Abstract
Chemotherapy constitutes a major part of modern-day therapy for infectious and chronic diseases. A drug is said to be effective if it can inhibit its target, induce stress, and thereby trigger an array of cell death pathways in the form of programmed cell death, autophagy, necrosis, etc. Chemotherapy is the only treatment choice against trypanosomatid diseases like Leishmaniasis, Chagas disease, and sleeping sickness. Anti-trypanosomatid drugs can induce various cell death phenotypes depending upon the drug dose and growth stage of the parasites. The mechanisms and pathways triggering cell death in Trypanosomatids serve to help identify potential targets for the development of effective anti-trypanosomatids. Studies show that the key proteins involved in cell death of trypanosomatids are metacaspases, Endonuclease G, Apoptosis-Inducing Factor, cysteine proteases, serine proteases, antioxidant systems, etc. Unlike higher eukaryotes, these organisms either lack the complete set of effectors involved in cell death pathways, or are yet to be deciphered. A detailed summary of the existing knowledge of different drug-induced cell death pathways would help identify the lacuna in each of these pathways and therefore open new avenues for research and thereby new therapeutic targets to explore. The cell death pathway associated complexities in metazoans are absent in trypanosomatids; hence this summary can also help understand the trigger points as well as cross-talk between these pathways. Here we provide an in-depth overview of the existing knowledge of these drug-induced trypanosomatid cell death pathways, describe their associated physiological changes, and suggest potential interconnections amongst them.
Keywords: Trypanosomatids, Regulated cell-death, Autophagy, Necrosis
Graphical abstract
1. Introduction
The trypanosomatid family harbor three kinetoplastids, namely Trypanosoma brucei, Trypanosoma cruzi, and Leishmania, which are the causative agents of African sleeping sickness, Chagas disease, and cutaneous, mucocutaneous, or visceral Leishmaniasis respectively, based on the infecting species. These neglected tropical diseases have plagued humankind for a long and owing to improper vector controls and ineffective vaccines; chemotherapy is the only choice against these diseases. Currently, no efficient chemotherapy for Chagas disease (CD) and sleeping sickness or Human African Trypanosomiasis (HAT) exists to eliminate the parasite without drawbacks. Limited drugs that are approved for CD, such as benznidole, and nifurtimox, depending on the parasite strains, have various disadvantages like long-term treatment, toxicity, variable sensitivity (Jackson et al., 2020, Viotti et al., 2009). Similar problems are exhibited by the clinically tested drugs for the treatment of sleeping sickness both in treating early and late stages of the disease. At present, nifurtimox–eflornithine combination treatment (NECT) is an effective option for African sleeping sickness. Recently developed drugs, fexinidazole and acoziborole, showed promising ability to combat both the early and late stages of HAT (Dickie et al., 2020, P De Koning, 2020).
In the context of Leishmaniasis, several drugs like sodium stibogluconate, miltefosine, pentamidine, Amphotericin B have been formulated, and more drug-repurposing trials are undergoing (de Menezes et al., 2015; Braga, 2019; Jones et al., 2018). A potent anti-leishmanial is one which is specific to the parasite and can effectively eliminate it. Owing to the similarity in metabolic pathways of Leishmania and its host, a specifically targeted drug having no effect on the host is not always available, but due to the rapid proliferation of Leishmania compared to the host, these drugs at non-cytotoxic doses with respect to the host, can effectively eliminate the parasite. These drugs, due to their lower dose and prolonged exposure time, induce morphological and biochemical features characteristic of regulated cell death in the parasites. With the advent of the TriTryp genome, many effector molecules and the linked pathways they trigger have been identified, but there is a lack of comprehensive knowledge on these pathways.
Many newly identified small molecules and inhibitors can effectively eliminate the parasites by activating regulated cell death mechanisms in a dose and time-dependent manner. The mode of such drug-induced cell death pathways depends on the type of stimuli, mode of action of the drug, the parasite subspecies, and its life-cycle stage. The unregulated and uncontrolled use of drugs has led to the emergence of chemo-resistance by overcoming drug-induced stress (Ponte-Sucre et al., 2017). Knowledge of the gradual development of chemo-resistance through overcoming the cell death pathways is essential to prevent such resistance. This makes the understanding of all discrete cell death mechanisms and their involved characteristics essential in studying resistance development and developing an effective therapeutic solution. By analysing all such available information, this study tries to draw analogies and perspectives, which would help mitigate these neglected tropical diseases.
2. The regulated cell death cascade
Based on current knowledge, programmed cell death or classical apoptosis and necrosis are considered physiological forms of regulated cell death (RCD). Other than classical apoptosis or PCD, other non-apoptotic RCD can be induced by drugs in metazoans, like necroptosis, pyroptosis, parthanatos, entotic, and netotic cell death, ferroptosis, lysosome-dependent cell death, autophagy-mediated death, alkaliptosis, and oxeiptosis. Apoptosis can be extrinsic or intrinsic, where death receptors like FAS and TNF receptor mediate extrinsic apoptosis and are driven by initiator caspase 8 and thereafter caspase 10 (Tang et al., 2019). Intrinsic apoptosis includes loss of mitochondrial outer membrane permeabilization (MOMP) triggered by pro-apoptotic factors that belong to Bcl-2 family proteins like Bid, BAX and BAK, that then leads to the release of mitochondrial proteins like cytochrome C, followed by activation of initiator caspase 9 (Tang et al., 2019). Caspase-independent apoptosis is activated by pro-apoptotic proteins that induce MOMP to release AIF or EndoG from mitochondria leading to chromatin condensation and DNA fragmentation. Another intriguing pathway involves PARP/AIF mediated apoptosis (Jiang et al., 2018).
Unicellular Trypanosomatids either lack homologs of many of these effectors that control RCD or are yet uncharacterized. However, the physiological outcome of these cell-death types remains largely similar. Several unknown effector molecules are known to be involved in the apoptosis-like or non-apoptotic cell death mechanisms in Leishmania. Various studies show that different anti-leishmanial drugs exhibit several physiological cell death features similar to metazoans. Hence a comprehensive study of these drug-induced cell death mechanisms and its associated features could unravel the regulated cell death cascade in Leishmania.
Apoptosis-like cell death in Leishmania exhibits perturbed Ca2+ homeostasis, mitochondrial dysfunction, phosphatidylserine externalization, nuclear condensation, DNA fragmentation, etc (Das et al., 2001). Upon entry into the host, Leishmania prosmastigotes exhibit an apoptotic mimicry by externalizing phosphatidylserine so as to get engulfed by the circulating neutrophils. This is part of their ‘Trojan horse’ mechanism of gaining access into macrophages, as these Leishmania containing neutrophils which due to their limited life span when undergoing apoptosis are engulfed by macrophages thus enabling the parasites to enter macrophage and thereby establish its niche. Once promastigote to amastigote differentiation has occurred inside macrophages and the amastigotes have proliferated, they burst of the macrophage to gain access to new macrophages and for this they also exhibit phosphatidylserine (El-Hani et al., 2012). The stated mechanism occurs due to the inherent property of macrophages as scavengers for apoptotic cells and entities and the externalization of phosphatidylserine is a chemotactic cue for such scavenging activity (Penberthy and Ravichandran, 2016).
Leishmania promastigotes exhibited a necrosis-like death with H2O2 doses above 4 mM, while doses below 4 mM exhibited an apoptosis-like death along with activation of effector proteases as well as cleavage of a PARP-like protein and activity of caspase-like proteases acting on caspase substrates (Das et al., 2001). It remains to be seen whether this apoptotic to necrotic trigger emanates from a rapid depletion of ATP levels with an increasing dose of H2O2. Camptothecin induced oxidative stress in L. donovani has been shown to increase intracellular Ca2+ levels, cause lipid peroxidation, nuclear breakage, cell volume loss, and GSH depletion (Sen et al., 2004). Interestingly H2O2 treated L. amazonensis promastigotes majorly exhibited an apoptosis-like death, but some persistor parasites showed a G2/M phase cell-cycle arrest. As a repair response to the nuclear damage in these persistor parasites, LaRPA1 relocalized from the G-quartet to a C-rich telomeric end to prevent telomeric shortening and thus genome integrity. These persistors later emanated into a resistant species (da Silva et al., 2017).
Studies show that sodium stibogluconate treated Leishmania amastigotes causes cell death by oxidative stress, with increased intracellular Ca2+ levels and DNA fragmentation (Moreira et al., 2011). Although MAPK-dependent pathways are one of the major cellular signaling cascades in mammals, it has scarcely been studied in Trypanosomatids. In a recent study, genistein (GEN) and chrysin (CHY) in a dose-dependent manner could inhibit the kinase activity of LdMAPK3 and thus exhibit anti-leishmanial activity (Raj et al., 2019). In another study, LdMAPK1 was down-regulated in antimony-resistant clinical isolates, and susceptibility to antimony increased upon overexpression of LdMAPK1 in these parasites. Interestingly, these LdMAPK1 down-regulated antimony-resistant parasites were susceptible to amphotericin B and miltefosine, which induced apoptosis-like death of these parasites (Garg and Goyal, 2015). Similarly, L. major MAPK2, by phosphorylating an influx pump, AQP1 can hinder antimony accumulation and exhibit antimony resistance (Garg and Goyal, 2015). Hence Leishmania MAPK proteins play an important role in drug-induced cell death and the concomitant emergence of drug-resistant parasites. Thus MAPKs might act as an effective drug target not only against antimony-resistant but other drug-resistant parasites as well.
Zinc (Zn) is a metabolically essential micronutrient for parasite survival, and its assimilation is carried out by ZIP3, which is a Zn transporter belonging to the ZRT/IRT-like Protein (ZIP) family (Carvalho et al., 2015). Like other micronutrients, increased amounts of zinc can lead to toxicity; hence high concentrations of zinc sulfate induced apoptotic-like death of L. major and L. tropica (Fattahi Bafghi et al., 2014). Conversely, the chelation of intracellular zinc using TPEN induces an apoptosis-like death, both in Sb(III) sensitive and resistant L. donovani promastigotes (Saini et al., 2017). Moreover, inhibition of the major Zn containing protease GP63 by another Zn chelator PHEN, induced the killing of these L. amazonensis promastigotes. Hence the effectiveness of zinc chelators in combination with other drugs needs to be tested in sensitive and resistant parasites as potential drug therapy.
Amphotericin B, an effective anti-fungal compound with high hepatic and renal toxicity in the free form, is the most effective anti-leishmanial after the wide emergence of antimony-resistant parasites. In one study, the synergistic action of MDR1 mediated drug efflux and up-regulated tryparedoxin led to scavenging of intracellular ROS, thus conferring AmB resistance (Suman et al., 2016). This suggests that AmB mediated cell death involves increased intracellular ROS. Recently KalsomeTM10, a new liposomal formulation of AmB containing ergosterol, was shown to effectively eliminate L. donovani promastigotes as well as intracellular amastigotes by causing loss of mitochondrial membrane potential, depletion of intracellular cellular ATP pool and GHS content, increase in lipid peroxidation and DNA fragmentation, all of which are hallmarks of apoptosis-like death. The caspase inhibitor, z-VAD-fmk, could prevent KalsomeTM10 mediated cell death of Leishmania, thus indicating the involvement of z-VAD-fmk sensitive caspase-like proteins in the process (Shadab et al., 2017). Contrary to mammalian cell membranes harboring cholesterol, parasite membrane harbor ergosterol. AmB interacts with this ergosterol/sterol component of the membrane, thus leading to the formation of transmembrane channels that alter solute permeability to cations and therefore swelling of these parasites, followed by their lysis (Cohen, 2010). Further studies are required to understand such dose-dependent triggers from apoptosis-like death to death-by-lysis and whether the later involves any necrotic feedback.
Miltefosine, an alkylphosphocholine, is an oral anti-leishmanial drug that targets cytochrome c oxidase (Luque-Ortega and Rivas, 2007), and on treatment, the parasites exhibit morphological and biochemical features like cell rounding up, cell shrinkage, phosphatidylserine externalization, which are apoptotic hallmarks (Paris et al., 2004). Susceptibility to miltefosine varies among Leishmania species, where L. major does not exhibit any apoptotic characteristics at concentrations sufficient to kill L. tropica. L. donovani displayed the highest susceptibility, while L. major exhibited the lowest susceptibility to miltefosine (Dorlo et al., 2012). Both miltefosine and BZTZ at low concentrations trigger an autophagic response from which the parasites recover, but when used in combination, a synergistic action of the two drugs overcomes the autophagic barrier and induces an apoptosis-like death of the parasites (Scariot et al., 2017). This is an interesting avenue of research, which would unravel the mechanism of progression from autophagy to either cell survival or apoptosis-like death in a simplistic manner avoiding the metazoan complexities. The sensitivity of a drug depends on its net accumulation regulated by the influx and efflux pathways. The decreased sensitivity of L. braziliensis to miltefosine was probably due to reduced expression of the plasma membrane proteins LdMT (a P-type ATPase) and LdRos3, which are involved in internalizing miltefosine (Weingärtner et al., 2010). Similarly, miltefosine resistance of L. infantum strains is due to impaired MT/ROS3 transporter, but it retains amphotericin B susceptibility (Weingärtner et al., 2010). Such studies would help in understanding the role of species-specific transporters for a particular drug as well as the parasite-specific expression pattern that governs drug transport. Miltefosine resistant trypanosomatids have been shown to up-regulate the antioxidant enzyme, FeSODA, to raise the intracellular level of reduced thiol to counteract the drug-induced oxidative stress (Piacenza et al., 2013; Veronica et al., 2019).
Topoisomerase I poisons have effective anti-leishmanial activity and exhibits apoptotic characteristics like surface blebbing, degranulation, and defragmentation (Das et al., 2006; Chowdhury et al., 2014; Mamidala et al., 2016; Saha et al., 2016). Since TopIB is a potential target, it compromises specificity due to the presence of TopIB in the host, but owing to the rapid proliferation of Leishmania as compared to the host, these drugs can nevertheless exhibit efficient parasite elimination at non-cytotoxic doses. Interestingly, the TriTryp genome harbors a prokaryotic TopA, which has been shown to be an essential enzyme in trypanosomes (Scocca and Shapiro, 2008). The absence of a homolog in the host makes it a potential parasite-specific target. Hence repurposing studies need to be carried out using clinical drugs targeting this prokaryotic homolog.
Owing to the chemo-resistance menace and lack of new drugs, researchers have used transporter inhibitors and parasite antioxidant inhibitors to resensitize the resistant parasites to the drugs. Sitamaquine, which inhibits ABC transporters LMDR1 and MRPA, can effectively revert miltefosine and antimony resistance, while buthionine sulfoximine (BSO), a biosynthesis-specific glutathione inhibitor, can revert the antimony-resistant phenotype (Pérez-Victoria et al., 2011; El Fadili et al., 2005). A mitochondrial ABC half transporter, LmABCB3, which plays a role in heme synthesis and cytosolic iron/sulphur clusters (ISC) biogenesis, is an essential gene whose disruption prevents the growth of Leishmania (Martínez-García et al., 2016), thus making it an effective anti-leishmanial target. To mitigate multidrug resistance, it is necessary to focus on influx activators or inhibit rapid efflux by effectively targeting ABC transporters.
Trypanosomatids also harbor several endo/exonucleases that are involved in apoptosis-like death. Baicalein, a TopIB inhibitor, induces a caspase-independent apoptosis-like death of the parasites by inducing the kinetoplast to nuclear translocation of EndoG followed by assembly of a DNA degradesome to cause DNA fragmentation (BoseDasgupta et al., 2008). Although the presence of apoptosis-like features and effector molecules imply “Regulated cell death” in Trypanosomatids, insufficient information on the canonical apoptosis-like death pathway is a drawback towards establishing this pathway in Trypanosomatids. Besides, it remains to be elucidated how one drug induces a caspase-like protease mediated cell death while another engages a DNA degradesome in a caspase-independent manner where all other hallmarks are similar to apoptotic cell death.
3. Autophagy associated cell-death pathways
Autophagy, a vesicular traffic engaging process, helps to transfer cellular components to lysosomes for degradation. Cellular homeostasis is maintained through autophagy, by degrading proteins and damaged organelles. In response to stress, autophagy acts as a cell survival mechanism, but it can also trigger cell death. The TriTryp genome reveals the presence of a number of autophagy related genes in varying numbers in these parasites where they could be playing a pivotal role in the maintenance of cellular homeostasis. Autophagy-related lysosome-like vacuoles were observed in a cysteine cathepsin inhibitor 13b treated L. major promastigotes at an early phase of cell death, and in the late phase they exhibited apoptosis-like death (Schurigt et al., 2010), thus indicating a progression from autophagy to apoptotic-cell death. Interestingly, Yangambin (a lignin) could induce autophagy in L. chagasi but apoptotic-cell death in L. amazonensis promastigotes (Monte Neto et al., 2011). This could be due to differential drug accumulation in the two species. But the intracellular trigger from autophagy to autophagic cell death or apoptotic cell death would be an important area of research and whether the drug type, drug dose, or dosage period plays a role in these needs to be determined. The alkaloid cryptolepine (CLP), which generates ROS inside L. donovani promastigotes, also forms MDC positive autophagic vacuoles and thereafter, results in DNA fragmentation and other hallmarks of apoptosis prior to cell-death (Sengupta et al., 2011). The combination therapy with drugs, known to induce both autophagy and apoptosis, includes thiosemicarbazone, BZTS, and miltefosine, respectively, that can generate a synergistic effect on L. amazonensis (Scariot et al., 2017). Recently miltefosine induced L. major cells were found to initiate early autophagy, but after prolonged stress, apoptotic cell-death was triggered (Basmaciyan et al., 2018). A β-carboline compound, C5 that has anti-leishmanial activity against Leishmania amazonensis, exhibits apoptotic features, acidic vesicular organelles (AVOs) as well as autophagic compartments (Mendes et al., 2016). Dihydroxyacetone causes G2/M cell cycle arrest and thereby inhibits the growth of T. brucei bloodstream forms, thus exhibiting morphological features like an increase in vesicular structures, formation of autophagy-like vacuoles and multivesicular bodies etc (Uzcátegui et al., 2007). From these studies, it is evident that autophagic vacuoles are a key hallmark of autophagy induction which are positively stained by monodansylcadaverine, and the same is prevented by PI3K inhibitors, 3-methyladenine or wortmannin (Giri and Shaha, 2019). But detailed biochemical pathway governing the autophagic process in trypanosomatids is yet to be deciphered.
Among the canonical macroautophagy, microautophagy, and chaperone-mediated autophagy in higher eukaryotes, macroautophagy has predominantly been observed in Leishmania species, but microautophagy has also been observed in a glycosome turnover study (Cull et al., 2014; Herman et al., 2008). Among Leishmania homologs of AuTophaGy (ATG) proteins, ATG4.1 and ATG4.2 have specific cleavage specificities towards 4 isoforms of ATG8 while the ATG5-ATG12 conjugation pathway has also been described (Williams et al., 2013). Hence, it is evident now that “macroautophagy” is an important process in regulating cell death in Leishmania. Mitochondrial translocation of ATG5 protein was evidenced earlier in L. major (Williams et al., 2012), while mitotoxic stress during early hours of cisplatin and etoposide treatment caused mitochondrial translocation of ATG8 (Giri and Shaha, 2019).
Higher eukaryotes have multiple mitochondria eliminated by mitophagy upon redundancy, and constant mitochondrial fission and fusion help to eliminate damaged mitochondria by autophagy (Ashrafi and Schwarz, 2013). In contrast, trypanosomatids harbor a single large reticulated mitochondrion whose turnover by autophagy is debatable. A berberine compound that triggers classical apoptosis-like cell death in L. donovani might induce mitochondrial fission in these parasites similarly in mammalian cells (De Sarkar et al., 2019). Additionally, the single dynamin-1-like protein of T. brucei (TbDLP) was shown to regulate the mitochondrial division and fission (Benz et al., 2017). Hence, DLP, a GTPase, was down-regulated in both miltefosine and sodium stibogluconate resistant L. infantum strains (Vincent et al., 2015), and decrease of DLP impacts the function and morphology of the mitochondrion in resistant strains. Depolarization below a certain Δψm is an indication of impaired mitochondrial function and a prerequisite for mitophagy in these resistant strains. Study of uncharacterized putative RBR genes in these parasites could reveal its role in ubiquitin metabolism for selective targeting of mitochondria to autophagosomes similarly to parkin mediated mitophagy in higher eukaryotes, where parkin E3 enzyme conjugates ubiquitin chains to outer mitochondrial membrane proteins that finally recruits mitophagy adaptors like p62/sequestosome1, orthologs of which can be identified by comparative genomics in trypanosomatids (Roca-Agujetas et al., 2019; Marín and Ferrús, 2002). Also based on an interesting finding in a higher eukaryote, regulation of mitophagy and apoptosis by mono or polyubiquitinated voltage-dependent anion channels (VDAC) might be explored in trypanosomatids as well (Ham et al., 2020; Pusnik et al., 2009). Thus it is intriguing to study the mechanism of stress-induced autophagy of the single large mitochondria of kinetoplastids.
Trypanosomes can efficiently uptake certain neuropeptides, that being cationic or amphiphilic can preferentially bind to negatively charged membrane and thereby exert trypanolytic activity by disrupting the lysosomes and accumulating glycolytic enzymes in the cytosol. This promotes the failure of energy metabolism and initiates an autophagy-like cell death of the parasites (Delgado et al., 2009). Bloodstream-form of parasites depend mainly on glycolysis for their ATP supply, and unlike mammals, they confine their glycolytic activities to the glycosome, which is absent in mammals. This exhibits an alternate avenue of triggering cell death in these trypanosomatids where the host would be unaffected. Besides, it also remains to be identified how disruption of glycosomal activities can trigger regulated cell death in these parasites and whether specific drugs can effectively induce this pathway. Bacteriocin As-48 kills Trypanosomes by autophagy-like cell death, evidenced by the appearance of double membraned vacuoles without any host cytotoxicity (Martínez-García et al., 2018). Additionally, disruption of glycosome biogenesis by a PEX14 inhibitor, pyrazolo[4,3-c]pyridine leads to the elimination of the parasites due to mislocalization and accumulation of glycosomal enzymes in the cytosol, and depletion of ATP levels (Kalel et al., 2017; Bauer and Morris, 2017).
Trypanosomatids are highly susceptible to mammalian mTOR and PI3K inhibitors, as TOR acts as a major regulator of cell growth and autophagy (Barquilla and Navarro, 2009, Diaz-Gonzalez et al., 2011). LmTOR3 mutants fail to survive within macrophages in vitro due to defects in acidocalcisome biogenesis (Madeira da Silva and Beverley, 2010). Proteins like ATG1and Beclin1/ATG6 homologs are yet to be found in Leishmania.
Little is known about the endosomal pathway and its role in Trypanosomatid cell death. The synergistic role of the autophagy pathway and the endosomal system in Leishmania is supported by defective transport to unusual multivesicular tubules (Besteiro et al., 2006; Hall et al., 2006). In response to stress, drug-induced autophagy is a survival strategy in Leishmania, failing which apoptosis-like death is triggered. Besides, parasite-specific pathways involving autophagy comes as an important avenue of further studies. In some instances, continued stress causes autophagy itself to eliminate the parasite from within through multivesicular structures.
4. ER-stress induced death mechanisms
ER responds to stress by minimizing protein translation through PERK signaling, up-regulating ER-resident chaperones through ATF-6, and activating ER-associated protein degradation (ERAD) through IRE1-signaling that curtail agglomeration of unfolded proteins (Adams et al., 2019). Altogether it is termed as Unfolded Protein Response (UPR), and prolonged UPR activation may predispose the cells toward oxidative stress generation at a lethal level.
Endoplasmic reticulum (ER) stress-induced apoptosis in Leishmania is completely unexplored. By inhibiting N-linked oligosaccharides biosynthesis, Tunicamycin (TM) can prevent the growth and alter the infectivity of different Leishmania species (Dolai et al., 2011). Although this apoptosis-like death depends on cellular calcium levels, it does not involve caspases. ER stress results in elevated cytosolic Ca2+ level due to the release of Ca2+ from internal stores, especially the ER and acidocalcisomes. Inhibition of Ca2+release by verapamil, which blocks voltage-gated cation channels, prevents Ca2+ release, and maintains mitochondrial membrane potential and intracellular ATP. Furthermore, ER stress-induced generation of ROS releases mitochondrial cytochrome c and endonuclease G to the cytosol, and subsequent translocation of endonuclease G into the nucleus has been observed in Leishmania (Dolai et al., 2011).
Upon Dithiothreitol (DTT) treatment of T. brucei, many classic UPR related genes were up-regulated, and additionally, genes involved in mitochondrial functions were also up-regulated by the increased half-life of chaperone protein DNAJ, protein disulfide isomerase (PDI), thioredoxin, and syntaxin. Few reports suggest that ER stress response reduces spliced leader RNA levels due to change in the amount and localization of SL RNA transcription factor tSNAP42 (Goldshmidt et al., 2010). When ER stress is persistent, SL-RNA depletion occurs, thus preventing matured mRNA production, which then triggers programmed cell death due to loss of Ca2+ homeostasis, mitochondrial membrane depolarization, PS externalization, and DNA fragmentation (Goldshmidt et al., 2010). It remains to be studied whether SL-RNA depletion mediated cell-death in Leishmania triggers UPR and ERAD.
Covalent attachment of ubiquitin (Ub) to proteins marks it for degradation via the proteasome (Lata et al., 2018). GNF6702, an azabenzoxazole non-competitively inhibits kinetoplastid proteasome without affecting the mammalian proteasome or its growth and thereby specifically inhibits the proliferation of L. donovani promastigotes and amastigotes (Khare et al., 2016). Another inhibitor GSK3494245, inhibits the chymotrypsin-like activity of the β5 subunit of L. donovani proteasome and therefore prevents the intramacrophage proliferation of amastigotes through the accumulation of ubiquitylated proteins (Winzeler and Ottilie, 2019). Thus Leishmania proteasome appears to be a potential drug target for specific elimination of the parasites, and the knowledge of the existing inhibitors would provide an added advantage.
Transfer of elongation factor 1-alpha (EF-1a) from the nucleus to the cytoplasm was observed in the dying Trypanosoma cruzi (Billaut-Mulot et al., 1996). Leishmania EF1-α plays a crucial role in parasite survival by regulating oxidative burst inside the host phagocytes. A cationic liposomal formulation of L. donovani EF1-α was shown to act as a potential antigen for host protective immunity (Sabur et al., 2018). The Leishmania genome database reveals the presence of SERCA and RYR/InsP3R orthologues but lacks CHOP and IRE1. ER-localized SERCA regulates parasite virulence in L. amazonensis by maintaining intracellular Ca2+ levels. ER stress-inducing agent thapsigargin induces the trans-autophosphorylation of ER-resident PERK and thereby represses general translation in Leishmania by phosphorylating eIF2alpha, thus suggesting that an ER transmembrane protein is capable of sensing ER stress. But whether its contribution to translation arrest during UPR is direct or indirect is yet to be deciphered (Chow et al., 2011). Endosomal PERK similar to the T. brucei kinase, TbeIF2αK2 control ROS levels in response to heme deprivation, while cytoplasmic GCN2 like kinase Ldke1 in L. donovani phosphorylates eIF2α and dampens protein translation during nutrient loss (da Silva Augusto et al., 2015; Rao et al., 2016). Hence extensive studies need to be carried out to reveal possible drug-induced ER stress response in Leishmania parasites and the key proteins involved in the process.
5. Induction of necrotic cell death
Necrotic death is characterized by cell swelling, loss of membrane integrity, and lysis. Organelle swelling is a major hallmark of necrosis wherein mitochondrial swelling depletes intracellular ATP levels, lysosomal rupture releases lytic enzymes, and plasma membrane protrusions appear. Nitroheteroaryl 1,3,4thiadiazole derivatives induce necrosis in parasites by reducing the specific activity of acid phosphatase, cleavage of a PARP-like protein, and DNA degradation (Ardestani et al., 2012). Betulinic acid, a DNA topoisomerase I and II inhibitor, can induce apoptosis-like death in Leishmania, but at the same dose, it induces necrosis in T. cruzi through the change of mitochondrial membrane potential, increased ROS production, and swelling of reservosomes (Sousa et al., 2017). This indicates that the drug-induced apoptosis-like death or necrosis predominantly depends on the drug dose. Therefore, these threshold boundaries from persistence to apoptosis to necrosis in terms of drug dose remain to be studied. Allicin treated Leishmania starts exhibiting few apoptotic characteristics, but a rapid loss of intracellular ATP triggers lysosomal swelling and rupture, thereby triggers necrosis and cell arrest at the premitotic G2/M phase (Corral et al., 2016). When Leishmania promastigotes are treated with Epigallocatechin-3-gallate (EGCG), it increases proton permeability across the inner mitochondrial membrane leading to decreased ATP levels and induction of parasite death by necrosis (Inacio et al., 2012). One obvious fact that emanates from the above studies is the extent and rapidity of ATP loss induced by a drug in a particular parasite will determine whether it will induce apoptosis or necrosis but whether the parasite or a specific drug determines the threshold for this need to be elucidated. The molecular switch between apoptosis and necrosis in metazoans is maintained by caspase3/7, which upon activation induces PARP cleavage, and the same is inhibited by z-VAD-fmk. Necrotic cell death also exhibits PARP cleavage initiated by lysosomal proteases cathepsins B, D, G, and this cleavage is unaffected by z-VAD-fmk (Gobeil et al., 2001). Interestingly inhibition of TbPARP does not inhibit cell growth, but when TcPARP is inhibited, it interferes with infection and proliferation (Haikarainen et al., 2017). The existence of PARP-like protein in Leishmania and its role in necrosis, if any, is yet to be deciphered.
6. The ferroptotic pathway
Ferroptosis, an iron-dependent pathway in mammals, results from inhibition of Glutathione Peroxidases, Gpx4, and is characterized by iron accumulation and cellular lipid peroxidation to lethal levels is suppressed by iron chelators or lipophilic antioxidants (Mou et al., 2019). While mammals harbor catalase, glutathione reductase, thioredoxin reductase, and selenium-dependent peroxidases in their antioxidant repertoire, Leishmania handles it through thiol-dependent peroxidases or heme-dependent ascorbate peroxidases (Moreira et al., 2018). Trypanosomes contain GPx-like glutathione (GSH) peroxidases (Px I–III), but they are type II tryparedoxin peroxidases that reduce tryparedoxin at much higher folds than GSH (Bogacz and Krauth-Siegel, 2018). The trypanosomes lacking this lipid hydroperoxide-detoxifying tryparedoxin peroxidases (PxI-III type) undergo rapid cell death when treated with certain drugs, and this is suppressed by iron chelation or by lipophilic antioxidants, thus signifying ferroptosis as the mode of cell-death (Bogacz and Krauth-Siegel, 2018). Interestingly, whether this iron accumulation or accumulation of lipid peroxides is a bystander to other apoptotic signatures or specifically occurring characteristics remains to be seen. Physiological substrates of peroxidases are H2O2, thymine hydroperoxide, and linoleic acid hydroperoxide. Leishmania also harbors a cysteine-homolog of glutathione peroxidase LmTDPX1which instead of GSH uses hydrogen peroxide and cumene hydroperoxide as the electron donor. But unlike 2-Cys peroxiredoxins, LmTryP, which is inactivated by over-oxidation LmTDPX1, is not sensitive to hydroperoxides. Phosphatidylcholine hydroperoxide or miltefosine may be targeting TXNPx's that use lipid hydroperoxides as their substrates (König and Fairlamb, 2007). Amphotericin B-resistant or potassium antimony-resistant isolates exhibit the up-regulation of cytoplasmic TXNPx in Leishmania species (Andrade and Murta, 2014) thus indicating a portion of AmB treated parasites undergoing ferroptosis only or in conjunction with apoptosis-like death. When trypanosomatids are treated with the mitochondrial complex I inhibitor Rotenone, there is a dose-dependent increase in both cytoplasmic and mitochondrial TXNPx expression (Das et al., 2017). Overall it appears that in trypanosomatids, targeted inhibition of peroxidases and tryparedoxins followed by mitochondrial dysfunction and unrepaired lipid peroxides result in ferroptosis mediated cell death. While overall dysregulation of the cell where these enzymes try to maintain homeostasis but fail, that then leads to DNA fragmentation emanate as apoptosis-like death.
7. Cell-death inducing proteases
Serine/Cysteine proteases: Although trypanosomatids harbor a huge repoitre of serine proteases, much less is studied about their role in cell death. A secretory serine protease of Leishmania is sensitive to different inhibitors such as aprotinin, benzamidine, N-tosyl-1-phenylalanine chloromethyl ketone, etc. Serine protease like metacaspases are involved in programmed cell death of Leishmania, and its activity is inhibited by trypsin inhibitors; leupeptin, antipain, and N(alpha)-tosyl-L-lysine-chloromethyl ketone(TLCK). Overexpression of metacaspases renders parasites more sensitive to hydrogen-peroxide (Lee et al., 2007; González et al., 2007; Geiger et al., 2016). Miltefosine and curcumin could induce a growth defect in wild type but not metacaspase deficient parasites. Metacaspases have also been implicated in autophagy (Casanova et al., 2015). Another serine protease in these parasites is Subtilisin, whose deletion interferes with the in vitro differentiation of promastigotes to amastigotes as it acts as a maturase for tryparedoxin peroxidases and also has a role in pentavalent antimony susceptibility (Swenerton et al., 2010). Leishmania ISPs are a promastigote specific protein involved in promastigote differentiation and flagellar homeostasis and therefore, an important therapeutic target. Trypanosomatid serine peptidases LdISP1 and LdISP2 are strong inhibitors of mammalian serine proteases trypsin and chymotrypsin.
Cathepsins (cysteine protease) are generally activated at low pH and are found inside lysosomes. Kinetoplastids harbor Cathepsin L and B like proteins where Cathepsin B, like proteases in L. donovani, T. cruzi, and T. brucei are inhibited by nicotinamide. Disruption of cathepsin B-like activity blocks the endocytic pathway and cytokinesis, which subsequently causes cell death (Unciti-Broceta et al., 2013). Lysosomal Cysteine peptidases CPA and CPB can degrade autophagosomes in Leishmania, thus making the parasites more susceptible to nutrient removal and prevents differentiation (Williams et al., 2006).
Calpains are known to be calcium-dependent proteases, but protozoan calpain-like proteins lack the calcium-binding EF-hand motifs. MDL28170, a calpain inhibitor, can interfere with macrophage infection, reduce the infection rate and intracellular multiplication of these parasites (Marinho et al., 2014). Miltefosine treated parasites pretreated with calpain inhibitors exhibit significant inhibition of NO-mediated PCD and apoptotic DNA fragmentation. SKCRP14.1, a calpain-related protein, is down-regulated in antimony and miltefosine resistant Leishmania strains, but it has the opposite effect in both the resistant strains. Over-expressed CALP markedly increases the sensitivity of the resistant strain to antimonials and promotes PCD while it protects against miltefosine-induced PCD (Branquinha et al., 2013). Hence an elaborated study could help in establishing Leishmania proteases as potential drug targets and its role in chemo-resistance.
8. Discussion
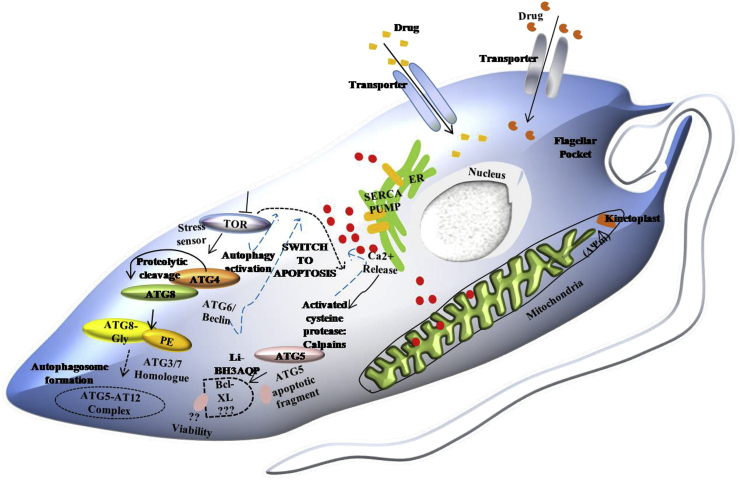
8.1. Switch between autophagy and apoptosis
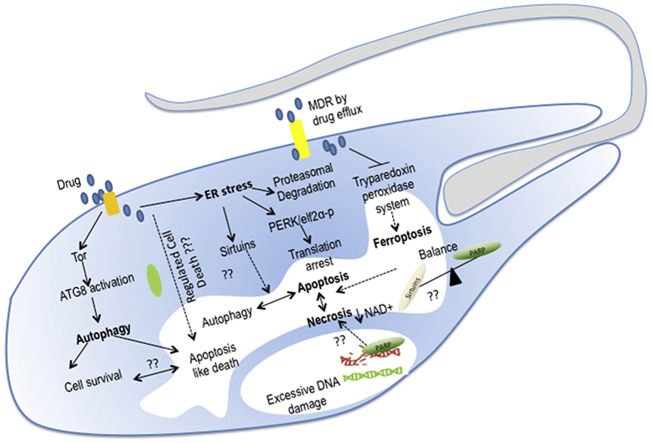
The conserved autophagosome formation process is initiated by two key initiation complexes: the ULK1 complex, which is the Atg1 complex in yeast, and the class III phosphatidylinositol-3 kinase complexes. Nutrient deprivation, Environmental stress or cellular damage like organelle damage, mitochondria dysfunction, constrained TOR kinase activity, and activates AMPK, which promote direct phosphorylation of ULK1/Atg1 and Atg13 and initiates downstream autophagic events. The alternative pathway of autophagy induction includes the TOR activated pathway involving Atg6 (Hurley and Young., 2017). Typical Atg1 proteins are not present in Trypanosomatids; instead, they harbor homologs of ATG3, ATG7, and ATG16. Leishmania TOR3 acts as a stress sensor and regulates of acidocalcisome biogenesis. Studies show that trypanosomatid ATG8 is cleaved by ATG4.1 and ATG4.2, where the later acts as a deconjugating enzyme for ATG8. Where ATG8a is associated with autophagosomes, ATG8b and ATG8c are located close to the flagellar pocket thus indicating their role in endocytosis or exocytosis, while ATG5 is essential for autophagosome formation. Overexpression of ATG8 renders the parasites resistant to stress, and it translocates to the region near the mitochondria in response to drug-induced stress. So autophagy might play a role in mitigating stress and exhibiting drug resistance. Although pro-apoptotic Bcl-2 proteins have not been characterized in Leishmania, expression of mammalian Bcl-XL inside Leishmania can revert the cell death process (Alzate et al., 2006). BH3 domain-containing Leishmania BH3AQP can specifically bind to Bcl-XL and cause reduced viability of Leishmania promastigotes treated with staurosporine or antimycin A (Genes et al., 2016). In higher eukaryotes, calpain-processed ATG5 does not require additional pro-apoptotic stimuli to induce apoptosis (Fan and Zong, 2013). The similar activity of trypanosomatid calpain like proteins can be proposed where stress-induced mitochondrial depolarization releases Ca2+ from mitochondria and increase intracellular Ca2+ conc. which might activate the calpain like proteins that could cleave ATG5 (Giese et al., 2008). Besides, the cellular level of calpains can modulate parasite susceptibility to drugs like antimony, and its overexpression promotes cell death (Yousefi et al., 2006). Hence it can be postulated that Leishmania harbors Bcl-XL like proteins, and interaction of calpain-mediated cleaved ATG5 and BH3AQP with Bcl-2 like proteins might be involved in switching autophagy to apoptosis. Autophagy has a crucial role in drug-induced stress regulation and cell death pathways. Since it is well documented that drug-induced stress in trypanosomatids shows autophagic characteristics (Menna-Barreto, 2019), it can be postulated that at lower drug doses it could initially activate autophagy like features as a survival mechanism, but to mitigate high stress level or persistent stress the cells switches from survival autophagy to either autophagic death, necrotic death or apoptosis-like death. Trypanosomatids do not harbor all the orthologs of mammalian autophagic proteins but based on the proteins characterized to date and functions of some of the orthologs in the mammalian system; we propose a schematic model of autophagy and various yet unknown factors that could signal cells to shift from autophagy to apoptosis-like death in Leishmania (Fig. 1).
Fig. 1.
Interplay between autophagy and apoptosis: An alternative to Atg1-dependent pathway could be the Tor-induced autophagy or Atg6/Beclin dependent autophagy. Initial low level stress detected by TOR might activate autophagy response by ATG4 mediated proteolytic cleavage of ATG8, and the exposed C-terminal glycine in ATG8 might be conjugated to PE(phosphatidylethanolamine) through the catalytic actions of the E1-like and E2-like enzymes of putative Leishmania homologs of ATG7 and ATG3 respectively. This is followed by the binding of ATG5-12 complex and autophagosome formation. The interplay between autophagy and apoptosis might be controlled by ATG6/Beclin or calpain processed ATG5 during high cellular level of stress. The presence of a BH3 domain-containing BH3AQP indicates the possible presence of Bcl-like protein whose interaction with cleaved ATG5 fragment might trigger apoptosis-like death in Leishmania.
8.2. Understanding ER stress in Leishmania
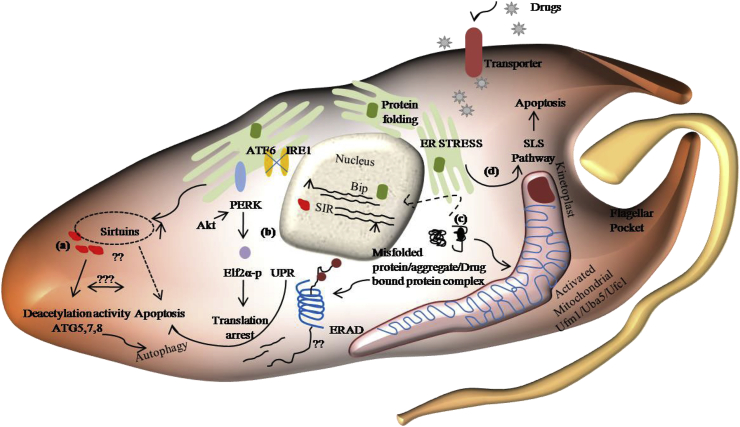
Maintenance of ER homeostasis in times of stress occurs through unfolded protein response (UPR), ER-associated degradation (ERAD), and autophagy. The latter two degrade misfolded proteins, which, if accumulated, would trigger UPR, thus increasing ER folding capacity, reducing ER protein input, activating ERAD, and possibly autophagy. IRE1, PERK, and increased cytosolic Ca2+ are ER stress linked autophagy inducers as activated PERK activates LC3/ATG8 and ATG5 (Senft and Ronai, 2015). Although Leishmania parasites lack IRE1-XBP1 and ATF6, DTT, or tunicamycin, treatment-induced ER stress caused the elevated expression of the stress chaperone BiP/GRP78 (Goldshmidt et al., 2010). SIR2, another UPR marker, is a histone deacetylase that is activated in Cryptolepine stressed Leishmania. Since ER stress in Leishmania promotes autophagy, it remains to be determined how GRP78 and SIR2 proteins can induce autophagy, thereafter how autophagosomes identify their targets and the cargos involved. Besides the involvement of trypanosomatid TOR kinases, if any, in the induction of ER stress induced autophagy also remains to be established. Unlike mammals, the Ufm1 system components in Leishmania localize to the mitochondria. This could be an adaptation to facilitate their intracellular survival by impeding the host protein degradation system. ER stresses can up-regulate the Ufm1 system, and loss of Ufmylation impairs ERAD (Zhang et al., 2012). Further studies need to be carried out to elucidate the trypanosomatids specific triggers for Ufmylation and whether specific drugs can influence this trigger.
Sirtuin 1 deacetylates essential components of autophagy such as ATG5, ATG7, LC3, and FOXO1 (Lee et al., 2008), thus negatively regulating ER stress, but deacetylation of XBP1 promotes cellular apoptosis. On the other hand, elevated SIRT1 can suppress apoptosis by deacetylating its own transcription factor, E2F1 (Koga et al., 2015). Also, ER-stress mediated SIRT1 expression is regulated by PI3K/Akt/GSK-3β signaling pathway. PERK, being a direct target of Akt, can activate UPR, and down-regulation of Akt/mTOR signaling by ER stress facilitates apoptosis while in some cases, it can promote autophagy associated cell death as well (Blaustein et al., 2013; Fu et al., 2017; Goan et al., 2019). In the case of trypanosomatids, cytoplasmic overexpression of SIR2 prevents programmed cell death. Also, the anti-trypanosomatid activity of certain Akt-like protein inhibitors suggests the anti-apoptotic and cell survival role of the TriTryp PI3K/AKT pathway, which has GSK-3 as a substrate (Varela-M et al., 2017; Tirado-Duarte et al., 2018; Ochoa et al., 2018). The anti-trypanosomatid activity of GSK3 inhibitors proposes selective inhibition of the GSK-3 pathway, which is necessary for cell survival (Ojo et al., 2008; Martínez de Iturrate et al., 2020). From these data summarized schematically in Fig. 2, it can be postulated that in Trypanosomatids at low levels of ER stress, Sirtuin 1 and Akt/PERK activates autophagy and UPR respectively as a pro-survival mechanism, while with increased stress levels, inactivation of the Akt pathway inhibits UPR and thereby promotes apoptotic cell death. Therefore it would be interesting to find out pro-survival and pro-death strategies of these parasites, which in a dose-dependent manner can exhibit the drug-induced autophagic response towards cell survival or to a higher dose-dependent switch to apoptosis.
Fig. 2.
Drug induced ER stress response regulation in trypanosomes: (a) Elevated expression of Sirtuins in ER stress might activate autophagy by deacetylating ATG proteins, and sirtuins regulate subsequent switch to apoptosis at a higher stress level. (b)Activated PERK phosphorylates elf2α that leads to translation arrest, while up-regulated GRP78/Bip and Sirtuins activates an Unfolded Protein response. (c,d) Drug bound protein complex or misfolded proteins might induce proteasomal degradation by regulating the unique mitochondrial conjugating system UFM1, which, when fails, could activate SL-RNA depletion leading to a cell-death pathway different from the classical apoptotic pathway.
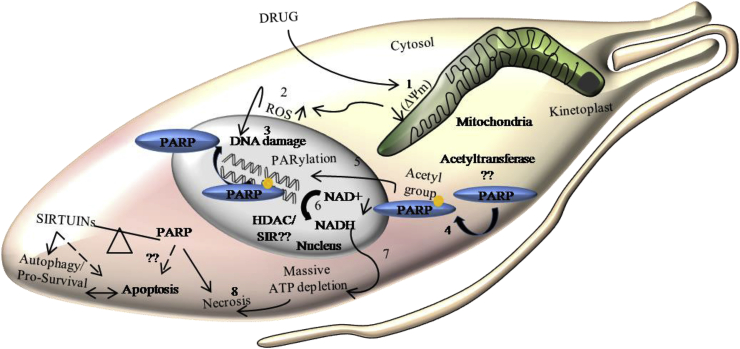
8.3. Balance between sirtuins and PARP
Sirtuins (SIRTs) and poly (ADP-ribose) polymerases (PARPs) are two different NAD + dependent proteins, which are associated with both cell survival and death. Sirtuins are deacetylases and, PARPs perform poly-ADP Ribosylation. Oxidative stress and DNA damage induced acetylation of PARP1 causes its nuclear translocation and PARylation of damaged DNA, which then activates the DNA repair pathway. But prolonged stress leads to excessive PARP activity and massive NAD loss leading to concomitant ATP depletion and, thereby, induction of cell death. From these data, PARP-1 emanates as a principle regulator of caspase-independent cell death. Overactivation of PARP1 suppresses SIRT1 activity by depleting cellular NAD levels and also induces mitochondria to nuclear translocation of AIF to degrade DNA. If SIRT1 is overexpressed before being deactivated by PARP1, it can promote cell survival by deacetylating PARP1 and maintaining cellular NAD levels. Additionally, SIRT1 could also block PARP1activity by deacetylating and inhibiting the acetyltransferase. Thus the counterbalance of SIRT1 and PARP1 determines the outcome of cell survival or cell death (Cantó et al., 2013). Trypanosomatid PARP is localized in the cytoplasm, which translocates to the nucleus upon genotoxic stress to trigger a DNA repair process. Based on the DNA template of trypanosomatid PARP1, it can be postulated that it is engaged in specific repair pathways and not a global repair protein in these parasites, but contrary to this T. cruzi PARP plays a global DNA repair role (Haikarainen et al., 2017). Although few studies exhibit PARP cleavage in Leishmania where the protein is of higher molecular weight than trypanosomatid counterpart, this protein is yet to be characterized for this parasite.
Trypanosomatid SIR2 exhibits a deacetylase activity, and in terms of localization, the Trypanosoma SIR2 resides in the cytoplasm while Leishmania SIR2 inside the nucleus (Mittal et al., 2017). Therefore it can be postulated that upon stress, trypanosomatid SIR2 deacetylates PARP1, which first deactivates SIR2 and then gets translocated to the nucleus in Leishmania or translocates via its N terminus to the nucleus in Trypanosoma. Once inside the nucleus, it can deactivate nuclear SIR2 and simultaneously initiate the DNA repair process. Based on the drug concentration, trypanosomatid PARP could trigger necrosis or apoptosis. Leishmania SIR2 being cytoplasmic is incapable of deactivating PARP1 when it has translocated to the nucleus, and therefore unrestricted PARP1 activity has more propensity of inducing necrotic or apoptotic death as that compared to mammalian PARP1 (Fig. 3). Hence the balance of PARP1 and SIR2 activity in trypanosomatids plays an important role in triggering necrosis or apoptosis, and it remains to be determined the threshold of this balance in terms of the drug used.
Fig. 3.
The Necrotic pathway: Oxidative stress-induced DNA damage could activate cytosolic PARP by acetylation, which upon translocating to the nucleus, performs NAD + dependent PARylation. Massive depletion in NAD+ and ATP leads to necrosis. Deacetylation of PARP by NAD + dependent deacetylase SIR2 or equivalent enzyme (HDAC) is necessary to inhibit PARP activity, thereby blocking necrosis.
8.4. Antioxidant systems
By scavenging intracellular ROS, antioxidant systems can prevent apoptotic cell death. One of the extensively studied antioxidant enzymes are the Superoxide dismutases (SODs) that remove toxic reactive oxygen species like superoxide radicals by converting them into hydrogen peroxide and oxygen. Leishmania harbors a unique FeSOD, which is overexpressed in the amastigotes, and since it is absent in the host, it is an effective drug target. To shield metabolic pathways from ROS induced oxidative damage, trypanosomatids compartmentalize glycolysis, fatty acid β-oxidation, ether lipid biosynthesis, and purine salvage pathways inside its Glycosome. FeSOD could play a key role in protecting glycosomal proteins from O2˙− toxicity; hence MIL-resistant Leishmania strains exhibit increased expression of FeSODA and LdSIR2 where the later can induce a pro-survival autophagic process and the former, upon being deacetylated, becomes hyperactivated and scavenges intracellular ROS. This also explains the cytoplasmic localization of Leishmania SIR2. Leishmania SIR2RP3 is a mitochondrial protein that possibly could be deacetylating mitochondrial FeSODA and thus activating it to scavenge mitochondrial ROS. Thus LdSIR2RP3 is crucial in maintaining the integrity of the kinetoplast DNA and the mitochondrial membrane potential. Hence it can be postulated that FeSODA and FeSODB reduce superoxides in their respective organelles, and other ROS are reduced by peroxidases.
Although kinetoplastids lack glutathione reductase, they contain substantial amount of glutathione, and both glutathione and tryparedoxin redox systems are protective mechanisms of Leishmania. The sensitivity of distinct Leishmania species to SNAP, a nitrogen-derived reactive species donor, is inversely related to their cellular glutathione levels. Application of buthionine sulfoximine, an inhibitor of glutathione synthesis, can preserve T. brucei infected mice by reducing the parasite's glutathione and thiol levels (Romão et al., 2006). Hence the emanating question is that if Trypanosomatid peroxidases are tryparedoxin dependent, then how GSH is regulated in these parasites. Ascorbate peroxidase (APx) overexpressing Leishmania promastigotes exhibited enhanced tolerance to oxidative stress-mediated apoptosis in AmB-resistant strains (Moreira et al., 2018). Peroxides and glutathione are known to keep mitochondrial cytochrome c in a reduced state, hence during oxidative stress, the release of cytochrome c into cytoplasm and its concomitant oxidation by cytochrome oxidase makes it bind to ER Ca2+ channels and disrupt intracellular Ca2+ homeostasis and thereby trigger cell death in either a caspase-dependent or independent mechanism. Therefore APx could be regulating the intracellular GSH levels, thereby maintaining the reduced state of Cytc and thus hindering the cell death processes. The absence of this redox pathway in the human host may be exploited for parasite-specific therapeutics.
9. Conclusion
We have highlighted the drug-induced cell death pathways, discussed their physiological features, targets, and effector molecules. Different pathways unique to trypanosomatids, which involve parasite specific enzymes, have been purported as effective targets for parasite elimination. Clearly, lack of evidence and knowledge imposes a big challenge for researchers to understand the basic mechanisms in the death processes of these parasites. We, however, based on discrete information, postulated few drug-dependent pathways in Leishmania that can be studied to unravel major contributors in its regulated cell death cascade. Furthermore, it is interesting to find features of pathways like ferroptosis, ERAD, and ER stress as part of regulated cell death of trypanosomatids that may be modulated by drug-dose and incubation time-dependent manner. Hence exploring and understanding the drug-induced mechanisms in these parasites help in their proper implementation through therapeutics to eliminate drug susceptible as well as resistant strains. Further, extensive studies by drug-repurposing could increase the availability and efficacy of therapeutic options against these neglected tropical diseases.
Funding
PD is supported by fellowship from DBT, SS is supported by fellowship from IIT Kharagpur, SBDG's position is supported by IIT Kharagpur through MoE. Laboratory research inputs are supported by INSA (SP/YSP/131/2016-1070) and DBT (BT/PR32810/Med/29/1468/2019-reg).
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
DBT for fellowship and Department of Biotechnology, IIT Kharagpur for laboratory and research facilities.
References
- Adams C.J., Kopp M.C., Larburu N., Nowak P.R., Ali M.M.U. Structure and molecular mechanism of ER stress signaling by the unfolded protein response signal activator IRE1. Front. Mol. Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzate J.F., Alvarez-Barrientos A., González V.M., Jiménez-Ruiz A. Heat-induced programmed cell death in Leishmania infantum is reverted by Bcl-X(L) expression. Apoptosis. 2006;11(2):161–171. doi: 10.1007/s10495-006-4570-z. [DOI] [PubMed] [Google Scholar]
- Andrade J.M., Murta S.M. Functional analysis of cytosolic tryparedoxin peroxidase in antimony-resistant and -susceptible Leishmania braziliensis and Leishmania infantum lines. Parasit Vectors. 2014;7:406. doi: 10.1186/1756-3305-7-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardestani S.K., Poorrajab F., Razmi S., Foroumadi A., Ajdary S., Gharegozlou B., Behrouzi-Fardmoghadam M., Shafiee A. Cell death features induced in Leishmania major by 1,3,4-thiadiazole derivatives. Exp. Parasitol. 2012;132(2):116–122. doi: 10.1016/j.exppara.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Ashrafi G., Schwarz T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquilla A., Navarro M. Trypanosome TOR as a major regulator of cell growth and autophagy. Autophagy. 2009;5(2):256–258. doi: 10.4161/auto.5.2.7591. [DOI] [PubMed] [Google Scholar]
- Basmaciyan L., Berry L., Gros J., Azas N., Casanova M. Temporal analysis of the autophagic and apoptotic phenotypes in Leishmania parasites. Microb Cell. 2018;5(9):404–417. doi: 10.15698/mic2018.09.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Morris M.T. Glycosome biogenesis in trypanosomes and the de novo dilemma. PLoS Neglected Trop. Dis. 2017;11(4) doi: 10.1371/journal.pntd.0005333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz C., Stříbrná E., Hashimi H., Lukeš J. Dynamin-like proteins in Trypanosoma brucei: a division of labour between two paralogs? PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besteiro S., Williams R.A., Morrison L.S., Coombs G.H., Mottram J.C. Endosome sorting and autophagy are essential for differentiation and virulence of Leishmania major. J. Biol. Chem. 2006;281(16):11384–11396. doi: 10.1074/jbc.M512307200. [DOI] [PubMed] [Google Scholar]
- Billaut-Mulot O., Fernandez-Gomez R., Loyens M., Ouaissi A. Trypanosoma cruzi elongation factor 1-alpha: nuclear localization in parasites undergoing apoptosis. Gene. 1996;174(1):19–26. doi: 10.1016/0378-1119(96)00254-5. [DOI] [PubMed] [Google Scholar]
- Blaustein M., Pérez-Munizaga D., Sánchez M.A., Urrutia C., Grande A., Risso G., Srebrow A., Alfaro J., Colman-Lerner A. Modulation of the Akt pathway reveals a novel link with PERK/eIF2α, which is relevant during hypoxia. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacz M., Krauth-Siegel R.L. Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death. Elife. 2018;7:e37503. doi: 10.7554/eLife.37503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoseDasgupta S., Das B.B., Sengupta S., Ganguly A., Roy A., Dey S., Tripathi G., Dinda B., Majumder H.K. The caspase-independent algorithm of programmed cell death in Leishmania induced by baicalein: the role of LdEndoG, LdFEN-1 and LdTatD as a DNA 'degradesome'. Cell Death Differ. 2008;15(10):1629–1640. doi: 10.1038/cdd.2008.85. [DOI] [PubMed] [Google Scholar]
- Braga S.S. Multi-target drugs active against leishmaniasis: a paradigm of drug repurposing. Eur. J. Med. Chem. 2019;183:111660. doi: 10.1016/j.ejmech.2019.111660. [DOI] [PubMed] [Google Scholar]
- Branquinha M.H., Marinho F.A., Sangenito L.S., Oliveira S.S., Goncalves K.C., Ennes-Vidal V., d'Avila-Levy C.M., Santos A.L. Calpains: potential targets for alternative chemotherapeutic intervention against human pathogenic trypanosomatids. Curr. Med. Chem. 2013;20(25):3174–3185. doi: 10.2174/0929867311320250010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C., Sauve A.A., Bai P. Crosstalk between poly(ADP-ribose) polymerase and sirtuin enzymes. Mol. Aspect. Med. 2013;34(6):1168–1201. doi: 10.1016/j.mam.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho S., Barreira da Silva R., Shawki A. LiZIP3 is a cellular zinc transporter that mediates the tightly regulated import of zinc in Leishmania infantum parasites. Mol. Microbiol. 2015;96(3):581–595. doi: 10.1111/mmi.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova M., Gonzalez I.J., Sprissler C., Zalila H., Dacher M., Basmaciyan L., Späth G.F., Azas N., Fasel N. Implication of different domains of the Leishmania major metacaspase in cell death and autophagy. Cell Death Dis. 2015;6(10) doi: 10.1038/cddis.2015.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C., Cloutier S., Dumas C., Chou M.N., Papadopoulou B. Promastigote to amastigote differentiation of Leishmania is markedly delayed in the absence of PERK eIF2alpha kinase-dependent eIF2alpha phosphorylation. Cell Microbiol. 2011;13(7):1059–1077. doi: 10.1111/j.1462-5822.2011.01602.x. [DOI] [PubMed] [Google Scholar]
- Chowdhury S., Mukherjee T., Chowdhury S.R., Sengupta S., Mukhopadhyay S., Jaisankar P., Majumder H.K. Disuccinyl betulin triggers metacaspase-dependent endonuclease G-mediated cell death in unicellular protozoan parasite Leishmania donovani. Antimicrob. Agents Chemother. 2014;58(4):2186–2201. doi: 10.1128/AAC.02193-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B.E. Amphotericin B membrane action: role for two types of ion channels in eliciting cell survival and lethal effects. J. Membr. Biol. 2010;238(1–3):1–20. doi: 10.1007/s00232-010-9313-y. [DOI] [PubMed] [Google Scholar]
- Corral M.J., Benito-Peña E., Jiménez-Antón M.D., Cuevas L., Moreno-Bondi M.C., Alunda J.M. Allicin induces calcium and mitochondrial dysregulation causing necrotic death in Leishmania. PLoS Neglected Trop. Dis. 2016;10(3) doi: 10.1371/journal.pntd.0004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull B., Prado Godinho J.L., Fernandes Rodrigues J.C., Frank B., Schurigt U., Williams R.A., Coombs G.H., Mottram J.C. Glycosome turnover in Leishmania major is mediated by autophagy. Autophagy. 2014;10(12):2143–2157. doi: 10.4161/auto.36438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Augusto L., Moretti N.S., Ramos T.C., de Jesus T.C., Zhang M., Castilho B.A., Schenkman S. A membrane-bound eIF2 alpha kinase located in endosomes is regulated by heme and controls differentiation and ROS levels in Trypanosoma cruzi. PLoS Pathog. 2015;11(2) doi: 10.1371/journal.ppat.1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva M.S., Segatto M., Pavani R.S., Gutierrez-Rodrigues F., Bispo V.D., de Medeiros M.H., Calado R.T., Elias M.C., Cano M.I. Consequences of acute oxidative stress in Leishmania amazonensis: from telomere shortening to the selection of the fittest parasites. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864(1):138–150. doi: 10.1016/j.bbamcr.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Das M., Mukherjee S.B., Shaha C. Hydrogen peroxide induces apoptosis-like death in Leishmania donovani promastigotes. J. Cell Sci. 2001;114(Pt 13):2461–2469. doi: 10.1242/jcs.114.13.2461. [DOI] [PubMed] [Google Scholar]
- Das B.B., Sen N., Roy A., Dasgupta S.B., Ganguly A., Mohanta B.C., Dinda B., Majumder H.K. Differential induction of Leishmania donovani bi-subunit topoisomerase I-DNA cleavage complex by selected flavones and camptothecin: activity of flavones against camptothecin-resistant topoisomerase I. Nucleic Acids Res. 2006;34(4):1121–1132. doi: 10.1093/nar/gkj502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S., Giri S., Sundar S., Shaha C. Functional involvement of Leishmania donovani tryparedoxin peroxidases during infection and drug treatment. Antimicrob. Agents Chemother. 2017;62(1):e00806–e00817. doi: 10.1128/AAC.00806-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Menezes J.P., Guedes C.E., Petersen A.L., Fraga D.B., Veras P.S. Advances in development of new treatment for leishmaniasis. BioMed Res. Int. 2015;2015:815023. doi: 10.1155/2015/815023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarkar S., Sarkar D., Sarkar A., Dighal A., Staniek K., Gille L., Chatterjee M. Berberine chloride mediates its antileishmanial activity by inhibiting Leishmania mitochondria. Parasitol. Res. 2019;118(1):335–345. doi: 10.1007/s00436-018-6157-3. [DOI] [PubMed] [Google Scholar]
- Delgado M., Anderson P., Garcia-Salcedo J.A., Caro M., Gonzalez-Rey E. Neuropeptides kill African trypanosomes by targeting intracellular compartments and inducing autophagic-like cell death. Cell Death Differ. 2009;16(3):406–416. doi: 10.1038/cdd.2008.161. [DOI] [PubMed] [Google Scholar]
- Diaz-Gonzalez R., Kuhlmann F.M., Galan-Rodriguez C., Madeira da Silva L., Saldivia M., Karver C.E., Rodriguez A., Beverley S.M., Navarro M., Pollastri M.P. The susceptibility of trypanosomatid pathogens to PI3/mTOR kinase inhibitors affords a new opportunity for drug repurposing. PLoS Negl. Trop. Dis. 2011;5(8) doi: 10.1371/journal.pntd.0001297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickie E.A., Giordani F., Gould M.K., Mäser P., Burri C., Mottram J.C., Rao S.P.S., Barrett M.P. New drugs for human african trypanosomiasis: a twenty first century success story. Trop Med Infect Dis. 2020;5(1):29. doi: 10.3390/tropicalmed5010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolai S., Pal S., Yadav R.K., Adak S. Endoplasmic reticulum stress-induced apoptosis in Leishmania through Ca2+-dependent and caspase-independent mechanism. J. Biol. Chem. 2011;286(15):13638–13646. doi: 10.1074/jbc.M110.201889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorlo T.P., Balasegaram M., Beijnen J.H., de Vries P.J. Miltefosine: a review of its pharmacology and therapeutic efficacy in the treatment of leishmaniasis. J. Antimicrob. Chemother. 2012;67(11):2576–2597. doi: 10.1093/jac/dks275. [DOI] [PubMed] [Google Scholar]
- El Fadili K., Messier N., Leprohon P., Roy G., Guimond C., Trudel N., Saravia N.G., Papadopoulou B., Légaré D., Ouellette M. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 2005;49(5):1988–1993. doi: 10.1128/AAC.49.5.1988-1993.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hani C.N., Borges V.M., Wanderley J.L., Barcinski M.A. Apoptosis and apoptotic mimicry in Leishmania: an evolutionary perspective. Front. Cell Infect Microbiol. 2012;2:96. doi: 10.3389/fcimb.2012.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y.J., Zong W.X. The cellular decision between apoptosis and autophagy. Chin. J. Canc. 2013;32(3):121–129. doi: 10.5732/cjc.012.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattahi Bafghi A., Noorbala M., Noorbala M.T., Aghabagheri M. Anti leishmanial effect of zinc sulphate on the viability of Leishmania tropica and L. Major promastigotes. Jundishapur J. Microbiol. 2014;7(9) doi: 10.5812/jjm.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.F., Liu X., Gao M., Zhang Y.N., Liu J. Endoplasmic reticulum stress induces autophagy and apoptosis while inhibiting proliferation and drug resistance in multiple myeloma through the PI3K/Akt/mTOR signaling pathway. Oncotarget. 2017;8(37):61093–61106. doi: 10.18632/oncotarget.17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M., Goyal N. MAPK1 of Leishmania donovani modulates antimony susceptibility by downregulating P-glycoprotein efflux pumps. Antimicrob. Agents Chemother. 2015;59(7):3853–3863. doi: 10.1128/AAC.04816-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger A., Bossard G., Sereno D., Pissarra J., Lemesre J.L., Vincendeau P., Holzmuller P. Escaping deleterious immune response in their hosts: lessons from trypanosomatids. Front. Immunol. 2016;7:212. doi: 10.3389/fimmu.2016.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genes C.M., de Lucio H., González V.M., Sánchez-Murcia P.A., Rico E., Gago F., Fasel N., Jiménez-Ruiz A. A functional BH3 domain in an aquaporin from Leishmania infantum. Cell Death Dis. 2016;2:16043. doi: 10.1038/cddiscovery.2016.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese V., Dallagiovanna B., Marchini F.K., Pavoni D.P., Krieger M.A., Goldenberg S. Trypanosoma cruzi: a stage-specific calpain-like protein is induced after various kinds of stress. Mem. Inst. Oswaldo Cruz. 2008;103(6):598–601. doi: 10.1590/s0074-02762008000600015. [DOI] [PubMed] [Google Scholar]
- Giri S., Shaha C. Leishmania donovani parasite requires Atg8 protein for infectivity and survival under stress. Cell Death Dis. 2019;10(11):808. doi: 10.1038/s41419-019-2038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goan Y.G., Wu W.T., Liu C.I., Neoh C.A., Wu Y.J. Involvement of mitochondrial dysfunction, endoplasmic reticulum stress, and the PI3K/AKT/mTOR pathway in nobiletin-induced apoptosis of human bladder cancer cells. Molecules. 2019;24(16):2881. doi: 10.3390/molecules24162881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S., Boucher C.C., Nadeau D., Poirier G.G. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 2001;8(6):588–594. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- Goldshmidt H., Matas D., Kabi A., Carmi S., Hope R., Michaeli S. Persistent ER stress induces the spliced leader RNA silencing pathway (SLS), leading to programmed cell death in Trypanosoma brucei. PLoS Pathog. 2010;6(1) doi: 10.1371/journal.ppat.1000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González I.J., Desponds C., Schaff C., Mottram J.C., Fasel N. Leishmania major metacaspase can replace yeast metacaspase in programmed cell death and has arginine-specific cysteine peptidase activity. Int. J. Parasitol. 2007;37(2):161–172. doi: 10.1016/j.ijpara.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Haikarainen T., Schlesinger M., Obaji E., Fernández Villamil S.H., Lehtiö L. Structural and biochemical characterization of poly-ADP-ribose polymerase from trypanosoma brucei. Sci. Rep. 2017;7(1):3642. doi: 10.1038/s41598-017-03751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B.S., Gabernet-Castello C., Voak A., Goulding D., Natesan S.K., Field M.C. TbVps34, the trypanosome orthologue of Vps34, is required for Golgi complex segregation. J. Biol. Chem. 2006;281(37):27600–27612. doi: 10.1074/jbc.M602183200. [DOI] [PubMed] [Google Scholar]
- Ham S.J., Lee D., Yoo H., Jun K., Shin H., Chung J. Decision between mitophagy and apoptosis by Parkin via VDAC1 ubiquitination. Proc. Natl. Acad. Sci. U. S. A. 2020;117(8):4281–4291. doi: 10.1073/pnas.1909814117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman M., Pérez-Morga D., Schtickzelle N., Michels P.A. Turnover of glycosomes during life-cycle differentiation of Trypanosoma brucei. Autophagy. 2008;4(3):294–308. doi: 10.4161/auto.5443. [DOI] [PubMed] [Google Scholar]
- Hurley J.H., Young L.N. Mechanisms of autophagy initiation. Annu. Rev. Biochem. 2017;86:225–244. doi: 10.1146/annurev-biochem-061516-044820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inacio J.D., Canto-Cavalheiro M.M., Menna-Barreto R.F., Almeida-Amaral E.E. Mitochondrial damage contribute to epigallocatechin-3-gallate induced death in Leishmania amazonensis. Exp. Parasitol. 2012;132(2):151–155. doi: 10.1016/j.exppara.2012.06.008. [DOI] [PubMed] [Google Scholar]
- Jackson Y., Wyssa B., Chappuis F. Tolerance to nifurtimox and benznidazole in adult patients with chronic Chagas' disease. J. Antimicrob. Chemother. 2020;75(3):690–696. doi: 10.1093/jac/dkz473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H.Y., Yang Y., Zhang Y.Y. The dual role of poly(ADP-ribose) polymerase-1 in modulating parthanatos and autophagy under oxidative stress in rat cochlear marginal cells of the stria vascularis. Redox Biol. 2018;14:361–370. doi: 10.1016/j.redox.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N.G., Catta-Preta C.M.C., Lima A.P.C.A., Mottram J.C. Genetically validated drug targets in Leishmania: current knowledge and future prospects. ACS Infect. Dis. 2018;4(4):467–477. doi: 10.1021/acsinfecdis.7b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalel V.C., Emmanouilidis L., Dawidowski M., Schliebs W., Sattler M., Popowicz G.M., Erdmann R. Inhibitors of glycosomal protein import provide new leads against trypanosomiasis. Microbial cell (Graz, Austria) 2017;4(7):229–232. doi: 10.15698/mic2017.07.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare S., Nagle A.S., Biggart A. Proteasome inhibition for treatment of leishmaniasis, Chagas disease and sleeping sickness. Nature. 2016;537(7619):229–233. doi: 10.1038/nature19339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Suico M.A., Shimasaki S., Watanabe E., Kai Y., Koyama K., Omachi K., Morino-Koga S., Sato T., Shuto T., Mori K., Hino S., Nakao M., Kai H. Endoplasmic reticulum (ER) stress induces sirtuin 1 (SIRT1) expression via the PI3K-Akt-GSK3β signaling pathway and promotes hepatocellular injury. J. Biol. Chem. 2015;290(51):30366–30374. doi: 10.1074/jbc.M115.664169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J., Fairlamb A.H. A comparative study of type I and type II tryparedoxin peroxidases in Leishmania major. FEBS J. 2007;274(21):5643–5658. doi: 10.1111/j.1742-4658.2007.06087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata S., Mishra R., Banerjea A.C. Proteasomal degradation machinery: favorite target of HIV-1 proteins. Front. Microbiol. 2018;9:2738. doi: 10.3389/fmicb.2018.02738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Gannavaram S., Selvapandiyan A., Debrabant A. Characterization of metacaspases with trypsin-like activity and their putative role in programmed cell death in the protozoan parasite Leishmania. Eukaryot. Cell. 2007;6(10):1745–1757. doi: 10.1128/EC.00123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.H., Cao L., Mostoslavsky R., Lombard D.B., Liu J., Bruns N.E., Tsokos M., Alt F.W., Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. U. S. A. 2008;105(9):3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luque-Ortega J.R., Rivas L. Miltefosine (hexadecylphosphocholine) inhibits cytochrome c oxidase in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2007;51(4):1327–1332. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira da Silva L., Beverley S.M. Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc. Natl. Acad. Sci. U. S. A. 2010;107(26):11965–11970. doi: 10.1073/pnas.1004599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamidala R., Majumdar P., Jha K.K., Bathula C., Agarwal R., Chary M.T., Majumder H.K., Munshi P., Sen S. Identification of Leishmania donovani Topoisomerase 1 inhibitors via intuitive scaffold hopping and bioisosteric modification of known Top 1 inhibitors. Sci. Rep. 2016;6:26603. doi: 10.1038/srep26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín I., Ferrús A. Comparative genomics of the RBR family, including the Parkinson's disease-related gene parkin and the genes of the ariadne subfamily. Mol. Biol. Evol. 2002;19(12):2039–2050. doi: 10.1093/oxfordjournals.molbev.a004029. [DOI] [PubMed] [Google Scholar]
- Marinho F.A., Gonçalves K.C., Oliveira S.S., Gonçalves D.S., Matteoli F.P., Seabra S.H., Oliveira A.C., Bellio M., Oliveira S.S., Souto-Padrón T., d'Avila-Levy C.M., Santos A.L., Branquinha M.H. The calpain inhibitor MDL28170 induces the expression of apoptotic markers in Leishmania amazonensis promastigotes. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0087659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Iturrate P., Sebastián-Pérez V., Nácher-Vázquez M., Tremper C.S., Smirlis D., Martín J., Martínez A., Campillo N.E., Rivas L., Gil C. Towards discovery of new leishmanicidal scaffolds able to inhibit Leishmania GSK-3. J. Enzym. Inhib. Med. Chem. 2020 Dec;35(1):199–210. doi: 10.1080/14756366.2019.1693704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García M., Campos-Salinas J., Cabello-Donayre M., Pineda-Molina E., Gálvez F.J., Orrego L.M., Sánchez-Cañete M.P., Malagarie-Cazenave S., Koeller D.M., Pérez-Victoria J.M. LmABCB3, an atypical mitochondrial ABC transporter essential for Leishmania major virulence, acts in heme and cytosolic iron/sulfur clusters biogenesis. Parasites Vectors. 2016;9(5):7. doi: 10.1186/s13071-015-1284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García M., Bart J.M., Campos-Salinas J. Autophagic-related cell death of Trypanosoma brucei induced by bacteriocin AS-48. Int. J. Parasitol. Drugs Drug Resist. 2018;8(2):203–212. doi: 10.1016/j.ijpddr.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes E.A., Desoti V.C., Silva Sde O., Ueda-Nakamura T., Dias Filho B.P., Yamada-Ogatta S.F., Sarragiotto M.H., Nakamura C.V. C5 induces different cell death pathways in promastigotes of Leishmania amazonensis. Chem. Biol. Interact. 2016;256:16–24. doi: 10.1016/j.cbi.2016.06.018. [DOI] [PubMed] [Google Scholar]
- Menna-Barreto R.F.S. Cell death pathways in pathogenic trypanosomatids: lessons of (over)kill. Cell Death Dis. 2019;10(2):93. doi: 10.1038/s41419-019-1370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N., Muthuswami R., Madhubala R. The mitochondrial SIR2 related protein 2 (SIR2RP2) impacts Leishmania donovani growth and infectivity. PLoS Neglected Trop. Dis. 2017;11(5) doi: 10.1371/journal.pntd.0005590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte Neto R.L., Sousa L.M., Dias C.S., Barbosa Filho J.M., Oliveira M.R., Figueiredo R.C. Morphological and physiological changes in Leishmania promastigotes induced by yangambin, a lignan obtained from Ocotea duckei. Exp. Parasitol. 2011;127(1):215–221. doi: 10.1016/j.exppara.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Moreira W., Leprohon P., Ouellette M. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2011;2(9) doi: 10.1038/cddis.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira D.S., Xavier M.V., Murta S.M.F. Ascorbate peroxidase overexpression protects Leishmania braziliensis against trivalent antimony effects. Mem. Inst. Oswaldo Cruz. 2018;113(12) doi: 10.1590/0074-02760180377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Y., Wang J., Wu J., He D., Zhang C., Duan C., Li B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J. Hematol. Oncol. 2019;12(1):34. doi: 10.1186/s13045-019-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa R., Rocha-Roa C., Marín-Villa M., Robledo S.M., Varela-M R.E. Search of allosteric inhibitors and associated proteins of an AKT-like kinase from Trypanosoma cruzi. Int. J. Mol. Sci. 2018;19(12):3951. doi: 10.3390/ijms19123951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo K.K., Gillespie J.R., Riechers A.J., Napuli A.J., Verlinde C.L., Buckner F.S., Gelb M.H., Domostoj M.M., Wells S.J., Scheer A., Wells T.N., Van Voorhis W.C. Glycogen synthase kinase 3 is a potential drug target for African trypanosomiasis therapy. Antimicrob. Agents Chemother. 2008;52(10):3710–3717. doi: 10.1128/AAC.00364-08. Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P De Koning H. The drugs of sleeping sickness: their mechanisms of action and resistance, and a brief history. Trav. Med. Infect. Dis. 2020;5(1):14. doi: 10.3390/tropicalmed5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paris C., Loiseau P.M., Bories C., Bréard J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2004;48(3):852–859. doi: 10.1128/AAC.48.3.852-859.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy K.K., Ravichandran K.S. Apoptotic cell recognition receptors and scavenger receptors. Immunol. Rev. 2016;269(1):44–59. doi: 10.1111/imr.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Victoria J.M., Bavchvarov B.I., Torrecillas I.R., Martínez-García M., López-Martín C., Campillo M., Castanys S., Gamarro F. Sitamaquine overcomes ABC-mediated resistance to miltefosine and antimony in Leishmania. Antimicrob. Agents Chemother. 2011;55(8):3838–3844. doi: 10.1128/AAC.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacenza L., Peluffo G., Alvarez M.N., Martínez A., Radi R. Trypanosoma cruzi antioxidant enzymes as virulence factors in Chagas disease. Antioxidants Redox Signal. 2013;19(7):723–734. doi: 10.1089/ars.2012.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte-Sucre A., Gamarro F., Dujardin J.C., Barrett M.P., López-Vélez R., García-Hernández R., Pountain A.W., Mwenechanya R., Papadopoulou B. Drug resistance and treatment failure in leishmaniasis: a 21st century challenge. PLoS Neglected Trop. Dis. 2017;11(12) doi: 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusnik M., Charrière F., Mäser P., Waller R.F., Dagley M.J., Lithgow T., Schneider A. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol. Biol. Evol. 2009;26(3):671–680. doi: 10.1093/molbev/msn288. [DOI] [PubMed] [Google Scholar]
- Raj S., Saha G., Sasidharan S., Dubey V.K., Saudagar P. Biochemical characterization and chemical validation of Leishmania MAP Kinase-3 as a potential drug target. Sci. Rep. 2019;9(1):16209. doi: 10.1038/s41598-019-52774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao S.J., Meleppattu S., Pal J.K. A GCN2-like eIF2α kinase (LdeK1) of Leishmania donovani and its possible role in stress response. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0156032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Agujetas V., de Dios C., Lestón L., Marí M., Morales A., Colell A. Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid. Med. Cell. Longev. 2019;2019:3809308. doi: 10.1155/2019/3809308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romão P.R., Tovar J., Fonseca S.G., Moraes R.H., Cruz A.K., Hothersall J.S., Noronha-Dutra A.A., Ferreira S.H., Cunha F.Q. Glutathione and the redox control system trypanothione/trypanothione reductase are involved in the protection of Leishmania spp. against nitrosothiol-induced cytotoxicity. Braz. J. Med. Biol. Res. 2006;39(3):355–363. doi: 10.1590/s0100-879x2006000300006. [DOI] [PubMed] [Google Scholar]
- Sabur A., Bhowmick S., Chhajer R., Ejazi S.A., Didwania N., Asad M., Bhattacharyya A., Sinha U., Ali N. Liposomal elongation factor-1α triggers effector CD4 and CD8 T cells for induction of long-lasting protective immunity against visceral leishmaniasis. Front. Immunol. 2018;9:18. doi: 10.3389/fimmu.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Acharya C., Pal U., Chowdhury S.R., Sarkar K., Maiti N.C., Jaisankar P., Majumder H.K. A novel spirooxindole derivative inhibits the growth of Leishmania donovani parasites both in vitro and in vivo by targeting type IB topoisomerase. Antimicrob. Agents Chemother. 2016;60(10):6281–6293. doi: 10.1128/AAC.00352-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini S., Bharati K., Shaha C., Mukhopadhyay C.K. Zinc depletion promotes apoptosis-like death in drug-sensitive and antimony-resistance Leishmania donovani. Sci. Rep. 2017;7(1):10488. doi: 10.1038/s41598-017-10041-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scariot D.B., Britta E.A., Moreira A.L., Falzirolli H., Silva C.C., Ueda-Nakamura T., Dias-Filho B.P., Nakamura C.V. Induction of early autophagic process on Leishmania amazonensis by synergistic effect of miltefosine and innovative semi-synthetic thiosemicarbazone. Front. Microbiol. 2017;8:255. doi: 10.3389/fmicb.2017.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J.R., Shapiro T.A. A mitochondrial topoisomerase IA essential for late theta structure resolution in African trypanosomes. Mol Microbiol. 2008;67(4):820–829. doi: 10.1111/j.1365-2958.2007.06087.x. [DOI] [PubMed] [Google Scholar]
- Schurigt U., Schad C., Glowa C., Baum U., Thomale K., Schnitzer J.K., Schultheis M., Schaschke N., Schirmeister T., Moll H. Aziridine-2,3-dicarboxylate-based cysteine cathepsin inhibitors induce cell death in Leishmania major associated with accumulation of debris in autophagy-related lysosome-like vacuoles. Antimicrob. Agents Chemother. 2010;54(12):5028–5041. doi: 10.1128/AAC.00327-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen N., Das B.B., Ganguly A. Camptothecin induced mitochondrial dysfunction leading to programmed cell death in unicellular hemoflagellate Leishmania donovani. Cell Death Differ. 2004;11(8):924–936. doi: 10.1038/sj.cdd.4401435. [DOI] [PubMed] [Google Scholar]
- Senft D., Ronai Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015;40(3):141–148. doi: 10.1016/j.tibs.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S., Chowdhury S., Bosedasgupta S., Wright C.W., Majumder H.K. Cryptolepine-induced cell death of Leishmania donovani promastigotes is augmented by inhibition of autophagy. Mol. Biol. Int. 2011;2011:187850. doi: 10.4061/2011/187850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadab M., Jha B., Asad M., Deepthi M., Kamran M., Ali N. Apoptosis-like cell death in Leishmania donovani treated with KalsomeTM10, a new liposomal amphotericin B. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0171306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa P.L., Souza R.O.D.S., Tessarolo L.D., de Menezes R.R.P.P.B., Sampaio T.L., Canuto J.A., Martins A.M.C. Betulinic acid induces cell death by necrosis in Trypanosoma cruzi. Acta Trop. 2017;174:72–75. doi: 10.1016/j.actatropica.2017.07.003. [DOI] [PubMed] [Google Scholar]
- Suman S.S., Equbal A., Zaidi A. Up-regulation of cytosolic tryparedoxin in Amp B resistant isolates of Leishmania donovani and its interaction with cytosolic tryparedoxin peroxidase. Biochimie. 2016;121:312–325. doi: 10.1016/j.biochi.2015.12.017. [DOI] [PubMed] [Google Scholar]
- Swenerton R.K., Knudsen G.M., Sajid M., Kelly B.L., McKerrow J.H. Leishmania subtilisin is a maturase for the trypanothione reductase system and contributes to disease pathology. J. Biol. Chem. 2010;285(41):31120–31129. doi: 10.1074/jbc.M110.114462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Kang R., Berghe T.V., Vandenabeele P., Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29(5):347–364. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirado-Duarte D., Marín-Villa M., Ochoa R., Blandón-Fuentes G., Soares M.J., Robledo S.M., Varela-Miranda R.E. The Akt-like kinase of Leishmania panamensis: as a new molecular target for drug discovery. Acta Trop. 2018;177:171–178. doi: 10.1016/j.actatropica.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Unciti-Broceta J.D., Maceira J., Morales S., García-Pérez A., Muñóz-Torres M.E., Garcia-Salcedo J.A. Nicotinamide inhibits the lysosomal cathepsin b-like protease and kills African trypanosomes. J. Biol. Chem. 2013;288(15):10548–10557. doi: 10.1074/jbc.M112.449207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzcátegui N.L., Carmona-Gutiérrez D., Denninger V., Schoenfeld C., Lang F., Figarella K., Duszenko M. Antiproliferative effect of dihydroxyacetone on Trypanosoma brucei bloodstream forms: cell cycle progression, subcellular alterations, and cell death. Antimicrob. Agents Chemother. 2007;51(11):3960–3968. doi: 10.1128/AAC.00423-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela R.E., Ochoa R., Muskus C.E., Muro A., Mollinedo F. Identification of a RAC/AKT-like gene in Leishmania parasites as a putative therapeutic target in leishmaniasis. Parasit Vectors. 2017;10(1):458. doi: 10.1186/s13071-017-2379-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronica J., Chandrasekaran S., Dayakar A. Iron superoxide dismutase contributes to miltefosine resistance in Leishmania donovani. FEBS J. 2019;286(17):3488–3503. doi: 10.1111/febs.14923. [DOI] [PubMed] [Google Scholar]
- Vincent I.M., Racine G., Légaré D., Ouellette M. Mitochondrial proteomics of antimony and miltefosine resistant Leishmania infantum. Proteomes. 2015;3(4):328–346. doi: 10.3390/proteomes3040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R., Vigliano C., Lococo B., Alvarez M.G., Petti M., Bertocchi G., Armenti A. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev. Anti Infect. Ther. 2009;7(2):157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- Weingärtner A., Drobot B., Herrmann A., Sánchez-Cañete M.P., Gamarro F., Castanys S., Günther Pomorski T. Disruption of the lipid-transporting LdMT-LdRos3 complex in Leishmania donovani affects membrane lipid asymmetry but not host cell invasion. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.A., Tetley L., Mottram J.C., Coombs G.H. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol. Microbiol. 2006;61(3):655–674. doi: 10.1111/j.1365-2958.2006.05274.x. [DOI] [PubMed] [Google Scholar]
- Williams R.A., Smith T.K., Cull B., Mottram J.C., Coombs G.H. ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major. PLoS Pathog. 2012;8(5) doi: 10.1371/journal.ppat.1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R.A., Mottram J.C., Coombs G.H. Distinct roles in autophagy and importance in infectivity of the two ATG4 cysteine peptidases of Leishmania major. J. Biol. Chem. 2013;288(5):3678–3690. doi: 10.1074/jbc.M112.415372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler E.A., Ottilie S. The proteasome as a target: how not tidying up can have toxic consequences for parasitic protozoa. Proc. Natl. Acad. Sci. U. S. A. 2019;116(21):10198–10200. doi: 10.1073/pnas.1904694116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefi S., Perozzo R., Schmid I., Ziemiecki A., Schaffner T., Scapozza T., Brunner T., Simon H.U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8(10):1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang M., Wu J., Lei G., Li H. Transcriptional regulation of the Ufm1 conjugation system in response to disturbance of the endoplasmic reticulum homeostasis and inhibition of vesicle trafficking. PloS One. 2012;7(11) doi: 10.1371/journal.pone.0048587. [DOI] [PMC free article] [PubMed] [Google Scholar]