Abstract
Objectives:
Alzheimer's disease (AD) is the most common cause of dementia worldwide in the older population. There is no disease-modifying therapy available for AD. The current standard of care drug therapy for AD is cholinesterase inhibitors, including donepezil. Bacopa monnieri or brahmi is used in traditional Indian medicine for memory loss. We conducted a phase 2b randomized controlled trial (RCT) to find out the efficacy of brahmi and donepezil in AD and mild cognitive impairment (MCI).
Patients and Methods:
The study was planned as a 52 week, randomized, double-blind, parallel-group, phase-2 single-center clinical trial comparing the efficacy and safety of Bacopa monnieri (brahmi) 300 mg OD and donepezil 10 mg OD for 12 months in 48 patients with AD and MCI-AD including cognitive and quality of life outcomes. The primary outcome was differences in the change from baseline of the neuropsychological tests [Alzheimer's disease assessment scale-cognitive subscale (ADAS-Cog) and postgraduate institute (PGI) memory scale] at 12 months between the intervention group (brahmi) and active comparison group (donepezil).
Results:
The study was terminated after 3 years and 9 months, after recruiting 34 patients, because of slow recruitment and a high dropout rate. Intention to treat analysis after adjusting for baseline confounders showed no difference in the rate of change in ADAS-Cog score from baseline at any time point, including the last follow-up. There was no difference in the rate of change in PGI Memory scale (PGIMS) at 3, 6, and 9 months. In the last follow-up, there was a significant difference in the change in total PGIMS score between brahmi and donepezil, while there was no difference in individual scores of the PGI memory scale.
Conclusion:
This phase-2 RCT on the efficacy of brahmi vs. donepezil showed no significant difference between them after 1 year of treatment. Larger phase-3 trials, preferably multicentric, are required to find the superiority of brahmi over donepezil.
Keywords: Alzheimer's disease, bacopa monnieri, brahmi, donepezil, dementia, mild cognitive impairment
INTRODUCTION
Alzheimer's disease (AD) is the most common cause of dementia worldwide in the older population.[1] The search for newer and effective therapy for AD is ongoing for many decades. There is no disease-modifying therapy available for AD. The current standard of care drug therapy for AD is cholinesterase inhibitors, including donepezil.[1] The role of cholinesterase inhibitors in mild cognitive impairment-AD (MCI-AD) is not proven.[2] But donepezil is widely used in clinical practice for MCI-AD since in routine clinical practice, it is challenging to differentiate mild AD from MCI-AD without a detailed neuropsychological evaluation including assessment of activities of daily living. Moreover, MCI-AD or amnestic MCI is 15 times more likely to develop AD on follow-up.[3]
Bacopa monnieri or brahmi is an herb belonging to plant family Scrophulariaceae. Indian traditional medicine (Ayurveda) literature reports its medicinal properties for the treatment of anxiety, intellect, and memory disorders.[4] Major active constituents in brahmi with possible action on memory and cognition are bacoside-A and bacoside-B apart from other phytochemicals like alkaloids, saponins, flavonoids, etc.[5] The postulated therapeutic effects of bacosides include enhancement of nerve impulse transmission, restoration of synaptic activity, and repair of damaged neurons by upregulating neuronal synthesis and kinase activity and maintaining neurotransmitter balance.[4,6] We had studied the efficacy of brahmi in reversing drug-induced amnesia in mice. Diazepam-induced amnesia was significantly reversed by brahmi.[7,8] Further research from our group found that brahmi reversed the L-NNA (N (w)-nitro-L-arginine)-induced anterograde and retrograde amnesia.[5,9] Even though there is good quality preclinical evidence on the utility of brahmi, peer-reviewed published data on the use of the brahmi in AD and MCI-AD is limited. There is a lack of high-quality evidence in the form of randomized control trials comparing brahmi and donepezil (common drug used in standard of care) in the management of AD/MCI-AD. We report the findings of a double-blind, phase-2 randomized control trial comparing the efficacy and safety of brahmi vs. donepezil, a common drug used in standard of care, in MCI-AD and AD patients.
PATIENTS AND METHODS
Institute ethics committee (Postgraduate Institute of Medical Education and Research, Chandigarh, reference number PGI/IEC/2012/1171) approved the protocol of the trial and consent forms. Informed consent was taken from all the participants. The trial is registered under Clinical Trials Registry, India (CTRI/2018/04/013388). The trial was registered retrospectively because of communication issues, but the patients were recruited only after approval from the ethics committee. The authors confirm that all ongoing and related trials for this drug/intervention are registered. The study was conducted between February 2013 and December 2016.
Study design
The study was planned as a randomized, double-blind, parallel-group, phase-2 single-center clinical trial comparing the efficacy and safety of Bacopa monnieri (brahmi) 300 mg OD and donepezil 10 mg OD for 52 weeks ( in each patient) in 48 patients with AD and MCI-AD including cognitive and quality of life outcomes. The trial was conducted in accordance with the declaration of Helsinki and the principles of good clinical practice. An independent data and safety monitoring board monitored all the adverse events. During screening, the eligible patients were those aged >50 years, diagnosed with MCI-AD or AD (Dubois criteria) with modified Hachinski ischemic scale score of less than 5 points, mini-mental status examination (MMSE) more than 10, available study informant, and having adequate vision and hearing for neuropsychological testing. Diagnosis of AD/MCI-AD was confirmed using magnetic resonance imaging (MRI) brain, fluorodeoxyglucose-positron emission tomography (FDG PET) brain, and cerebrospinal fluid “(CSF) Ab amyloid and total tau. Patients who were already on a cholinesterase inhibitor were excluded. Other exclusion criteria included young onset dementia, vascular dementia, frontotemporal dementia, and other secondary acquired causes of dementia.
Randomization and masking
The randomization sequence was generated by independent personnel from department of pharmacology who were not involved in the study. Brahmi (300 mg of standardized mixture, which consists of bacoside-A and bacoside-B) and donepezil (10 mg) filled in identical capsules. The capsules were packaged identically and coded in a random sequence. The enrolled subjects were sequentially assigned to receive either brahmi or donepezil starting from the lowest allocation number. The subjects, treating neurologists, outcome assessors, and data analysts were blinded to the allocation sequence. No blindness evaluation was planned in the study.
Procedure
After the initial screening procedure, those patients satisfying the inclusion and exclusion criteria were enrolled in the study after informed consent. Patients were randomly assigned in 1:1 ratio, as and when they visited the department, to receive either brahmi 300 mg or donepezil 10 mg for 1 year (52 weeks). The participants underwent standard evaluation protocol for dementia during the screening period including MRI brain, FDG PET brain, electrolytes, liver and renal function tests, complete blood counts, venereal disease research laboratory, HIV, and Vitamin B12. The outpatient visits included screening, baseline, 3, 6, 9, and 12 months. Every attempt was made to follow up patients who withdrew from the study. During each visit, adverse event monitoring and neuropsychological evaluation were done. Adverse effect (AE) was defined as “any newly occurred undesirable syndromes of the enrolled patients during the trial, which is not necessarily a consequence of the intervention but could also be the result of the condition being treated.” Severe adverse effect (SAE) is defined as death, stroke of all cause, and vegetative state. All AEs and SAEs were recorded in the case record form. Investigators notified the SAEs to the Data and Safety Monitoring Board (DSMB) within 24 h. Neuropsychological evaluation included the MMSE, Alzheimer's disease assessment scale (Cognitive), postgraduate institute (PGI) memory scale, verbal fluency-controlled oral word test (phonemic) and animal names test (categorical), quality of life-AD, and Alzheimer's disease cooperative study (ADCS)—activities of daily living inventory and Hamilton depression rating scale.
Outcome
The primary outcome was differences in the change from baseline of the neuropsychological tests (ADAS-Cog and PGI memory scale) at 3, 6, 9, and 12 months between the intervention group (brahmi) and active comparison group (donepezil).
The secondary outcomes were change from baseline of the neuropsychological tests (verbal fluency—controlled oral word test (phonemic) and animal names test (categorical), quality of life—AD, ADCS—activities of daily living inventory), compliance to treatment, and number of adverse events.
Statistical analysis
The sample size of 48 participants (24 each) was calculated for phase-2 trial (using Simon's randomized phase-2 design) for intervention and active comparison groups with least expected baseline response rate of 12% and assumed difference in response rate between the best treatment and the other treatments as 20%. Intention to treat (ITT) analysis was conducted and all patients randomized were included in the final analysis. Multiple imputations were used to handle missing data because of lost to follow up. Changes in neuropsychological parameters between the 2 groups were measured by unpaired t-test and Mann–Whitney U test for nonparametric variables. The outcomes were adjusted for baseline age, sex, baseline neuropsychological test score, and duration of disease.
Amendments in primary protocol
The protocol registered in Clinical Trial Registry of India is the original protocol of the study. The study had two primary outcomes ADAS-Cog and PGI memory scale at 3, 6, 9, and 12 months. Due to four different time points, effectively this led to eight primary outcomes. Hence, we had decided to keep the time point of 12 months or last follow-up as the time point for primary outcome.
RESULTS
Patient disposition is characterized in Figure 1.
Figure 1.
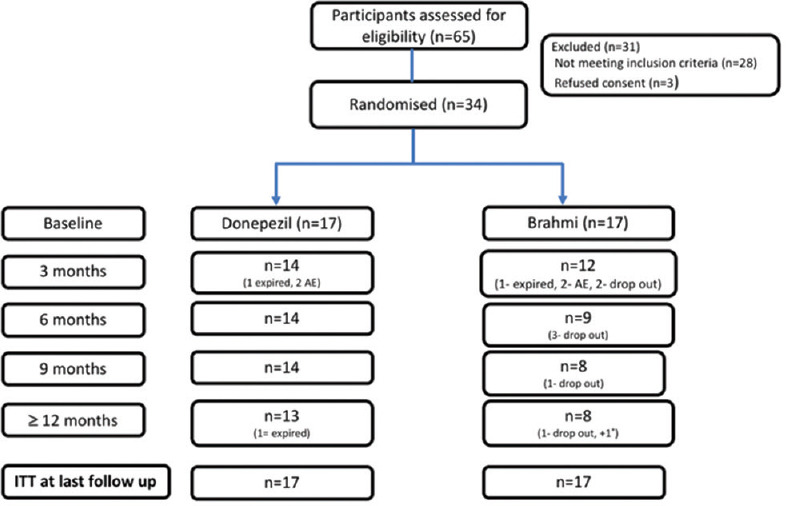
CONSORT flow chart of the trial
Between February 2013 and December 2016, a total of 65 patients were screened at the Dementia Clinic/Neurology outpatient services, Postgraduate Institute of Medical Education and Research, Chandigarh, India, of whom 34 enrolled into the study and were randomized to either brahmi or donepezil. The study was terminated after 3 years and 9 months, in view of slow recruitment and high dropout rate. The initial screen failure included those not satisfying inclusion and exclusion criteria. Three patients refused consent. The discontinuation rates were numerically more in brahmi arm. The reasons for discontinuation were side effects, poor socioeconomic status, and inability to come for follow-up (living alone/no caregiver support). The drop-out rates due to adverse events were similar in both arms (two each). The baseline characteristics including demographics, risk factor profile, and neuropsychological scores are described in Table 1. The mean age of the patients was similar in both groups. There was no significant difference in other risk factors like hypertension, diabetes, coronary artery disease, stroke, and dyslipidemia. None of the patients were taking any antidementia medications prior to during the study. There were more patients with AD in donepezil group and more patients with MCI in brahmi group. The median duration of disease was more in brahmi group and more severe scores of baseline neuropsychological scales in donepezil group. These baseline imbalances in prognostic variables were adjusted by analysis of covariance. ITT analysis was done for primary and secondary outcomes using multiple imputations. The imaging and FDG PET scan findings will be discussed in a separate publication.
Table 1.
Baseline characteristics of the participants
| Donepezil | Brahmi | |
|---|---|---|
| Age | 68.71 (9.54 SD) | 70.18 (6.73 SD) |
| Gender | ||
| Male | 09 (52.9%) | 13 (76.5%) |
| Female | 08 (47.1%) | 04 (23.5%) |
| Education | ||
| Illiterate | 06 (35.3%) | 01 (5.9%) |
| Up to 12 | 07 (41.2%) | 08 (47.1%) |
| Graduate | 04 (23.5%) | 08 (47.1%) |
| Alzheimer’s Disease | 10 (58.8%) | 05 (29.4%) |
| MCI-AD 7 | (41.2%) | 12 (70.6%) |
| Hypertension | 5 (29.4%) | 10 (58.8%) |
| Diabetes Mellitus | 4 (23.5%) | 7 (41.2%) |
| CAD | 2 (11.8%) | 3 (17.6%) |
| Stroke | 0 | 0 |
| Dyslipidemia | 2 (11.8%) | 5 (29.4%) |
| Median duration of disease (months) | 12 (IQR:12-30) | 24 (IQR:12-42) |
| ADAS (mean±SE) | 17.09±1.63 | 12.61±1.03 |
| CDR (mean±SE) | 0.85±0.09 | 0.67±0.07 |
| Animal naming test (mean±SE) | 6.35±0.65 | 7.35±0.77 |
| COWAT (mean±SE) | 3.23±0.59 | 5.55±0.62 |
| Quality of Life (Patient) (mean±SE) | 35.18±1.96 | 33.88±1.74 |
| Quality of Life (Informant) (mean±SE) | 34.06±1.90 | 31.88±1.02 |
| Activities of daily living (mean±SE) | 53.76±2.85 | 56.88±2.83 |
| MMSE (mean±SE) | 18.06±1.50 | 23.76±0.97 |
| PGI Memory Scale (mean±SE) | 41.11±4.28 | 54.94±3.60 |
| Remote Memory (mean±SE) | 4.00±0.36 | 4.82±0.37 |
| RECENT MEMORY (mean±SE) | 2.82±0.39 | 3.82±0.38 |
| Mental Balance (mean±SE) | 3.76±0.77 | 6.23±0.63 |
| Attention and Concentration (mean±SE) | 7.06±0.51 | 8.05±0.59 |
| Delayed Recall (mean±SE) | 4.11±0.57 | 5.23±0.53 |
| Immediate Recall (mean±SE) | 4.24±0.60 | 5.88±0.55 |
| Retention for Similar Pairs (mean±SE) | 3.29±0.28 | 4.05±0.29 |
| Retention for Dissimilar Pairs (mean±SE) | 4.18±0.87 | 6.05±0.90 |
| Visual Retention (mean±SE) | 2.65±0.75 | 4.23±0.87 |
| Visual Recognition (mean±SE) | 5.00±0.70 | 6.53±0.58 |
Efficacy analysis
ITT analysis after adjusting for baseline confounders showed no difference in rate of change in ADAS-Cog score from baseline at any time point including the last follow-up. There was no difference in rate of change in PGIMS at 3, 6, and 9 months [Table 2]. In the last follow-up, there was a significant difference in change in total PGIMS score between brahmi and donepezil, while there was no difference in individual scores of PGI Memory scale [Table 3]. The analysis of the secondary outcomes (CDR, MMSE, ADCS-ADL, QOL, animal naming test, and controlled oral word association test ) also showed no difference in ITT analysis except MMSE at 3 months which showed improvement in donepezil arm. Only 46.1% in brahmi arm completed the course of treatment, while 76.1% completed the treatment in donepezil arm.
Table 2.
Change from baseline in cognitive and functional scores
| Change from baseline in cognitive and functional scores | ||||
|---|---|---|---|---|
| Neuropsychological scales | 3 months | 6 months | 9 months | 12 months |
| ADAS-Cog | ||||
| Donepezil | 0.24 (4.42) | 0.73 (4.41) | 0.23 (3.84) | 2.27 (5.65) |
| Brahmi | 1.59 (4.42) | 0.55 (4.41) | 1.02 (3.84) | 0.51 (5.65) |
| Mean Difference corrected* | −1.35 | 0.18 | −0.79 | 1.76 |
| P | 0.39 | 0.91 | 0.57 | 0.39 |
| PGIMS | ||||
| Donepezil | −1.03 (8.32) | −2.99 (9.97) | −4.05 (8.40) | −0.46 (10.96) |
| Brahmi | −0.69 (8.32) | 0.72 (9.97) | −2.32 (8.40) | 7.94 (10.96) |
| Mean Difference corrected* | −0.34 | −3.72 | −1.73 | −8.40 |
| P | 0.91 | 0.31 | 0.57 | 0.04 |
| CDR | ||||
| Donepezil | 0.14 (0.27) | 0.01 (0.35) | 0.01 (0.29) | −0.27 (0.47) |
| Brahmi | 0.17 (0.27) | 0.21 (0.35) | 0.14 (0.29) | −0.07 (0.47) |
| Mean Difference corrected* | 0.16 | 0.19 | 0.13 | 0.20 |
| P | 0.10 | 0.11 | 0.21 | 0.23 |
| MMSE | ||||
| Donepezil | 0.72 (3.13) | 0.68 (3.21) | −0.09 (3.67) | 1.59 (4.45) |
| Brahmi | −2.02 (3.13) | 0.55 (3.21) | −0.03 (3.67) | 2.31 (4.45) |
| Mean Difference corrected* | 2.74 | 0.13 | −0.06 | −0.72 |
| P | 0.02 | 0.91 | 0.97 | 0.66 |
| ADCS-ADL | ||||
| Donepezil | 0.43 (9.33) | 1.94 (9.76) | 1.45 (8.45) | 2.35 (11.7) |
| Brahmi | −0.31 (9.33) | 0.11 (9.76) | 0.75 (8.45) | 4.47 (11.7) |
| Mean Difference corrected* | 0.75 | 1.83 | 0.69 | −2.12 |
| P | 0.82 | 0.59 | 0.81 | 0.60 |
| QOL-Patient | ||||
| Donepezil | −1.86 (3.63) | −0.86 (4.94) | −2.52 (4.50) | 0.29 (4.99) |
| Brahmi | −2.91 (3.63) | −1.09 (4.94) | −2.84 (4.50) | 0.37 (4.99) |
| Mean Difference corrected* | 1.05 | 0.23 | 0.33 | −0.08 |
| P | 0.41 | 0.89 | 0.83 | 0.96 |
| QOL-Informant | ||||
| Donepezil | −0.64 (4.30) | 0.52 (4.78) | 0.56 (4.57) | 2.92 (5.11) |
| Brahmi | −1.04 (4.30) | 0.54 (4.78) | 0.43 (4.57) | 0.81 (5.11) |
| Mean Difference corrected* | 0.40 | −0.02 | 0.12 | 2.11 |
| P | 0.79 | 0.99 | 0.94 | 0.24 |
| Animal Naming test | ||||
| Donepezil | −0.03 (2.60) | 0.07 (2.18) | 0.78 (2.31) | 0.72 (2.52) |
| Brahmi | −1.27 (2.60) | 0.75 (2.18) | 0.09 (2.31) | 0.62 (2.52) |
| Mean Difference corrected* | 1.24 | −0.68 | 0.69 | 0.09 |
| P | 0.18 | 0.38 | 0.40 | 0.91 |
| COWAT (Controlled Oral Word Association Test) | ||||
| Donepezil | 0.16 (2.09) | −0.19 (2.18) | 0.18 (2.02) | 0.26 (2.10) |
| Brahmi | −0.69 (2.09) | −0.76 (2.18) | −0.64 (2.02) | 0.55 (2.10) |
| Mean Difference corrected* | 0.85 | 0.57 | 0.81 | −0.29 |
| P | 0.26 | 0.47 | 0.27 | 0.70 |
Table 3.
Change from baseline in PGI memory scale
| PGI memory scale | Change from baseline in cognitive and functional scores | |||
|---|---|---|---|---|
| 3 months Mean (SD) | 6 months Mean (SD) | 9 months Mean (SD) | 12 months Mean (SD) | |
| Remote Memory | ||||
| Donepezil | 0.65 (1.67) | 0.76 (1.54) | 0.58 (1.43) | 0.34 (1.39) |
| Brahmi | −0.25 (1.67) | 0.48 (1.54) | 0.24 (1.43) | 0.78 (1.39) |
| Mean Difference corrected* | 0.9 | 0.28 | 0.34 | −0.44 |
| P | 0.13 | 0.61 | 0.5 | 0.38 |
| Recent Memory | ||||
| Donepezil | −0.19 (1.23) | 0.33 (1.33) | 0.09 (1.36) | 1.23 (1.40) |
| Brahmi | 0.17 (1.23) | 0.24 (1.33) | 0.4 (1.36) | 1.29 (1.40) |
| Mean Difference corrected* | −0.36 | 0.09 | −0.31 | −0.06 |
| P | 0.41 | 0.85 | 0.52 | 0.9 |
| Mental Balance | ||||
| Donepezil | 0.18 (2.21) | 0.522 (2.11) | 0.68 (1.99) | 1.13 (2.19) |
| Brahmi | 0.51 (2.21) | −0.09 (2.11) | 0.09 (1.99) | 1.66 (2.19) |
| Mean Difference corrected* | −0.33 | 0.612 | 0.59 | −0.53 |
| P | 0.68 | 0.42 | 0.42 | 0.5 |
| Attention and Concentration | ||||
| Donepezil | −0.09 (1.96) | 0.03 (1.98) | 0.19 (1.80) | 0.49 (2.02) |
| Brahmi | −0.59 (1.96) | 0.04 (1.98) | −0.57 (1.80) | 1.22 (2.02) |
| Mean Difference corrected* | 0.5 | −0.01 | 0.76 | −0.73 |
| P | 0.47 | 0.99 | 0.23 | 0.31 |
| Delayed Recall | ||||
| Donepezil | −0.82 (2.31) | −0.23 (2.30) | −0.47 (2.02) | −0.68 (2.08) |
| Brahmi | −0.26 (2.31) | −0.52 (2.30) | −1.25 (2.02) | −0.49 (2.08) |
| Mean Difference corrected* | −0.56 | 0.29 | 0.78 | −0.19 |
| P | 0.49 | 0.72 | 0.27 | 0.8 |
| Immediate Recall | ||||
| Donepezil | 0.57 (2.10) | −0.34 (2.35) | −0.55 (2.76) | −0.29 (2.55) |
| Brahmi | −0.34 (2.10) | −0.86 (2.35) | −0.92 (2.76) | −0.14 (2.55) |
| Mean Difference corrected* | 0.91 | 0.52 | 0.37 | −0.15 |
| P | 0.23 | 0.53 | 0.71 | 0.87 |
| Similar Pairs | ||||
| Donepezil | −0.08 (1.11) | 0.39 (1.40) | −0.03 (1.01) | 0.45 (1.55) |
| Brahmi | −0.08 (1.11) | 0.43 (1.40) | 0.15 (1.01) | 0.52 (1.55) |
| Mean Difference corrected* | 0 | −0.04 | −0.18 | −0.07 |
| P | 1 | 0.93 | 0.62 | 0.9 |
| Dissimilar Pairs | ||||
| Donepezil | −0.88 (2.76) | −1.14 (2.29) | −2.61 (2.55) | −1.26 (2.84) |
| Brahmi | −0.24 (2.76) | −0.45 (2.29) | −0.74 (2.55) | −0.55 (2.84) |
| Mean Difference corrected* | −0.64 | −0.69 | −1.87 | −0.71 |
| P | 0.51 | 0.39 | 0.05 | 0.48 |
| Visual Retention | ||||
| Donepezil | 0.73 (2.18) | 0.33 (2.61) | 0.47 (2.43) | 0.23 (2.39) |
| Brahmi | −0.36 (2.18) | −0.16 (2.61) | −0.34 (2.43) | 0.65 (2.39) |
| Mean Difference corrected* | 1.09 | 0.49 | 0.81 | −0.42 |
| P | 0.16 | 0.59 | 0.35 | 0.62 |
| Visual Recognition | ||||
| Donepezil | 0.17 (2.18) | −0.39 (1.85) | −0.61 (2.18) | 0.11 (2.52) |
| Brahmi | −0.22 (2.18) | −0.42 (1.85) | −0.55 (2.18) | 1.15 (2.52) |
| Mean Difference corrected* | 0.39 | 0.03 | −0.06 | −1.04 |
| P | 0.61 | 0.97 | 0.94 | 0.25 |
| Total | ||||
| Donepezil | −1.03 (8.36) | −2.99 (9.97) | −4.05 (8.42) | −0.46 (10.96) |
| Brahmi | −0.69 (8.36) | 0.72 (9.97) | −2.32 (8.42) | 7.94 (10.96) |
| Mean Difference corrected* | −0.34 | −3.7 | −1.73 | −8.4 |
| P | 0.91 | 0.31 | 0.57 | 0.04 |
Safety analysis
There was no significant difference in the number of participants with one or more adverse events [Table 4]. There were three mortalities (two in donepezil and one in brahmi) due to cardiovascular causes (myocardial infarction). The treatment-emergent adverse events were nausea, diarrhea, asthenia, arthralgia, headache, dizziness, anxiety, restlessness, insomnia, and crying [Table 4].
Table 4.
No. (%) of individuals in Donepezil and Brahmi group experiencing adverse events
| Symptoms | Donepezil (n=17) | Brahmi (n=17) |
|---|---|---|
| Nausea | 01 (5.9) | 02 (11.8) |
| Diarrhea | 02 (11.8) | 03 (17.6) |
| Asthenia | 02 (11.8) | 01 (5.9) |
| Arthralgia | 00 (00) | 03 (17.6) |
| Headache | 01 (5.9) | 04 (23.5) |
| Dizziness | 02 (11.8) | 02 (11.8) |
| Anxiety | 04 (23.5) | 02 (5.9) |
| Restlessness | 04 (23.5) | 03 (17.6) |
| Insomnia | 07 (41.2) | 04 (23.5) |
| Crying | 05 (29.4) | 02 (11.8) |
DISCUSSION
The phase-2 trial showed that brahmi treatment for 12 months was not superior to donepezil on the effect of progression of symptoms (ADAS-Cog scores) in individuals with MCI-AD or mild-to-moderate AD. At the last follow-up, on the PGI memory scale (total score), donepezil arm had lesser progression compared to brahmi, while there was no difference in individual scores of PGI memory scale. No brahmi-donepezil differences were observed on the change in any secondary outcome scores at last follow-up.
A meta-analysis of randomized control trials on the cognitive effects of brahmi concluded that “Bacopa monnieri has potential to improve cognition, particularly speed of attention but only a large well designed 'head-to-head' trial against an existing medication will provide definitive data on its efficacy on healthy or dementia patients using a standardized preparation.”[10] They included nine studies in their meta-analysis of which seven studies were done on healthy subjects[11,12,13,14,15,16,17] and two trials were on patients with memory impairment.[18,19]
The two trials which enrolled patients (Barbhaiya et al. and Raghav et al.) also did not include AD.[18,19] Raghav et al. included participants with age-associated memory impairment (AAMI) which is defined in persons aged >50 years with subjective memory complaint, objective evidence of recent memory impairment (i.e., ≥ 1 standard deviation below the mean performance of young adults).[20] AAMI is a concept which preceded the MCI without the specifications of sparing of activities of daily living as in MCI. Barbhaiya et al. included subjects (50–75 years) with subjective memory impairment of at least 1-year duration and MMSE >24.[18] Hence, both these studies may have included MCI and some normal healthy subjects also. Since the data on involvement of ADL is not available, inclusion of mild AD cannot be ascertained.
In our study, we have included MCI-AD (amnestic MCI) and AD. Most of the AD were excluded during the screening since they were severe AD and neuropsychological evaluation was not possible. Our study is the first randomized controlled trial (RCT) assessing the efficacy of brahmi in MCI-AD and AD, proven by CSF biomarkers (Abeta42 amyloid and T-Tau) and FDG-PET imaging. We have used detailed neuropsychological testing including ADAS-Cog, PGIMS, ADCS, QOL scales, and verbal fluency tests. The duration of drug intake was 1 year unlike most other studies which used drugs for lesser duration.
ADAS-Cog showed no difference between brahmi and donepezil. Even though there was statistical difference in the total PGI memory scale, there was no difference in the individual scale subsets and, hence, there was little clinical significance. Although the primary outcome time point was at 12 months, many patients did not come for the last evaluation on time and there was a gap between end of study drug intake and final evaluation. We had included them in the ITT analysis. But when 9-month follow-up assessment is considered, there is no significant difference between two arms. Though we cannot claim noninferiority between brahmi and donepezil based on the results, it gives enough data for a larger phase-3 trial to detect superiority of brahmi over donepezil.
LIMITATIONS OF THE STUDY
The sample size was small for any strong conclusions on the efficacy of brahmi. As expected in an RCT with small sample size, there were significant differences in the baseline characteristics though they were adjusted in the final analysis. We did not mention the P values for baseline characteristics, as a value of <0.05 or >0.05 will not be meaningful to interpret in either way when number of participants are small. The dropout rate was high and recruitment rate was poor and, hence, trial had to be stopped prior to attaining the sample size. The study demonstrates the practical problems of conducting an RCT on dementia in a country like India. Many patients were staying alone or from poor socioeconomic background. They could not come for regular follow-up. The caregivers were also not willing to bring most patients as they had to lose that day's wages and many were manual labourers with no fixed income. The duration of drug intake was 1 year in this study which is longer than other trials of brahmi. But ideally a 3-year follow-up is essential to document the progression, especially in MCI.
IMPLICATIONS FOR FUTURE TRIAL
A multicenter phase-3 RCT should be done including MCI and mild AD patients.
CONCLUSIONS
This phase-2 RCT on efficacy of brahmi vs. donepezil showed no significant difference between them after 1 year of treatment. Larger phase-3 trials, preferably multicentric, are required to find the actual difference in the efficacy of brahmi over donepezil and/or other commonly used drugs in MCI and mild AD.
Trial registration
Clinical Trial Registry India (CTRI/2018/04/013388).
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Intramural funds, Department of Neurology, PGIMER Chandigarh.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to acknowledge Dr. Ashok Kumar for statistical analysis and Ms. Geetika Sikand for assistance in neuropsychological testing.
REFERENCES
- 1.Birks JS, Harvey RJ. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev. 2018;6:CD001190. doi: 10.1002/14651858.CD001190.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tricco AC, Soobiah C, Berliner S, Ho JM, Ng CH, Ashoor HM, et al. Efficacy and safety of cognitive enhancers for patients with mild cognitive impairment: A systematic review and meta-analysis. CMAJ. 2013;185:1393–401. doi: 10.1503/cmaj.130451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns A, Iliffe S. Dementia. BMJ. 2009;338:b75. doi: 10.1136/bmj.b75. [DOI] [PubMed] [Google Scholar]
- 4.Singh HK, Dhawan BN. Neuropsychopharmacological effects of the Ayurvedic nootropic Bacopa monnieri Linn.(Brahmi) Indian J Pharmacol. 1997;29:359. [Google Scholar]
- 5.Mathur D, Goyal K, Koul V, Anand A. The molecular links of re-emerging therapy: A review of evidence of Brahmi (Bacopa monniera) Front Pharmacol. 2016;7:44. doi: 10.3389/fphar.2016.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Charles PD, Ambigapathy G, Geraldine P, Akbarsha MA, Rajan KE. Bacopa monniera leaf extract up-regulates tryptophan hydroxylase and serotonin transporter expression: Implications in memory formation. J Ethnopharmacol. 2011;134:55–61. doi: 10.1016/j.jep.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar S, Saraf MK, Pandhi P, Anand A. Bacopa monniera exerts antiamnesic effect on diazepam induced anterograde amnesia in mice. Psychopharmacology (Berl) 2008;200:27–37. doi: 10.1007/s00213-007-1049-8. [DOI] [PubMed] [Google Scholar]
- 8.Saraf MK, Prabhakar S, Pandhi P, Anand A. Bacopa monniera ameliorates amnesic effects of diazepam qualifying behavioral-molecular partitioning. Neuroscience. 2008;155:476–84. doi: 10.1016/j.neuroscience.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 9.Saraf MK, Prabhakar S, Anand A. Bacopa monniera alleviates N (omega)-nitro-L-arginine arginine-induced but not MK-801-induced amnesia: A mouse watermaze study. Neuroscience. 2009;160:149–55. doi: 10.1016/j.neuroscience.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Kongkeaw C, Dilokthornsakul P, Thanarangsarit P, Limpeanchob N, Norman Scholfield C. Meta-analysis of randomized controlled trials on cognitive effects of Bacopa monnieri extract. J Ethnopharmacol. 2014;151:528–35. doi: 10.1016/j.jep.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Calabrese C, Gregory WL, Leo M, Kraemer D, Bone K, Oken B. Effects of a standardized Bacopa monnieri extract on cognitive performance, anxiety, and depression in the elderly: A randomized, double-blind, placebo-controlled trial. J Altern Complement Med N Y N. 2008;14:707–13. doi: 10.1089/acm.2008.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan A, Stevens J. Does Bacopa monnieri improve memory performance in older persons.Results of a randomized, placebo-controlled, double-blind trial? J Altern Complement Med N Y N. 2010;16:753–9. doi: 10.1089/acm.2009.0342. [DOI] [PubMed] [Google Scholar]
- 13.Peth-Nui T, Wattanathorn J, Muchimapura S, Tong-Un T, Piyavhatkul N, Rangseekajee P, et al. Effects of 12-week bacopa monnieri consumption on attention, cognitive processing, working memory, and functions of both cholinergic and monoaminergic systems in healthy elderly volunteers. Evid-Based Complement Altern Med ECAM. 2012;2012:606424. doi: 10.1155/2012/606424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roodenrys S, Booth D, Bulzomi S, Phipps A, Micallef C, Smoker J. Chronic effects of Brahmi (Bacopa monnieri) on human memory. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2002;27:279–81. doi: 10.1016/S0893-133X(01)00419-5. [DOI] [PubMed] [Google Scholar]
- 15.Sathyanarayanan V, Thomas T, Einöther SJL, Dobriyal R, Joshi MK, Krishnamachari S. Brahmi for the better.New findings challenging cognition and anti-anxiety effects of Brahmi (Bacopa monniera) in healthy adults? Psychopharmacology (Berl) 2013;227:299–306. doi: 10.1007/s00213-013-2978-z. [DOI] [PubMed] [Google Scholar]
- 16.Stough C, Downey LA, Lloyd J, Silber B, Redman S, Hutchison C, et al. Examining the nootropic effects of a special extract of Bacopa monniera on human cognitive functioning: 90 day double-blind placebo-controlled randomized trial. Phytother Res PTR. 2008;22:1629–34. doi: 10.1002/ptr.2537. [DOI] [PubMed] [Google Scholar]
- 17.Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, et al. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl) 2001;156:481–4. doi: 10.1007/s002130100815. [DOI] [PubMed] [Google Scholar]
- 18.Barbhaiya HC, Desai RP, Saxena VS, Pravina K, Wasim P, Geetharani P, et al. Efficacy and tolerability of BacoMind®on memory improvement in elderly participants-A double blind placebo controlled study. J Pharmacol Toxicol. 2008;3:425–34. [Google Scholar]
- 19.Raghav S, Singh H, Dalal PK, Srivastava JS, Asthana OP. Randomized controlled trial of standardized Bacopa monniera extract in age-associated memory impairment. Indian J Psychiatry. 2006;48:238–42. doi: 10.4103/0019-5545.31555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crook TH, Ferris SH. Age associated memory impairment. BMJ. 1992;304:714. doi: 10.1136/bmj.304.6828.714-b. [DOI] [PMC free article] [PubMed] [Google Scholar]


