Abstract
Mangosteen (Garcinia mangostana L) fruit contains many xanthones in its pericarp, such as α-mangostin. Here, we aimed to elucidate the physiological effect of α-mangostin and the mechanism on melanogenesis in mouse B16F10 cells. The melanin production in B16F10 cells was decreased by α-mangostin treatment. α-Mangostin also suppressed the enzymatic activity of tyrosinase, the critical enzyme for melanin synthesis. Furthermore, Western blot analysis revealed that α-mangostin down-regulated the protein quantity of tyrosinase, tyrosinase relative protein (TRP)-2, and microphthalmia-associated transcription factor (MITF). We also used inhibitors of the extracellular signal-regulated kinase (ERK), and glycogen synthase kinase 3 (GSK-3β) to identify the upstream signaling cascade of MITF. Results showed us GSK3β plays a more important role in α-mangostin regulated melanogenesis. Further, the de-pigmentation effect on normal human epidermal melanocytes (NHEMs) of α-mangostin was also confirmed. These results suggested that α-mangostin is a reagent for depigmentation and it has the potential to be applied as a component of cosmetics or pharmaceuticals for the therapy of spots, chloasma, or melanosis.
Keywords: Melanogenesis, α-Mangostin, Tyrosinase, MITF, GSK-3β
Graphical abstract
Highlights
α-Mangostin suppressed the melanin production in B16F10 cells.
α-Mangostin suppressed the activity of tyrosinase.
α-Mangostin suppressed the protein expression of tyrosinase, TRP-2 and MITF.
GSK3β is involved in α-mangostin-regulated melanogenesis.
α-Mangostin suppressed the melanin production in normal human melanocytes.
1. Introduction
The epidermis is composed mainly of keratinocytes and melanocytes. Melanocytes are the factory for melanin synthesis, which locate in the lower layer of keratinocytes [1]. Melanin, synthesized in organelle melanosomes, is distributed to other melanocytes and keratinocytes through the dendrites of melanocytes, causing pigmentation in the skin.
In the melanin synthesis process, tyrosinase is the most important and rate-limiting enzyme [2]. Tyrosinase promotes the conversion of tyrosine to l-DOPA, which is a substrate for melanin synthesis, and l-DOPA to DOPA quinone. Tyrosinase-related protein (TRP)-1 and TRP-2 (also known as dopachrome tautomerase [DCT]) are present in melanosomes and participate in melanin synthesis catalytic reaction [2]. Tyrosinase is involved in the synthesis of both known melanin types produced in mammalian cells, pheomelanin and eumelanin. In contrast, TRP-1 and TRP-2 only catalyze the reaction of eumelanin, which is black or brown color [3].
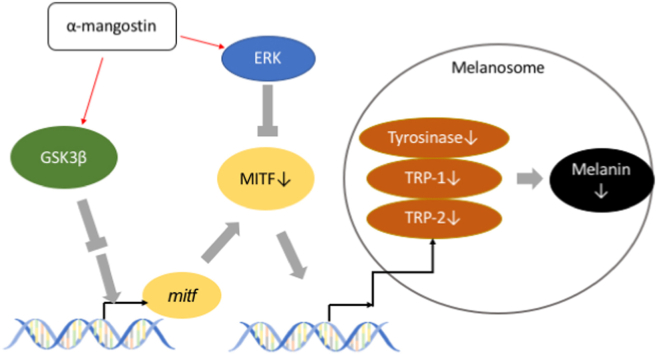
The microphthalmia-associated transcription factor (MITF) controls the expression of tyrosinase, TRP-1, and TRP-2 in melanocytes [4,5]. The ERK and GSK3β locate upstream of MITF, which induce MITF degradation or suppress its transcriptional activity [1]. The ERK pathway triggers a signal by α-MSH via the receptor MC1R. Subsequently, MEK (MAPK/ERK kinase), which is activated by RAS and RAF activation, phosphorylates ERK, and pERK promotes MITF degradation via the phosphorylation of serine 73 of MITF [6,7]. It is also known that phosphorylated GSK-3β regulated by PI3k/Akt signaling cascade inhibits the binding of MITF to the tyrosinase promoter region [8,9], which decreases tyrosinase gene expression and down-regulate melanogenesis.
Skin melanin protects internal cells and the body by absorbing ultraviolet rays (UVs) from sunlight [10]; however, excessive melanin production is accompanied by pigmentation abnormalities such as melasma and blotches [2]. Suppression of melanin production in melanocytes helps prevent such abnormalities and is thought to result in a whitening effect. Therefore, we want to investigate natural products that could reduce melanogenesis.
Mangosteen (Garcinia mangostana L) is a tropical evergreen tree originating in Southeast Asia [11]. The tree has reddish-purple fruits (~5 cm diameter). Because of the taste, it is also called the “queen of fruits” [12]. In Indonesia and Thailand, mangosteen is additionally used to treat certain conditions. For example, the extracts of mangosteen fruit pericarp have been used as antipyretic agents and cystitis remedies, the dried peels have been used to treat dysentery and diarrhea [[11], [12], [13]]. Mangosteen peel also can be used to make ointments for skin diseases such as eczema, skin malfunction, and wounds. Mangosteen fruit contains over 60 kinds of xanthones, like α-mangostin (α-mg), β-mangostin, γ-mangostin, and gartanin [14]. Among them, α-mg is the predominant xanthone in the mangosteen peel, and its content accounts for 78% of all xanthones [15]. α-mg shows antioxidant, anti-cancer, and active oxygen removal effects and can be applied as a therapeutic agent for various diseases [14]. So, α-mg was recognized as a candidate for suppressing melanogenesis.
In this study, we identify the melanin suppress the effect of α-mg, and investigated the GSK-3β signaling cascade that plays a critical role in α-mg regulated melanogenesis.
2. Materials and methods
2.1. Materials
The mouse melanoma cell line B16F10 was purchased from RIKEN Cell Bank (Tsukuba, Japan), and NHEMs were purchased from KURABO (Osaka, Japan). RPMI-1640, l-DOPA, α-MSH, U 0126, and BIO were from Sigma-Aldrich (St. Louis, MO, USA). The DermaLife® Basal Medium of Lifeline (MD, USA) and the DermaLife® M LifeFactors kit were used together as the medium for the NHEMs. α-mangostin was purchased from Wako (Osaka, Japan). Trypan blue (0.4%) was from Logos Biosystem (Gyeonggi-do, South Korea). Antibodies against tyrosinase (Santa Cruz Biotechnology, Dallas, TX, USA), TRP-2 (Santa Cruz Biotechnology), MITF, ERK, p-ERK, GSK3β, p-GSK3β, β-actin (Cell Signaling Technology, Danvers, MA, USA) were also obtained.
α-mangostin was dissolved in dimethyl sulfoxide (DMSO) for B16F10 cells and 70% EtOH for NHEMs. The adjusted α-mg sample was sterilized using a 0.20 μm filter and stored at −80 °C.
2.2. Cell culture
B16F10 cells were cultured at 37 °C and 5% CO2, using RPMI-1640 (Sigma-Aldrich) containing 10% FBS. Normal human epidermal melanocytes (NHEM) were also cultured at 37 °C and 5% CO2, but the culture medium was DermaLifeR Basal Medium (MD, USA). Cells were grown in a 10 cm dish, collected using 0.25% trypsin solution before they reached confluence, and seeded on dishes or plates for each experiment. After 1 or 2 days, the α-mg was administered after the cells proliferated sufficiently. During the cell culture period, the medium was changed every 2 days.
2.3. MTT assay
B16F10 cells were seeded in 24-well plates at 1.0 × 104 cells/well, and after 24 h, the cells were treated with α-mg for 72 h. After 72 h, a medium containing 10% MTT was added, and the cells were cultured at 37 °C for a further 4 h. Then the medium was removed and isopropanol containing 0.04 M HCl was added to dissolve synthesized formazan. Subsequently, the absorbance of the solution at 570 nm relative to 630 nm was measured using a microplate reader. The cell viability was calculated as follows:
NHEMs were seeded at 2.0 × 103 cells/well in a 96-well plate and cultured for 72 h. Then, the medium containing 10% MTT was added, and the cells were cultured at 37 °C for 6 h. Subsequently, 10% SDS was added, and the cells were further cultured overnight. Absorbance at 570 nm against 630 nm was measured using a microplate reader. The effect on cell proliferation was calculated as above.
2.4. Trypan blue assay
Cells were seeded at 5.0 × 103 cells/well in a 24-well plate and cultured with α-mg. After 72 h, the cells were collected by trypsinization, the cell solution and 0.4% trypan blue were mixed at a ratio of 1:1, and the living and dead cells were counted.
2.5. Cell growth
Cells were seeded at 3.0 × 103 cells/well in a 24-well plate, and the next day was set as day 0. α-mg was continuously administered for 6 days, starting from day 0 to day 5, and the number of cells was measured every day.
2.6. Melanin content
Cells were seeded at 3.4 × 104 cells/well in a 6-well plate and cultured with α-mg. Cells collected by trypsin treatment were lysed with 1 M NaOH and warmed for 1 h at 80 °C. The lysate was transferred to a 96-well plate, and the absorbance was measured at 475 nm. α-MSH (10 μM) was administered together with α-mg and was used as a positive control for melanin synthesis. The data were normalized by the total protein level measured by the BCA kit.
2.7. Tyrosinase activity assay
Cells were seeded as 3.4 × 104 cells/well in a 6-well plate and cultured with α-mg. Then, cells were recovered with phosphate buffer (0.1 M, pH 6.8, containing 1% Triton X-100), disrupted using an ultrasonic homogenizer, and centrifuged. l-DOPA (10%) was added to the supernatant, and the absorbance (475 nm) at 0 min and 40 min was measured. The value obtained by subtracting the absorbance at the start from that after 40 min was calculated as tyrosinase activity. α-MSH (10 μM) was administered together with α-mg and was used as a positive control. All values were normalized by the total protein amount.
2.8. Western blotting
B16F10 cells were seeded in a 6-cm dish at 4 × 105 cells/dish and cultured with α-mg. The cells were washed twice with RIPA buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% Na-deoxycholate, 1 mM EDTA, 10 mM NaF, 1 mM Na3VO4, 0.8 μM Aprotinin [Wako], 50 μM Bestatin [Wako], 20 μM Leupeptin [Wako], 10 μM Pepstatin A [Wako] and 1 mM AEBSF [Wako]). Then, cells were disrupted using an ultrasonic homogenizer and centrifuged to obtain a supernatant for western blotting. After the antibody reaction, the membrane was treated with LuminoGLO reagent (Cell Signaling Technology) to cause the band of the target protein to emit light, and the band was detected and photographed using AE-9300H Ez-Capture MG (ATTO Corporation, Tokyo, Japan). Each protein detection was performed at least 3 times replication.
2.9. Statistical analysis
Differences were assessed via a Student's t-test, using SPSS software (IBM, NY, USA). P < 0.05 as *, p < 0.01 as ** and p < 0.005 as ***. Additionally, to distinguish the comparison subjects, p < 0.05 was indicated as # and p < 0.01 as ## according to need.
3. Results
3.1. The effect of α-mg on cell viability
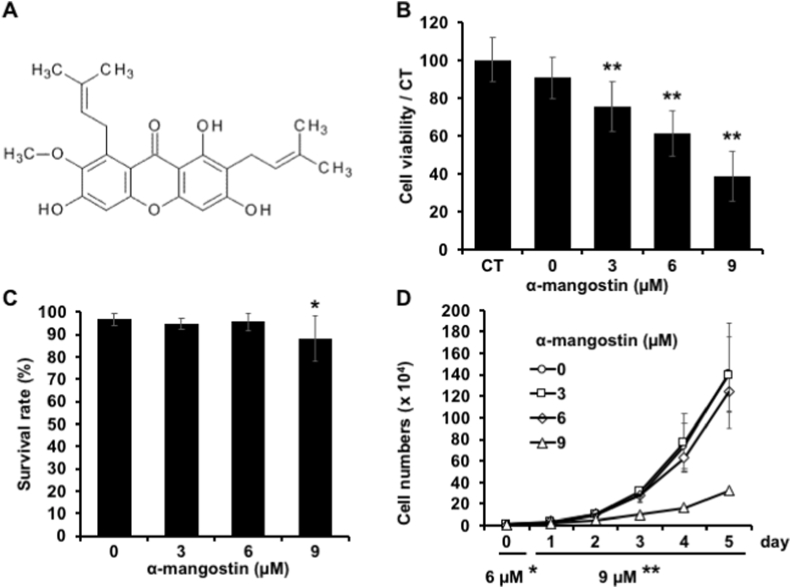
α-mg is a kind of xanthones compound contained in mangosteen, which has a structure shown in Fig. 1A. Firstly, we used the MTT assay to verify the cytotoxicity of α-mg (Fig. 1B). As shown in the results, cell viability dose-dependently decreased by the a-mg (0, 3, 6, 9 μM) treatment. This result seems to indicate that a-mg can significantly inhibit the cell viability of B16F10. While there was no apparent visual increase in cell death happened in cell culture plates. Therefore, we next used trypan blue and cell proliferation assay to confirm the cytotoxicity of α-mg to B16F10.
Fig. 1.
Effect of α-mangostin (α-mg) on cell viability, survival rate, and cell proliferation. α-mg (0, 3, 6, 9 μM) was administered to B16F10 cells for 72 h. Cell viability was analyzed by MTT assay (A). After 72 h treatment, medium and cells were collected, and the cell pellet was finally put together. The cell survival rate was examined by trypan blue staining (B). CT indicates cells cultured in the normal medium; since the α-mg was dissolved in DMSO, we also added DMSO in 0 μM α-mg groups. Besides, α-mg (3, 6, 9 μM) was administered to B16F10 cells and the number of cells was measured daily, in a state where α-mg was kept treating for 6 days from day 0 to day 5 and the effect on cell proliferation was investigated (C). Mean ± SD; **p < 0.01 vs. CT (A); *p < 0.05, **p < 0.01 vs. 0 μM (B, C). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Depend on the trypan blue assay, the cell survival rate in the groups treated with 3 and 6 μM α-mg was similar to the control group (Fig. 1B). However, cells treated with 9 μM α-mg showed a lower survival rate than control, which means the 9 μM a-mg actually a little bit toxic to B16F10 cells, but not to the extent observed with the MTT assay. Further, we examined the effect of α-mg on cell proliferation in 6 days (Fig. 1C). In 3 and 6 μM α-mg groups, the growth curve almost had no change compared to the control groups, whereas 9 μM α-mg suppressed the growth from the third day onwards. Therefore, we speculated that the cell viability decreased by 9 μM α-mg in the MTT assay is mainly due to the decrease in cell number caused by the inhibition of cell proliferation. Nevertheless, because 9 μM α-mg also showed a high cell survival rate, α-mg of 0, 3, 6, and 9 μM was used in further experiments.
3.2. α-mg suppresses melanogenesis in B16F10 melanoma cells
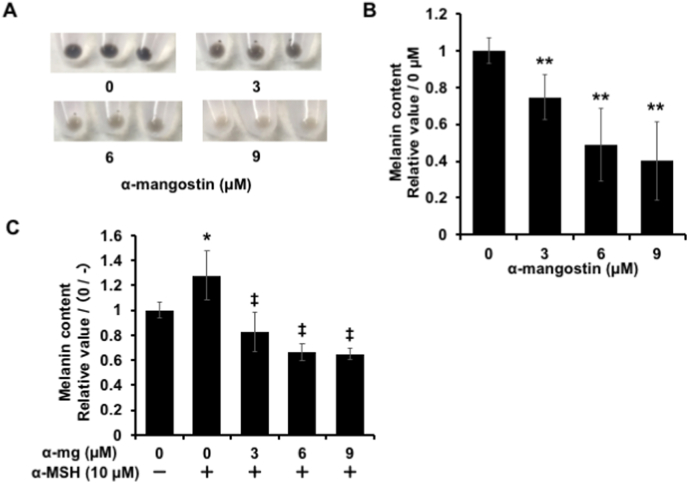
The effect of α-mg on melanogenesis in B16F10 cells was measured. The cells were treated with α-mg (0, 3, 6, 9 μM) for 72 h, and then centrifuged to obtain pellets. Comparing with the control group, α-mg treatment dose-dependently lightened the melanin content in cell pellets (Fig. 2A). The relative melanin quantity was measured by absorbance at 475 nm wavelength. Data also showed that α-mg markedly suppressed the amount of melanin in a dose-dependent manner (Fig. 2B). When α-MSH, the promoter for melanogenesis, was administered to the cells as a positive control, the amount of melanin increased by 1.3 times compared with the non-treatment groups (Fig. 2C). In the presence of α-MSH, α-mg also suppressed melanin production. Therefore, we concluded that α-mg is an effective reagent that can suppress melanin synthesis.
Fig. 2.
Effect of α-mg on melanin synthesis. B16F10 cells were cultured with α-mg (0, 3, 6, 9 μM) administered for 72 h. Cells were collected by trypsinization and centrifuged to obtain pellets (A). The pellets were dissolved in 1 M NaOH, and the relative amount of melanin in each experimental group was determined by measuring the absorbance at 475 nm. α-mg dose-dependently decreased the melanin content in cells (B). α-MSH (10 μM) was used as a positive control for melanin synthesis, which was administered at the same time as α-mg. α-mg also decreased melanin content with the presence of α-MSH (C). Mean ± SD; **p < 0.01 vs. 0 μM (B); *p < 0.05 vs. 0 μM (-α-MSH), p < 0.01 vs. 0 μM (+α-MSH) (C).
3.3. α-mg suppresses intercellular tyrosinase activity through down-regulating tyrosinase and MITF gene expression
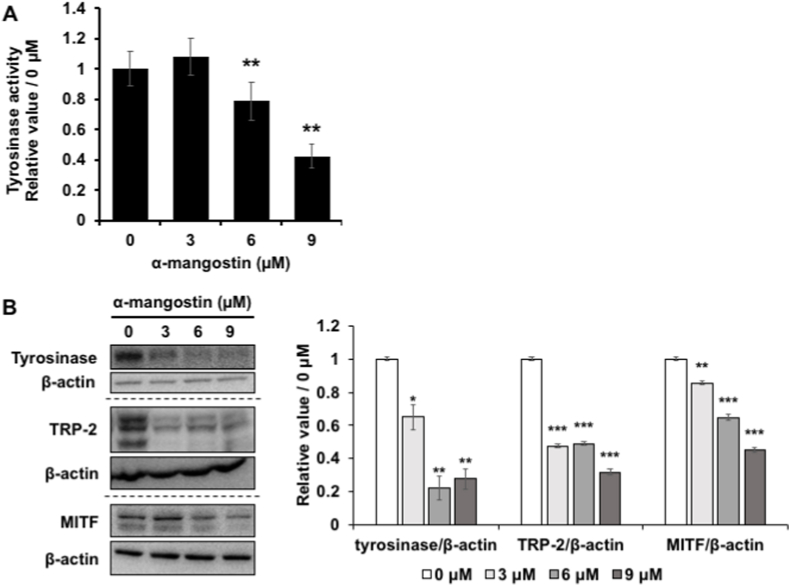
As tyrosinase plays an important role in the melanin synthesis reaction, we next measured the activity of tyrosinase. We mixed the cell lysate with l-DOPA which is the substrate for melanin synthesis catalyzed by tyrosinase. The change of absorbance at 475 nm in 40 min is considered as the tyrosinase activity. The results showed that the amount of melanin was suppressed, especially in the group treated with 6 and 9 μM α-mg compared with the control (Fig. 3A). Therefore, we concluded that α-mg decreased melanin synthesis through suppressing tyrosinase activity.
Fig. 3.
α-mg suppresses intercellular tyrosinase activity through down-regulating tyrosinase and MITF gene expression. B16F10 cells were cultured with α-mg (0, 3, 6, 9 μM) administered for 72 h. The same quantity of cells lysate was mixed with l-DOPA, the melanin synthesized in 40 min, measured by plate-reader at 475 nm wavelength, was recognized as tyrosinase activity. α-mg decreased the tyrosinase activity over 6 μM (A). the protein level was tested by Western blot. All these three genes were decreased by α-mg treatment (B). Mean ± SD; **p < 0.01, ***p < 0.005 vs. 0 μM.
To investigate whether the decrease in tyrosinase activity is due to the decrease in the gene expression of tyrosinase, the protein levels of tyrosinase, TRP-1, TRP-2, and MITF were examined by western blotting (Fig. 3B). The expression levels of tyrosinase and TRP-2 involved in the melanin synthesis reaction were significantly decreased by administration of α-mg, while TRP-1 had no big difference treated by α-mg (data not shown). MITF, the transcription factor for tyrosinase and TRP-1 and -2, was also down-regulated by α-mg treatment. We investigated that α-mg decreased melanin synthesis by down-regulating the key factor MITF and tyrosinase gene expression.
3.4. The effect of α-mg is mediated through the ERK and GSK3β pathways
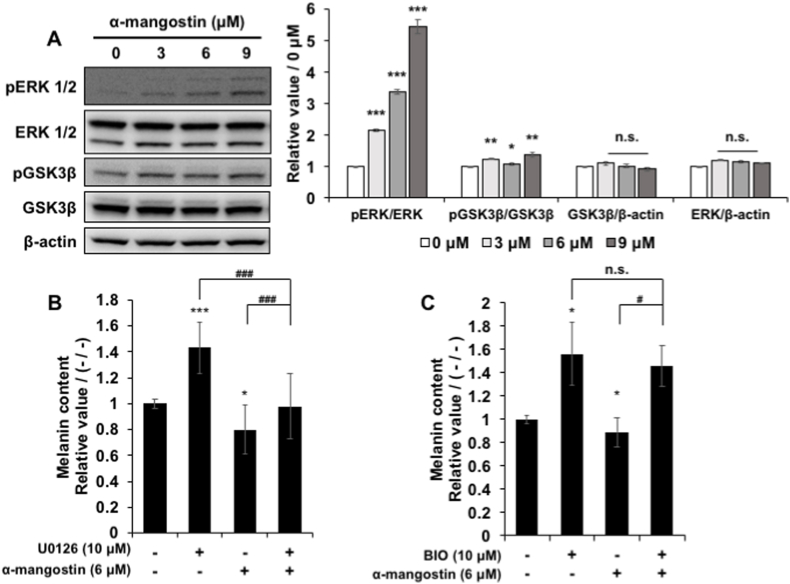
Since we have found that α-mg inhibited melanin synthesis through down-regulating melanogenesis related gene like tyrosinase and MITF, we want to find key factors upstream of MITF in α-mg suppression melanin. It is known that ERK and GSK3β can regulate MITF activity directly or indirectly [[6], [7], [8], [9]], we measured their expression and phosphorylation by Western blot. Results showed that both GSK3β and ERK phosphorylation were up-regulated by α-mg treatment, while without regulating the expression of ERK and GSK3β. (Fig. 4 A).
Fig. 4.
The effect of α-mg is mediated through the ERK and GSK3β pathways.
B16F10 cells were cultured with α-mg (0, 3, 6, 9 μM) administered for 72 h. α-mg treatment increased pERK and pGSK3β without regulating ERK and GSK3β gene expression (A). U0126 was used to treat cells, and it increased melanin in cells. Melanin in U0126 cotreatment with α-mg group lower than U0126 group and higher than α-mg group (B). BIO was used to treat cells, and it also increased melanin in cells. Melanin in BIO cotreatment with α-mg group had no significant difference with BIO (C). Mean ± SD; *p < 0.05, **p < 0.01, ***p < 0.005 vs. non-treatment group (-/-); #p < 0.05, ###p < 0.005 vs. co-treatment group (+/+); n. s. non-significance.
To confirm the importance of these two factors in α-mg regulated melanogenesis, we used the inhibitor for these two factors. BIO and U0126 were used to inhibit the GSK3β and ERK phosphorylation, respectively. Consistent with our expectations, BIO and U0126 promoted intracellular melanin content (Fig. 4 B, C). In the U0126 group, the melanin content in U0126 and α-mg cotreatment group was significantly lower than the U0126 group, but it was significantly higher than that in the α-mg group (Fig. 4 B). This result means that although the ERK pathway is included in the melanin down-regulation process of α-mg, it is not the most important. In the BIO group, we found that in cells present in BIO, α-mg could not down-regulate the melanin content (Fig. 4C), which means GSK3β is more important in α-mg regulated melanogenesis. Therefore, we established that α-mg down-regulates melanogenesis through GKS3β and ERK signaling pathways.
3.5. α-mg inhibits melanogenesis in NHEMs
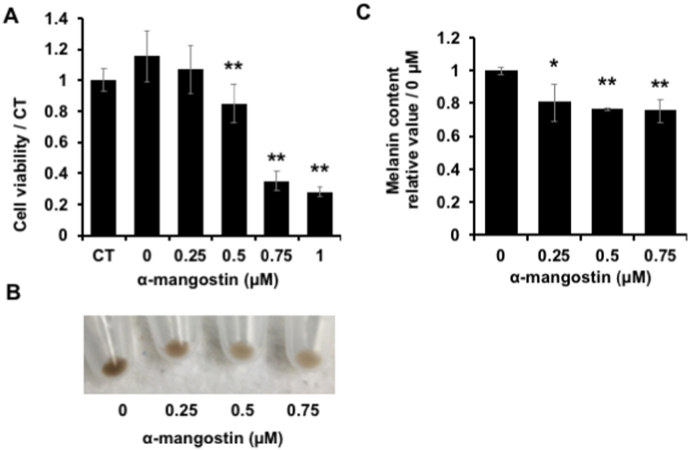
Because of the depigmenting effect of α-mg on murine melanoma cells, we also examined the effect of α-mg on normal human epidermal melanocytes (NHEMs) melanogenesis. Initially, the MTT test was conducted to investigate the effect of α-mg on the proliferation of NHEMs (Fig. 5A). We also tested more dose from 0.1 μM to 9 μM and we found the α-mg had strong cytotoxicity higher than 1 μM. Although it was shown that 0.75 μM groups had very low cell viability, we also confirmed the cell survival rate by trypan blue assay. A similar result to low concentration was got, so we considered that there was little toxic on cell at 0.75 μM. In the following experiments, NHEMs were exposed to α-mg for 72 h, and the cells were collected and centrifuged to obtain pellets (Fig. 5B). The pellet color decreased depending on the α-mg concentration. While the amount of melanin depending on absorbance was shown to decrease at a similar level (Fig. 5C). Therefore, we investigated that α-MG had a similar whitening effect on normal human melanocytes.
Fig. 5.
Effect of α-mg on cell proliferation and melanin synthesis in NHEMs. NHEMs were cultured with α-mg (0, 0.25, 0.5, 0.75, 1 μM) for 72 h, and cell viability was examined by MTT assay (A). CT means experiment group cultured in medium alone; 0 μM α-mg in medium containing 0.07% EtOH as a solvent. The cells were collected by trypsin and centrifuged to obtain pellets (B). The pellet was dissolved in 1 M NaOH and the amount of melanin in each experimental group was determined by measuring the absorbance at 475 nm (C). Mean ± SD; **p < 0.01 vs. CT (A); *p < 0.05, **p < 0.01 vs. 0 μM (C).
4. Discussion
In this study, it was revealed that α-mg, a xanthone, has an inhibitory effect on melanin synthesis in B16F10 cells (Fig. 2). Administration of α-mg (3, 6, 9 μM) to the cells decreased the amount of melanin produced in a dose-dependent manner. α-MSH, a ligand of MC1R that increases the intracellular cAMP level to promote melanin synthesis, is widely used as a stimulator of melanin production in many research studies [1,16]. In the skin, synthesis of α-MSH is increased by ultraviolet (UV) irradiation, and α-MSH promotes melanin synthesis to protect the inside of the skin from UV damage [17]. So far, many studies on the regulation of melanin production have used α-MSH as a positive inducer [18]. In this study as well, α-MSH induced melanogenesis (Fig. 2C). In contrast, the administration of α-mg significantly reduced melanin production when compared with α-MSH treatment groups (Fig. 2C). This result suggested that α-mg is an effective reagent markedly suppressing melanin synthesis even with the α-MSH.
Tyrosinase is known as a rate-limiting enzyme essential for melanin synthesis [2]. Therefore, a decrease in tyrosinase activity is associated with inhibition of melanin production [19]. Previous studies have reported many cases in which components isolated from natural products inhibited tyrosinase activity and consequently inhibited melanin production [20]. Previously, α-mangostin was also proved that can down-regulate the mushroom tyrosinase activity by in vitro. assay [21]. Many similar studies about natural extracts or compounds have shown that they directly inhibit the catalytic activity of tyrosinase by regulating the molecular structure, but cell lysate included not only tyrosinase but also TRP-1 and TRP-2. In this study, the suppression in tyrosinase activity by α-mg was investigated (Fig. 3A). Further, we decided to clarify the mechanism of action of suppressing melanin production by α-mg, was through regulating the expression level of the protein involved in melanin synthesis (Fig. 3B). TRP-2, one of the enzymes involved in melanin synthesis, catalyzes the reaction from DOPA chrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA) [22]. Therefore, like tyrosinase, a decrease in the protein expression level of TRP-2 is considered a cause of melanin production suppression [23]. MITF is the transcription factor of tyrosinase, TRP-1, and TRP-2; and it is known as the most important transcription factor in melanin production [24,25]. Protein expression analysis revealed that the expression levels of tyrosinase and TRP-2 were significantly decreased (Fig. 3B). Furthermore, the expression of MITF was also decreased. These data suggest that α-mg suppresses tyrosinase activity, based on the downregulation of tyrosinase and MITF expression levels, resulting in the suppression of melanin synthesis.
Several signaling pathways are involved in melanin synthesis [1]. In this study, we focused on the ERK and GSK3β pathways, which promote the degradation of MITF or inhibit the transcriptional activity; and examined their relationship with the α-mg inhibitory effect on melanin production. In this experiment, we identified that the most important factor in the α-mg down-regulating effect was GSK3β, while ERK (MAPK) was partially involved. PI3k/AKT signaling pathway locates the upstream and regulates the GSK3β activity [8,26]. We also measured the gene expression and phosphorylation of AKT by Western blot. However, we didn't find any significant changes in AKT. PI3k inhibitor LY 294002 was also used to figure out the role of AKT. We found the pGSK3β was down-regulated by LY294002 treatment and the intracellular melanin showed a similar tendency as BIO treatment in Fig. 4C (data not shown). Therefore, we thought that PI3k/AKT signaling is the upstream factor for GSK3β, but it is not necessarily included in α-mg induced depigmentation process.
In this experiment, we found that 9 μM of α-mg showed a strong cell proliferation inhibition effect and weak cytotoxicity. In some previous studies, the effect of α-mg on time- or dose-dependently inhibiting cell proliferation on different types of cells has also been reported [27,28], which is also consistent with our results. Also, we found that many studies suggest that a-mg can induce cancer cell apoptosis [28,29]; while it protects normal cells, such as nerve cells from chemical induced-apoptosis [30]. In our experiment, we used B16F10 melanoma cells, so we speculated that the weak cytotoxicity caused by 9 μM α-mg was caused by the apoptosis-inducing effect of α-mg on cancer cells.
In some previous studies, α-mg has been proven to inhibit melanin synthesis in the keratinocyte/melanocyte co-culture system [31]. And the use of α-mg for the treatment of pigment disorders has been patented. In this experiment, we not only verified the effect of a-mg on the depigmentation of mouse B16F10 cells, which is consistent with the results of the previous studies; we also preliminarily explained the regulation mechanism of α-mg on melanogenesis in B16F10 mouse melanoma cells. Further, we verified the depigmentation effect of α-mg in normal human epidermal melanocytes. However, some recent studies have also shown that there are interspecies differences in the regulation mechanism of melanogenesis [32,33]. Therefore, in order to better analyze and explain the mechanism of α-mg regulating melanin synthesis in cells of different species, and the difference in regulation between normal cells and cancer cells, it is necessary to use some other types or species of normal melanocytes and melanoma cells for further experiments and analysis.
Some previous studies have shown that α-mg has anticarcinogenic [34] and antioxidant effects [35]. α-mg showed a different regulatory effect on normal cells and cancer cells as mentioned above [[27], [28], [29], [30]]. The current research in our laboratory has also showed that a-mg can promote the differentiation of mouse myoblast cells [36]. Due to the multiple physiological activities of α-mg, it is necessary and will be a very interesting topic to investigate the comprehensive physiological effects of α-mg in vivo.
With the view of facilitating human application in the future, we investigated the effect of α-mg treatment on melanin production in NHEMs and found that α-mg suppressed melanin production in this cell line. This result suggested that although a higher dose of α-mg has some cytotoxicity, it still had the potential as an effective whitening reagent for human application.
5. Conclusion
In this study, it was revealed that α-mg markedly suppresses melanin production in B16F10 cells. Besides, it was shown that this effect was due to suppressing the expression of tyrosinase and MITF. Moreover, given that α-mg also inhibited melanin production in NHEMs, it could be applied as a component of cosmetics and pharmaceuticals for the treatment of spots, chloasma, and melanosis. Furthermore, given that the mangosteen fruit peel, which contains α-mg, has traditionally been used for healing of skin diseases and wounds1, α-mg may show similar effects in vivo, including the promotion of skin metabolism. For the eventual human application of α-mg, initial validation in vivo using mouse and zebrafish models should be the subject of future study. Since α-mg inhibited the melanin production of NHEMs, it may be applied as a component of cosmetic and pharmaceutical products for the treatment of spots, flushing, and melanosis.
Author statement
This manuscript has not been published or presented elsewhere in part or in entirety and is not under consideration by another journal. We have read and understood your journal's policies, and we believe that neither the manuscript nor the study violates any of these. There are no conflicts of interest to declare.
Declaration of competing interest
There are no conflicts of interest to declare.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research and Education from the University of Tsukuba, Japan.
References
- 1.D'Mello S.A., Finlay G.J., Baguley B.C., Askarian-Amiri M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016;17(7):E1144. doi: 10.3390/ijms17071144. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando H., Kondoh H., Ichihashi M., Hearing V.J. Hearing, Approaches to identify inhibitors of melanin biosynthesis via the quality control of tyrosinase. J. Invest. Dermatol. 2007;127(4):751–761. doi: 10.1038/sj.jid.5700683. [DOI] [PubMed] [Google Scholar]
- 3.Ito S., Wakamatsu K., Ozeki H. Chemical analysis of melanins and its application to the study of the regulation of melanogenesis. Pigm. Cell Res. 2000;13(Suppl 8):103–109. doi: 10.1034/j.1600-0749.13.s8.19.x. [DOI] [PubMed] [Google Scholar]
- 4.Yasumoto K., Yokoyama K., Shibata K., Tomita Y., Shibahara S. Microphthalmia-associated transcription factor as a regulator for melanocyte-specific transcription of the human tyrosinase gene. Mol. Cell Biol. 1994;14(12):8058–8070. doi: 10.1128/mcb.14.12.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy C., Khaled M., Fisher D.E. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006;12(9):406–414. doi: 10.1016/j.molmed.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Imokawa G., Ishida K. Inhibitors of intracellular signaling pathways that lead to stimulated epidermal pigmentation: perspective of anti-pigmenting agents. Int. J. Mol. Sci. 2014;15(5):8293–8315. doi: 10.3390/ijms15058293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu W., Gong L., Haddad M.M., Bischof O., Campisi J., Yeh E.T., Medrano E.E. Regulation of microphthalmia-associated transcription factor MITF protein levels by association with the ubiquitin-conjugating enzyme hUBC9. Exp. Cell Res. 2000;255(2):135–143. doi: 10.1006/excr.2000.4803. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K., Takemoto C., Kobayashi I. Ser 298 of MITF, a mutation site in Waardenburg syndrome type 2, is a phosphorylation site with functional significance. Hum. Mol. Genet. 2000;9(1):125–132. doi: 10.1093/hmg/9.1.125. [DOI] [PubMed] [Google Scholar]
- 9.Li H., Min Y.S., Park K.C., Kim D.S. Inhibition of melanogenesis by Xanthium strumarium L. Biosci. Biotechnol. Biochem. 2012;76(4):767–771. doi: 10.1271/bbb.110894. [DOI] [PubMed] [Google Scholar]
- 10.Videira I.F., Moura D.F., Magina S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013;88(1):76–83. doi: 10.1590/S0365-05962013000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamid M.A., Sarmidi M.R., Park C.S. Mangosteen leaf extract increases melanogenesis in B16F1 melanoma cells by stimulating tyrosinase activity in vitro and by up-regulating tyrosinase gene expression. Int. J. Mol. Med. 2012;29(2):209–217. doi: 10.3892/ijmm.2011.840. [DOI] [PubMed] [Google Scholar]
- 12.Shibata M.A., Iinuma M., Morimoto J. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent xenograft model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011;9:69. doi: 10.1186/1741-7015-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Si H., Liu D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014;25(6):581–591. doi: 10.1016/j.jnutbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obolskiy D., Pischel I., Siriwatanametanon N., Heinrich M. Garcinia mangostana L: a phytochemical and pharmacological review. Phytother Res. 2009;23(8):1047–1065. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- 15.Beninati S., Oliverio S., Cordella M. Inhibition of cell proliferation, migration and invasion of B16-F10 melanoma cells by α-mangostin. Biochem. Biophys. Res. Commun. 2014;450(4):1512–1527. doi: 10.1016/j.bbrc.2014.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Yang Y.M., Son Y.O., Lee S.A. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother Res. 2011;25(8):1166–1173. doi: 10.1002/ptr.3417. [DOI] [PubMed] [Google Scholar]
- 17.Le Pape E., Passeron T., Giubellino A. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proc. Natl. Acad. Sci. U. S. A. 2009;106(6):1802–1807. doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An S.M., Kim H.J., Kim J.E., Boo Y.C. Flavonoids, taxifolin and luteolin attenuate cellular melanogenesis despite increasing tyrosinase protein levels. Phytother Res. 2008;22(9):1200–1207. doi: 10.1002/ptr.2435. [DOI] [PubMed] [Google Scholar]
- 19.Boissy R.E., Visscher M., DeLong M.A. DeoxyArbutin: a novel reversible tyrosinase inhibitor with effective in vivo skin lightning potency. Exp. Dermatol. 2005;14(8):601–608. doi: 10.1111/j.0906-6705.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 20.Chang T.S. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. Materials. 2012;5(9):1661–1685. [Google Scholar]
- 21.Hassan W.N.A.W., Zulkifli R.M., Basar N., Ahmad F., Yunus M.A.C. Antioxidant and tyrosinase inhibition activities of α- mangostin and Garcinia mangostana Linn. pericarp extracts. J. Appl. Pharmaceut. Sci. 2015;5(9):37–40. [Google Scholar]
- 22.Yang S.H., Tsatsakis A.M., Tzanakakis G. Soyasaponin Ag inhibits α-MSH-induced melanogenesis in B16F10 melanoma cells via the downregulation of TRP-2. Int. J. Mol. Med. 2017;40(3):631–636. doi: 10.3892/ijmm.2017.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim S.S., Kim M.J., Choi Y.H. Down-regulation of tyrosinase, TRP-1, TRP-2 and MITF expressions by citrus press-cakes in murine B16F10 melanoma. Asian Pac J Trop Biomed. 2013;3(8):617–622. doi: 10.1016/S2221-1691(13)60125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao H.C., Najjaa H., Villareal M.O. Arthrophytum scoparium inhibits melanogenesis through the down-regulation of tyrosinase and melanogenic gene expressions in B16 melanoma cells. Exp. Dermatol. 2013;22(2):131–136. doi: 10.1111/exd.12089. [DOI] [PubMed] [Google Scholar]
- 25.Lee H.J., Lee W.J., Chang S.E., Lee G.Y. Hesperidin, a popular antioxidant inhibits melanogenesis via Erk 1/2 mediated MITF degradation. Int. J. Mol. Sci. 2015;16(8):18384–18395. doi: 10.3390/ijms160818384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu C., Aisa H.A. Upregulation of melanogenesis and tyrosinase activity: potential agents for vitiligo. Molecules. 2017;22(8):E1303. doi: 10.3390/molecules22081303. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J.J., Long Z.J., Xu D.F. Inhibition of autophagy augments the anticancer activity of α-mangostin in chronic myeloid leukemia cells. Leuk. Lymphoma. 2014 Mar;55(3):628–638. doi: 10.3109/10428194.2013.802312. Epub 2013 Jul 18. [DOI] [PubMed] [Google Scholar]
- 28.Kritsanawong S., Innajak S., Imoto M., Watanapokasin R. Antiproliferative and apoptosis induction of α-mangostin in T47D breast cancer cells. Int. J. Oncol. 2016 May;48(5):2155–2165. doi: 10.3892/ijo.2016.3399. Epub 2016 Feb 18. [DOI] [PubMed] [Google Scholar]
- 29.Lee H.N., Jang H.Y., Kim H.J. Antitumor and apoptosis-inducing effects of α-mangostin extracted from the pericarp of the mangosteen fruit (Garcinia mangostana L.) in YD-15 tongue mucoepidermoid carcinoma cells. Int. J. Mol. Med. 2016 Apr;37(4):939–948. doi: 10.3892/ijmm.2016.2517. Epub 2016 Mar 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janhom P., Dharmasaroja P. Neuroprotective effects of alpha-mangostin on MPP(+)-Induced apoptotic cell death in neuroblastoma SH-SY5Y cells. J. Toxicol. 2015;2015:919058. doi: 10.1155/2015/919058. Epub 2015 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.H., Chen H., Kolev V. High-throughput, high-content screening for novel pigmentation regulators using a keratinocyte/melanocyte co-culture system. Exp. Dermatol. 01 Feb 2014;23(2):125–129. doi: 10.1111/exd.12322. PMID: 24438532, PMCID: PMC3977999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hou L., Pavan W.J. Transcriptional and signaling regulation in neural crest stem cell-derived melanocyte development: do all roads lead to Mitf? Cell Res. 2008 Dec;18(12):1163–1176. doi: 10.1038/cr.2008.303. [DOI] [PubMed] [Google Scholar]
- 33.Hou L., Arnheiter H., Pavan W.J. Interspecies difference in the regulation of melanocyte development by SOX10 and MITF. Proc. Natl. Acad. Sci. U. S. A. 2006 Jun 13;103(24):9081–9085. doi: 10.1073/pnas.0603114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang K.J., Gu Q.L., Yang K. Anticarcinogenic effects of α-mangostin: a review. Planta Med. 2017 Feb;83(3–04):188–202. doi: 10.1055/s-0042-119651. Epub 2016 Nov 4. [DOI] [PubMed] [Google Scholar]
- 35.Chen G., Li Y., Wang W. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: a review. Expert Opin. Ther. Pat. 2018 May;28(5):415–427. doi: 10.1080/13543776.2018.1455829. Epub 2018 Apr 3. [DOI] [PubMed] [Google Scholar]
- 36.Lin M., Zhou S., Sakamoto K. Alpha Mangostin promotes myogenic differentiation of C2C12 mouse myoblast cells. Biochem. Biophys. Res. Commun. 2020 Jul 12;528(1):193–198. doi: 10.1016/j.bbrc.2020.04.128. Epub 2020 May 28. [DOI] [PubMed] [Google Scholar]