1.Lee J.H., Giannikopoulos P., Duncan S.A., Wang J., Johansen C.T., Brown J.D., Plutzky J., Hegele R.A., Glimcher L.H., Lee A.H. The transcription factor cyclic AMP-responsive element-binding protein H regulates triglyceride metabolism. Nat Med. 2011;17:812–815. doi: 10.1038/nm.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omori Y., Imai J., Watanabe M., Komatsu T., Suzuki Y., Kataoka K., Watanabe S., Tanigami A., Sugano S. CREB-H: a novel mammalian transcription factor belonging to the CREB/ATF family and functioning via the box-B element with a liver-specific expression. Nucleic Acids Res. 2001;29:2154–2162. doi: 10.1093/nar/29.10.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang K., Shen X., Wu J., Sakaki K., Saunders T., Rutkowski D.T., Back S.H., Kaufman R.J. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 4.Danno H., Ishii K.A., Nakagawa Y., Mikami M., Yamamoto T., Yabe S., Furusawa M., Kumadaki S., Watanabe K., Shimizu H., Matsuzaka T., Kobayashi K., Takahashi A., Yatoh S., Suzuki H., Yamada N., Shimano H. The liver-enriched transcription factor CREBH is nutritionally regulated and activated by fatty acids and PPARalpha. Biochem Biophys Res Commun. 2010;391:1222–1227. doi: 10.1016/j.bbrc.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 5.Luebke-Wheeler J., Zhang K., Battle M., Si-Tayeb K., Garrison W., Chhinder S., Li J., Kaufman R.J., Duncan S.A. Hepatocyte nuclear factor 4alpha is implicated in endoplasmic reticulum stress-induced acute phase response by regulating expression of cyclic adenosine monophosphate responsive element binding protein H. Hepatology. 2008;48:1242–1250. doi: 10.1002/hep.22439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steinmetz A., Utermann G. Activation of lecithin: cholesterol acyltransferase by human apolipoprotein A-IV. J Biol Chem. 1985;260:2258–2264. [PubMed] [Google Scholar]
- 7.Chen C.H., Albers J.J. Activation of lecithin: cholesterol acyltransferase by apolipoproteins E-2, E-3, and A-IV isolated from human plasma. Biochim Biophys Acta. 1985;836:279–285. doi: 10.1016/0005-2760(85)90131-6. [DOI] [PubMed] [Google Scholar]
- 8.Fournier N., Atger V., Paul J.L., Sturm M., Duverger N., Rothblat G.H., Moatti N. Human ApoA-IV overexpression in transgenic mice induces cAMP-stimulated cholesterol efflux from J774 macrophages to whole serum. Arterioscler Thromb Vasc Biol. 2000;20:1283–1292. doi: 10.1161/01.atv.20.5.1283. [DOI] [PubMed] [Google Scholar]
- 9.Steinmetz A., Barbaras R., Ghalim N., Clavey V., Fruchart J.C., Ailhaud G. Human apolipoprotein A-IV binds to apolipoprotein A-I/A-II receptor sites and promotes cholesterol efflux from adipose cells. J Biol Chem. 1990;265:7859–7863. [PubMed] [Google Scholar]
- 10.Duverger N., Tremp G., Caillaud J.M., Emmanuel F., Castro G., Fruchart J.C., Steinmetz A., Denefle P. Protection against atherogenesis in mice mediated by human apolipoprotein A-IV. Science. 1996;273:966–968. doi: 10.1126/science.273.5277.966. [DOI] [PubMed] [Google Scholar]
- 11.Cohen R.D., Castellani L.W., Qiao J.H., Van Lenten B.J., Lusis A.J., Reue K. Reduced aortic lesions and elevated high density lipoprotein levels in transgenic mice overexpressing mouse apolipoprotein A-IV. J Clin Invest. 1997;99:1906–1916. doi: 10.1172/JCI119358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostos M.A., Conconi M., Vergnes L., Baroukh N., Ribalta J., Girona J., Caillaud J.M., Ochoa A., Zakin M.M. Antioxidative and antiatherosclerotic effects of human apolipoprotein A-IV in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1023–1028. doi: 10.1161/01.atv.21.6.1023. [DOI] [PubMed] [Google Scholar]
- 13.Kim H., Mendez R., Zheng Z., Chang L., Cai J., Zhang R., Zhang K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor alpha to regulate metabolic hormone FGF21. Endocrinology. 2014;155:769–782. doi: 10.1210/en.2013-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakagawa Y., Satoh A., Yabe S., Furusawa M., Tokushige N., Tezuka H., Mikami M., Iwata W., Shingyouchi A., Matsuzaka T., Kiwata S., Fujimoto Y., Shimizu H., Danno H., Yamamoto T., Ishii K., Karasawa T., Takeuchi Y., Iwasaki H., Shimada M., Kawakami Y., Urayama O., Sone H., Takekoshi K., Kobayashi K., Yatoh S., Takahashi A., Yahagi N., Suzuki H., Yamada N., Shimano H. Hepatic CREB3L3 controls whole-body energy homeostasis and improves obesity and diabetes. Endocrinology. 2014;155:4706–4719. doi: 10.1210/en.2014-1113. [DOI] [PubMed] [Google Scholar]
- 15.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F., Wu J., Kharitonenkov A., Flier J.S., Maratos-Flier E., Spiegelman B.M. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Z., Pan X., Wu F., Ye D., Zhang Y., Wang Y., Jin L., Lian Q., Huang Y., Ding H., Triggle C., Wang K., Li X., Xu A. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015;131:1861–1871. doi: 10.1161/CIRCULATIONAHA.115.015308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokkinos J., Tang S., Rye K.A., Ong K.L. The role of fibroblast growth factor 21 in atherosclerosis. Atherosclerosis. 2017;257:259–265. doi: 10.1016/j.atherosclerosis.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 18.Liu S.Q., Roberts D., Kharitonenkov A., Zhang B., Hanson S.M., Li Y.C., Zhang L.Q., Wu Y.H. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Sci Rep. 2013;3:2767. doi: 10.1038/srep02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan X., Shao Y., Wu F., Wang Y., Xiong R., Zheng J., Tian H., Wang B., Wang Y., Zhang Y., Han Z., Qu A., Xu H., Lu A., Yang T., Li X., Xu A., Du J., Lin Z. FGF21 prevents angiotensin ii-induced hypertension and vascular dysfunction by activation of ACE2/angiotensin-(1-7) axis in mice. Cell Metab. 2018;27:1323–1337 e5. doi: 10.1016/j.cmet.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Park J.G., Xu X., Cho S., Lee A.H. Loss of transcription factor CREBH accelerates diet-induced atherosclerosis in Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2016;36:1772–1781. doi: 10.1161/ATVBAHA.116.307790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu X., Park J.G., So J.S., Hur K.Y., Lee A.H. Transcriptional regulation of apolipoprotein A-IV by the transcription factor CREBH. J Lipid Res. 2014;55:850–859. doi: 10.1194/jlr.M045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthan N.R., Giovanni A., Schaefer E.J., Brown B.G., Lichtenstein A.H. Impact of simvastatin, niacin, and/or antioxidants on cholesterol metabolism in CAD patients with low HDL. J Lipid Res. 2003;44:800–806. doi: 10.1194/jlr.M200439-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi T., Orihara K., Oikawa F., Han S.I., Kuba M., Okuda K., Satoh A., Osaki Y., Takeuchi Y., Aita Y., Matsuzaka T., Iwasaki H., Yatoh S., Sekiya M., Yahagi N., Suzuki H., Sone H., Nakagawa Y., Yamada N., Shimano H. Intestinal CREBH overexpression prevents high-cholesterol diet-induced hypercholesterolemia by reducing Npc1l1 expression. Mol Metab. 2016;5:1092–1102. doi: 10.1016/j.molmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa Y., Oikawa F., Mizuno S., Ohno H., Yagishita Y., Satoh A., Osaki Y., Takei K., Kikuchi T., Han S.I., Matsuzaka T., Iwasaki H., Kobayashi K., Yatoh S., Yahagi N., Isaka M., Suzuki H., Sone H., Takahashi S., Yamada N., Shimano H. Hyperlipidemia and hepatitis in liver-specific CREB3L3 knockout mice generated using a one-step CRISPR/Cas9 system. Sci Rep. 2016;6:27857. doi: 10.1038/srep27857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chanda D., Kim Y.H., Li T., Misra J., Kim D.K., Kim J.R., Kwon J., Jeong W.I., Ahn S.H., Park T.S., Koo S.H., Chiang J.Y., Lee C.H., Choi H.S. Hepatic cannabinoid receptor type 1 mediates alcohol-induced regulation of bile acid enzyme genes expression via CREBH. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiang J.Y. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlein C., Talukdar S., Heine M., Fischer A.W., Krott L.M., Nilsson S.K., Brenner M.B., Heeren J., Scheja L. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab. 2016;23:441–453. doi: 10.1016/j.cmet.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Lo Sasso G., Murzilli S., Salvatore L., D'Errico I., Petruzzelli M., Conca P., Jiang Z.Y., Calabresi L., Parini P., Moschetta A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang Y., He S., Li J.Z., Seo Y.K., Osborne T.F., Cohen J.C., Hobbs H.H. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci U S A. 2010;107:7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang H., Zhao M., Sud N., Christian P., Shen J., Song Y., Pashaj A., Zhang K., Carr T., Su Q. Glucagon regulates hepatic lipid metabolism via cAMP and Insig-2 signaling: implication for the pathogenesis of hypertriglyceridemia and hepatic steatosis. Sci Rep. 2016;6:32246. doi: 10.1038/srep32246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang T., Espenshade P.J., Wright M.E., Yabe D., Gong Y., Aebersold R., Goldstein J.L., Brown M.S. Crucial step in cholesterol homeostasis: sterols promote binding of SCAP to INSIG-1, a membrane protein that facilitates retention of SREBPs in ER. Cell. 2002;110:489–500. doi: 10.1016/s0092-8674(02)00872-3. [DOI] [PubMed] [Google Scholar]
- 32.Satoh A., Han S.I., Araki M., Nakagawa Y., Ohno H., Mizunoe Y., Kumagai K., Murayama Y., Osaki Y., Iwasaki H., Sekiya M., Konishi M., Itoh N., Matsuzaka T., Sone H., Shimano H. CREBH improves diet-induced obesity, insulin resistance, and metabolic disturbances by FGF21-dependent and FGF21-independent mechanisms. iScience. 2020;23:100930. doi: 10.1016/j.isci.2020.100930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa Y., Satoh A., Tezuka H., Han S.I., Takei K., Iwasaki H., Yatoh S., Yahagi N., Suzuki H., Iwasaki Y., Sone H., Matsuzaka T., Yamada N., Shimano H. CREB3L3 controls fatty acid oxidation and ketogenesis in synergy with PPARalpha. Sci Rep. 2016;6:39182. doi: 10.1038/srep39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X., Qi Y.F., Chang J.R., Lu W.W., Zhang J.S., Wang S.P., Cheng S.J., Zhang M., Fan Q., Lv Y., Zhu H., Xin M.K., Lv Y., Liu J.H. Possible role of fibroblast growth factor 21 on atherosclerosis via amelioration of endoplasmic reticulum stress-mediated apoptosis in apoE mice. Heart and Vessels. 2014 doi: 10.1007/s00380-014-0557-9. [DOI] [PubMed] [Google Scholar]
- 35.Zheng Z., Kim H., Qiu Y., Chen X., Mendez R., Dandekar A., Zhang X., Zhang C., Liu A.C., Yin L., Lin J.D., Walker P.D., Kapatos G., Zhang K. CREBH couples circadian clock with hepatic lipid metabolism. Diabetes. 2016;65:3369–3383. doi: 10.2337/db16-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min A.K., Jeong J.Y., Go Y., Choi Y.K., Kim Y.D., Lee I.K., Park K.G. cAMP response element binding protein H mediates fenofibrate-induced suppression of hepatic lipogenesis. Diabetologia. 2013;56:412–422. doi: 10.1007/s00125-012-2771-2. [DOI] [PubMed] [Google Scholar]
- 37.Karasawa T., Takahashi A., Saito R., Sekiya M., Igarashi M., Iwasaki H., Miyahara S., Koyasu S., Nakagawa Y., Ishii K., Matsuzaka T., Kobayashi K., Yahagi N., Takekoshi K., Sone H., Yatoh S., Suzuki H., Yamada N., Shimano H. Sterol regulatory element-binding protein-1 determines plasma remnant lipoproteins and accelerates atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1788–1795. doi: 10.1161/ATVBAHA.110.219659. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi S., Brown M.S., Goldstein J.L., Gerard R.D., Hammer R.E., Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hotta Y., Nakamura H., Konishi M., Murata Y., Takagi H., Matsumura S., Inoue K., Fushiki T., Itoh N. Fibroblast growth factor 21 regulates lipolysis in white adipose tissue but is not required for ketogenesis and triglyceride clearance in liver. Endocrinology. 2009;150:4625–4633. doi: 10.1210/en.2009-0119. [DOI] [PubMed] [Google Scholar]
- 40.Yakar S., Liu J.L., Stannard B., Butler A., Accili D., Sauer B., LeRoith D. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96:7324–7329. doi: 10.1073/pnas.96.13.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Madison B.B., Dunbar L., Qiao X.T., Braunstein K., Braunstein E., Gumucio D.L. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 42.Shimano H., Shimomura I., Hammer R.E., Herz J., Goldstein J.L., Brown M.S., Horton J.D. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nakagawa Y., Shimano H., Yoshikawa T., Ide T., Tamura M., Furusawa M., Yamamoto T., Inoue N., Matsuzaka T., Takahashi A., Hasty A.H., Suzuki H., Sone H., Toyoshima H., Yahagi N., Yamada N. TFE3 transcriptionally activates hepatic IRS-2, participates in insulin signaling and ameliorates diabetes. Nat Med. 2006;12:107–113. doi: 10.1038/nm1334. [DOI] [PubMed] [Google Scholar]
- 44.Okazaki M., Usui S., Ishigami M., Sakai N., Nakamura T., Matsuzawa Y., Yamashita S. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol. 2005;25:578–584. doi: 10.1161/01.ATV.0000155017.60171.88. [DOI] [PubMed] [Google Scholar]
- 45.Ide T., Shimano H., Yahagi N., Matsuzaka T., Nakakuki M., Yamamoto T., Nakagawa Y., Takahashi A., Suzuki H., Sone H., Toyoshima H., Fukamizu A., Yamada N. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol. 2004;6:351–357. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- 46.Yoshikawa T., Ide T., Shimano H., Yahagi N., Amemiya-Kudo M., Matsuzaka T., Yatoh S., Kitamine T., Okazaki H., Tamura Y., Sekiya M., Takahashi A., Hasty A.H., Sato R., Sone H., Osuga J., Ishibashi S., Yamada N. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism: I—PPARs suppress sterol regulatory element binding protein-1c promoter through inhibition of LXR signaling. Mol Endocrinol. 2003;17:1240–1254. doi: 10.1210/me.2002-0190. [DOI] [PubMed] [Google Scholar]
- 47.Ide T., Shimano H., Yoshikawa T., Yahagi N., Amemiya-Kudo M., Matsuzaka T., Nakakuki M., Yatoh S., Iizuka Y., Tomita S., Ohashi K., Takahashi A., Sone H., Gotoda T., Osuga J., Ishibashi S., Yamada N. Cross-talk between peroxisome proliferator-activated receptor (PPAR) alpha and liver X receptor (LXR) in nutritional regulation of fatty acid metabolism: II—LXRs suppress lipid degradation gene promoters through inhibition of PPAR signaling. Mol Endocrinol. 2003;17:1255–1267. doi: 10.1210/me.2002-0191. [DOI] [PubMed] [Google Scholar]
- 48.Uchida A., Whitsitt M.C., Eustaquio T., Slipchenko M.N., Leary J.F., Cheng J.X., Buhman K.K. Reduced triglyceride secretion in response to an acute dietary fat challenge in obese compared to lean mice. Frontiers in Physiology. 2012;3:26. doi: 10.3389/fphys.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fujimoto Y., Nakagawa Y., Satoh A., Okuda K., Shingyouchi A., Naka A., Matsuzaka T., Iwasaki H., Kobayashi K., Yahagi N., Shimada M., Yatoh S., Suzuki H., Yogosawa S., Izumi T., Sone H., Urayama O., Yamada N., Shimano H. TFE3 controls lipid metabolism in adipose tissue of male mice by suppressing lipolysis and thermogenesis. Endocrinology. 2013;154:3577–3588. doi: 10.1210/en.2013-1203. [DOI] [PubMed] [Google Scholar]