Abstract
The Coronavirus disease 2019 (COVID-19) pandemic has changed the way patients seek medical attention and how medical services are provided. We sought to compare characteristics, clinical course, and outcomes of patients presenting with acute myocardial infarction (AMI) during the pandemic compared with before it. This is a multicenter, retrospective cohort study of consecutive COVID-19 negative patients with AMI in Lithuania from March 11, 2020 to April 20, 2020 compared with patients admitted with the same diagnosis during the same period in 2019. All patients underwent angiography. Six-month follow-up was obtained for all patients. A total of 269 patients were included in this study, 107 (40.8%) of whom presented during the pandemic. Median pain-to-door times were significantly longer (858 [quartile 1=360, quartile 3 = 2,600] vs 385.5 [200, 745] minutes, p <0.0001) and post-revascularization ejection fractions were significantly lower (35 [30, 45] vs 45 [40, 50], p <0.0001) for patients presenting during vs. prior to the pandemic. While the in-hospital mortality rate did not differ, we observed a higher rate of six-month major adverse cardiovascular events for patients who presented during versus prior to the pandemic (30.8% vs 13.6%, p = 0.0006). In conclusion, 34% fewer patients with AMI presented to the hospital during the COVID-19 pandemic, and those who did waited longer to present and experienced more 6-month major adverse cardiovascular events compared with patients admitted before the pandemic.
Coronavirus disease 2019 (COVID-19) has been a matter of international concern since it was first reported in December 2019.1 As the World Health Organization (WHO) declared COVID-19 a pandemic on March 11, 2020,2 European governments responded with strict “stay at home” policies in attempts to slow the spread of the virus.3 Beginning March 11, 2020, the Lithuanian government imposed some of the most restrictive measures compared with countries with similar infection levels. Though these protocols aided in slowing the growth of the pandemic in Lithuania, they also had immediate negative impacts on the treatment of other important diseases, such as acute myocardial infarction (AMI).4 As the repercussions of the pandemic are still evolving, it is the purpose of this paper to examine the 6-month outcomes of the COVID-19 pandemic lockdown in terms of major cardiovascular adverse events (MACE) among noninfected AMI patients.
Methods
This is a multicenter, retrospective cohort study including 6 out of 10 administrative regions in the republic of Lithuania. The study involved consecutive patients with AMI (NSTEMI or STEMI) who received a negative test result for COVID-19 infection from March 11, 2020 to April 20, 2020 and underwent invasive angiography at the Hospital of the Lithuanian University of Health Sciences Kaunas Clinics or the Republican Hospital of Panevezys. The data were compared with patients admitted with the same diagnosis during the same period in 2019. Patients with AMI and a COVID-19 positive test were excluded from the study (there were 5 instances of this). Patients were followed until whichever occurred first: death or 6 months following hospital discharge. This study received approval from the Research Ethics Committee of the region of Kaunas and Panevezys.
Data collected included patient demographics, co-morbidities, medications, cardiac catheterization procedural characteristics, echocardiography results, and clinical course (length of stay, ischemic or hemorrhagic stroke, cardiopulmonary resuscitation, hypotensive shock, endotracheal intubation). We considered a composite primary end point of MACE within 6 months of hospital discharge, and also examined its individual components, as well as all-cause mortality. MACE was defined as follows: cardiovascular death, nonfatal myocardial infarction, target vessel revascularization, recurrent hospitalization due to decompensated heart failure, and stroke (ischemic or hemorrhagic).
STEMI and NSTEMI were defined according to fourth universal definition of myocardial infarction.5 Cardiogenic shock was defined as persistent hypotension (systolic blood pressure < 90 mm Hg or a mean arterial pressure 30 mm Hg below baseline) with evidence of decreased organ perfusion caused by severe right, left or biventricular dysfunction despite adequate fluid administration.6 Successful PCI was defined as the reduction of coronary artery lesion stenosis to <20%. Dyslipidemia was defined as fasting low density lipoprotein cholesterol ≥100 mg/dl. Pain-to-door time was defined as the duration (in minutes) from onset of symptoms to first medical contact at the PCI center. Door-to-wire time was defined as the time (in minutes) from first medical contact at the facility to crossing the culprit lesion with a coronary wire. Post-reperfusion left ventricular ejection fraction was assessed via echocardiography imaging within 24 hours after coronary reperfusion using Simpson's biplane method. Six-month follow-up information was obtained via telephone interview or a visit at a participating outpatient clinic.
Continuous variables were skewed and are presented as median [quartile 1, quartile 3]. Categorical variables are presented as frequency and percentage. Differences in patient and clinical characteristics between those admitted in the pandemic period versus the prepandemic period were assessed via the Wilcoxon Rank Sum Test and Chi-Square or Fisher's Exact Test, as appropriate. We examined the association between pain-to-door time and after-revascularization ejection fraction using Spearman's correlation coefficient. Differences in the primary outcome of MACE, its individual components, and all-cause mortality between study periods were assessed via Chi-Square or Fisher's Exact Test, as appropriate. We utilized multivariable logistic regression only for the composite outcome of MACE because the small number of events that occurred in this study largely prohibited statistical adjustment for other outcomes. Factors identified as having a significant association with study period (Table 1 and Table 2 ) were considered for use in the multivariable model. We utilized stepwise selection in order to identify jointly significant factors while preserving the degrees of freedom, due to the small number of events. We considered an interaction term between study period (pandemic/pre-pandemic) and AMI type (NSTEMI/STEMI).
Table 1.
Characteristics of patients presenting with acute myocardial infarction during compared with prior to the COVID-19 pandemic
| Overall |
NSTEMI |
STEMI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Pandemic (n = 107) |
Prepandemic (n = 162) | p-value | Pandemic (n = 40) |
Prepandemic (n = 76) |
p-value | Pandemic (n = 67) |
Prepandemic (n =86) |
P-value |
| Men | 71 (66.4%) | 108 (66.7%) | 0.9577 | 27 (67.5%) | 47 (61.8%) | 0.5467 | 44 (65.7%) | 61 (70.9%) | 0.4868 |
| Age (years) | 68 [61, 76] | 67 [59, 80] | 0.7499 | 69.5 [64, 80] | 70 [61, 80.5] | 0.8932 | 67 [59, 76] | 66 [59, 80] | 0.7953 |
| Obesity | 47 (43.9%) | 61 (37.7%) | 0.3045 | 20 (50.0%) | 32 (42.1%) | 0.4164 | 27 (40.3%) | 29 (33.7%) | 0.4021 |
| Killip Score | 0.0654 | 0.0691 | |||||||
| 1 | 38 (35.5%) | 70 (43.2%) | 15 (37.5%) | 35 (46.1%) | 23 (34.3%) | 35 (40.7%) | |||
| 2 | 60 (56.1%) | 66 (40.7%) | 22 (55%) | 31 (40.8%) | 38 (56.7%) | 35 (40.7%) | |||
| 3 | 4 (3.7%) | 15 (9.3%) | 3 (7.5%) | 6 (7.9%) | 1 (1.5%) | 9 (10.5%) | |||
| 4 | 5 (4.7%) | 10 (6.2%) | 0 (0%) | 3 (3.9%) | 5 (7.5%) | 7 (8.1%) | |||
| Dyslipidemia | 93 (86.9%) | 132 (81.5%) | 0.2382 | 37 (92.5%) | 67 (88.2%) | 0.5403 | 56 (83.6%) | 65 (75.6%) | 0.2273 |
| Hypertension | 95 (88.8%) | 146 (90.1%) | 0.725 | 38 (95%) | 71 (93.4%) | 1 | 57 (85.1%) | 75 (87.2%) | 0.7034 |
| Smoker | 21 (19.6%) | 33 (20.4%) | 0.8814 | 7 (17.5%) | 14 (18.4%) | 0.9025 | 14 (20.9%) | 19 (22.1%) | 0.8582 |
| Diabetes mellitus | 27 (25.2%) | 33 (20.4%) | 0.3484 | 11 (27.5%) | 19 (25%) | 0.7701 | 16 (23.9%) | 14 (16.3%) | 0.24 |
| Coronary artery disease | 45 (42.1%) | 53 (32.7%) | 0.1192 | 21 (52.5%) | 29 (38.2%) | 0.1382 | 24 (35.8%) | 24 (27.9%) | 0.2953 |
| Prior CABG | 13 (12.1%) | 5 (3.1%) | 0.0036 | 9 (22.5%) | 3 (3.9%) | 0.0032 | 4 (6%) | 2 (2.3%) | 0.2492 |
| COPD | 1 (0.9%) | 5 (3.1%) | 0.4074 | 0 (0%) | 3 (3.9%) | 0.5502 | 1 (1.5%) | 2 (2.3%) | 1 |
| Peripheral arterial disease | 4 (3.7%) | 3 (1.9%) | 0.4414 | 3 (7.5%) | 1 (1.3%) | 0.1176 | 1 (1.5%) | 2 (2.3%) | 1 |
| Cerebrovascular disease | 9 (8.4%) | 12 (7.4%) | 0.7639 | 4 (10%) | 6 (7.9%) | 0.7352 | 5 (7.5%) | 6 (7%) | 1 |
| Dementia | 1 (0.9%) | 1 (0.6%) | 1 | 0 (0%) | 0 (0%) | - | 1 (1.5%) | 1 (1.2%) | 1 |
Obesity = Body Mass Index ≥ 30 kg/m2; Dyslipidemia = fasting low density lipoprotein cholesterol ≥100 mg/dl; CABG = Coronary artery bypass Graft surgery; COPD = Chronic obstructive pulmonary disease
Table 2.
Clinical course and in-hospital clinical outcomes of patients presenting with acute myocardial infarction during compared with prior to the COVID-19 pandemic
| Overall |
NSTEMI |
STEMI |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Pandemic (n = 107) |
Prepandemic (n = 162) | p-value | Pandemic (n = 40) |
Prepandemic (n = 76) |
p value | Pandemic (n = 67) |
Prepandemic (n =86) |
p value | |
| Pain to door time (mins) | 858 [360, 2600] | 385.5 [200, 745] | <.0001 | 2021 [960, 5746] | 558 [369, 882.5] | <.0001 | 582 [180, 1212] | 262 [120, 525] | 0.0003 | |
| Door to wire time (mins) | 101 [64, 273] | 94 [49, 194] | 0.137 | 302.5 [179, 600] | 200.5 [98, 434.5] | 0.0948 | 75 [53.5, 106.5] | 71 [43, 119] | 0.2257 | |
| Number of narrowed coronary arteries | 2 [1, 3] | 2 [1, 3] | 0.9455 | 2 [2, 3] | 2 [1, 3] | 0.4242 | 2 [1, 3] | 2 [1, 3] | 0.3836 | |
| Ejection fraction after PCI (%) | 35 [30, 45] | 45 [40, 50] | <.0001 | 37.5 [25, 45] | 47 [40, 50] | 0.0032 | 35 [30, 45] | 42 [35, 50] | 0.0003 | |
| Reperfusion strategy | 0.0741 | 0.0832 | ||||||||
| CABG | 4 (3.7%) | 17 (10.5%) | 2 (5%) | 14 (18.4%) | 2 (3%) | 3 (3.5%) | ||||
| PCI | 98 (91.6%) | 133 (82.1%) | 34 (85%) | 51 (67.1%) | 64 (95.5%) | 82 (95.3%) | ||||
| Medical therapy | 5 (4.7%) | 12 (7.4%) | 4 (10%) | 11 (14.5%) | 1 (1.5%) | 1 (1.2%) | ||||
| PCI access | 0.1658 | |||||||||
| Femoral | 22 (20.6%) | 24 (14.8%) | 11 (27.5%) | 9 (11.8%) | 11 (16.4%) | 15 (17.4%) | ||||
| Right radial | 79 (73.8%) | 134 (82.7%) | 23 (57.5%) | 66 (86.8%) | 56 (83.6%) | 68 (79.1%) | ||||
| Left proximal radial | 6 (5.6%) | 4 (2.5%) | 6 (15%) | 1 (1.3%) | 0 (0%) | 3 (3.5%) | ||||
| Culprit vessel 2,7,1,6,1,1 | 0.0083 | 0.2639 | 0.0092 | |||||||
| No culprit | 9 (8.4%) | 19 (11.7%) | 5 (12.5%) | 17 (22.4%) | 4 (6%) | 2 (2.3%) | ||||
| Right coronary artery | 25 (23.4%) | 38 (23.5%) | 6 (15%) | 6 (7.9%) | 19 (28.4%) | 32 (37.2%) | ||||
| Left main | 4 (3.7%) | 24 (14.8%) | 3 (7.5%) | 12 (15.8%) | 1 (1.5%) | 12 (14%) | ||||
| LAD | 49 (45.8%) | 46 (28.4%) | 14 (35%) | 19 (25%) | 35 (52.2%) | 27 (31.4%) | ||||
| Left circumflex | 18 (16.8%) | 28 (17.3%) | 11 (27.5%) | 16 (21.1%) | 7 (10.4%) | 12 (14%) | ||||
| Successful PCI0,6,0,5,0,1 | 93 (86.9%) | 129 (79.6%) | 0.3536 | 33 (82.5%) | 52 (68.4%) | 0.2687 | 60 (89.6%) | 77 (89.5%) | 0.8316 | |
| Troponin I (µg/l) | 7.8 [2.6, 37.2] | 4.5 [1.1, 25.4] | 0.013 | 2.7 [1.5, 10.4] | 2.5 [0.6, 9.7] | 0.3248 | 25.0 [4.9, 61.8] | 9.0 [2.2, 32.5] | 0.0535 | |
| Length of stay (days) | 6 [5, 7] | 7 [5, 7] | 0.4931 | 6 [5, 7] | 6 [4, 8] | 0.4488 | 6 [5, 8] | 7 [5, 7] | 0.0755 | |
| In-hospital hemorrhagic stroke | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - | |
| In-hospital ischemic stroke | 3 (2.8%) | 0 (0%) | 0.0619 | 2 (5%) | 0 (0%) | 0.1169 | 1 (1.5%) | 0 (0%) | 0.4379 | |
| In-hospital CPR | 6 (5.6%) | 9 (5.6%) | 0.9855 | 3 (7.5%) | 4 (5.3%) | 0.6306 | 3 (4.5%) | 5 (5.8%) | 1 | |
| Cardiogenic shock | 10 (9.3%) | 12 (7.4%) | 0.5702 | 5 (12.5%) | 6 (7.9%) | 0.5088 | 5 (7.5%) | 6 (7%) | 1 | |
| In-hospital re-infarction | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - | 0 (0%) | 0 (0%) | - | |
| In-hospital death | 6 (5.6%) | 9 (5.6%) | 0.9855 | 3 (7.5%) | 4 (5.3%) | 0.6909 | 3 (4.5%) | 5 (5.8%) | 1 | |
CABG = coronary artery bypass graft surgery; CPR = cardiopulmonary resuscitation; PCI = percutaneous coronary intervention; LAD = left anterior descending. Numbers in superscripts indicate missing data in each of the 6 groups; missing data for successful PCI indicates PCI was not attempted.
Results
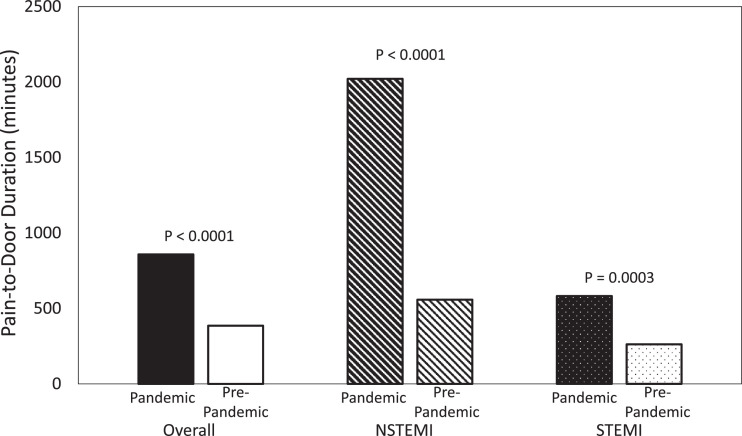
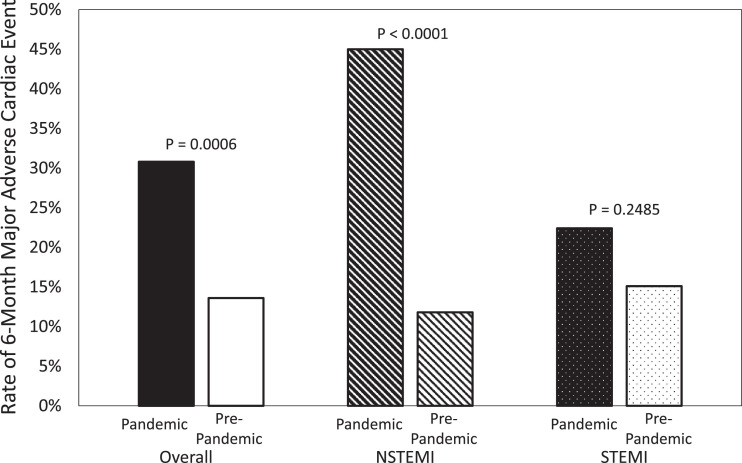
A total of 269 patients were analyzed in this study, with 107 (40.8%) presenting in the pandemic period and 162 (60.2%) presenting in the pre-pandemic period. Neither age, nor obesity, nor male prevalence, nor co-morbidity burden differed significantly between the two time periods (Table 1); however, pain-to-door times (Table 2; Figure 1 ) and troponin I levels were significantly higher for patients presenting during the pandemic compared with before it. Post-revascularization ejection fraction was significantly lower for patients during the pandemic (Table 2). Additionally, we detected a weak negative correlation between pain-to-door and post-revascularization ejection fraction (Spearman r = -0.21, p = 0.0005). In-hospital mortality outcomes did not differ significantly between the two study timeframes (Table 2). We observed a significantly higher rate of 6-month MACE for patients treated during the pandemic compared to those treated before the pandemic (Table 3 ; Figure 2 ).
Figure 1.
Median durations of pain-to-door displayed by study period and type of acute myocardial infarction.
Table 3.
Six-month clinical outcomes of patients presenting with acute myocardial infarction during compared with prior to the COVID-19 pandemic
| Overall |
NSTEMI |
STEMI |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pandemic (n = 107) |
Prepandemic (n = 162) | p-value | Pandemic (n = 40) |
Prepandemic (n = 76) |
pvalue | Pandemic (n = 67) |
Prepandemic (n =86) |
p Value | |
| Ischemic or hemorrhagic stroke | 2 (1.9%) | 1 (0.6%) | 0.565 | 2 (5%) | 0 (0%) | 0.1169 | 0 (0%) | 1 (1.2%) | 1 |
| Nonfatal MI | 5 (4.7%) | 3 (1.9%) | 0.2719 | 1 (2.5%) | 2 (2.6%) | 1 | 4 (6%) | 1 (1.2%) | 0.1688 |
| Cardiovascular death | 11 (10.3%) | 11 (6.8%) | 0.3066 | 7 (17.5%) | 5 (6.6%) | 0.1054 | 4 (6%) | 6 (7%) | 1 |
| TVR | 7 (6.5%) | 5 (3.1%) | 0.2299 | 3 (7.5%) | 2 (2.6%) | 0.3382 | 4 (6%) | 3 (3.5%) | 0.6997 |
| Decompensated HF requiring hospitalization | 23 (21.5%) | 4 (2.5%) | <.0001 | 12 (30%) | 1 (1.3%) | <.0001 | 11 (16.4%) | 3 (3.5%) | 0.0059 |
| MACE | 33 (30.8%) | 22 (13.6%) | 0.0006 | 18 (45%) | 9 (11.8%) | <0.0001 | 15 (22.4%) | 13 (15.1%) | 0.2485 |
| All-cause mortality | 11 (10.3%) | 13 (8%) | 0.5253 | 7 (17.5%) | 6 (7.9%) | 0.1328 | 4 (6%) | 7 (8.1%) | 0.7565 |
HF = Heart failure; MACE = The composite of stroke; MI = Myocardial infarction; TVR = Target vessel revascularization; nonfatal myocardial infarction, revascularization, heart failure hospitalization, and cardiovascular death
Figure 2.
Rates of major adverse cardiac events displayed by study period and type of acute myocardial infarction.
The model building process for the multivariable logistic regression (for the outcome of 6-month MACE) confirmed the findings discussed above and in Table 2; ejection fraction after-revascularization was closely associated with time period (pandemic or prepandemic), and pain-to-door was closely associated with time period and type of AMI (NSTEMI/STEMI). As such, including all four of these variables in the model was redundant and caused instability (Hosmer-Lemeshow p < 0.05). We found that, while the model preferentially selected the continuous variables of ejection fraction and pain-to-door instead of the categorical variables of AMI type and time period, the model did not fit well (Hosmer-Lemeshow p < 0.05) with them in it, so we proceeded with the categorical variables. We found evidence of a differential effect of presentation during the COVID-19 pandemic on MACE according to the type of AMI (interaction p = 0.0393), with patients presenting with NSTEMI during the pandemic having the worst outcomes (odds ratio (OR) for patients with NSTEMI = 6.0, 95% confidence interval (CI) = (2.36 - 15.28), OR for patients with STEMI = 1.62, 95% CI = (0.71 - 3.69)). The overall effect of admission during the COVID-19 pandemic yielded more than a doubling of risk for 6-month MACE (OR = 2.36, 95% CI = (1.26 - 4.43)) compared with admission before the pandemic.
A total of 116 patients with NSTEMI were analyzed in this study, with 40 (34.5%) in the pandemic period and 76 (65.5%) in the prepandemic period. Patients had a significantly prolonged pain-to-door time during the pandemic than prior to it, and post-revascularization ejection fraction was lower for patients presenting during the pandemic (Table 2). Pain-to-door and door-to-revascularization durations were associated with MACE (p < 0.0001, p = 0.0139, respectively). NSTEMI patients during the pandemic generally had worse outcomes at 6 months than patients before the pandemic (Table 3, Supplemental Figure 1); there were 18 (45%) and 9 (11.8%) patients who had MACE within 6 months of admission during and before the pandemic, respectively (p < 0.0001) (Table 3, Figure 2).
A total of 153 patients with STEMI were analyzed in this study, with 67 (43.8%) in the pandemic period and 86 (56.2%) in the pre-pandemic period. While patients waited significantly longer at home before presenting to the hospital during the pandemic, there was no evidence to suggest that the door-to-wire times differed (Table 2, Figure 1). We detected moderate negative correlations between post-revascularization ejection fraction and pain-to-door and door-to-wire times (spearman r = -0.452, -0.283, p <0.0001, p = 0.0005, respectively); both of these variables were significantly associated with MACE (p's < 0.0001). There was not a significant difference in rates of MACE between those admitted during vs. before the pandemic (p = 0.2485); however, patients admitted during the pandemic had a higher rate of re-hospitalization for heart failure (16.4% vs 3.5%, p = 0.0059) (Table 3, Figures 2 and Supplemental Figure 1).
Discussion
In this study of 269 AMI patients, we observed a 34.0% decline in admissions during the early phase of the pandemic compared with the same period a year prior. This decrease may be partly attributed to the movement restrictions which were instituted by governments, and to the extensive media coverage which amplified patients’ fear of contracting COVID-19 and precluded them from seeking timely medical care.9 , 8 STEMI admissions dropped by 22.1%, while NSTEMI dropped by 47.4%. We hypothesize that the drop in NSTEMI presentations was larger than that of STEMI because STEMI patients tend to have more acute and intense symptoms compared with NSTEMI patients.7 Similarly, this hypothesis explains why the median pain-to-door time more than doubled for STEMI patients during the pandemic and increased by more than fourfold for NSTEMI patients (Table 2, Figure 1). Our findings are consistent with previous studies conducted in Europe.4 , 10 , 11
Health care facilities around the world have had to adopt pandemic-specific procedures to slow the spread of the disease. In order to increase safety in the healthcare delivery setting, pandemic-specific protocols have been implemented to protect both patients and healthcare professionals by preventing the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).12 , 13 For example, new protocols have proposed quick revascularization for patients diagnosed with STEMI (i.e., without waiting for COVID-19 test results), while patients with stable NSTEMI are treated less swiftly (i.e., entering the catheterization lab after receiving COVID-19 test results).12 , 14 Our findings suggest that these protocols likely contributed to longer door-to-wire times in NSTEMI patients, while not significantly affecting the door-to-wire times in STEMI patients.
Furthermore, we detected a significant decrease in post-revascularization ejection fraction in AMI patients during the pandemic era compared with the year prior (Table 2). This is likely attributed to a prolonged ischemic state, as evidenced by longer pain-to-door time and door-to-wire time. This is consistent with results from previous reports, which revealed that longer ischemia duration is associated with greater infarct size and lower ejection fraction levels.15, 16, 17 Similarly, Cerrato et al. (2017) reported that AMI patients with delays in presentation had lower left ventricular systolic function and higher rates of acute decompensated heart failure.18
In parallel, our data showed that patients who presented during the pandemic (with a significantly longer median pain-to-revascularization time) had worse left ventricular systolic function, and a higher rate of MACE and readmission due to decompensated acute heart failure compared with patients who presented before the pandemic, indicating a worse prognosis for AMI patients during the pandemic era compared with the pre-pandemic (Tables 2 and 3, Figures 1 and 2 and Supplemental Figure 1)
In this study, we observed more than a doubling in overall risk for MACE during the pandemic period compared with the year prior; this finding was exaggerated for patients with NSTEMI, with the risk increasing by nearly four- fold (Table 3, Figure 2). Additionally, cardiovascular mortality rates were higher during the pandemic vs. pre-pandemic for patients with NSTEMI, but not for patients with STEMI. This may be partially explained by the fact that STEMI patients waited less than NSTEMI patients, and were provided with special care, extensive follow-up, and a prolonged rehabilitation program, regardless of pandemic.19 Moreover, the re-hospitalization rates due to decompensated heart failure were significantly higher for patients who had AMI during the COVID-19 pandemic, which was to be expected, given the lower left ventricular systolic function observed after reperfusion (Tables 2 and 3, Supplemental Figure 1). We observed a twenty-fold increase in risk of heart failure hospitalization during the pandemic period compared with the year prior for patients treated for NSTEMI; this finding was less amplified for patients with STEMI who had a four-fold increase in risk of heart failure hospitalization (Table 3, Supplemental Figure 1).
This study has all the limitations of a small retrospective, observational, database study. The relatively small sample size, particularly when considering subgroups, may have corresponded to a lack of statistical power to detect meaningful differences. Similarly, the patients in this study experienced a relatively small number of adverse events, which limited our ability to examine statistical models with adjustment for confounding variables; however, the cohorts were fairly similar in terms of demographics and baseline risk. Further, due to the nature of this study, we are unable to draw conclusions of causality, and while we offer a possible explanation of patients experiencing prolonged ischemia by waiting at home for a longer period of time before presenting to the hospital during the pandemic as a contributing factor for worse clinical outcomes, we are unclear if there are additional factors (e.g., change in diet, inability to exercise, mental and/or emotional impact of social isolation) that may have emerged during the pandemic to also affect that relationship.
In conclusion, this multicenter study of patients treated in the republic of Lithuania reveals that the lockdown during the early period of the COVID-19 pandemic may be associated with a significant decline in hospital admissions for patients with AMI, as well as a significantly longer time of ischemia. Longer ischemic times were associated with worse left ventricular systolic function after-revascularization. Patients presenting with AMI during the pandemic were at higher risk for developing 6-month MACE and for being re-hospitalized for decompensated heart failure within 6-months.
Author Contribution
Ali Aldujeli M.D., M.Sc: Supervision, Conceptualization, Data curation, Methodology, Writing-Original Draft preparation, Writing- Reviewing and Editing. Anas Hamadeh M.D.: Supervision, Conceptualization, Investigation, Validation, Writing- Reviewing and Editing. 4Kristen M. Tecson Ph.D.: Supervision, Formal Analysis, Conceptualization, Writing- Reviewing and Editing, Visualization. Zilvinas Krivickas: Investigation- Data collection, Validation, Writing- Reviewing and Editing. Laurynas Maciulevicius: Investigation- Data collection, Validation, Writing- Reviewing and Editing. Simas Stiklioraitis: Investigation- Data collection, Writing- Reviewing and Editing. Marius Sukys M.D., M.Sc: Conceptualization, Formal Analysis, Writing- Reviewing and Editing. Kasparas Briedis M.D., M.Sc: Investigation- Data collection, Writing- Reviewing and Editing. Montazar Aldujeili M.D.: Writing- Reviewing and Editing. Kamilija Briede M.D. M.Sc: Writing- Reviewing and Editing. Rima Braukyliene, M.D., M.Sc: Investigation- Data collection, Writing- Reviewing and Editing. Andrius Pranculis M.D., M.Sc., Ph.D.: Writing- Reviewing and Editing. Ramunas Unikas M.D., M.Sc., Ph.D: Writing- Reviewing and Editing. Diana Zaliaduonyte M.D., M.Sc., PhD.: Writing- Reviewing and Editing. Peter A. McCullough M.D., M.P.H: Writing- Reviewing and Editing.
Disclosures
The authors have no disclosures.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded, in part, by the Baylor Health Care System Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2021.01.043.
Appendix. Supplementary materials
Supplemental Figure 1. Rates of individual major adverse cardiac events displayed by study period and type of acute myocardial infarction
REFERENCES
- 1.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, Yuan Q, Xiao X. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39:1011–1019. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020:m1036. doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 3.Taghrir MH, Akbarialiabad H, Ahmadi Marzaleh M. Efficacy of mass quarantine as leverage of health system governance during COVID-19 outbreak: a mini policy review. Arch Iran Med. 2020;23:265–267. doi: 10.34172/aim.2020.08. [DOI] [PubMed] [Google Scholar]
- 4.Aldujeli A, Hamadeh A, Briedis K, Tecson KM, Rutland J, Krivickas Z, Stiklioraitis S, Briede K, Aldujeili M, Unikas R, Zaliaduonyte D, Zaliunas R, Vallabhan RC, McCullough PA. Delays in presentation in patients with acute myocardial infarction during the COVID-19 pandemic. Cardiol Res. 2020;11:386–391. doi: 10.14740/cr1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018) Circulation. 2018;138 doi: 10.1161/CIR.0000000000000617. Available at: [DOI] [PubMed] [Google Scholar]
- 6.Giannuzzi P, Imparato A, Temporelli PL, Vito F de, Silva PL, Scapellato F, Giordano A. Doppler-derived mitral deceleration time of early filling as a strong predictor of pulmonary capillary wedge pressure in postinfarction patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 1994;23:1630–1637. doi: 10.1016/0735-1097(94)90667-x. [DOI] [PubMed] [Google Scholar]
- 7.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng Z-J. NRMI investigators for the. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parmet WE, Sinha MS. Covid-19 — the law and limits of quarantine. N Engl J Med. 2020;382:e28. doi: 10.1056/NEJMp2004211. [DOI] [PubMed] [Google Scholar]
- 9.Wong L, Hawkins J, Langness S, Murrell KL, Iris P, Sammann A. Where are all the patients? Addressing Covid-19 fear to encourage sick patients to seek emergency care. NEJM Catal Innov Care Del. 2020 doi: 10.1056/CAT.20.0193. [DOI] [Google Scholar]
- 10.Wu J, Mamas M, Rashid M, Weston C, Hains J, Luescher T, Belder MA de, Deanfield JE, Gale CP. Patient response, treatments, and mortality for acute myocardial infarction during the COVID-19 pandemic. Eur Heart J Qual Care Clin Outcomes. 2020:qcaa062. doi: 10.1093/ehjqcco/qcaa062. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJ. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852–1853. doi: 10.1093/eurheartj/ehaa314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briedis K, Aldujeli A, Aldujeili M, Briede K, Zaliunas R, Hamadeh A, Stoler RC, McCullough PA. Considerations for management of acute coronary syndromes during the SARS-CoV-2 (COVID-19) pandemic. Am J Cardiol. 2020;131:115–119. doi: 10.1016/j.amjcard.2020.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmud E, Dauerman HL, Welt FGP, Messenger JC, Rao SV, Grines C, Mattu A, Kirtane AJ, Jauhar R, Meraj P, Rokos IC, Rumsfeld JS, Henry TD. Management of acute myocardial infarction during the COVID -19 pandemic. Catheter Cardiovasc Interv. 2020;96:336–345. doi: 10.1002/ccd.28946. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Zeng H, Jiang H, Yang Y, Yuan Z, Cheng X, Jing Z, Liu B, Chen J, Nie S, Zhu J, Li F, Ma C. CSC expert consensus on principles of clinical management of patients with severe emergent cardiovascular diseases during the COVID-19 epidemic. Circulation. 2020;141:e810–e816. doi: 10.1161/CIRCULATIONAHA.120.047011. [DOI] [PubMed] [Google Scholar]
- 15.Guerchicoff A, Brener SJ, Maehara A, Witzenbichler B, Fahy M, Xu K, Gersh BJ, Mehran R, Gibson CM, Stone GW. Impact of delay to reperfusion on reperfusion success, infarct size, and clinical outcomes in patients with ST-segment elevation myocardial infarction. JACC: Cardiovasc Interv. 2014;7:733–740. doi: 10.1016/j.jcin.2014.01.166. [DOI] [PubMed] [Google Scholar]
- 16.Denktas AE, Anderson HV, McCarthy J, Smalling RW. Total ischemic time. JACC: Cardiovascular Interventions. 2011;4:599–604. doi: 10.1016/j.jcin.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 17.McNair PW, Bilchick KC, Keeley EC. Very late presentation in ST elevation myocardial infarction: predictors and long-term mortality. IJC Heart & Vasculature. 2019;22:156–159. doi: 10.1016/j.ijcha.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cerrato E, Forno D, Ferro S, Chinaglia A. Characteristics, in-hospital management and outcome of late acute ST-elevation myocardial infarction presenters. J Cardiovasc Med. 2017;18:567–571. doi: 10.2459/JCM.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 19.Hall M, Laut K, Dondo TB, Alabas OA, Brogan RA, Gutacker N, Cookson R, Norman P, Timmis A, Belder M de, Ludman PF, Gale CP. Patient and hospital determinants of primary percutaneous coronary intervention in England, 2003–2013. Heart. 2016;102:313–319. doi: 10.1136/heartjnl-2015-308616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Rates of individual major adverse cardiac events displayed by study period and type of acute myocardial infarction