Abstract
Nodular regenerative hyperplasia (NRH) of the liver may lead to noncirrhotic portal hypertension with subsequent development of portosystemic shunts. While extrahepatic and macrovascular shunts are readily visualized with imaging or endoscopy, there is no standard technique to detect intrahepatic microvascular portosystemic shunting and quantitatively assess shunt burden. We present a case of a 53-year-old female with suspected NRH and hepatopulmonary syndrome with inconclusive liver biopsies and absent portosystemic shunts per abdominal imaging. A percutaneous transportal infusion of Technetium-99m labeled macroaggregated albumin (99mTc-MAA) successfully identified intrahepatic microvascular portosystemic shunting and quantified a lung shunt fraction of more than 30%. NRH was subsequently confirmed with a surgical wedge biopsy and the patient was successfuly treated with a liver transplant. Transportal 99mTc-MAA could be used to both identify and quantify otherwise occult microvascular portosystemic shunts in patients with clinical sequelae of portal hypertension.
Keywords: 99mTc-MAA, portal hypertension, portosystemic shunting, liver transplantation, nodular regenerative hyperplasia
Abbreviations: NRH, Nodular regenerative hyperplasia; HPS, Hepatopulmonary syndrome; 99mTc-MAA, Technetium-99m labeled macroaggregated albumin; LT, Liver transplantation; ROI, Regions of interest; LSF, Lung shunt fraction
Introduction
Nodular regenerative hyperplasia (NRH) is a non-neoplastic disorder that arises as a response to alterations in hepatic blood flow, such as obstructive portal venopathy or systemic arteritis [1]. Histologically, NRH is characterized by diffuse nodules consisting of intermixed hyperplastic and atrophic hepatocytes with minimal or absent fibrosis [1]. NRH represents a diagnostic challenge as laboratory abnormalities are nonspecific, and histopathological confirmation is confounded by heterogeneous tissue involvement which may generate a false negative biopsy.
Although commonly asymptomatic, NRH may lead to noncirrhotic portal hypertension and acquired portosystemic shunting which may manifest as encephalopathy, hepatopulmonary syndrome (HPS), portopulmonary hypertension, and nephropathy [2]. The degree of shunting is conventionally ascertained with qualitative measures, as there is no accepted study to quantitatively assess shunt burden.
This report will present a patient with suspected NRH and symptomatic evidence of HPS in whom intrahepatic microvascular portosystemic shunting was identified and quantified using a transportal Technetium-99m labeled macroaggregated albumin (99mTc-MAA) infusion. Marked radiotracer deposition was detected in the lungs, supporting the clinically evident hepatopulmonary pathophysiology. NRH was subsequently confirmed with a surgical wedge biopsy and resolution of symptoms was achieved after treatment with liver transplantation (LT).
Case report
A 53-year-old woman presented with progressive shortness of breath, exertional dyspnea, and a nonproductive cough which eventually required supplemental oxygen. Her past medical history included congenital asplenia, nephrolithiasis, a cholecystectomy, and chronic anemia with elevated monocyte and lymphocyte counts. Laboratory tests revealed a mild transaminitis and alkaline phosphatase elevation. Autoantibody positivity was noted for antinuclear and anticentromere IgG antibodies yet were negative for double-stranded DNA, antimitochondrial, and smooth muscle antibodies. Viral hepatitis testing was negative. A contrast-enhanced computed tomogram demonstrated a nodular liver without portosystemic shunts. A transjugular liver biopsy with wedged portal pressure demonstrated prominent sinusoidal lymphocytosis, sinusoidal dilation, focal periportal fibrosis without cirrhosis, and an estimated portosystemic gradient of 10 mm Hg. These findings were suggestive but not diagnostic for NRH, as the characteristic parenchymal nodularity was not visualized.
Contrast echocardiography with agitated saline suggested intrapulmonary shunting without structural disease. A 6-minute walk test resulted in an oxygen desaturation from 93% at rest on room air to 87%. Pulmonary function tests and a ventilation perfusion scan were normal. A reduced diffusion capacity (40%-60%) suggested the presence of pulmonary parenchymal or vascular disease.
Portosystemic shunting was suspected as the cause of the apparent hepatopulmonary physiology. Given the absence of portal venous shunts per contrast-enhanced computed tomography, percutaneous hepatic and portal venography was performed. This confirmed the absence of extrahepatic shunts, but direct portal and hepatic venous pressure assessment revealed more severe portal hypertension with a gradient of 16 mm Hg. An additional percutaneous core needle biopsy obtained at the time of portal venography was inconclusive.
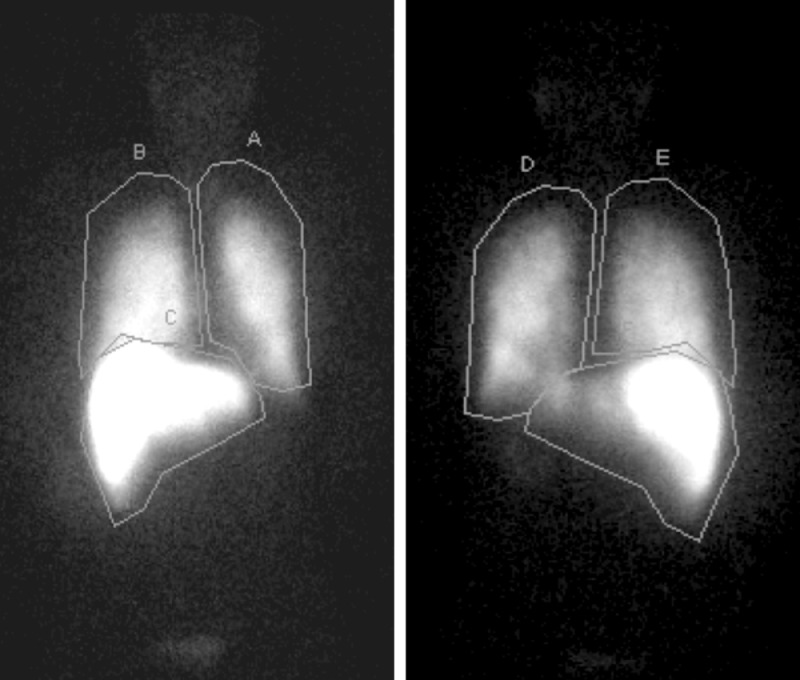
To search for the presence of intrahepatic microvascular portosystemic shunts, transportal administration of 4.4 mCi 99mTc-MAA (DRAXIMAGE MAA, Jubilant Radiopharma, Yardley, PA) was performed via the main portal vein. The patient was transferred to nuclear medicine where planar scintigraphy was obtained using a gamma camera (Fig. 1). Regions of interest (ROI) were drawn for both liver and lungs in anterior and posterior projections. The lung shunt fraction (LSF) was calculated using the following equation: LSF = (Total lung count)/(Total lung count + total abdomen count) × 100. The LSF from anterior and posterior projections demonstrated shunting of 30.3% and 37.3%, respectively.
Fig. 1.
Anterior and posterior planar scintigraphy demonstrating radiotracer activity within pulmonary (ROI A and D = Left lung, B and E = Right lung) and hepatic parenchyma (ROI C = liver). Note the high intensity of pulmonary activity relative to the liver.
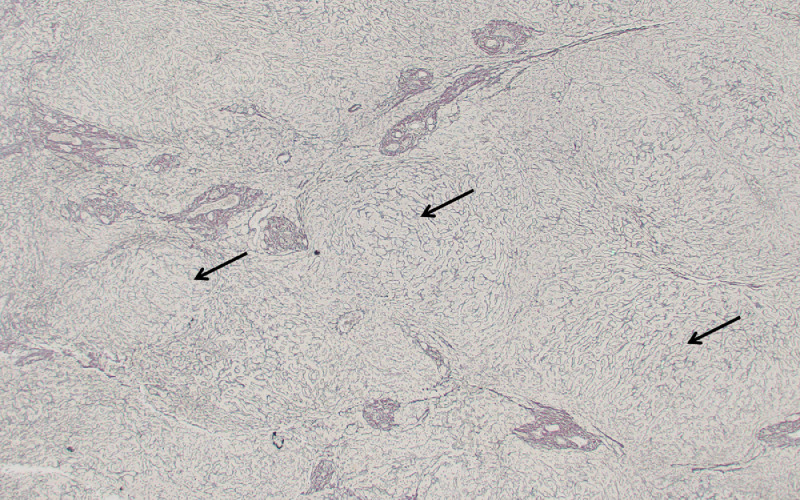
As a result of these findings, the patient underwent a surgical wedge biopsy which confirmed the diagnosis of NRH. She was listed for LT secondary to advanced HPS. Prior to LT 7 months after her diagnosis, the patient had also developed portopulmonary hypertension, an additional complication of portosystemic shunting, which required temporary management with both ambrisentan and sildenafil in the post-transplant setting. Explant liver tissue analysis with a reticulin stain demonstrated the classical findings of NRH (Fig. 2). The patient had clinical improvement with resolution of respiratory symptoms 2 months after transplant and eventually discontinued medical treatment for pulmonary hypertension. At last follow-up, 25 months after LT, normal liver and pulmonary function was documented.
Fig. 2.
Histopathological analysis of explant tissue confirmed NRH. Reticulin stain demonstrates the presence of parenchymal nodularity (arrows) in the absence of cirrhosis.
Discussion
Portosystemic shunts are aberrant vascular communications between the portal venous system and lower-pressure systemic veins. As blood bypasses the liver, neurotoxins and vasoactive substances travel to the brain and lung, and predispose the patient to the development of hepatic encephalopathy, HPS, and portopulmonary hypertension [3].
Extrahepatic and macrovascular portosystemic shunts (eg, paraumbilical, splenorenal, mesenteric, and gastroesophageal varices) are readily detected with imaging modalities such as Doppler ultrasonography, computed tomography, magnetic resonance imaging, and endoscopy [4]. There is no standard test for the detection of intrahepatic microvascular shunts, which are occult to imaging studies, and may not be evident on biopsy as in the present case.
The radiotracer 99mTc-MAA has been historically utilized for lung perfusion scans to characterize regional circulation and assess for right to left shunts [5]. While 99mTc-MAA has been previously utilized as a peripheral IV infusion to detect intrapulmonary shunting in patients with HPS [6], to our knowledge, it has not been used to detect intrahepatic veno-venous portosystemic shunts. Intra-arterial injection of 99mTc-MAA has become a widely adopted and well-tolerated method for planning locoregional therapies in patients with intrahepatic malignancies [7]. This technique is used to visualize radiotracer deposition as a surrogate for Yttrium-90 micropsheres and calculate the estimated arteriovenous LSF to prevent radiation pneumonitis [8].
Administration of 99mTc-MAA via the portal vein has been performed previously, as pre-procedural mapping for a patient that underwent transportal radioembolization as salvage therapy for hepatocellular carcinoma [9]. The infusion was conducted safely and revealed radiotracer deposition confined to the liver, with a portosystemic LSF of 3% in this particular patient [9].
In the presented case, intrahepatic microvascular shunts arose in the context of portal hypertension from NRH. By allowing visualization of portosystemic shunting, transportal infusion of 99mTc-MAA provided evidence to support an otherwise occult intrahepatic origin of HPS and eventually porto-pulmonary hypertension. Utilization of this technique may both identify and quantify occult intrahepatic portosystemic shunts in patients with other related sequelae, such as hepatic encephalopathy, and provide an objective measure of disease burden. Transportal 99mTc-MAA scintigraphy could assist in the diagnosis of patients with suspected intrahepatic portosystemic shunting and an otherwise negative work-up.
Furthermore, transportal administration of 99mTc-MAA could be utilized to characterize both macro- and microvascular shunts in patients undergoing evaluation for LT. Measurement of portosystemic shunt fractions may conceptually contribute a quantifiable selection metric in patients with sequelae of portal hypertension, such as encephalopathy or HPS. Limitations to this report include the lack of normal portosystemic LSF comparisons outside of the previous case report and the absence of shunt vessel caliber characterization relative the the MAA particles used in this case, which could effect LSF. Further studies should be performed to provide normative data for intrahepatic portosystemic shunt fractions and efforts to optimize 99mTc-MAA particle sizes for this indication.
Informed consent
Informed consent was obtained from the patient.
Footnotes
Declaration of competing interest: BB Toskich - Advisor to Boston Scientific, Johnson and Johnson, Astra Zeneca, HistoSonics. Other authors – none to disclose.
References
- 1.Wanless IR. Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2,500 autopsies and a new classification of benign hepatocellular nodules. Hepatology. 1990;11(5):787–797. doi: 10.1002/hep.1840110512. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, Elias JE, Eapen CE, Ramakrishna B, Elias E. Idiopathic non-cirrhotic intrahepatic portal hypertension (NCIPH)-newer insights into pathogenesis and emerging newer treatment options. J Clin Exp Hepatol. 2014;4(3):247–256. doi: 10.1016/j.jceh.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidal-González J, Quiroga S, Simón-Talero M, Genescà J. Spontaneous portosystemic shunts in liver cirrhosis: new approaches to an old problem. Therap Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820961287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bandali MF, Mirakhur A, Lee EW. Portal hypertension: Imaging of portosystemic collateral pathways and associated image-guided therapy. World J Gastroenterol. 2017;23(10):1735–1746. doi: 10.3748/wjg.v23.i10.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhodes BA, Stern HS, Buchanan JA, Zolle I, Wagner HN. Lung scanning with 99mTc-microspheres. Radiology. 1971;99(3):613–621. doi: 10.1148/99.3.613. [DOI] [PubMed] [Google Scholar]
- 6.Abrams GA, Nanda NC, Dubovsky EV., Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114(2):305–310. doi: 10.1016/S0016-5085(98)70481-0. [DOI] [PubMed] [Google Scholar]
- 7.Garin E, Rolland Y, Laffont S, Edeline J. Clinical impact of 99mTc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with 90Y-loaded microspheres. Eur J Nucl Med Mol Imaging. 2016;43(3):559–575. doi: 10.1007/s00259-015-3157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert B, Mertens J, Sturm EJ, Stienaers S, Defreyne L, D'Asseler Y. 99mTc-labelled macroaggregated albumin (MAA) scintigraphy for planning treatment with 90Y microspheres. Eur J Nucl Med Mol Imaging. 2010;37(12):2328–2333. doi: 10.1007/s00259-010-1566-2. [DOI] [PubMed] [Google Scholar]
- 9.Toskich BB, Tabriz DM, Zendejas I, Cabrera R, Geller B. Transportal radioembolization as salvage hepatocellular carcinoma therapy to maintain liver transplant candidacy. J Vasc Interv Radiol. 2015;26(10):1479–1483. doi: 10.1016/j.jvir.2015.06.029. [DOI] [PubMed] [Google Scholar]