Abstract
Background:
Nusinersen has recently been approved and more widely used as first-line treatment of spinal muscular atrophy (SMA). This study aimed to evaluate the real-world experience of nusinersen use for patients with a broad spectrum of SMA.
Methods:
We reviewed consecutive patients with SMA treated with nusinersen from April 2018 to April 2020. Data collected included clinical and diagnostic characteristics, molecular genetics, functional motor outcomes, and adverse events.
Results:
Seven patients including four with SMA type 1 and three with SMA type 2 were treated with nusinersen. The median disease duration at the time of the first dose and the median follow-up duration were 37 months (range: 0.5–254 months) and 6.1 months (range: 2.1–22.1 months), respectively. Of the 41 lumbar punctures (LPs), seven fluoroscopy-guided LPs were successfully performed for two patients without sedation. All patients showed improvement in motor function even though the current tools for motor assessment seemed unable to detect subtle subjective improvement. All patients maintained a stable respiratory status. No patient has experienced a severe adverse event or discontinued treatment so far.
Conclusion:
Although the number of patients in this study was small, our results suggest that nusinersen is effective even in patients with a later stage of the disease. Additional long-term prospective studies with more number of patients having a broad spectrum of diseases are needed to identify meaningful improvement in the motor function and quality of life after nusinersen treatment.
Keywords: Adolescents, child, nusinersen, spinal muscular atrophy
INTRODUCTION
Spinal muscular atrophy (SMA) is a common fatal autosomal recessive disorder that occurs 1 in 6000–10,000 people.[1] It is characterized by motor neuron loss in the spinal cord and lower brainstem and is the leading genetic cause of infant death.[2] SMA is caused by the deletion or mutation of survival motor neuron 1 (SMN1), resulting in the degeneration of alpha motor neurons and loss of anterior horn cells in the brainstem and spinal cord because of low levels of full-length survival motor neuron (SMN) protein.[3] This progressive degeneration causes weakness of the bulbar, trunk, and limb muscles, resulting in respiratory failure and death.[4,5] Because of a deficiency in the functional SMN1 gene, the patient relies on its paralogous gene, called survival motor neuron 2 (SMN2), which encodes approximately 10% of the full-length SMN protein. Thus, the number of SMN2 gene copies strongly correlates with the severity of SMA.[6] SMA is classified into four major phenotypes based on the onset and clinical symptoms of the disease.[7] SMA type I is the most severe life-threatening form of SMA. Children with SMA type I produce very little SMN protein. Hence, they are unable to sit without support and generally do not survive past the age of 2 years because of respiratory failure. Individuals with later-onset SMA types 2 and 3 produce greater amounts of SMN protein and have less severe but still clinically significant forms of SMA,[1,6] whereas SMA type 4 is the adult-onset form.[6]
In December 2016, nusinersen (Spinraza) gained approval from the US Food and Drug Administration as the first pharmacologic treatment for SMA.[8,9,10,11] Since April 2019, it has been commercially available in Korea for patients with SMA who develop symptoms before 3 years of age. Nusinersen is an antisense oligonucleotide that promotes SMN2 exon 7 inclusion, resulting in increased production of full-length SMN protein.[8,9,10,11,12] It has been reported to improve motor function in infant and late-onset SMA, who are in the early and middle symptomatic phases.[13,14] In addition, results from a phase-2 clinical trial demonstrated the potential benefit of treatment initiation in presymptomatic infants who were genetically diagnosed with SMA.[15] Recent real-world data demonstrated the efficacy of nusinersen in a heterogenic patient population of SMA type 1, 2, and 3.[16] An Italian group showed 12 months' changes after treatment with nusinersen in a cohort of type 1 patients having a much wider range of age and severity than those in the clinical trial.[17] However, there is a lack of research on the real-world effectiveness of nusinersen in SMA patients who have a more severe phenotype, such as scoliosis, contracture, or respiratory difficulty. Therefore, we report our clinical experience using nusinersen for the treatment of patients with SMA types 1 and 2 with a broad spectrum of disease.
METHODS
We retrospectively analyzed the data of SMA patients treated with nusinersen at Kyungpook National University Hospital in Korea. At the hospital, a dedicated team of health professionals, including a pediatric neurologist, adult neurologist, rehabilitation doctor, orthopedic surgeon, neurosurgeon, radiologist, and nutritionists, routinely worked together in the care of SMA patients. Throughout their treatment, all patients were managed according to the standard-of-care consensus guideline on SMA.[18,19] Intrathecal injections of nusinersen were administered using a loading dose (dosing on days 1, 15, 29, and 64) followed by a maintenance dose once every 4 months thereafter. Topical local anesthesia was used in all patients before the injections. Motor function and motor milestone assessment were performed during the pretreatment period, on day 64, and at every maintenance dosing by a pediatric neurologist (L.Y.J.) and a rehabilitation physician (K.A.R.). Evaluators were not blinded to the treatment with nusinersen. SMA type 1 patients were assessed using the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND), which includes 16 items with a total score between 0 and 64,[20] and the Hammersmith Infant Neuromuscular Examination (HINE) section 2 motor milestones, which is composed of the following eight milestones: head control, sitting, voluntary grasp, ability to kick, rolling, crawling, standing, and walking.[21] Patients with later-onset SMA were assessed using the Hammersmith Functional Motor Scale Expanded (HFMSE),[22] which includes 33 items with a total score between 0 and 66; the Revised Upper Limb Module (RULM),[23] which is used to investigate upper-limb abilities in SMA patients and includes 19 items with a maximum score of 37; and the World Health Organization (WHO) motor milestone criteria. The following data were collected: age at diagnosis and at initial nusinersen injection, SMA type, SMN2 copy number, presence of scoliosis, ventilator support (modality, number, and hours/day), pulmonary function test findings, method of delivery of nusinersen, adverse events, and pre and posttreatment functional assessment including CHOP INTEND, HFMSE, RULM, and HINE section 2 and WHO motor milestone criteria. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Institutional Review Board of Kyungpook National University Hospital (No. 2020-03-16). Informed consent was obtained from the patients and their parents in the study.
RESULTS
Patient characteristics
Seven patients, including four with SMA type 1 and three with SMA type 2 (median age, 44.7 months; range: 1.1 months to 22 years 1 month), received nusinersen injection during a median follow-up duration of 6.1 months (range: 2.1–22.1 months). The median duration of the disease (from symptom onset to initial injection) was 37.0 months (range: 0.5–254 months). Five patients were previously diagnosed and two were newly diagnosed with SMA. The baseline characteristics of the patients are shown in Table 1. All patients had homozygous SMN1 deletion. Four SMA type 1 patients had two copies of SMN2, and three SMA type 2 patients had three copies of SMN2. Scoliosis was observed in two patients (patients 4 and 7; Figure 1), hip dislocation in two (patients 6 and 7), and elbow, knee, and ankle joint contractures in three patients (patients 4, 6, and 7). None of our patients underwent scoliosis or orthopedic surgery prior to treatment. Two patients with SMA type 1 (patients 3 and 4) had been receiving 24-h invasive mechanical ventilation (IMV) via tracheostomy and feeding via gastrostomy before the initial injection. Nusinersen was used in two patients with SMA type 1 (patients 3 and 4) by expanded access program before it was commercially available in Korea. Two patients newly diagnosed with SMA received nusinersen injection within 1 month after symptom onset (patients 1 and 2).
Table 1.
Demographic and clinical characteristics of SMA patients
| Pt No | Sex/age (Year) | SMA type | SMN2 copy | Age at symptom onset | Age at diagnosis (month) | Disease duration (month) | Scoliosis | Hip dislocation | Joint contracture | Respiratory status | Nutrition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/0.2 | 1 | 2 | 15 d | 1 | 0.5 | - | - | - | NIV <8 h/d | OF |
| 2 | F/0.3 | 1 | 2 | 7 d | 1.4 | 1.0 | - | - | - | NIV <8 h/d | OF |
| 3 | F/3.1 | 1 | 2 | 5 m | 6 | 14 | - | - | - | 24-h IMV | EF |
| 4 | M/5.5 | 1 | 2 | 1 m | 3 | 43 | + | - | + | 24-h IMV | EF |
| 5 | F/4.6 | 2 | 3 | 9 m | 24 | 37 | - | - | - | SB | OF |
| 6 | M/9.3 | 2 | 3 | 8 m | 8 | 98 | - | + | + | SB | OF |
| 7 | M/22.6 | 2 | 3 | 15 m | 23 | 254 | + | + | + | SB | OF |
EF–enteral feeding; NIV–noninvasive ventilation; IMV–invasive mechanical ventilation; OF–oral feeding; SB–spontaneous breathing; SMA–spinal muscular atrophy; SMN2–survival motor neuron 2
Figure 1.
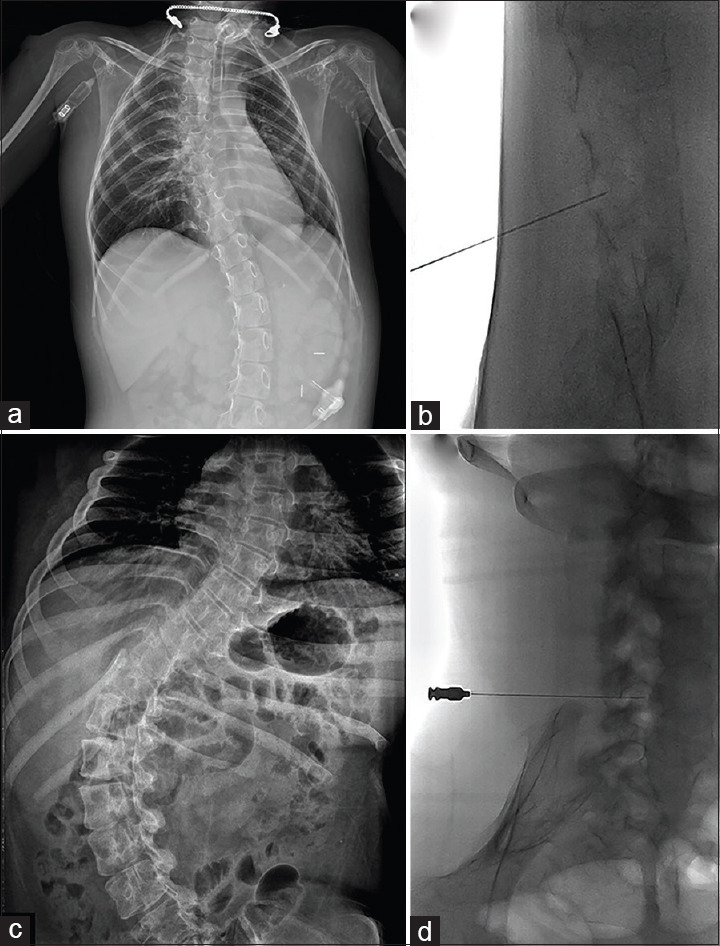
The fluoroscopy-guided approach in patients with scoliosis. (a, c) Plain radiograph of a 5-year-old patient with spinal muscular atrophy (SMA) type 1 (patient 4) (a) and a 22-year-old patient with SMA type 2 (patient 7) (c). (b, d) The fluoroscopy-guided approach in patient 4 (b) and patient 7 (d) with intrathecal needle placement at the level of L4/5
Administration of nusinersen
A total of 41 lumbar punctures (LPs) were performed in seven patients. All seven patients completed the loading regimen of the treatment. Two patients received injection via the fluoroscopy-guided approach (patient 4 and 7). One patient received the conventional LP approach at loading dose despite scoliosis; however, we changed the conventional approach to a fluoroscopy-guided approach after recurrent failures [Figure 1a and b]. The other patients with severe scoliosis received the fluoroscopy-guided approach after an initial injection [Figure 1c and d]. A total seven fluoroscopy-guided LPs were technically successful without sedation for these patients. Two patients required mild sedation because they feared the procedures. There were no reports of any adverse events after LPs, including headache, infection, site pain, hemorrhage, and leg pains. We documented stable vital signs before and after intrathecal administration of the drug in all patients. Treatment was not discontinued in any of the patients.
Functional outcome
The functional outcome is summarized in Figure 2 and Table 2. Among the four patients with SMA type 1, three patients showed an increase of at least four points from baseline in the CHOP INTEND. They showed improvement in ankle dorsiflexion strength, knee and elbow movement, and handgrip. Although the CHOP INTEND score did not change in one patient (patient 4) who had 0 points at the baseline assessment, there was a subtle change in the hip external/internal rotation movement and loud vocalization, which was not included in the scoring system. Two SMA type 1 patients (patients 1 and 2), who started treatment within 1 month from symptom onset, had much better improvement as compared with the other patients [Figure 2]. All patients with SMA type 2 showed an increased at least 2 points from baseline in the HFMSE. They had a longer time to sit independently. Patient 5 in particular showed improvement in rollover and propping on forearms with head up by the time of the fourth injection. Similar to SMA type 1 patients, SMA type 2 patients with shorter disease duration (patients 5 and 6) tended to have higher scores on the motor function assessment.
Figure 2.
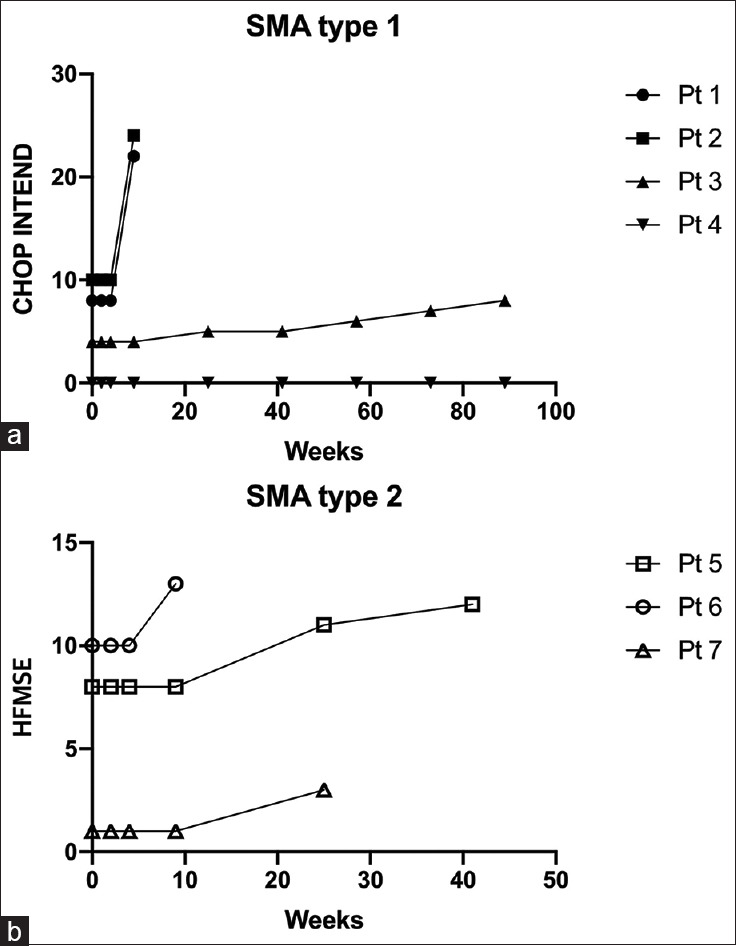
Functional assessments after nusinersen injection. (a) CHOP INTEND score in spinal muscular atrophy (SMA) type 1 patients and (b) Hammersmith Functional Motor Scale Expanded score in SMA type 2
Table 2.
Functional outcome of SMA patients
| Pt No | Age at first LP (month) | Motor functions score* | Motor milestone score† | Respiratory status | Feeding | Adverse reactions | Extra admission (n) | Follow-up duration (month)/Numbers of injection (n) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Before first dose | Last follow-up | Before first dose | Last follow-up | |||||||
| SMA type 1 | ||||||||||
| 1 | 1.1 | 8 | 22 | N | Improved head control (wobble) Kicks leg horizontally | NIV <6 h/d | OF | N | - | 2.1/4 |
| 2 | 1.4 | 10 | 24 | N | Voluntary grasp using whole hand | NIV <6 h/d | OF | N | - | 2.1/4 |
| 3 | 15.1 | 4 | 8 | N | Voluntary grasp using whole hand Kicks leg horizontally | 24-h IMV | EF | N | 3: pneumonia | 21.9/9 |
| 4 | 44.7 | 0 | 0 | N | N | 24-h IMV | EF | N | 1: febrile illness | 22.1/9 |
| SMA type 2 | ||||||||||
| 5 | 46.7 | 8 | 12 (12‡) | Sitting without support | Sitting without support | SB | OF | N | - | 8.0/6 |
| 6 | 106 | 10 | 13 (17‡) | Sitting with support | Sitting without support | SB | OF | N | 1: AGE | 5.2/4 |
| 7 | 269.4 | 1 | 3 (15‡) | Sitting with support | Sitting without support | SB | OF | N | - | 6.1/5 |
*SMA types 1 and 2 patients were assessed using CHOP INTEND score and HFSME, respectively. †SMA types 1 and 2 patients were assessed using HINE section 2 and WHO motor milestone, respectively. ‡RULM score. AGE–acute gastroenteritis; EF–enteral feeding; IMV–invasive mechanical ventilation; N–none; NIV–noninvasive ventilation; OF–oral feeding; SB–spontaneous breathing
The respiratory status of most patients remained stable. Two patients with SMA type 1 (patients 1 and 2) using noninvasive ventilation (NIV) (<8 h/day) to prevent respiratory failure and chest wall distortion showed a reduction of required hours of NIV to 6 or less hours/day by the time of the fourth injection. For two SMA type 1 patients using IMV, ventilator support could not be reduced but remained stable. A follow-up pulmonary function test was performed in one SMA type 2 patient (patient 7) and showed no change before and after 6 months of treatment. With the exception of two type 1 patients with enteral feeding via gastrostomy, all patients were on oral feeding without swallowing difficulty. Three patients had a history of hospitalization during treatment due to pneumonia, acute gastroenteritis, and febrile illness, which did not need intensive care.
DISCUSSION
Since it was approved as the first therapeutic agent for SMA, nusinersen has been used to treat patients worldwide. However, there are only limited studies of the real-world experience of nusinersen use in older patients with chronic phenotypes such as joint contracture, scoliosis, and muscle atrophy.[16,17,24,25,26] This study aimed to share the clinical experience of nusinersen treatment in SMA type 1 and type 2 patients within a broad spectrum of disease from April 2018 to April 2020 and to analyze its safety and efficacy.
Our study revealed that all patients had varying degrees of improvement in motor function, although one patient with a score of 0 at baseline did not demonstrate an increase in motor function assessment. SMA type 1 patients showed improvements in voluntary grasp and kicking, and SMA type 2 patients showed improvement in sitting endurance. In addition, most patients showed improvements that were not included in the rating scale, such as louder vocalization, endurance, and more effective coughing. These findings are consistent with a previous report suggesting that patients benefit from nusinersen even at a later stage of the disease, as demonstrated in 8-year-old patients with SMA type 1.[24] Similarly, a recent preliminary study reported significant improvement in CHOP INTEND and HINE-2 assessment scores after 12 months of nusinersen treatment in 85 SMA type 1 patients between 2 months and 15 years of age.[17] In a study with a heterogonous patient cohort of SMA type 1, 2, and 3, nusinersen showed similar efficacy and safety throughout all ages and severity of the diseases, as seen in the clinical trials.[16] Because patients with more than 16 h of ventilation dependency or with tracheostomy cannula were excluded from the treatment and the proportions of three copies of SMN2 gene in the type 2 group were relatively high (30%) in this study group, it is possible that less severe type 1 patients were included and their functional outcomes could be better than those in our patients. However, in SMA patients with a long disease duration, there is a lack of studies on the clinically meaningful changes in the functional assessment score indicating an effect on activities of daily living and quality of life. Even if the HFMSE score is low in SMA patients with severe phenotype, better upper limb function might be related to better activities of daily living. Therefore, the efficacy of nusinersen in older patients should be measured not only using a motor function assessment but also using other measures such as caregiver burden, activity of daily living, and self-reported quality of life.[27]
Nevertheless, in the case of patients 1 and 2, who began treatment right after diagnosis, the improvement of motor function was apparent within 3 months [Figure 2]. This result is consistent with previous findings indicating that greater improvements in motor function after nusinersen treatment were observed in those with relatively shorter duration of disease at treatment initiation.[13,14] In the interim analysis from the phase-2 clinical trial, many infants with SMA treated during the presymptomatic period achieved motor milestones closer to normal development.[15] These findings emphasize the need for early identification of SMA patients and immediate treatment with nusinersen.
LPs were performed successfully in all cases, even in patients with severe scoliosis, and no adverse events occurred. Unlike our experience, a recent study reported that 81.5% of their patients had at least one adverse event, including headache, back pain, nausea, constipation, and dizziness in 28 adult SMA patients.[28] This difference can be explained by a limitation of communication in young SMA type 1 patients, as in patients 1 and 2. Nevertheless, our data provide further evidence for the safety and tolerability of intrathecal injection using the fluoroscopy-guided approach in chronic patients with scoliosis and joint contracture.[25,28] However, because nusinersen is administered over a long term, young patients, who receive treatment via fluoroscopy or computed tomography-guided injection may be exposed to radiation for a long period, resulting in higher lifetime accumulation of radiation.[29] Therefore, radiation exposure should be monitored to identify organs at risk for this potentially lifelong therapy.
The duration of NIV use was shown to be decreasing in SMA type 1 patients (patients 1 and 2). However, follow-up data analysis will be required, because the therapeutic effect of nusinersen on respiratory function has not been well established. We were unable to observe objective respiratory improvement in chronic patients on IMV (patients 3 and 4). This result is in line with a recent study reporting that none of the SMA type 1 patients on IMV (n = 46) showed improvement at 10 months after treatment.[30] Generally, in qualitative interviews with parents and caregivers, respiratory stability was perceived as a subjective improvement after treatment.[30] Moreover, there is a report of deterioration of respiratory ability after nusinersen treatment.[24] Further longitudinal follow-up data on the effect of nusinersen on respiratory function should be provided.
Our results confirmed the clinical benefit of nusinersen treatment in SMA patients of various ages and disease spectrum. Not only infants and children but also older patients with severe phenotypes showed improvements in motor function. Our results provide additional data that will be helpful for predicting the effectiveness of nusinersen based on disease severity. This study has limitations, including the small number of patients and its retrospective nature. Future long-term prospective studies including many patients with a broad spectrum of disease should investigate the maximum therapeutic effect and the impact of nusinersen on the quality of life of both patients and caregivers. However, we believe that the results of this study will play a positive role in other institutions and countries by sharing the experience and therapeutic results of the actual clinical application of nusinersen in SMA patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pearn J. Incidence, prevalence, and gene frequency studies of chronic childhood spinal muscular atrophy. J Med Genet. 1978;15:409–13. doi: 10.1136/jmg.15.6.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash LA, Burns JK, Chardon JW, Kothary R, Parks RJ. Spinal muscular atrophy: More than a disease of motor neurons? Curr Mol Med. 2016;16:779–92. doi: 10.2174/1566524016666161128113338. [DOI] [PubMed] [Google Scholar]
- 3.Messina S, Pane M, Sansone V, Bruno C, Catteruccia M, Vita G et al. Expanded access program with Nusinersen in SMA type I in Italy: Strengths and pitfalls of a successful experience. Neuromuscul Disord. 2017;27:1084–6. doi: 10.1016/j.nmd.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Nurputra DK, Lai PS, Harahap NIF, Morikawa S, Yamamoto T, Nishimura N, et al. Spinal muscular atrophy: From gene discovery to clinical trials. Ann Hum Genet. 2013;77:435–63. doi: 10.1111/ahg.12031. [DOI] [PubMed] [Google Scholar]
- 5.Boyer JG, Bowerman M, Kothary R. The many faces of SMN: Deciphering the function critical to spinal muscular atrophy pathogenesis. Future Neurol. 2010;5:873–90. [Google Scholar]
- 6.Feldkötter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: Fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70:358–68. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al. ; Participants of the International Conference on SMA Standard of Care.Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22:1027–49. doi: 10.1177/0883073807305788. [DOI] [PubMed] [Google Scholar]
- 8.Neil EE, Bisaccia EK. Nusinersen: A novel antisense oligonucleotide for the treatment of spinal muscular atrophy. J Pediatr Pharmacol Ther. 2019;24:194–203. doi: 10.5863/1551-6776-24.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farrar MA, Park SB, Vucic S, Carey KA, Turner BJ, Gillingwater TH, et al. Emerging therapies and challenges in spinal muscular atrophy. Ann Neurol. 2017;81:355–68. doi: 10.1002/ana.24864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paton DM. Nusinersen: Antisense oligonucleotide to increase SMN protein production in spinal muscular atrophy. Drugs Today (Barc) 2017;53:327–37. doi: 10.1358/dot.2017.53.6.2652413. [DOI] [PubMed] [Google Scholar]
- 11.Goodkey K, Aslesh T, Maruyama R, Yokota T. Nusinersen in the treatment of spinal muscular atrophy. Methods Mol Biol. 2018;1828:69–76. doi: 10.1007/978-1-4939-8651-4_4. [DOI] [PubMed] [Google Scholar]
- 12.Zingariello CD, Brandsema J, Drum E, Henderson AA, Dubow S, Glanzman AM, et al. A multidisciplinary approach to dosing nusinersen for spinal muscular atrophy. Neurol Clin Pract. 2019;9:424–32. doi: 10.1212/CPJ.0000000000000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–32. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 14.Mercuri E, Darras BT, Chiriboga CA, Day JW, Campbell C, Connolly AM, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378:625–35. doi: 10.1056/NEJMoa1710504. [DOI] [PubMed] [Google Scholar]
- 15.De Vivo DC, Bertini E, Swoboda KJ, Hwu WL, Crawford TO, Finkel RS, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: Interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29:842–56. doi: 10.1016/j.nmd.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szabó L, Gergely A, Jakus R, Fogarasi A, Grosz Z, Molnár MJ, et al. Efficacy of nusinersen in type 1, 2 and 3 spinal muscular atrophy: Real world data from Hungarian patients. Eur J Paediatr Neuro. 2020 doi: 10.1016/j.ejpn.2020.05.002. S1090.3798 (20) 30099.4. doi: 10.1016/j.ejpn. 2020.05.002. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Pane M, Coratti G, Sansone VA, Messina S, Bruno C, Catteruccia M, et al. Nusinersen in type 1 spinal muscular atrophy: Twelve-month real-world data. Ann Neurol. 2019;86:443–51. doi: 10.1002/ana.25533. [DOI] [PubMed] [Google Scholar]
- 18.Mercuri E, Finkel RS, Muntoni F, Wirth B, Montes J, Main M, et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28:103–15. doi: 10.1016/j.nmd.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Finkel RS, Mercuri E, Meyer OH, Simonds AK, Schroth MK, Graham RJ, et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2018;28:197–207. doi: 10.1016/j.nmd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Glanzman AM, Mazzone E, Main M, Pelliccioni M, Wood J, Swoboda KJ, et al. The Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP INTEND): Test development and reliability. Neuromuscul Disord. 2010;20:155–61. doi: 10.1016/j.nmd.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Sanctis R, Coratti G, Pasternak A, Montes J, Pane M, Mazzone ES, et al. Developmental milestones in type I spinal muscular atrophy. Neuromuscul Disord. 2016;26:754–9. doi: 10.1016/j.nmd.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glanzman AM, O'Hagen JM, McDermott MP, Martens WB, Flickinger J, Riley S, et al. Pediatric Neuromuscular Clinical Research Network for Spinal Muscular Atrophy (PNCR), Muscle Study Group (MSG).Validation of the expanded hammersmith functional motor scale in spinal muscular atrophy type II and III. J Child Neurol. 2011;26:1499–507. doi: 10.1177/0883073811420294. [DOI] [PubMed] [Google Scholar]
- 23.Mazzone ES, Mayhew A, Montes J, Ramsey D, Fanelli L, Young SD, et al. Revised upper limb module for spinal muscular atrophy: Development of a new module. Muscle Nerve. 2017;55:869–74. doi: 10.1002/mus.25430. [DOI] [PubMed] [Google Scholar]
- 24.Aragon-Gawinska K, Seferian AM, Daron A, Gargaun E, Vuillerot C, Cances C, et al. Nusinersen in patients older than 7 months with spinal muscular atrophy type 1: A cohort study. Neurology. 2018;91:e1312–8. doi: 10.1212/WNL.0000000000006281. [DOI] [PubMed] [Google Scholar]
- 25.Veerapandiyan A, Eichinger K, Guntrum D, Kwon J, Baker L, Collins E, et al. Nusinersen for older patients with spinal muscular atrophy: A real-world clinical setting experience. Muscle Nerve. 2020;61:222–6. doi: 10.1002/mus.26769. [DOI] [PubMed] [Google Scholar]
- 26.Pane M, Palermo C, Messina S, Sansone VA, Bruno C, Catteruccia M, et al. Nusinersen in type 1 SMA infants, children and young adults: Preliminary results on motor function. Neuromuscul Disord. 2018;28:582–5. doi: 10.1016/j.nmd.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Mercuri E, Messina S, Montes J, Muntoni F, Sansone VA. all participants and the SMA PROM working group. Patient and parent oriented tools to assess health-related quality of life, activity of daily living and caregiver burden in SMA.Rome, 13 July 2019. Neuromuscul Disord. 2020;30:431–6. doi: 10.1016/j.nmd.2020.02.019. [DOI] [PubMed] [Google Scholar]
- 28.Stolte B, Totzeck A, Kizina K, Bolz S, Pietruck L, Mönninghoff C, et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther Adv Neurol Disord. 2018;11:1756286418803246. doi: 10.1177/1756286418803246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kizina K, Stolte B, Totzeck A, Bolz S, Fleischer M, Monninghoff C, et al. Clinical implication of dosimetry of computed tomography- and fluoroscopy-guided intrathecal therapy with nusinersen in adult patients with spinal muscular atrophy. Front Neurol. 2019;10:1166. doi: 10.3389/fneur.2019.01166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sansone VA, Pirola A, Albamonte E, Pane M, Lizio A, D'Amico A, et al. Respiratory needs in patients with Type 1 spinal muscular atrophy treated with nusinersen. J Pediatr. 2020;219:223–8. doi: 10.1016/j.jpeds.2019.12.047. [DOI] [PubMed] [Google Scholar]


