Abstract
The most important task of the olfactory system is to generate a precise representation of odor information under different brain and behavioral states. As the first processing stage in the olfactory system and a crucial hub, the olfactory bulb plays a key role in the neural representation of odors, encoding odor identity, intensity, and timing. Although the neural circuits and coding strategies used by the olfactory bulb for odor representation were initially identified in anesthetized animals, a large number of recent studies focused on neural representation of odorants in the olfactory bulb in awake behaving animals. In this review, we discuss these recent findings, covering (1) the neural circuits for odor representation both within the olfactory bulb and functional connections between the olfactory bulb and higher order processing centers; (2) how related factors such as sniffing affect and shape the representation; (3) how the representation changes under different states; and (4) recent progress on the processing of temporal aspects of odor presentation in awake, behaving. We highlight discussion of the current views and emerging proposals on the neural representation of odorants in the olfactory bulb.
Keywords: olfactory bulb, odor representation, physiological states, feedback modulation, temporal information
1. Introduction
Olfactory perception begins when volatile chemical molecules dissolved in the air are inhaled into the nasal cavity and interact with olfactory receptors expressed in the cilia extending from the dendrites of the olfactory sensory neurons (OSN). In rodents there exist more than 1000 odor receptors to enable the efficient reception of more than 1 million types of odors and their combinations in the natural environment, and each OSN expresses only one receptor1. The OSNs project their axons to the glomeruli of the olfactory bulb (OB), the first information processing center of the olfactory system. In the glomeruli, OSNs project excitatory synapses to, among others, the mitral and tufted cells (M/Ts), which are the major projection neurons of the OB. After complex neural processing by the circuits in the OB, M/Ts send processed information to higher olfactory centers such as piriform cortex, olfactory tubercle and anterior olfactory nuclei.
In order to yield appropriate response, it is critical for the olfactory system to perceive odor information accurately and precisely in the ever-changing external world. This process is rather complex regarding the need to process parallel input from ~1000 olfactory receptors in rodents in a turbulent odor plume2. However, increasing evidence indicates that the OB has the ability to represent most aspects of the odor information, such as odor identity, intensity and timing3–7. In general, two strategies have been proposed for odor representation in the OB: spatial coding and temporal coding. A given odor evokes specific activation of a subset of glomeruli, forming a unique spatial odor map that links odor identity / intensity to the pattern of activated glomeruli8,9. Spatial coding of odors via these glomerular odor maps is highly conserved across species and has been demonstrated through a variety of imaging techniques, including 2-DG uptake, intrinsic optical imaging, and fMRI8,9. Temporal coding, on the other hand, focuses on the timing properties of M/T cell firing in response to odors. The latency to the first spike, the firing pattern and local field potential (LFP) theta oscillations across a short time window such as a sniff, and the overall firing pattern from M/T ensembles are all critical for representation of odor identity and intensity4,6,10–12. The temporal coding strategy is supported by evidence from electrophysiological recordings in both anesthetized and awake rodents13–16.
Both spatial and temporal coding strategies face the challenge that odor perception is dynamic and varies under different brain states. Wakefulness, attention, experience, metabolism status, and the value of the odor for the subject are important factors that can change the perception of the same odor17–20. The underlying mechanisms by which the OB represents odor information precisely under different brain and behavioral states remain elusive, although recent studies have provided relevant data and some hypotheses have been established6,21. In this review, we will first discuss the neural circuits for odor representation both within the OB and between the OB and higher centers, and how external factors such as sniffing affect and shape the representation. Then we will focus on the odor representation under different brain and behavioral states, namely anesthetized, awake, learning, active/passive odor sampling, and rewarded. We will also describe recent progress on the coding of information related to odor timing in awake, behaving rodents, such as the duration of odor presentation and the time between two odors.
2. Major components and key neural circuits of the OB
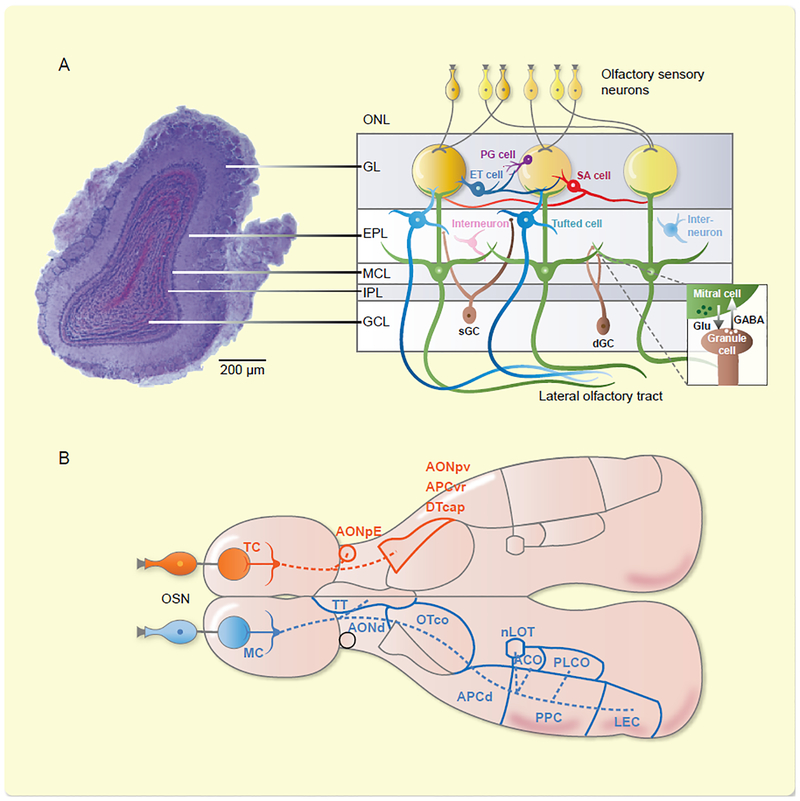
The OB is a typical laminar structure containing a diversity of neurons (Fig. 1)22. The main output neurons, the M/Ts, receive direct excitatory input from the OSNs at the glomeruli23. There are large numbers of GABAergic interneurons in almost all of the layers, as well as dopaminergic interneurons in the glomerular layer, and both are involved in the neural circuits that mediate transmission from the OSNs to the M/Ts and shape the firing properties of M/Ts in response to odors24,25. Two important neural circuits for processing olfactory information are located in the glomerular layer and in the external plexiform layer26. Interestingly, a recent study has identified a mirror-symmetric excitatory connection between the two bulbs and this inter-bulb circuits could enable odor perceptual unity27. Thus, odor information is encoded in the firing of the M/Ts after complex processing by the neural circuits within the OB.
Fig 1.
Organization of the OB. A. Left, photomicrograph of a coronal section through the mouse OB. ONL, olfactory nerve layer; GL, glomerular layer; EPL, external plexiform layer; MCL, mitral cell layer; IPL, internal plexiform layer; GCL, granule cell layer. Right, diagram of the OB network. LOT, lateral olfactory tract. Modified from22. B. Output projections of MC (orange) and TC (blue). OSN: olfactory sensory neuron, TC: tufted cell, AONpE: AON pars externa , AONpv: posteroventral part of the AON, APCvr: the ventrorostral part of the APC, OTcap: the cap part of the olfactory tubercle, MC: mitral cell, TT: tenia tecta, AONd: the dorsal part of the AON, OTco: cortical part of the olfactory tubercle, APCd: dorsal part of the APC, PPC: posterior piriform cortex, LEC: lateral entorhinal cortex, nLOT: nucleus of the lateral olfactory tract, ACO: anterior cortical amygdaloid nucleus, PLCO: posterolateral cortical amygdaloid nucleus.
2.1. Neural circuits in the glomerular layer
Neurons in the glomerular layer are morphologically heterogeneous and can be classified into three identified types: external tufted cells (glutamatergic), periglomerular cells (GABAergic), and superficial short-axon cells (combined GABAergic and dopaminergic)25. Dendrites of external tufted cells project to higher areas of olfactory cortex and also provide extensive feedforward excitation to local glomerular interneurons and M/Ts28. The feedback pathway may play a role in augmenting and/or affecting the dynamics of the reaction of M/T cells to OSN input. This is extremely important since direct inputs from OSNs to M/Ts are often too weak to evoke action potentials. Thus, the multi-step excitation mediated by external tufted cells may affect odor-evoked responses of M/Ts as a vital node transferring odorant information to cortical areas28.
One-third of periglomerular cells receive direct input from the OSNs and drive presynaptic inhibition of OSNs via presynaptic GABAB and D2 receptors29. The remaining periglomerular cells receive indirect excitatory input from external tufted cells, mitral cells (MCs), and tufted cells (TCs), and send inhibitory output to all of them. GABA release from a single periglomerular cells is sufficient to trigger peripheral activation of homotypic periglomerular cells in the same glomerulus through GABAergic periglomerular cells–periglomerular cells synapses30. Thus, periglomerular cells contribute to recurrent inhibition of a single activated M/T and odor-evoked suppression of M/T firing25,26.
Despite being a small population, superficial short-axon cells powerfully regulate sensory-evoked activity in the OB25. The majority (~70%) of superficial short-axon cells receive sensory input indirectly, mediated by the external tufted cells, while the remainder appear to receive sensory input directly from the OSNs. GABAergic and dopaminergic interglomerular projections and gap junctions between superficial short-axon cells and external tufted cells and/or M/Ts drive gain control, contrast enhancement, and possibly lateral inhibition of M/Ts in the glomerular layer31. It’s likely that centrifugal input to periglomerular cells and superficial short-axon cells modulates their activity across distinct brain states since odors evoke stronger excitation in the awake state than in anesthetized state30.
2.2. Neural circuits in the external plexiform layer
In the external plexiform layer, the lateral M/T dendrites form elaborate dendrodendritic synapses with at least two types GABAergic interneuron: granule cells (GCs) and parvalbumin-positive (PV+) interneurons32–34. The superficial GCs and deep GCs form reciprocal dendrodentritic synapses with the lateral dendrites of TCs and MCs, respectively35. The GCs receive abundant inhibitory inputs from deep short-axon cells in the granule cell layer and basal forebrain centrifugal GABAergic projections. The GC dendrodentritic synapses are traditionally considered the basis for recurrent inhibition and lateral inhibition of the M/Ts, and important for the coding of odor identity34,35. However, recent studies found that lateral inhibition between heterotypic M/Ts in the external plexiform layer is not predominantly mediated by GCs, but by the external plexiform layer interneurons, which show remarkably high rates of reciprocal synapses with M/Ts (around 50%)32,33. Furthermore, the external plexiform layer interneurons also mediate recurrent inhibition of M/Ts and play an important role in driving gamma-frequency (40–100 Hz) synchronization of M/Ts and broad gain control among heterotypic M/Ts25,32,33.
2.3. Comparison between MCs and TCs
At the output level, MCs and TCs, that process in parallel afferent olfactory sensory information23, are two distinct channels of OB output and could encode complementary aspects of olfactory information35,36. In terms of morphology MCs are located in the MCL of OB, and the secondary dendrites are distributed in the deep external plexiform layer, while TCs are smaller, located throughout the external plexiform layer, the secondary dendrites distributed in the superficial external plexiform layer36. In electrophysiological properties, TCs exhibited more sensitive, broadly tuned, lower responsive threshold, concentration invariant responses, and earlier responsive in the sniff cycle36–38. Therefore, TCs are presumably more efficient at distinguishing similar odorants at low concentration35. These functional differences between MCs and TCs are likely due to the different inhibitory effects from the glomerular layer and granule cell layer10,35. In addition, MCs and TCs project their axons to many non-overlapping regions: axonal projections of TCs are restricted to the rostral structures, while MCs cover entire olfactory cortex uniformly and extend to caudal regions37. Thus, the functional difference between MCs and TCs provides important neuronal basis for representation of multiple aspects of olfactory information at the OB and higher brain centers.
3. Feedback and centrifugal modulation of the OB
The OB receives dense modulation from higher brain areas, including both feedback and centrifugal inputs. Piriform cortex and the anterior olfactory nuclei are the major source of feedback inputs39, and other modulatory centrifugal inputs include cholinergic, noradrenergic, and serotonergic innervation40. Specific optogenetic manipulation of these circuits has demonstrated that all of these projections dramatically modulate cell activity and odor information processing and representation in the OB, and also affect olfactory-related behavior.
3.1. Piriform cortex
Piriform cortex is the most important cortical region for olfaction and projects directly to the OB. It has the ability to encode information about the identity, intensity, and timing of odors41–43. More importantly, piriform cortex plays a major role in odor preference learning, odor pattern separation, olfactory learning, odor fear memory, and the processing of odor objects44–46. Odor-evoked activation has been observed directly in pyramidal axons projecting from piriform cortex to the OB47,48. The feedback projection from piriform cortex to the OB is ipsilateral and diffuse, and targets mainly the granule cell layer, and the axonal response to odors is sparse and somewhat odor-specific47–49. Optogenetic activation of these piriform cortex–OB axons indirectly inhibits MCs by directly exciting the GCs, which in turn inhibit MCs via dendrodendritic connections49. Piriform centrifugal innervation can also modulate the activity of short-axon cells, which drive feedforward inhibition of GCs. Activation of piriform cortex–OB axons has only a weak effect on spontaneous activity of the M/Ts, but strongly inhibits odor-evoked responses in vivo. In general, although the projection from the piriform cortex to the OB has diverse and complex effects on the OB microcircuits, it has been postulated that the major net effect on the M/Ts is an amplification of odor-evoked inhibition49.
3.2. Anterior olfactory nucleus
Neurons in the anterior olfactory nucleus send axons to the contralateral and the ipsilateral OB and APC50. Functionally, the anterior olfactory nucleus is involved in processing the differences in odor concentration between the two nostrils and is crucial for the localization of odor sources51. Odor stimulation elicits strong increases or decreases in activity in axons that project from the anterior olfactory nucleus to the OB, suggesting odor-dependent modulation of OB circuits by the anterior olfactory nucleus52. Interestingly, GABA type B receptors on AON-OB / piriform-OB afferents gate excitatory transmission in a target-specific manner and thus shape how the OB integrates sensory inputs and top-down information53. Optogenetic activation of the axons from the anterior olfactory nucleus in the OB revealed that this feedback modulation inhibits the activity of the MCs by indirect activation of the GCs or short axon cells, or excites the MCs by direct depolarization54. Activation of these feedback axons in vivo inhibits both spontaneous and odor evoked M/Ts responses, and a sparse, excitation effect is manifested as accurately timed spikes. Thus, the feedback from the anterior olfactory nucleus plays a role in suppressing background activity and odor-evoked excitation of MCs and also permits precisely timed spikes in a narrow time window during specific periods of behavior54.
3.3. Cholinergic modulation
The major source of cholinergic input to the OB is the horizontal limb of the diagonal band of Broca (HDB) in the basal forebrain, whose activity is correlated with attention, learning, and memory in almost all sensory systems. A recent study showed that this projection co-expresses markers for GABAergic transmission55. Both nicotinic and muscarinic receptors are found in the OB and play crucial and somewhat different roles in neural and behavioral odor discrimination56–59. Cell-specific recording of M/T calcium signals showed that acetylcholine (ACh) in the OB increases glomerular sensitivity to odors and decreases the M/T activation threshold56. The majority of presumed M/Ts were excited by electrical stimulation of the HDB, which activates both cholinergic and GABAergic neurons, and muscarinic receptors in the OB were required for this effect60. Specific activation of cholinergic cell bodies in the HDB inhibits the spontaneous activity of M/Ts, periglomerular cells, and GCs, sharpens the olfactory tuning curves of most M/Ts, and broadly increases the odor-evoked responses of periglomerular cells and GCs61. However, optogenetic activation of the axons of cholinergic neurons that project to the OB increases both spontaneous and odor-evoked spiking in M/Ts. The enhancement of the M/T odor response is strong and broad, indicating that the modulation adds general excitatory effects to M/Ts62. Thus, slight differences in the stimulation paradigm (light on cell bodies versus light on axons) result in totally different, contradictory observations, suggesting that cholinergic modulation of OB circuits is complex and precise61,62. It is important to note that all of these studies were performed on anesthetized animals60–62; how the neurons of the OB are modulated by cholinergic input in awake, behaving animals remains an open question. Interestingly, a recent study has demonstrated that Ach released to the OB during the prolonged odor stimulation modulated habituated odor responses and odor salience, and further caused mice to suddenly investigate a previous ignored odor, indicating the importance of Ach in the process of odor habituation and dishabituation63. In addition to cholinergic input from the HDB, some cholinergic interneurons have been found in both the main and accessory olfactory bulbs64. It will be interesting to investigate the functions of these intrinsic cholinergic neurons in the future.
3.4. Noradrenergic modulation
The OB receives significant noradrenergic inputs from the locus coeruleus, which is known to play an important role in arousal, attention, and emotional state. Norepinephrine dramatically changes the neural activity in a number of different bulbar cell types, including MCs, GCs, and external tufted cells, etc65–67. It activates multiple receptor sub-types in the OB in a concentration-dependent manner68. Behaviorally, norepinephrine is involved in olfactory associative learning in neonatal animals during sensitive periods, as well as in odor detection and discrimination in mature rodents69. Electrical activation of the locus coeruleus under anesthesia causes long-lasting suppression of M/T responses to both food odors and urine. Moreover, specific behavioral effects of this stimulation are observable after recovery from anesthesia, suggesting that locus coeruleus-mediated olfactory neural plasticity is able to store an individual recognition memory70. Furthermore, odor-evoked activities in the glomerular layer are persistently weakened after locus coeruleus activation due to suppression of presynaptic input, indicating that noradrenaline released from the locus coeruleus has an effect on odor representation even at the earliest stage in the olfactory system71. A recent important study combined direct application of noradrenaline to the OB with electrical stimulation of the locus coeruleus. The authors proposed that noradrenaline enhances odor responses not through direct potentiation of the afferent signal per se, but by reducing the intrinsic noise in the system66. Although specific manipulation of noradrenergic neurons by optogenetics is feasible, surprisingly only one study has used this technique to date. This study finds that Optogenetic inhibition of adrenergic fibers alters odorant-induced changes in power of oscillations in the olfactory bulb in mice learning to discriminate odorants72. Information is still lacking about the effects of optogenetic activation of noradrenergic inputs on odor-related spiking in the OB in awake animals.
3.5. Serotonergic modulation
Serotonergic neurons in the raphe nuclei have widespread projections in the brain and are thus involved in a variety of brain functions, including regulation of mood and anxiety, the sleep–wake cycle, reward, patience during decision making, and sexual preference73,74. Serotonergic projections from the dorsal and medial raphe nucleus innervate the OB densely, where they may modulate the initial representation of olfactory information22,75. Although elimination of most of the serotonergic neurons from the forebrain has no detectable effect on the ability of mice to perform a go/no-go olfactory discrimination task76, the activity of almost all neuron types in the OB is dramatically modulated by serotonin75,77,78. Optogenetic activation of serotonergic neurons increases spontaneous firing in both mitral and tufted cells, and it also bidirectionally modulates odor-evoked firing in mitral cells, resulting in improved pattern separation of odors78,79. Since serotonergic neurons are related to reward and odor-evoked elicits significant firing change of serotonergic neurons in the dorsal raphe during a go / no go task where the odorant is associated with a reward80, it will be interesting to study how the serotonergic inputs to the OB modulate the response of M/Ts to rewarded / unrewarded odors in behaving animals in the future.
In conclusion, understanding of odor representations in the OB is complex because the neural activity of the M/Ts is dynamically modulated by strong feedback and centrifugal input. In the awake state, the weight of these inputs changes from moment to moment and modulate odor representation dynamically. It’s important to study how these inputs affect odor representation in awake behaving animals and how different sources of the inputs (eg. serotonergic input and feedback from piriform cortex) work in a consorted manner in the future.
4. Sniffing: active sampling of odor
In mammals, odors are sampled through sniffing / respiration and retronasal air flow into the nasal cavity for dynamic detection by OSNs. Sniffing represents active sampling of the odors with high frequency of respiration (> 4Hz)81 coordinated with other orofacial motor actions such as movement of whiskers, chewing, licking and lateral displacement of the nostrils82. This respiration / sniffing controls the access of odors to the OSNs and plays an important role in olfactory discrimination and perception81. Exploratory sniffing is reliably evoked by novel odorant stimuli, and is dominant during rapid odor-source localization in rodents83. Interestingly, sniffing is also involved in other behavior-related processes, such as internal action models and during social interactions. For example, it has been reported that, compared with typically developing controls, children with autism spectrum disorder had a profoundly altered sniff response pattern to odors with different values84. In rodents, investigation by one rat toward the facial region of a conspecific often elicits a decrease in sniffing frequency in the conspecific, indicating that they use sniffing to communicate information85. Furthermore, a recent study has demonstrated that a steady sniffing (4 Hz) is critically involved in conditioned fear-induced freezing behavior of rodents, and the neural circuits between olfactory pathway and prefrontal cortex have been identified86. Finally, the retronasal mode of olfaction that takes place when odorants enter the nose through the mouth during chewing plays an essential role in perception of flavors87.
4.1. OSN activation is suppressed during exploratory high-frequency sniffing
In the OB, activity of different types of neurons, as well as the neural circuits are modulated dramatically by sniffing. The OSNs respond to mechanical stimulation suggesting that sniffing can activate the OSNs88,89. Thus, in the glomerular layer, the activity of some OSN terminals and glomeruli can be driven by sniffing, even in the absence of an odor11,89. During odor stimulation, a greater number of activated glomeruli are locked to sniffing. However, in calcium-imaging studies, this type of odor-evoked, sniffing-locked response pattern changes to a sustained response pattern if the frequency of sniffing is higher than 4 Hz81, suggesting that this may be an important mechanism to selectively suppress OSN activation by background odors during exploratory high-frequency sniffing.
4.2. Odor representation by M/Ts is shaped and modulated dramatically by sniffing
One common property of a subset of the M/Ts is that their spontaneous and/or odor-evoked firing is locked to a specific phase of the sniffing or respiration cycle, in both anesthetized and awake animals36. This type of phasic M/T firing likely plays an important role in the coding strategy for odor representation in awake animals6,13. In awake, head-fixed rats, the firing pattern, rather than firing rate, carries more information about odor identity6,11,14. The coherence between M/T firing and theta oscillations, which are highly correlated with sniffing, is also crucial for the representation of odor identity in behaving, free-moving mice12. Recent studies found that respiration gates the sensory input response of the M/Ts11,90, and sustained odorant sampling at higher frequencies leads to increasing decorrelation of the M/T cell population response pattern over time3. Strikingly, data from the in vivo whole-cell patch-clamp recording have demonstrated that the plasticity of odor-evoked M/Ts responses during the go / no go task could be contributed to the sniffing strategies developed during the learning91. Therefore, at the output level of the OB, the neural representation of an odor can be shaped and modulated dramatically by sniffing.
4.3. Firing pattern of GCs is modulated by sniffing
It is difficult to study how sniffing modulates the interneurons of the OB with in vivo electrophysiological recordings because of technical challenges. However, juxtacellular ‘loose-patch’ method has been used to identify GC firing92. Although GC firing is strongly coupled with respiration in anesthetized mice, the firing is desynchronized and independent of respiration in awake mice. Similar results have been found with in vivo patch-clamp recordings in awake mice sniffing at different frequencies: synaptic input to GCs is strongly phase modulated during basal respiration, but this subthreshold phase tuning of the membrane potential becomes heterogeneous during higher respiratory frequencies93. Therefore, GCs likely shape the response of M/Ts through broad lateral interactions that are relatively independent of sniffing in awake animals. It will be interesting to discover how sniffing modulates the activity of other types of interneurons in the glomerular and external plexiform layers in future studies. Interestingly, oscillations of the local field potential in the OB are locked to lick patterns in animals undergoing fast sniffing in the go/no go task72 raising the question whether there is a motor relationship between the theta LFP, sniff and licking in this behavioral state82.
5. Neural representation of odor under different states: anesthetized, awake, behaving, and reward
5.1. Neural representation of odor under anesthetized v.s. awake state
The phenomenon that neural activity in the OB is largely dependent on brain state was initially reported in 1950 by Adrian, who found that the depth of anesthesia dramatically affected both ongoing spontaneous neural activity and odor-evoked responses in the OB94. In fact, the OB response to the same odor changes even with different depths of anesthesia95, or with transitions between up and down states, indicating that odor representation in the OB are rather sensitive to slight changes in brain state. While odorants do induce strong changes in M/T firing under anesthesia, the firing of these units remains largely constant when the animal is awake14,96,97, although one imaging study of glomerular OSN input showed dense representation of natural odors in awake head-fixed mice98. Similar to M/Ts, the basal firing rate of inhibitory GABAergic neurons in the OB is higher in the awake state than the anesthetized state. However, unlike M/Ts, odor-evoked changes in firing are much stronger in these cells in the awake state than the anesthetized state17,92, indicating that GCs are more actively involved in shaping the properties of odor representation in the OB in the awake state99.
The mechanism underlying why the odorant induced firing changes of M/Ts are weak and sparse in the awake animal remains elusive, although several hypotheses have been proposed. It is possible that the activity of M/T cells is being modulated when the animal is focusing on discrimination of odorants to optimize representation of a subset of stimuli. This could be the reason why the centrifugal and feedback modulation from higher brain centers under awake state is more active97, resulting in higher activity of the GCs, which is likely the key factor that contributes the firing properties of M/Ts in awake state17,92,99. In addition, anesthetics could influence activity of the neurons in the OB directly by depressing the glutamatergic and / or increasing the GABAergic synaptic activities. It is still not clear how the extrinsic and intrinsic effects of the anesthetics on shaping the firing and odor response properties of the M/Ts contribute to their effects on OB circuit activity.
While only weak total firing changes of M/Ts is induced by odor stimulation under the awake state, one important question is how the odor identity is represented. Recent studies showed that many M/Ts changed their spike timing in an odor-specific manner while the total firing rate remained constant14. The firing rate showed transit changes within a respiratory cycle other than averaged from all the duration of the odor presentation15, and fine temporal structures within a respiration cycle conveys information on odor identity13. Thus, temporal coding with the respiratory cycle, or higher oscillatory frequencies, carry odor information in awake animals6,11,12,81. Furthermore, odor information could also be represented by the evolving dynamics in an ensemble of neurons. The mitral cell ensemble activity contains information at different timescales that could be separately or complementarily exploited by downstream brain centers to attain odor discrimination5,100.
5.2. Neural representation of odor during odor discrimination tasks
Interestingly, in behaving animals performing odor discrimination tasks, the firing of M/Ts changes with behavioral events other than odors96. Both the number of units responding to odors and the number of units showing divergent response to S+ (rewarded odor) and S- (unrewarded odor) increases, after the animals learned to discriminate odors (Fig.2)12,101, suggesting strong plasticity of M/Ts responding to odors during learning102. Similar plasticity has been also found for oscillations of local field potentials in behaving rodents12,72,103. Furthermore, whether this type of plasticity exists in the olfactory sensory neurons is controversial104,105. Finally, in addition to learning, recent studies show that early odor exposure could also dramatically change odor evoked M/Ts responses and elicit incorporation of new GCs through neurogenesis from the subventricular zone106.
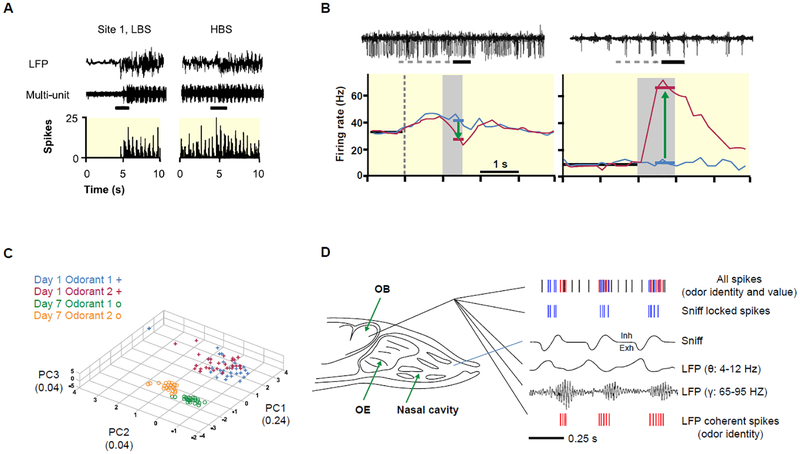
Fig 2.
Odor response of M/Ts under different anesthesia levels (brain states). A. Odor response of M/Ts under low brain state (LBS) and high brain state (HBS). Raw LFP signals (top), raw multiple-unit (middle) and histogram of spiking (bottom) at one recording site with 2s odor stimulation under LBS and HBS. The black bars represent odor stimulation. Modified from95 with permission. B. Odor response of M/Ts under awake (left) and anesthetized mouse (right). Top, raw traces of spiking recording from the same location in awake behaving and anesthetized mouse. The solid horizontal bars indicate time of odorant exposure (citral). The dashed bars indicate the time of final valve activation that in the behavioral paradigm corresponds to the time the mouse spend in the port before odor delivery. Modified from97, copyright 2006 Society for Neuroscience. C. Mitral cell population responses pooled across all animals in the difficult discrimination task, plotted for day 1 (animals can’t discriminate odors) and day 7 (animals learned to discriminate odors) in the first three principal component axes. Note the increase in separation of odorant 1 and odorant 2 trials with training. Modified from101 with permission. D. The diagram shows a model that the firing of action potentials in the M/Ts carries information for odor reward, and the odor feature that carries information to differentiate between odors regardless of associated outcome, which could be odor identity or intensity, is carried by coherence between spike firing and gamma LFP. OE, olfactory epithelium. Inh, inhalation. Exh, exhalation. Modified from12 with permission.
Importantly, the synchronized firing between two different M/Ts increased when responding to rewarded odor, and decreased in firing when responding to unrewarded odor, regardless of odor identity107. Thus, the positive response evoked by odor doesn’t reflect its identity, but rather whether that odor is rewarded as opposed to unrewarded. That means the synchronized firing in these M/Ts conveys information on odor value instead of odor identity107. The recent study has revealed that the information on odor identity during the go / no go odor discrimination task is likely carried by the coherence between the gamma oscillations of LFP and spikes of the M/Ts since it differentiates between odors irrespective of associated outcome (Fig.2)12. Why are there substantial changes in odorant representation in M/T firing or OB circuit oscillations during learning resulting in representation of odorant value in this early sensory circuit? It is possible that behavioral tasks that motivate the animal to focus on particular odorants elicits optimization of the ensemble difference in neural response between the stimuli, focusing the ability to discriminate by excluding other inputs, a process that would be analogous to the cocktail party effect in the auditory system108. This possibility needs to be evaluated in future studies.
6. Trail-to-trial variability of odor response of M/Ts in awake behaving animals
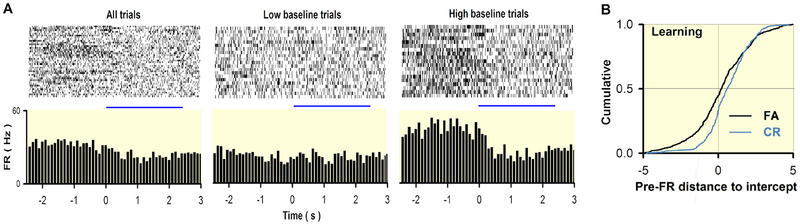
Neurons in the brain usually change their firing rate from trial to trial when the animals perform a specific behaving task, and the sensory response and behavioral output are dependent on the ongoing firing rate19. In the OB, slice recordings have revealed that individual M/Ts switch between states with low and high baseline firing rate and exhibit glomerulus-wide long-lasting depolarizations109. A recent in vivo study found that ‘silent’ M/Ts with no or weak baseline firing rate increased the firing rate dramatically responding to odors, while these M/Ts with high baseline firing rate showed weak or decreased responses110. However, another explanation of this observation is that for the same neuron, the odor response depends on the baseline firing rate, with increased response under low baseline firing rate and decreased response under high baseline firing rate. This hypothesis is supported by extracellular recordings in awake behaving mice (Fig.3)111, and consistent with findings in a subset of the previous studies showing that the responsiveness to odorants differed depending on the behavioral status of the animal17,107. More importantly, the baseline firing rate is affected by the behavioral status with larger values in the passive task compared with the active task where odorant valence plays a role in decision making (Fig.3)111. In addition, the trail-to-trial baseline firing rate is different when an animal makes a mistake in the active associative learning task suggesting that it reflects an anticipatory cue111,112. Thus, the odor representation in the awake behaving state likely depends on the ongoing baseline firing, which varies from trial-to-trial. In the future it is important to study the neural mechanisms underlying the trial-to-trial changes in the baseline firing rate; it is likely that centrifugal feedback is involved.
Fig. 3.
Trial-to-trial variability of odor evoked M/Ts firing rate (FR, A) and the association between baseline firing rate and behavior output (B). A. Example of odorant responses for a single unit. Top, raster plot (bottom to top: first to last trial); bottom, PSTH. Left to right, All, low and high pre-FA trials (high pre-FR ≥ mean pre-FR). B. Cumulative probability for the distance along the pre-FR axis between a point and the intercept between the best-fit line and odor FR = pre-FR. Dividing by the SD of pre-FR normalized this distance. Correct rejection (CR) versus false alarm (FA) for the learning segment (Kolmogorov-Smirnov, P < 0.001, number of trials: 233 CRs, 667 FAs). Modified from111 with permission.
7. Precise representation of timing
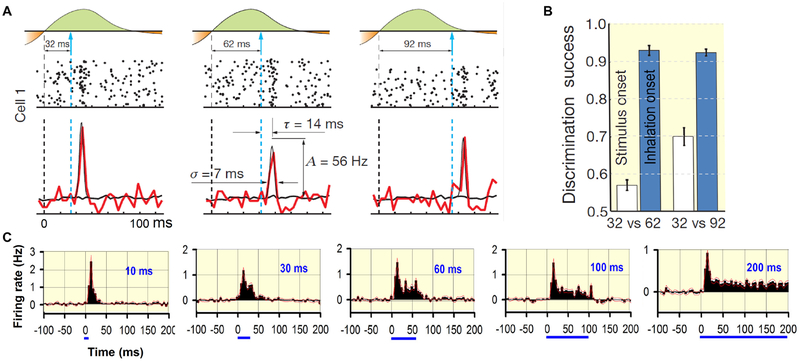
Compared with vision and audition, olfaction is considered a slow sensory system with poor temporal resolution, mainly because the process of odor sampling through sniffing and detection and transduction of odorant detection in OSNs take place in the 10–200 msec time frame113. However, the neural processing of the central olfactory system is precise likely because temporally accurate detection is needed to process information in odorant plumes2. Rats were able to use internasal time differences as short as 50 milliseconds to locate odor source and this capability is consistent with the firing properties of the M/Ts114. Direct optogenetic activation of the OSNs with precise duration has revealed that the mice have the ability to discriminate differences of 10 milliseconds in duration and some M/Ts in the OB of awake animals convey information on stimulus duration by responding tonically (Fig.4)115. The mice also have an impressive ability to perceive the timing of olfactory activation relative to the sniff cycle; they can discriminate between light-evoked inputs that are shifted in the sniff cycle by as little as 10 milliseconds, and individual M/Ts encode this timing by the changing the firing properties including firing timing and firing rate (Fig.4)116. Furthermore, direct optogenetic stimulation of M/Ts by patterned activation triggered by specific sniffing phases found that virtual odors that differed by as little as 13 milliseconds could be distinguishable by mice, and the imaging studies indicated that the different activation patterns evoked distinct dynamics of the calcium response of the mitral cells117. This is consistent with a related study which optogenetically stimulated a specific glomerulus118. Interestingly, information on timing of the stimulus response is likely decoded by the downstream piriform cortex6,119. Therefore, animals can discriminate olfactory related temporal information precisely, and the neurons, especially M/Ts in the OB, have the ability to represent this information, although the details of the mechanism remain elusive.
Fig 4.
Representation of temporal information by M/Ts. A. Response of M/Ts to light stimulation with different latencies after inhalation onset. Top, light application. Middle and bottom, raster plots + PSTH for one representative M/T’s responses to light at three latencies after inhalation onset. Colored and gray lines are PSTHs for light responses and spontaneous activity, respectively. Thin black lines are Gaussian fits of the difference between PSTHs for stimulated and unstimulated sniffs. The fit parameters yield measures of response width (σ), latency (τ) and amplitude (A). B. Classification performance for the neuronal population response discriminating between 32 and 62 ms and between 32 and 92 ms light stimulation latency. Responses are aligned to the stimulus onset (yellow) and inhalation onset (green). A and B are modified from116, reprinted by permission from Springer Nature. C. Mean change in firing rate for all units recorded from normalized by dividing by the firing rate before stimulation with light. Top to bottom, light stimulation with different durations. Modified from115 with permission.
8. Conclusions and future directions
A key question on olfactory research is how the brain represents odor information in a real environment with turbulent odorant plumes emanating from different sources, the cocktail party effect for smell. The OB is the first relay station of the olfactory system and plays important roles in information processing and representation of the odors. In the past decade, data have been accumulated and hypotheses have been built on how the OB represents odor information in awake animals under different behavioral and brain states. A rather complicated scenario has been revealed and the consensus is that the ability of the OB to represent the odor not only relies on the complex neural circuits within the OB, but that massive centrifugal innervation controlled by brain areas relevant to olfactory behavior such as sniffing are also involved. Although there has been substantial progress in understanding OB neural processing future studies are necessary to unveil neural mechanisms underlying olfaction in natural settings.
The following are bullet points regarding future developments:
Functions of interneurons in the OB. The interneurons in the glomerular layer and granule cell layer have been investigated intensively and most functions of these neurons have been identified. However, the function of most different types of interneurons in the external plexiform layer and their effect on the M/Ts representation of odors are still not clear25,26. Although recent studies have revealed the functions of PV-positive interneurons32,33, the circuit interaction of other interneurons, such as VIP-positive and CB-positive neurons, need further investigations.
Other cortical feedback and potential functions. Besides piriform cortex and anterior olfactory nucleus, other olfactory cortical regions which receive direct input from the OB, such as cortical amygdala120 and olfactory tubercle121, likely play crucial roles in odor representation in the OB. Although no clear input from the olfactory tubercle to the OB is found in a recent study122, whole-brain mapping of the inputs and outputs of the medial part of the olfactory tubercle has revealed massive projections123, however, the function is still not clear. Furthermore, since the olfactory tubercle is related to the odor valance121, it’s important to study how this circuit contributes to the representation of odor value in awake behaving animals.
Behavioral state-dependent centrifugal modulation. The state-dependent changes in odor representation of M/Ts is likely due to the feedback and centrifugal projections to the OB, but which projections and how they modulate the OB circuit under a specific brain states are not clear. In addition, as discussed above, during the go / no go task, the spiking of M/Ts conveys information on odor value rather than odor identity12,107. Since the serotonergic neurons also carry information on the value of the stimulus including rewarded odor80, and excitatory effect of the serotonergic input on the M/Ts22,78, it is likely that firing properties of the M/Ts responding to rewarded odors is shaped by serotonergic input. However, direct evidence is needed in future studies which should combine the electrophysiological recording and optical calcium recordings of the axons projecting to the OB in behaving animals.
Studying odor representation by monitoring multi-site olfactory centers. While the OB is an important olfactory center for odor representation, other olfactory centers are also involved6,41,124. Monitoring the neural activity from multiple olfactory centers could provide important information on how the whole system represents odors. Previous studies have successfully recorded LFP from multiple brain areas in awaking behaving rats and information on how olfactory related brain areas cooperated in different situations has been investigated125. In future studies, it will be important to record simultaneously the spiking of the neurons from different brain areas to reveal how the odors are represented by the synchronized activity from distant neuronal populations and to compare to the results obtained within the OB107.
Comparison of the different odor discrimination tasks and different conditions during the task. The go / no go and two-alternative choice are the tasks that have provided information on odor representation of M/Ts in awaking behaving animals13,107,111. However, large difference between these two tasks have been found in recent studies, including time needed to learn the tasks, odor sampling strategy, and temporal integration126. Although the data of LFP recordings from the OB have revealed correlations between OB oscillations and behavioral states, further studies focusing on comparing the strategies of the odor representation by the M/Ts for mice under different behavioral status are needed. Furthermore, the odor representation is likely dependent on the conditions of the animals during the task, e.g. free-moving v.s. head-fixed12,14,101,111. Under these two conditions, behavioral performance of animals appears similar127 but the neural activity likely differs due to differences in behavioral status and the level of stress. Besides, odor sampling is vastly different under the head-fix condition compared to the freely-moving animal. Furthermore, neural processing of odorant search in a turbulent odor plume likely involves different neural processes2 as well as coordination with oromotor82 and locomotor neural activity128. Therefore, it’s important to compare the strategy for neural representation under different well-defined and well-characterized behavioral paradigms to reveal the neural mechanisms underlying how the OB conveys odor information.
Acknowledgement
This work was supported by National Natural Science Foundation of China (NSFC, 31571082, 31872771), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (16KJA180007) and NIH grant DC000566. We Thank Dr. Dejuan Wang for assistance with the figure preparation and comments on the manuscript.
Footnotes
Conflict of interests
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
This article does not contain any studies with human participants.
References
- 1.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65(1):175–187. [DOI] [PubMed] [Google Scholar]
- 2.Gire DH, Kapoor V, Arrighi-Allisan A, Seminara A, Murthy VN. Mice Develop Efficient Strategies for Foraging and Navigation Using Complex Natural Stimuli. Curr Biol. 2016;26(10):1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diaz-Quesada M, Youngstrom IA, Tsuno Y, Hansen KR, Economo MN, Wachowiak M. Inhalation Frequency Controls Reformatting of Mitral/Tufted Cell Odor Representations in the Olfactory Bulb. J Neurosci. 2018;38(9):2189–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gire DH, Restrepo D, Sejnowski TJ, Greer C, De Carlos JA, Lopez-Mascaraque L. Temporal Processing in the Olfactory System: Can We See a Smell? Neuron. 2013;78(3):416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gschwend O, Beroud J, Vincis R, Rodriguez I, Carleton A. Dense encoding of natural odorants by ensembles of sparsely activated neurons in the olfactory bulb. Scientific reports. 2016;6:36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uchida N, Poo C, Haddad R. Coding and Transformations in the Olfactory System. Annu Rev Neurosci. 2014;37:363–385. [DOI] [PubMed] [Google Scholar]
- 7.Arneodo EM, Penikis KB, Rabinowitz N, et al. Stimulus dependent diversity and stereotypy in the output of an olfactory functional unit. Nat Commun. 2018;9(1):1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson BA, Leon M. Chemotopic odorant coding in a mammalian olfactory system. J Comp Neurol. 2007;503(1):1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2003;100(19):11029–11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geramita M, Urban NN. Differences in Glomerular-Layer-Mediated Feedforward Inhibition onto Mitral and Tufted Cells Lead to Distinct Modes of Intensity Coding. J Neurosci. 2017;37(6):1428–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwata R, Kiyonari H, Imai T. Mechanosensory-Based Phase Coding of Odor Identity in the Olfactory Bulb. Neuron. 2017;96(5):1139–1152 e1137. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Gire DH, Restrepo D. Upsilon spike-field coherence in a population of olfactory bulb neurons differentiates between odors irrespective of associated outcome. J Neurosci. 2015;35(14):5808–5822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cury KM, Uchida N. Robust Odor Coding via Inhalation-Coupled Transient Activity in the Mammalian Olfactory Bulb. Neuron. 2010;68(3):570–585. [DOI] [PubMed] [Google Scholar]
- 14.Gschwend O, Beroud J, Carleton A. Encoding Odorant Identity by Spiking Packets of Rate-Invariant Neurons in Awake Mice. PloS one. 2012;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci. 2011;14(8):1039–U1136. [DOI] [PubMed] [Google Scholar]
- 16.Wilson CD, Serrano GO, Koulakov AA, Rinberg D. A primacy code for odor identity. Nat Commun. 2017;8(1):1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato HK, Chu MW, Isaacson JS, Komiyama T. Dynamic Sensory Representations in the Olfactory Bulb: Modulation by Wakefulness and Experience. Neuron. 2012;76(5):962–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross JM, Fletcher ML. Learning-Dependent and -Independent Enhancement of Mitral/Tufted Cell Glomerular Odor Responses Following Olfactory Fear Conditioning in Awake Mice. J Neurosci. 2018;38(20):4623–4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontanini A, Katz DB. Behavioral states, network states, and sensory response variability. J Neurophysiol. 2008;100(3):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson KS, Gadziola MA, Dauster ES, Wesson DW. Selective Attention Controls Olfactory Decisions and the Neural Encoding of Odors. Curr Biol. 2018;28(14):2195–2205 e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong E, Rinberg D. Behavioral readout of spatio-temporal codes in olfaction. Curr Opin Neurobiol. 2018;52:18–24. [DOI] [PubMed] [Google Scholar]
- 22.Wang DJ, Zhou Y, Cao TT, Li AA. Progresses of Modulatory Effects of Serotonergic Projections From The Raphe Neuclei on The Olfactory Bulb. Prog Biochem Biophys. 2017;44(2):117–128. [Google Scholar]
- 23.Vaaga CE, Westbrook GL. Parallel processing of afferent olfactory sensory information. The Journal of physiology. 2016;594(22):6715–6732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaaga CE, Yorgason JT, Williams JT, Westbrook GL. Presynaptic gain control by endogenous cotransmission of dopamine and GABA in the olfactory bulb. J Neurophysiol. 2017;117(3):1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burton SD. Inhibitory circuits of the mammalian main olfactory bulb. J Neurophysiol. 2017;118(4):2034–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagayama S, Homma R, Imamura F. Neuronal organization of olfactory bulb circuits. Front Neural Circuit. 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grobman M, Dalal T, Lavian H, et al. A Mirror-Symmetric Excitatory Link Coordinates Odor Maps across Olfactory Bulbs and Enables Odor Perceptual Unity. Neuron. 2018;99(4):800–813 e806. [DOI] [PubMed] [Google Scholar]
- 28.Gire DH, Franks KM, Zak JD, et al. Mitral Cells in the Olfactory Bulb Are Mainly Excited through a Multistep Signaling Path. J Neurosci. 2012;32(9):2964–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGann JP. Presynaptic inhibition of olfactory sensory neurons: new mechanisms and potential functions. Chem Senses. 2013;38(6):459–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parsa PV, D’Souza RD, Vijayaraghavan S. Signaling between periglomerular cells reveals a bimodal role for GABA in modulating glomerular microcircuitry in the olfactory bulb. Proc Natl Acad Sci U S A. 2015;112(30):9478–9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Puche AC, Shipley MT. The Interglomerular Circuit Potently Inhibits Olfactory Bulb Output Neurons by Both Direct and Indirect Pathways. J Neurosci. 2016;36(37):9604–9617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato HK, Gillet SN, Peters AJ, Isaacson JS, Komiyama T. Parvalbumin-Expressing Interneurons Linearly Control Olfactory Bulb Output. Neuron. 2013;80(5):1218–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamichi K, Shlomai-Fuchs Y, Shu M, Weissbourd BC, Luo LQ, Mizrahi A. Dissecting Local Circuits: Parvalbumin Interneurons Underlie Broad Feedback Control of Olfactory Bulb Output. Neuron. 2013;80(5):1232–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pressler RT, Strowbridge BW. Direct Recording of Dendrodendritic Excitation in the Olfactory Bulb: Divergent Properties of Local and External Glutamatergic Inputs Govern Synaptic Integration in Granule Cells. J Neurosci. 2017;37(49):11774–11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geramita MA, Burton SD, Urban NN. Distinct lateral inhibitory circuits drive parallel processing of sensory information in the mammalian olfactory bulb. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AT. Two distinct channels of olfactory bulb output. Neuron. 2012;75(2):320–329. [DOI] [PubMed] [Google Scholar]
- 37.Igarashi KM, Ieki N, An M, et al. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci. 2012;32(23):7970–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kikuta S, Fletcher ML, Homma R, Yamasoba T, Nagayama S. Odorant response properties of individual neurons in an olfactory glomerular module. Neuron. 2013;77(6):1122–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Padmanabhan K, Osakada F, Tarabrina A, et al. Diverse Representations of Olfactory Information in Centrifugal Feedback Projections. J Neurosci. 2016;36(28):7535–7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Linster C, Cleland TA. Neuromodulation of olfactory transformations. Curr Opin Neurobiol. 2016;40:170–177. [DOI] [PubMed] [Google Scholar]
- 41.Bolding KA, Franks KM. Complementary codes for odor identity and intensity in olfactory cortex. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roland B, Deneux T, Franks KM, Bathellier B, Fleischmann A. Odor identity coding by distributed ensembles of neurons in the mouse olfactory cortex. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolding KA, Franks KM. Recurrent cortical circuits implement concentration-invariant odor coding. Science. 2018;361(6407). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72(4):506–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaffer ES, Stettler DD, Kato D, Choi GB, Axel R, Abbott LF. Odor Perception on the Two Sides of the Brain: Consistency Despite Randomness. Neuron. 2018;98(4):736–742 e733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meissner-Bernard C, Dembitskaya Y, Venance L, Fleischmann A. Encoding of Odor Fear Memories in the Mouse Olfactory Cortex. Curr Biol. 2019;29(3):367–380 e364. [DOI] [PubMed] [Google Scholar]
- 47.Boyd AM, Kato HK, Komiyama T, Isaacson JS. Broadcasting of Cortical Activity to the Olfactory Bulb. Cell Rep. 2015;10(7):1032–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Otazu GH, Chae H, Davis MB, Albeanu DF. Cortical Feedback Decorrelates Olfactory Bulb Output in Awake Mice. Neuron. 2015;86(6):1461–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical Feedback Control of Olfactory Bulb Circuits. Neuron. 2012;76(6):1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan Z, Tan J, Qin C, Lu Y, Ding C, Luo M. Precise circuitry links bilaterally symmetric olfactory maps. Neuron. 2008;58(4):613–624. [DOI] [PubMed] [Google Scholar]
- 51.Kikuta S, Sato K, Kashiwadani H, Tsunoda K, Yamasoba T, Mori K. From the Cover: Neurons in the anterior olfactory nucleus pars externa detect right or left localization of odor sources. Proc Natl Acad Sci U S A. 2010;107(27):12363–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rothermel M, Wachowiak M. Functional imaging of cortical feedback projections to the olfactory bulb. Front Neural Circuit. 2014;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazo C, Lepousez G, Nissant A, Valley MT, Lledo PM. GABA(B) Receptors Tune Cortical Feedback to the Olfactory Bulb. J Neurosci. 2016;36(32):8289–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76(6):1175–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Case DT, Burton SD, Gedeon JY, Williams SG, Urban NN, Seal RP. Layer- and cell type-selective co-transmission by a basal forebrain cholinergic projection to the olfactory bulb. Nat Commun. 2017;8(1):652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bendahmane M, Ogg MC, Ennis M, Fletcher ML. Increased olfactory bulb acetylcholine bi-directionally modulates glomerular odor sensitivity. Scientific reports. 2016;6:25808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan W, Singh S, Keshav T, et al. Mice Lacking M1 and M3 Muscarinic Acetylcholine Receptors Have Impaired Odor Discrimination and Learning. Frontiers in synaptic neuroscience. 2017;9:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Almeida L, Idiart M, Dean O, Devore S, Smith DM, Linster C. Internal Cholinergic Regulation of Learning and Recall in a Model of Olfactory Processing. Frontiers in cellular neuroscience. 2016;10:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamamoto M, Kiyokage E, Sohn J, Hioki H, Harada T, Toida K. Structural Basis for Cholinergic Regulation of Neural Circuits in the Mouse Olfactory Bulb. J Comp Neurol. 2017;525(3):574–591. [DOI] [PubMed] [Google Scholar]
- 60.Zhan XP, Yin PB, Heinbockel T. The basal forebrain modulates spontaneous activity of principal cells in the main olfactory bulb of anesthetized mice. Front Neural Circuit. 2013;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma M, Luo M. Optogenetic activation of basal forebrain cholinergic neurons modulates neuronal excitability and sensory responses in the main olfactory bulb. J Neurosci. 2012;32(30):10105–10116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rothermel M, Carey RM, Puche A, Shipley MT, Wachowiak M. Cholinergic Inputs from Basal Forebrain Add an Excitatory Bias to Odor Coding in the Olfactory Bulb. J Neurosci. 2014;34(13):4654–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ogg MC, Ross JM, Bendahmane M, Fletcher ML. Olfactory bulb acetylcholine release dishabituates odor responses and reinstates odor investigation. Nat Commun. 2018;9(1):1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marking S, Krosnowski K, Ogura T, Lin W. Dichotomous Distribution of Putative Cholinergic Interneurons in Mouse Accessory Olfactory Bulb. Front Neuroanat. 2017;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li G, Linster C, Cleland TA. Functional differentiation of cholinergic and noradrenergic modulation in a biophysical model of olfactory bulb granule cells. J Neurophysiol. 2015;114(6):3177–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manella LC, Petersen N, Linster C. Stimulation of the Locus Ceruleus Modulates Signal-to-Noise Ratio in the Olfactory Bulb. J Neurosci. 2017;37(48):11605–11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou FW, Dong HW, Ennis M. Activation of beta-noradrenergic receptors enhances rhythmic bursting in mouse olfactory bulb external tufted cells. J Neurophysiol. 2016;116(6):2604–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandipati S, Gire DH, Schoppa NE. Adrenergic receptor-mediated disinhibition of mitral cells triggers long-term enhancement of synchronized oscillations in the olfactory bulb. J Neurophysiol. 2010;104(2):665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Escanilla O, Alperin S, Youssef M, Ennis M, Linster C. Noradrenergic but not cholinergic modulation of olfactory bulb during processing of near threshold concentration stimuli. Behavioral neuroscience. 2012;126(5):720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shea SD, Katz LC, Mooney R. Noradrenergic induction of odor-specific neural habituation and olfactory memories. J Neurosci. 2008;28(42):10711–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckmeier D, Shea SD. Noradrenergic plasticity of olfactory sensory neuron inputs to the main olfactory bulb. J Neurosci. 2014;34(46):15234–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramirez-Gordillo D, Ma M, Restrepo D. Precision of Classification of Odorant Value by the Power of Olfactory Bulb Oscillations Is Altered by Optogenetic Silencing of Local Adrenergic Innervation. Frontiers in cellular neuroscience. 2018;12:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kraus C, Castren E, Kasper S, Lanzenberger R. Serotonin and neuroplasticity - Links between molecular, functional and structural pathophysiology in depression. Neurosci Biobehav Rev. 2017;77:317–326. [DOI] [PubMed] [Google Scholar]
- 74.Luo M, Li Y, Zhong W. Do dorsal raphe 5-HT neurons encode “beneficialness”? Neurobiol Learn Mem. 2016;135:40–49. [DOI] [PubMed] [Google Scholar]
- 75.Huang Z, Thiebaud N, Fadool DA. Differential serotonergic modulation across the main and accessory olfactory bulbs. The Journal of physiology. 2017;595(11):3515–3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carlson KS, Whitney MS, Gadziola MA, Deneris ES, Wesson DW. Preservation of Essential Odor-Guided Behaviors and Odor-Based Reversal Learning after Targeting Adult Brain Serotonin Synthesis. eNeuro. 2016;3(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brill J, Shao Z, Puche AC, Wachowiak M, Shipley MT. Serotonin increases synaptic activity in olfactory bulb glomeruli. J Neurophysiol. 2016;115(3):1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brunert D, Tsuno Y, Rothermel M, Shipley MT, Wachowiak M. Cell-Type-Specific Modulation of Sensory Responses in Olfactory Bulb Circuits by Serotonergic Projections from the Raphe Nuclei. J Neurosci. 2016;36(25):6820–6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kapoor V, Provost AC, Agarwal P, Murthy VN. Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels. Nat Neurosci. 2016;19(2):271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu ZX, Zhou JF, Li Y, et al. Dorsal Raphe Neurons Signal Reward through 5-HT and Glutamate. Neuron. 2014;81(6):1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wachowiak M All in a Sniff: Olfaction as a Model for Active Sensing. Neuron. 2011;71(6):962–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Moore JD, Kleinfeld D, Wang F. How the brainstem controls orofacial behaviors comprised of rhythmic actions. Trends Neurosci. 2014;37(7):370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan AG, Sarangi M, Bhalla US. Rats track odour trails accurately using a multi-layered strategy with near-optimal sampling. Nat Commun. 2012;3:703. [DOI] [PubMed] [Google Scholar]
- 84.Endevelt-Shapira Y, Perl O, Ravia A, et al. Altered responses to social chemosignals in autism spectrum disorder. Nat Neurosci. 2017. [DOI] [PubMed] [Google Scholar]
- 85.Wesson DW. Sniffing Behavior Communicates Social Hierarchy. Curr Biol. 2013;23(7):575–580. [DOI] [PubMed] [Google Scholar]
- 86.Moberly AH, Schreck M, Bhattarai JP, Zweifel LS, Luo W, Ma M. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat Commun. 2018;9(1):1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rebello MR, Kandukuru P, Verhagen JV. Direct behavioral and neurophysiological evidence for retronasal olfaction in mice. PloS one. 2015;10(2):e0117218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Connelly T, Yu YQ, Grosmaitre X, et al. G protein-coupled odorant receptors underlie mechanosensitivity in mammalian olfactory sensory neurons. P Natl Acad Sci USA. 2015;112(2):590–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu R, Liu Y, Wang L, Li B, Xu F. Activity Patterns Elicited by Airflow in the Olfactory Bulb and Their Possible Functions. J Neurosci. 2017;37(44):10700–10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Short SM, Morse TM, McTavish TS, Shepherd GM, Verhagen JV. Respiration Gates Sensory Input Responses in the Mitral Cell Layer of the Olfactory Bulb. PloS one. 2016;11(12):e0168356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jordan R, Fukunaga I, Kollo M, Schaefer AT. Active Sampling State Dynamically Enhances Olfactory Bulb Odor Representation. Neuron. 2018;98(6):1214–1228 e1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cazakoff BN, Lau BY, Crump KL, Demmer HS, Shea SD. Broadly tuned and respiration-independent inhibition in the olfactory bulb of awake mice. Nat Neurosci. 2014;17(4):569–576. [DOI] [PubMed] [Google Scholar]
- 93.Youngstrom IA, Strowbridge BW. Respiratory modulation of spontaneous subthreshold synaptic activity in olfactory bulb granule cells recorded in awake, head-fixed mice. J Neurosci. 2015;35(23):8758–8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2(4):377–388. [DOI] [PubMed] [Google Scholar]
- 95.Li A, Gong L, Xu F. Brain-state-independent neural representation of peripheral stimulation in rat olfactory bulb. Proc Natl Acad Sci U S A. 2011;108(12):5087–5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2(11):1003–1009. [DOI] [PubMed] [Google Scholar]
- 97.Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26(34):8857–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vincis R, Gschwend O, Bhaukaurally K, Beroud J, Carleton A. Dense representation of natural odorants in the mouse olfactory bulb. Nat Neurosci. 2012;15(4):537–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koulakov AA, Rinberg D. Sparse incomplete representations: a potential role of olfactory granule cells. Neuron. 2011;72(1):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bathellier B, Buhl DL, Accolla R, Carleton A. Dynamic ensemble odor coding in the mammalian olfactory bulb: Sensory information at different timescales. Neuron. 2008;57(4):586–598. [DOI] [PubMed] [Google Scholar]
- 101.Chu MW, Li WL, Komiyama T. Balancing the Robustness and Efficiency of Odor Representations during Learning. Neuron. 2016;92(1):174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gschwend O, Abraham NM, Lagier S, Begnaud F, Rodriguez I, Carleton A. Neuronal pattern separation in the olfactory bulb improves odor discrimination learning. Nat Neurosci. 2015;18(10):1474–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kay LM. Olfactory system oscillations across phyla. Curr Opin Neurobiol. 2015;31:141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abraham NM, Vincis R, Lagier S, Rodriguez I, Carleton A. Long term functional plasticity of sensory inputs mediated by olfactory learning. Elife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chu MW, Li WL, Komiyama T. Lack of Pattern Separation in Sensory Inputs to the Olfactory Bulb during Perceptual Learning. eNeuro. 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liu A, Urban NN. Prenatal and Early Postnatal Odorant Exposure Heightens Odor-Evoked Mitral Cell Responses in the Mouse Olfactory Bulb. eNeuro. 2017;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doucette W, Gire DH, Whitesell J, Carmean V, Lucero MT, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron. 2011;69(6):1176–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McLachlan N, Wilson S. The central role of recognition in auditory perception: a neurobiological model. Psychol Rev. 2010;117(1):175–196. [DOI] [PubMed] [Google Scholar]
- 109.Heyward P, Ennis M, Keller A, Shipley MT. Membrane bistability in olfactory bulb mitral cells. J Neurosci. 2001;21(14):5311–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kollo M, Schmaltz A, Abdelhamid M, Fukunaga I, Schaefer AT. ‘Silent’ mitral cells dominate odor responses in the olfactory bulb of awake mice. Nat Neurosci. 2014;17(10):1313–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li A, Guthman EM, Doucette WT, Restrepo D. Behavioral Status Influences the Dependence of Odorant-Induced Change in Firing on Prestimulus Firing Rate. J Neurosci. 2017;37(7):1835–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gardner MP, Fontanini A. Encoding and tracking of outcome-specific expectancy in the gustatory cortex of alert rats. J Neurosci. 2014;34(39):13000–13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schoenfeld TA, Cleland TA. The anatomical logic of smell. Trends Neurosci. 2005;28(11):620–627. [DOI] [PubMed] [Google Scholar]
- 114.Rajan R, Clement JP, Bhalla US. Rats smell in stereo. Science. 2006;311(5761):666–670. [DOI] [PubMed] [Google Scholar]
- 115.Li A, Gire DH, Bozza T, Restrepo D. Precise detection of direct glomerular input duration by the olfactory bulb. J Neurosci. 2014;34(48):16058–16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smear M, Shusterman R, O’Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature. 2011;479(7373):397–U149. [DOI] [PubMed] [Google Scholar]
- 117.Rebello MR, McTavish TS, Willhite DC, Short SM, Shepherd GM, Verhagen JV. Perception of odors linked to precise timing in the olfactory system. PLoS biology. 2014;12(12):e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Smear M, Resulaj A, Zhang J, Bozza T, Rinberg D. Multiple perceptible signals from a single olfactory glomerulus. Nat Neurosci. 2013;16(11):1687–1691. [DOI] [PubMed] [Google Scholar]
- 119.Haddad R, Lanjuin A, Madisen L, Zeng H, Murthy VN, Uchida N. Olfactory cortical neurons read out a relative time code in the olfactory bulb. Nat Neurosci. 2013;16(7):949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Root CM, Denny CA, Hen R, Axel R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature. 2014;515(7526):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xiong A, Wesson DW. Illustrated Review of the Ventral Striatum’s Olfactory Tubercle. Chem Senses. 2016;41(7):549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.In ‘t Zandt EE, Cansler HL, Denson HB, Wesson DW. Centrifugal Innervation of the Olfactory Bulb: A Reappraisal. eNeuro. 2019;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Z, Zhang H, Wen P, et al. Whole-Brain Mapping of the Inputs and Outputs of the Medial Part of the Olfactory Tubercle. Front Neural Circuits. 2017;11:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stern M, Bolding KA, Abbott LF, Franks KM. A transformation from temporal to ensemble coding in a model of piriform cortex. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kay LM, Beshel J. A beta oscillation network in the rat olfactory system during a 2-alternative choice odor discrimination task. J Neurophysiol. 2010;104(2):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Frederick DE, Brown A, Brim E, Mehta N, Vujovic M, Kay LM. Gamma and Beta Oscillations Define a Sequence of Neurocognitive Modes Present in Odor Processing. J Neurosci. 2016;36(29):7750–7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abraham NM, Guerin D, Bhaukaurally K, Carleton A. Similar odor discrimination behavior in head-restrained and freely moving mice. PloS one. 2012;7(12):e51789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Felsen G, Mainen ZF. Neural substrates of sensory-guided locomotor decisions in the rat superior colliculus. Neuron. 2008;60(1):137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]