Abstract
A variety of neurologic manifestations of COVID-19 infections have been reported. Here, we present a case of steroid-responsive MOG-antibody associated encephalitis, characterized by cognitive decline, headaches, fever, unilateral FLAIR-hyperintensities, and leptomeningeal enhancement, that occurred in the setting of recent COVID-19 infection.
Keywords: MOG-associated disease, Encephalitis, SARS-CoV-2, Coronavirus
1. Case report
A 23-year old man with a history of childhood non-febrile seizures, presented to our hospital because of cognitive slowing and personality changes. Five weeks prior, he gradually developed a moderate but nearly constant, left-sided headache associated with dysesthesias. During this timeframe, he had known exposure to SARS-CoV-2 infected coworkers. A nasopharyngeal rapid antigen test one week after known exposure was negative but nasopharyngeal PCR testing was positive for SARS-CoV-2. He did not have systemic symptoms, including fever, cough, or dyspnea. Two weeks after testing positive for COVID-19, he had three generalized seizures. He was brought to the emergency room, where brain MRI was normal. Lumbar puncture (LP) revealed 589 red blood cells (RBCs)/µL, one white blood cell (WBC)/µL, and normal protein (36 mg/dL) and glucose (78 mg/dL). Repeat SARS-CoV-2 nasopharyngeal swab PCR was still positive. He was discharged on levetiracetam. Over the ensuing two weeks, he developed worsening headache, fatigue, inattention, and cognitive slowing and ultimately presented to our tertiary care hospital for further evaluation.
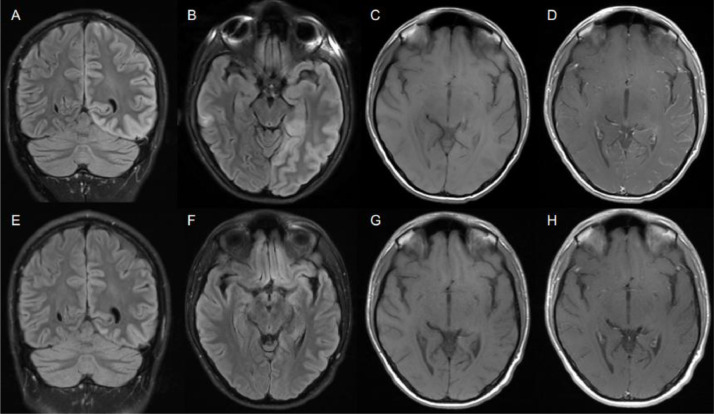
At presentation, his neurologic exam was notable for difficulty naming low-frequency objects, decreased verbal fluency, mild inattention, and impaired delayed recall. Montreal Cognitive Assessment (MOCA) score was 20/30. The remainder of his neurologic exam, including dilated ophthalmic evaluation, was normal. He had intermittent fevers as high as 102.1°F. MRI brain showed diffuse left-hemispheric cortical T2-fluid-attenuated inversion recovery (FLAIR) hyperintensity, most pronounced in the left occipital and posterior temporal lobes, as well as left-hemispheric leptomeningeal enhancement (Fig. 1 a-d). Continuous EEG monitoring showed frequent left posterior temporal rhythmic delta activity and epileptiform spikes that became less prevalent on an increased dose of levetiracetam. Repeat LP revealed an elevated opening pressure (32cm H2O), 0 RBCs/µL, 57 WBCs/µL (50% lymphocytes, 35% granulocytes, and 15% monocytes), normal protein (40 mg/dL), and normal glucose (60 mg/dL). CSF IgG and IgG index were increased (3.9 mg/dL and 0.84, respectively) but cerebrospinal fluid (CSF) restricted oligoclonal bands were absent. CSF viral testing included Eastern Equine virus antibodies and West Nile virus, Varicella zoster virus, Herpes simplex virus, enterovirus and SARS-CoV-2 PCR (Kudo et al., 2020), all of which were negative. Serum myelin oligodendrocyte glycoprotein (MOG) IgG antibody via Fluorescence-Activated Cell Sorting (FACS) was positive at a titer of 1:100 (Mayo Clinic). CSF autoimmune encephalopathy panel (Mayo Clinic) was negative.
Fig. 1.
MRI brain before treatment (A-D) showing left-sided T2-FLAIR hyperintensity (A, B) and leptomeningeal enhancement (C, D) compared to MRI after treatment (E-H) showing improvement in T2-FLAIR signal (E, F) and resolution of leptomeningeal enhancement (G, H).
He was treated with intravenous methylprednisolone 1 gram daily for 5 days with resolution of his headache and fevers and marked improvement in his cognitive symptoms. MOCA score after steroids and prior to discharge improved to 27/30, and he was discharged home on an oral steroid taper. MRI brain two weeks after discharge showed improvement in the left-hemispheric FLAIR hyperintensity and resolution of the leptomeningeal enhancement (Fig. 1e-h). He noted continued cognitive improvement but he continued to have impaired delayed recall at follow-up three weeks after hospital discharge. His subjective cognitive symptoms resolved and he had normal delayed recall 8 weeks after hospital discharge.
2. Discussion
A host of neurological syndromes have been associated with SARS-CoV-2 infection, including inflammatory CNS disorders, ischemic stroke, and Guillain-Barre syndrome (Paterson et al., 2020). Recently, other cases of MOG-associated optic neuritis, myelitis, and inflammatory vasculopathy have been reported in association with COVID-19 infection (Zhou et al., 2020; Pinto et al., 2020). Case series that have systematically assessed CSF in patients with neurological symptoms associated with COVID-19 only rarely report SARS-CoV-2 in the CSF, suggesting that mechanisms other than viral replication in the CNS contribute to neuropathology (Neumann et al., 2020). One possible mechanism for neurological damage during and after COVID-19 is the presence of autoreactive antibodies targeting the CNS. This is supported by a recent study demonstrating a high frequency of CSF autoantibodies in patients with COVID-19 associated neurological symptoms (Franke et al., 2021), and by a prior case report of NMDA-receptor autoimmune encephalitis in a patient with COVID-19 (Panariello et al., 2020).
Here we report a case of MOG-antibody associated encephalitis in a patient recently infected with SARS-CoV-2. We speculate that SARS-CoV-2 infection may have triggered an autoimmune response, leading to the development of MOG-antibodies and a post-infectious encephalitis syndrome. A previous study showed that infection with the murine coronoavirus MHV (mouse hepatitis virus) A59 worsened the immunopathology in mice with MOG autoantibodies (Burrer et al., 2007). This suggests that viral infections like COVID-19 may unmask previously quiescent autoimmune pathologies. It is unclear if vaccination against SARS-CoV-2 will produce similar inflammatory responses that could potentially unmask an autoimmune response against CNS antigens. Reassuringly, previous studies have shown no evidence of other vaccines inducing CNS demyelination events at higher than expected rates (Baxter et al., 2016). It remains to be seen if this holds true with currently available vaccines against SARS-CoV-2 given their unique mechanisms of action.
Our patient did not display any of the common, respiratory symptoms of SARS-CoV-2 infection, raising the possibility that his initial presentation of COVID-19, which consisted only of severe headache, may have represented CNS inflammation during the period of acute infection. The clinical syndrome he eventually developed, consisting of unilateral FLAIR-hyperintensities, leptomeningeal enhancement, seizures, headache, and fever has previously been reported with MOG-antibody associated disease (Budhram et al., 2019). Further studies are needed to assess whether the presence of neurological symptoms during acute COVID-19 associate with later post-infectious neurological disorders.
This case expands the spectrum of parainfectious neurological consequences associated with SARS-CoV-2 infection and highlights possible links between SARS-CoV-2 and autoimmune neurologic disease.
3. Study funding
This work was supported by NIH, K23MH118999 (SFF) and K23107624 (EEL), R01AI157488 (SFF), Race to Erase MS (EEL), NWO Rubicon 019.181EN.004 (CBFV) .
4. Disclosures
J. Peters reports no disclosures relevant to the manuscript; S. Alhasan reports no disclosures relevant to the manuscript; C.B.F. Vogels reports no disclosures relevant to the manuscript; S. Farhadian reports no disclosures relevant to the manuscript, E.E. Longbrake reports no disclosures relevant to the manuscript.
References
- Baxter R., Lewis E., Goddard K., et al. Acute demyelinating events following vaccines: a case-centered analysis. Clin. Infect. Dis. 2016;63(11):1456–1462. doi: 10.1093/cid/ciw607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhram A., Mirian A., Le C., et al. Unilateral cortical FLAIR-hyperintense Lesions in anti-MOG-associated encephalitis with seizures (FLAMES): characterization of a distinct clinico-radiographic syndrome. J. Neurol. 2019;266:2481–2487. doi: 10.1007/s00415-019-09440-8. [DOI] [PubMed] [Google Scholar]
- Burrer R., Buchmeier M.J., Wolfe T., et al. Exacerbated pathology of viral encephalitis in mice with central nervous system-specific autoantibodies. Am J Pathol. 2007;170(2):557–566. doi: 10.2353/ajpath.2007.060893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, C., Ferse, C., Kreye, J., et al. 2021 High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. medRxiv 2020.07.01.20143214. [DOI] [PMC free article] [PubMed]
- Kudo E., Israelow B., Vogels C.B.F., et al. Detection of SARS-CoV-2 RNA by multiplex RT-qPCR. PLoS Biol. 2020;18 doi: 10.1371/journal.pbio.3000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann B., Schmidbauer M.L., Dimitriadis K., et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J. Neurol. Sci. 2020;418 doi: 10.1016/j.jns.2020.117090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panariello A., Bassetti R., Radice A., et al. Anti-NMDA receptor encephalitis in a psychiatric Covid-19 patient: a case report. Brain Behav. Immun. 2020;87:179–181. doi: 10.1016/j.bbi.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020:awaa240. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto A.A., Carroll L.S., Nar V., Varatharaj A., Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol. Neuroimmunol. Neuroinflamm. 2020;7(5):e813. doi: 10.1212/NXI.0000000000000813. Published 2020 Jun 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Jones-Lopez E.C., Soneji D.J., Azevedo C.J., Patel V.R. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J. Neuroophthalmol. 2020;40(3):398–402. doi: 10.1097/WNO.0000000000001049. [DOI] [PMC free article] [PubMed] [Google Scholar]