Abstract
A novel infectious disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was detected in December 2019 and declared as a global pandemic by the World Health. Approximately 15% of patients with COVID-19 progress to severe pneumonia and eventually develop acute respiratory distress syndrome (ARDS), septic shock and/or multiple organ failure with high morbidity and mortality. Evidence points towards a determinant pathogenic role of members of the renin–angiotensin system (RAS) in mediating the susceptibility, infection, inflammatory response and parenchymal injury in lungs and other organs of COVID-19 patients. The receptor for advanced glycation end-products (RAGE), a member of the immunoglobulin superfamily, has important roles in pulmonary pathological states, including fibrosis, pneumonia and ARDS. RAGE overexpression/hyperactivation is essential to the deleterious effects of RAS in several pathological processes, including hypertension, chronic kidney and cardiovascular diseases, and diabetes, all of which are major comorbidities of SARS-CoV-2 infection. We propose RAGE as an additional molecular target in COVID-19 patients for ameliorating the multi-organ pathology induced by the virus and improving survival, also in the perspective of future infections by other coronaviruses.
Keywords: SARS-CoV-2, COVID-19, RAGE, HMGB1, Renin-angiotensin system (RAS)
Graphical abstract
1. Introduction
Several members of the Coronaviridae family (i.e. HIV, hepatitis B and C, and influenza viruses), constantly circulate in the human population usually causing mild respiratory diseases [1]. In contrast, the severe acute respiratory syndrome coronavirus (SARS-CoV) is transmitted from animals to humans and causes severe consequences in affected individuals [1,2]. SARS emerged for the first time in 2002 in China where the human transmission to horseshoe bats, which are the natural reservoir hosts for SARS-CoV [3], was extremely facilitated by intermediate hosts like civets, cats and raccoon dogs, which are frequently sold as food sources in Chinese wet markets [4]. In December 2019, a new infectious respiratory disease called coronavirus disease 2019 (COVID-19), which is caused by SARS-CoV-2, emerged in Wuhan (Hubei, China) and rapidly spread all over the world, forcing the World Health Organization to officially declare a global pandemic [5,6].
Although most patients with COVID-19 exhibit mild to moderate symptoms, approximately 15% develop severe pneumonia, acute respiratory distress syndrome (ARDS), septic shock and/or multiple organ failure [5,7] with high morbidity and mortality.
Unfortunately, at the beginning of the pandemic neither vaccines or antiviral drugs were available to treat the first SARS pandemic, which has been being counteracted with conventional control measures, including travel restrictions, interpersonal distance, and patient isolation. Consequently, from April 2020 the Food and Drug Administration (FDA) has created a special emergency program for possible coronavirus therapies, i.e. the Coronavirus Treatment Acceleration Program [8]. Nowadays, five different pharmacologic managements have been approved by FDA to treat COVID-19 patients, based on the severity of disease. For mild-to-moderate COVID-19 patients who are at high risk of severe progression, but not requiring hospitalization or supplemental oxygen, two monoclonal antibodies with different mechanisms of action have been approved [9,10]: Bamlanivimab (LY-CoV555) binds to the receptor binding domain (RBD) of the SARS-CoV-2 spike protein, whereas Casirivimab plus imdevimab (REGN-COV2) binds to non-overlapping regions of the SARS-CoV-2 RBD [9,10]. For COVID-19 patients requiring hospitalization, but not supplemental oxygen, the antiviral drug Remdesivir (Veklury), which inhibits the RNA polymerase essential for viral replication, has been approved [11]. When COVID-19 patients require supplemental oxygen (but not invasive ventilation), a combination of Veklury and dexamethasone is recommended [12]. Lastly, to treat hospitalized COVID-19 patients requiring mechanical ventilation, dexamethasone treatment has been approved, since it might modulate inflammation-mediated lung injury and thereby reduces the progression towards respiratory failure and death [12].
Recently, FDA has emanated an Emergency Use Authorization (EUA) to permit the use of three vaccines by Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273) and AstraZeneca (ChAdOx1-S or AZD1222) for the prevention of COVID-19 [[13], [14], [15]]. The vaccines by Pfizer-BioNTech and Moderna are based on a new technology using a messenger RNA (mRNA) that encodes the full-length SARS-CoV-2 spike protein, which enables the virus to enter cells [3,13,14]. AstraZeneca vaccine is a conventional replication-deficient simian adenovirus vector (ChAdOx1) containing the full-length codon-optimized coding sequence of the spike protein [15]. After vaccination, the spike protein is produced prompting the immune system to attack the coronavirus in future infections. The vaccines are administered by two intramuscular injections and are suitable in people over 16 for Pfizer-BioNTech or over 18 years for Moderna and AstraZeneca.
In addition to approved strategies, other options can be envisaged to control or prevent emerging infections of SARS-CoV-2, including renin-angiotensin system (RAS) signalling inhibitors. Indeed, the binding of the spike viral protein with the recognized receptor, angiotensin-converting enzyme 2 (ACE2) and subsequent viral-dependent ACE2 down-regulation, leads to uncontrolled accumulation of angiotensin (Ang) II, which mediates the inflammatory response and parenchymal injury in lungs and other organs of COVID-19 patients through the type 1 angiotensin receptor (AT1R) [[16], [17], [18]].
In this context, we focus on the receptor for advanced glycation end-products (RAGE), a member of the immunoglobulin superfamily, as a possible molecular target to alleviate the pathology induced by SARS-CoV-2 and improve the survival of infected patients. RAGE was characterized in 1992 in endothelial cells and owes its name to its ability to bind advanced glycation end-products (AGEs), which are adducts formed by glycoxidation accumulating in several disorders [19]. However, RAGE is a receptor able to bind multiple ligands, many of which can be defined as pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) [20]. Contrary to the developmental stage, during which RAGE is highly expressed in many tissues, in the majority of healthy adult tissues RAGE is expressed at a very low basal level or not expressed at all, and up-regulation of RAGE in the adulthood has been associated with a wide range of pathological events [21]. Uniquely, adult skin [22] and pulmonary tissue express remarkably high basal levels of RAGE, and numerous studies indicate important roles for RAGE in both lung physiology and pathological states, including pulmonary fibrosis, pneumonia and ARDS [23,24], which are all major consequences of SARS-CoV-2 infection. An important functional crosstalk has been reported between RAGE and AT1R pathways in several pathogenic processes, including diabetic, atherosclerosis and nephropathy, and endothelial and cardiac dysfunction, in which RAGE overexpression/hyperactivation seems to be essential to the deleterious effects of RAS [[25], [26], [27]]. In this review we summarize the hypotheses and the experimental evidences about the crucial role of RAGE axis in COVID-19 pathogenesis [28,29], and propose a potential molecular mechanism used by the receptor to induce the hallmarks of severe COVID-19 in susceptible subjects infected by SARS-CoV-2. RAGE hyperactivity might impose unresolved lung inflammation leading to ARDS, but also might induce multi-organ damage effects by sustaining RAS hyperactivation in different tissues. We propose targeting RAGE as a novel therapeutic strategy to restrain or prevent severe COVID-19, and to reduce the adverse effects of RAS hyperactivation, avoiding compromising the physiological role of RAS in the maintenance of body homeostasis, which limits the use of conventional RAS blockade.
2. SARS-CoV-2 pathogenesis
The main pathogenic feature of COVID-19 is severe pneumonia evolving in ARDS [5,30], which is characterized by diffuse alveolar damage with formation of hyaline membranes, infiltration of mononuclear cells and macrophages in air spaces, and diffuse thickening of the alveolar wall. Viral particles were detected in type I and type II pneumocytes in foci of diffuse alveolar damage, and in ciliated epithelial cells of nasal, bronchial and bronchiolar mucosae [[31], [32], [33]]. SARS-CoV-2 infectious virus particles have been isolated also from faeces and urine of COVID-19 patients [33,34], and human testes are considered an additional target and reservoir of SARS-CoV-2 [6]. In addition to lung damage, RNAemia, ground-glass opacities in lung X-ray films, acute cardiac injury, spleen atrophy, hilar lymph node necrosis, focal hemorrhage in the kidney, enlarged liver with inflammatory cell infiltration, oedema and scattered degeneration of central neurons were detected in some patients [31].
In mild COVID-19 patients, resident lung macrophages initiate an efficient innate and adaptive inflammatory response that is able to curb the SARS-CoV-2 replication and infection, so that the patients with either no or only mild pneumonia recover quickly. In severe or critical COVID-19 cases, alveolar macrophages and/or epithelial cells, which result basally sub-clinical activated by the presence of various comorbidities/conditions underpinning the susceptibility to the virus infection, produce excessive levels of proinflammatory cytokines and chemokines that massively attract monocytes and neutrophils to the infection site to clear the virus particles and infected cells, resulting in an uncontrolled innate inflammatory response. This unsuccessful response seems due to the delay in the production/action of type I interferons (IFNs) cytokines machinery, which is essential for a successful virus depletion in early phase of the infection and the induction of effective adaptive immune response, i.e. activation of T cell response. The defective IFNs signalling activation leads to abnormal production of cytokines that seems the principal mechanism at the basis of SARS-CoV-2 escape. In this condition, SARS-CoV-2 disrupts the integrity of the epithelial–endothelial (air–blood) barrier by attacking epithelial cells as well as lung capillary endothelial cells, leading to a large amount of plasma exudate in the alveolar cavity and consequent respiratory distress [35,36].
In accordance with this hypothesis, no severe cases were reported in children, in which the inflammatory system is not basally activated and innate immune response is highly effective, whereas adults with underlying pro-inflammatory chronic status due to a setting of comorbidities, including hypertension, diabetes, coronary heart disease and cerebrovascular disease, especially in elderly, are particularly susceptible to virus infection and evolution to a severe or fatal COVID-19 [5,35,36].
Most severe COVID-19 patients exhibit substantially elevated serum levels of proinflammatory cytokines and chemokines, including IL (interleukin)-6 and IL-1ß, as well as IL-2, IL-8, IL-17, G-CSF (granulocyte-colony stimulating factor), GM-CSF (granulocyte-macrophage colony-stimulating factor), IP10 (interferon gamma-induced protein 10), MCP (monocyte chemoattractant protein) 1, MIP (macrophage inflammatory protein) 1a and TNF (tumor necrosis factor)-α, that define a cytokine storm. High proinflammatory cytokines cause shock and tissue damage locally in the lung and systemically in the heart, liver and kidney, leading to respiratory failure and multiple organ failure. Also, C-reactive protein and D-dimer are found abnormally high [5,[35], [36], [37]]. The observed spleen atrophy and lymph node necrosis found in deceased COVID-19 patients are indicative of immune-mediated damage [35].
Due to the “virus escape” from adaptive immune response and the chronic pulmonary recruitment of circulating immune cells, lymphopenia is a common feature in patients with severe but not mild or recovered COVID-19, with drastically reduced numbers of CD4+ T cells, CD8+ T cells, B cells, and natural killer (NK) cells, as well as reduced percentages of circulating monocytes, eosinophils and basophils [5,[35], [36], [37]]. Antibodies against the SARS-CoV-2 spike glycoprotein has been detected in recovered or convalescent patients [38]. Notably, AIDS patients with deficient immune system are somewhat resistant to SARS infection, raising the possibility that an excessive immune response is responsible for the lethality in patients who die of SARS [39].
Besides specific vaccines to counter SARS-CoV-2 morbidity and mortality [[13], [14], [15]], several approaches to treat COVID-19 are under active investigation waiting to be validated and standardized. The combination of different strategies pointing to prevent the virus entry, to block viral replication, or to reduce excessive immune response to avoid ARDS, may represent a more efficacious approach against SARS-CoV-2 than using single modalities, also in consideration of the virus variants spreading [40,41].
3. SARS-CoV-2 mechanism of action
Similar to previous SARS-CoV, superficial spike proteins of SARS-CoV-2 recognize and bind the human, pig, civet (but not mouse) angiotensin-converting enzyme 2 (ACE2), which is a type I transmembrane metallocarboxypeptidase enzyme within the RAS cascade, to gain entry into ACE2 expressing cells [42] (Fig. 1 ). Indeed, treatment of Vero-E6 cells, a monkey kidney cell line that permits SARS-CoV replication, with a specific anti-ACE2 antibody blocked the entry of SARS-CoV-2 spike proteins. The affinity of SARS-CoV-2 for ACE2 is 10–20-fold higher than that of SARS-CoV, which could explain its higher transmissibility. Binding of the spike protein to ACE2, along with proteolytic cleavage of ACE2 by transmembrane serine protease 2 (TMPRSS2), facilitates the entry of the virus into cells, viral replication and cell-to-cell transmission [42].
Fig. 1.
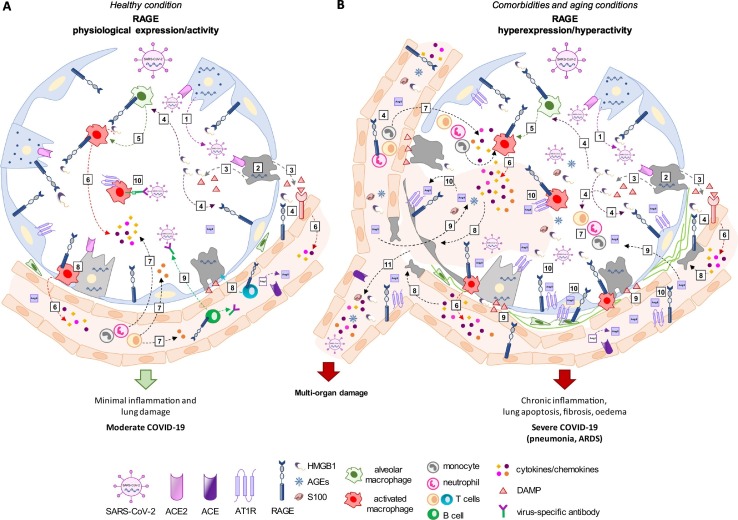
Overview of RAGE (receptor for advanced glycation end-products) activity in coronavirus disease 2019 (COVID-19).
(A) SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) enters healthy subjects through the respiratory tract and infects secretory lung cells expressing the surface receptor ACE2 (angiotensin-converting enzyme 2) (1). The replication and release of virus cause pyroptosis of host cells (2) and consequent release of DAMPs (damage-associated molecular patterns), including HMGB1 (high mobility group 1), which are recognized by receptors, including RAGE (3,4), expressed in neighbouring epithelial cells, endothelial cells and alveolar macrophages. RAGE/HMGB1 signalling sustains the activation of alveolar macrophages and endothelial cells (5) triggering the generation of pro-inflammatory cytokines and chemokines (6), which attract monocytes, neutrophils and T cells to the site of infection promoting further inflammation (7). Alveolar macrophages and virus-specific T cells eliminate apoptotic and infected cells, respectively (8), before the virus spreads. Neutralizing antibodies produced by B cells (9) can block viral infection, and alveolar macrophages phagocyte the neutralized virus (10). This positive inflammatory feedback leads to the clearance of the virus and minimal lung damage, resulting in recovery. (B) In aged subjects or in patients with preexisting comorbidities a basal state of inflammation and high levels of circulating RAGE ligands i.e. S100 proteins, AGEs (advanced glycation end-products) and HMGB1, occur. In these conditions, SARS-CoV-2 infects, replicates and induces apoptosis of ACE2-expressing lung cells, which in turn release DAMPs (including HMGB1) (1–3). The massive presence of RAGE ligands (4) leads to over-expression and hyperactivation of RAGE in alveolar, endothelial and immune cells, including activated alveolar macrophages (5). The excessive release of cytokines and chemokines (6) may lead to abnormal accumulation of immune cells in the lung, i.e. pneumonia (7) and to a strong cytokine-dependent parenchymal epithelial and endothelial damage (8). The consequent disruption of the air-blood barrier leads to release of considerable amount of DAMPs and biomarkers of endothelial injury, such as angiotensin (Ang) II, in the alveoli and blood (9). Ang II combines with AT1R (type 1 angiotensin receptor) (10), which is over-expressed and sustained by RAGE signalling in alveolar epithelial cells, inflammatory cells, endothelial cells and fibroblasts leading to increased capillary permeability, vasoconstriction, interstitial pulmonary oedema and fibrosis, additional pulmonary and vessel cell apoptosis, and chronic inflammatory cell recruitment and activation, i.e. ARDS (acute respiratory distress syndrome). The disruption of microcapillary integrity causes the entry in the bloodstream of a cytokine storm and RAGE ligands, resulting in widespread inflammation and Ang II-dependent multi-organ damage (11). In addition, the excessive pro-inflammatory response inhibits the type I interferons (IFNs)-mediated activation of T cells leading to non-neutralizing antibodies production by B cells and reduced removal of infected cells. The RAGE-HMGB1 axis may be involved in the virus immune escape leading to virus diffusion (11) and massive virus infection of ACE2-expressing cells, culminating in patient's death.
ACE2 is expressed in vascular endothelial cells [43], renal tubular epithelium [43,44], lung (especially in type 2 alveolar epithelial cells (AT-2) and Clara cells) [45], and the gastrointestinal tract [46], in which it functions as a co-receptor for nutrient uptake, justifying intestine as the putative entry site of SARS-CoV-2 after eating food at the Wuhan market, and in Leydig cells in the testes [47,48], explaining why these cells can serve as a reservoir for viral invasion [48]. ACE2 is also found at different extent in heart, thyroid, adipose tissue, liver, bladder, adrenal gland, blood, spleen, bone marrow, brain, and muscle tissue [43,44], explaining the symptoms and multi-organ dysfunction observed in critical COVID-19 patients [5]. The involvement of ACE2 in the pathogenesis of SARS-CoV infection has been proved by several reports [49]. Overexpression of human ACE2 enhanced disease severity in a mouse model of SARS-CoV infection [50].
ACE2 degrades Ang I into Ang 1–9, and Ang II into Ang 1–7, which act on the Mas proto-oncogene receptor expressed on a variety of cell lineages including AT-2, thereby negatively counter-regulating components of the RAS (Fig. 2 ). Indeed, ACE2 exhibits a protective function in the cardiovascular system and other organs by modestly lowering blood pressure through vasodilation and by promoting kidney sodium and water excretion, but also attenuates inflammation through the production of nitric oxide [51]. Ang 1–9 also shows beneficial biological effects via the angiotensin type 2 receptor (AT2R) resulting in cardioprotection [52,53]. These effects directly oppose those induced by ACE–Ang II signalling, whereby ACE converts the inactive peptide Ang I into Ang II, which acts principally on AT1R to increase blood pressure by inducing vasoconstriction, kidney reabsorption of sodium and water and oxidative stress, and to promote inflammation and fibrosis [44,51] (Fig. 2). Indeed, Ang II acts also as a pro-inflammatory cytokine via AT1R by activation of the nuclear factor-κB (NF-κB) and the metalloprotease, ADAM17 which generates the mature form of epidermal growth factor receptor ligands, TNF-α and soluble form of IL-6 leading to a loop of NF-kB-STAT3 stimulation [54,55]. Although capillary blood vessels in the lung are one of the major sites of ACE expression and Ang II production in the human body [56], the components of both deleterious and protective RAS cascade are co-expressed in the majority of tissues and act in both paracrine and autocrine manner in several organs; the balance between these cascades determines whether or not tissue injury will occur in response to a stimulus, especially in heart and kidney [44,51].
Fig. 2.
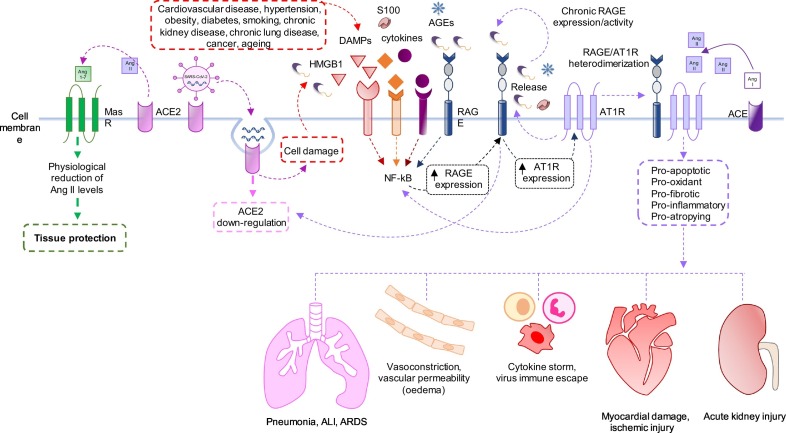
Potential mechanism underlying the interaction between RAGE (receptor for advanced glycation end-products) signalling and RAS (renin–angiotensin system) cascade in SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection.
The abnormal activation of RAS is associated with the pathogenesis of many diseases, such as hypertension, myocardial infarction, heart failure, diabetes and inflammatory lung disease [17]. Regardless to lung injury, Ang II induces pulmonary vasoconstriction and hypertension also in response to hypoxia; increases vascular permeability thus facilitating pulmonary oedema; induces pulmonary fibrosis by the activation of smooth muscle cell or fibroblast proliferation; directly induces apoptosis of human lung alveolar-derived carcinoma cell line, A549, and in primary type I alveolar (AT-1) cells [[56], [57], [58]] (Fig. 1, Fig. 2). Interestingly, in SARS-CoV spike-treated mice, Ang II levels were increased and the worsened ARDS symptoms could be partially reversed by treatment with AT1R blockers [59]. In a cohort of twelve COVID-19 patients, circulating Ang II levels were markedly elevated compared to healthy controls, and linearly correlated with viral load, providing a direct link between tissue ACE2 downregulation and systemic RAS imbalance, and facilitating the development of multi-organ damage from SARS-CoV-2 infection [60].
On the other hand, pulmonary ACE2 showed a strong protective effect in several animal models of acute lung injury (ALI) and ARDS that can be triggered by SARS [16,61,62], by regulating the circulating levels of Ang II and Ang 1–7. In experimental models of lung disease, catalytically active ACE2 alleviated pulmonary injury and vascular damage [61,63], prevented pulmonary hypertension, decreased lung fibrosis and arterial remodelling, and improved right ventricular performance [64] due to a combination of direct action in the lungs and the activation of an ACE2-dependent gut-lung axis [65]. Moreover, 1) ACE2 knock-out mice displayed more severe symptoms of ARDS compared with wild-type controls [61]; 2) recombinant ACE2 protein has been proved effective in improving lung pathologies associated with ALI and ARDS induced by influenza viruses [66,67]; and, 3) recombinant spike protein did not cause ARDS in ACE2 knockout mice [16,59]. Notably, a recombinant human ACE2 (APN01) resulted safe, with no negative hemodynamic effects in healthy volunteers and in a small cohort of ARDS patients in which its administration rapidly decreased the levels of Ang II and inflammation [68]. Interestingly, SARS-CoV2 rapidly disappeared from the nasal cavity, lung and serum of one patient with severe COVID-19 after APN01 therapy, without interference with the generation of neutralizing antibodies [69].
Thus, RAS appears to play a critical role in the pathogenesis of ALI and ARDS, and the role of ACE2 is of particular significance in pathological conditions where RAS is overstimulated, including acute lung failure in several diseases. Noteworthy, SARS-CoV infection induced a marked downregulation of ACE2 expression in lungs of wild-type mice and the treatment with recombinant SARS-CoV spike protein downregulated ACE2 expression in vitro and in vivo [59,70]. SARS-CoV-2 infection might lead to the suppression of ACE2 expression, as a consequence of increased internalization and shedding of ACE2 from the cell surface, leading to significant loss of the ACE2 physiological function. This subsequently would lead to dwindling levels of Ang 1–7 and elevated Ang II production. The abnormal increase of Ang II/AT1R activity might drive the inflammatory response in lungs and potentially induce direct parenchymal injury as well as pathological effects in other organs (Fig. 1).
Manipulation of RAS to limit SARS-CoV-2 infection and confer protection against lung and other tissue damages in COVID-19 patients is currently under investigation [17]. The use of ACE2-derived peptides, small molecule inhibitors, ACE2 antibody or single chain antibody fragment against ACE2 might potentially prevent the binding of human ACE2 to SARS-CoV-2. Interestingly, in two phase II clinical trials, administration of ACE2 translated into reduced systemic inflammation and shifted the RAS peptide balance away from Ang II towards Ang 1–7 [68,70]. Selective receptor antagonists, such as L-158809 or losartan, and PD-123319 or PD-126055, which are respectively inhibitors of AT1R and AT2R, represent additional tools to limit RAS activation during SARS-CoV-2 infection [71].
4. RAGE
4.1. Structure and ligands
RAGE is a member of the immunoglobulin (Ig) superfamily [72]. Protein homology studies revealed that RAGE is present only in mammals and it has the highest homology with genes coding for Ig cell adhesion molecules (IgCAMs), including ALCAM, BCAM and NCAM [73]. Full-length RAGE is composed of an extracellular region containing one “V”-type connected to two “C”-type Ig domains. These extracellular domains are followed by a hydrophobic transmembrane spanning domain that in turn neighbours a highly charged, short cytoplasmic domain essential for RAGE intracellular signalling. The V domain of RAGE is essential for binding most RAGE ligands [74,75].
RAGE was identified as a receptor for advanced glycation end-products (AGEs) [72], which are products of nonenzymatic glycation and oxidation of lipids, proteins and other macromolecules that appear, in particular, under conditions of increased availability of reducing sugars and/or enhanced oxidative stress, especially when molecules turn over slowly and aldose levels are elevated, as in the diabetic state [76].
Due to its ability to recognize three-dimensional structures rather than specific amino acid sequences, RAGE can interact with a wide range of endogenous ligands such as HMGB1 (high mobility group 1), S100 family members/Calgranulins, amyloid-β peptides (Aβ) and nucleic acids (DNA, RNA), which are considered DAMPs; exogenous ligands such as bacterial lipopolysaccharide (LPS) and dietary AGEs; and cell adhesion related ligands, i.e. collagen I/IV and macrophage-1 antigen (Mac)-1 [77,78].
Twenty splice variants of RAGE have been identified in humans, and seventeen exist in mice; the tissue type dictates which splice form is expressed [79,80]. The soluble isoform, sRAGE is found in the extracellular space and results from alternative splicing of Ager mRNA or cleavage from the membrane RAGE by proteolysis. sRAGE lacks the transmembrane and cytosolic domains and, consequently, is capable of binding RAGE ligands prior to their interaction with full-length RAGE thereby acting as a decoy receptor [81].
4.2. RAGE expression
RAGE is highly expressed in many embryonal tissues, but its expression decreases or stops in adult tissues, exceptions being represented by lung and skin [21,22], suggesting that RAGE may have lung- and skin-specific functions distinct from those in other adult tissues. RAGE is highly expressed in the basolateral membrane of AT-1 cells, for which it represents a specific marker [24,82], and where it acts as an adhesion molecule to mediate the contact between AT-1 cells and the alveolar basal lamina, probably contributing to their flat and spread morphology, essential for gas exchange [83]. RAGE is expressed also in AT-2 epithelial cells, in bronchiolar epithelia, and in alveolar macrophages [84].
Although RAGE knockout does not affect lung development [85], several studies have illustrated that RAGE possesses a number of important physiological roles in the lung, including modulation of cell spreading, adhesion to extracellular matrix components, proliferation and migration [83]. Indeed, decreased RAGE expression in lung has been associated with decrease of biomechanical power, lung cancer and pulmonary fibrosis [[86], [87], [88]].
RAGE is expressed at low levels in vascular smooth muscle cells, endothelial cells, pericytes, airway smooth muscle cells, neurons, and in circulating adaptive and innate immune cells, such as macrophages, dendritic cells, eosinophils, T cells and B cells [[89], [90], [91]]. In these cell types RAGE expression is increased in certain conditions as inflammation, ageing, upon injury or in the presence of elevated amounts of RAGE ligands [21,78,92].
4.3. RAGE and inflammation
Due to its ability to bind and mediate the cellular responses to a range of DAMPs (including HMGB1 and S100 proteins) and to act as an innate immune sensor of microbial PAMPs (including bacterial endotoxin, viruses and microbial DNA), RAGE is viewed as a pattern recognition receptor (PRR) and has emerged as a key regulator of the innate immune response and the inflammatory process [77,78].
Activation of cell-surface RAGE by HMGB1, S100 proteins and AGEs results in many signalling cascades leading to activation of the NF-kB, a transcription factor with a crucial role in a variety of immune and inflammatory processes [[93], [94], [95]]. An autoregulatory positive feedback loop has been proposed whereby RAGE is up-regulated and overexpressed via its ligands by NF-κB (Fig. 2), thereby converting a brief pulse of cellular activation to sustained cellular perturbation and tissue injury. In this way, engagement of cell-surface RAGE by ligands can lead to increased expression of proinflammatory cytokines, chemokines and adhesion molecules as well as enhanced oxidative stress that may culminate in local inflammation or neurodegeneration [91,96,97].
RAGE signalling is upregulated in conditions of chronic inflammation, including diabetes, cardiovascular diseases (CVD), obesity, rheumatoid arthritis, osteoarthritis, atherosclerosis, chronic renal disease, sepsis, vasculitis, cancer, Alzheimer's disease (AD), cancer cachexia, muscular dystrophies, and a range of host- and pathogen-induced inflammatory diseases, where RAGE contributes to the development, progression and complications of the pathological states [93,[98], [99], [100]].
An interesting interplay between RAGE/HMGB1 and the adhesion molecule β2 integrin, Mac-1 has been reported in leukocytes, required for HMGB1-mediated inflammatory cell recruitment [93].
S100-RAGE interactions might have important consequences in inflammatory and degenerative events, since they determine the activation of endothelial and vascular smooth muscle cells, neurons, astrocytes, microglia/macrophages, and other cell types, inducing the activation of different signalling pathways leading to production of proinflammatory cytokines and adhesion molecules [95,101]. Certain S100 proteins, namely S100A4, S100A8/S100A9 heterodimer and S100B, show a critical role in the pathophysiology of obesity by promoting macrophage-based inflammation via RAGE and/or toll-like receptor 4 [102]. In general, S100 proteins have to be present at high levels to generate these effects, except for S100B, which activates RAGE in neurons at low and high concentrations with trophic and toxic effects, respectively, and in macrophages with antiinflammatory and proinflammatory effects at low and high S100B concentrations, respectively [101,103].
Evidence is accumulating that RAGE strongly contributes to inflammaging, i.e. the chronic low-grade and sterile inflammation that appears in ageing and whose absence is considered the best predictor of successful longevity [104]. Together with the accumulation of its ligands, RAGE sustains the pro-inflammatory response and leads to stress events favouring mitochondrial dysfunction or cellular senescence. By promoting chronic inflammation, RAGE activity is closely linked to age-related diseases, including retinopathy, neuropathy, nephropathies, type 2 diabetes, cancer, osteoarthritis, CVD and AD [104]. Deletion or blockade of RAGE in mice leads to a pro-longevity phenotype in which the features of ageing are significantly diminished in vessels and kidneys, and is associated with a decreased overall inflammatory burden [105]. Interestingly, SARS-CoV-2 infection is characterized by increased mortality in elderly people with age-related comorbidities [106], and RAGE activity might be proposed as a key mechanism responsible for the high susceptibility to the virus in the presence of age-related disorders.
4.4. RAGE in lung diseases
Although further studies are needed to better unravel the physiological function(s) of pulmonary RAGE, the disturbance of RAGE expression/activity in lung might give rise to non-infective and infective pathological states, including pulmonary fibrosis, ALI, ARDS, pneumonia, cystic fibrosis (CF) and bronchopulmonary dysplasia [23,24]. Studies on different animal models of fibrosis have produced conflicting results. In bleomycin, asbestos or silica-treated animals, both membrane RAGE and sRAGE protein levels were found reduced [23,86,107]. RAGE-deficient (Ager −/−) mice develop more severe fibrosis upon treatment with asbestos compared to control mice, and spontaneously develop fibrosis-like alterations in lungs during ageing [86]. Accordingly, RAGE protein levels were found reduced in lung homogenates, and lower concentration of sRAGE was found in bronchoalveolar lavage fluid (BALF) of patients suffering from idiopathic pulmonary fibrosis (IPF) [108]. However, other researcher groups by the use of bleomycin-induced fibrosis reported that i) Ager −/− mice were resistant to lung injury, showing enhanced survival rates and lower fibrotic scores; ii) HMGB1 and AGEs levels increased in the serum of injected mice; iii) HMGB1 was able to induce epithelial-mesenchymal transition (EMT) in AT-2 cells via RAGE; and iv) blockade formation of AGEs by treatment with aminoguanidine attenuated bleomycin-induced fibrosis [109,110]. Moreover, overexpression of RAGE was found in reactive pneumocytes, bronchiolar metaplastic epithelium, endothelium and, especially, in fibroblastic foci in lungs of IPF patients [111]. These findings are in accordance with the traditional view of RAGE as a profibrotic receptor [112].
It has been suggested that some S100 proteins mediate their proinflammatory effects in CF via RAGE. S100A12 is locally expressed by infiltrating neutrophils in the lungs of CF patients [113]. Furthermore, the levels of S100A12 are extremely high in the sputum of CF patients, serum S100A12 is increased during acute infectious exacerbations, and the intravenous antibiotic treatment successfully reduced S100A12 levels [114]. In addition, RAGE expression in CF neutrophils was significantly enhanced along with a reduction in sRAGE levels [114], pointing to a role of RAGE in neutrophil-associated airway inflammation in CF. Polymorphisms of the AGER gene have been associated with increased disease severity in CF. AGER − 429 T/C variant is associated with decreased FEV1 (forced expiratory volume in 1 s) and increased AGER promoter activity, leading to increased RAGE expression and worsening lung function [115], and AGER − 374 T/A variant is associated with increased RAGE expression and results in increased IgE levels in lungs [116]. The authors hypothesized that CF patients with this RAGE-mediated increase in allergic airway inflammation are more susceptible to sensitization by environmental allergens and pathogens [116].
The RAGE activity has been implicated also in ALI and ARDS, which are characterized by hallmarks typical of severe COVID-19 [5,7]. In several mouse models of ALI and in patients with ALI/ARDS, sRAGE levels were increased in BALF and plasma correlating with the degree of lung injury [24,[117], [118], [119], [120]]. Interestingly, mice lacking RAGE were protected from hyperoxia-induced ALI and mortality [118], suggesting that RAGE signalling sustains lung inflammation and alveolar destruction in ALI. In humans, systemic and alveolar levels of HMGB1, S100A12 and sRAGE from damaged AT-1 cells are increased in ARDS patients [119]. Since the ALI/ARDS resolves in patients, plasma sRAGE levels fall, sRAGE can serve not only as a marker of disease severity and prognosis but also as a marker of resolving disease [121].
Several studies support the role of HMGB1 as an effector and regulator of ALI sustaining oxidative stress, neutrophils infiltration, pulmonary hemorrhage, and inflammatory cytokine release [122]. The levels of HMGB1 were significantly increased in plasma and lungs of a mouse model of LPS-induced ALI [123]; HMGB1 is a critical mediator of bacteria-induced pyroptosis in lung endothelial cells by directly binding LPS outside the cells and efficiently delivering LPS into the cytoplasm via RAGE [124]. HMGB1 directly decreases transendothelial electrical resistance (TER) in human pulmonary artery endothelial cells, suggesting a direct role in vascular barrier disruption in sepsis-induced ALI [125]; HMGB1 induces a dose-dependent increase in paracellular gap formation in concert with loss of peripheral organized actin fibres, dissociation of cell-cell junctional cadherins, and development of central stress fibres, a phenotypic change associated with increased contractile activity and increased endothelial permeability, principally by RAGE-depended activation of p38 MAP kinase and Hsp27 [125].
In pneumonia, the role of RAGE is closely linked to the type of infective pathogen [20]. Murine pneumonia induced by bacteria (i.e., S. pneumoniae and K. pneumoniae) is associated with an upregulation of intra-alveolar membrane RAGE expression and high levels of HMGB1 in BALF from infected patients [126,127]. In the case of S. pneumoniae infection, mice lacking RAGE had reduced lung injury and neutrophil recruitment, a better survival rate together with a lower pulmonary bacterial load and decreased bacterial dissemination to blood and spleen compared to wild-type mice, due to the improvement of killing capacity of Ager −/− alveolar macrophages leading to early bacterial removal [126]. Blockade of the RAGE activity and the subsequent inflammatory response might be an explanation for the less severe pulmonary damage in Ager −/− mice during S. pneumoniae infection. In contrast, RAGE plays a beneficial role in mice during the host response to K. pneumoniae, where RAGE deficiency was associated with increased mortality and bacterial outgrowth and dissemination [127]. In this case, RAGE deficiency impaired host effective antibacterial defences, likely neutrophils' phagocytising capacity.
Recent findings provide a central role for RAGE in cigarette smoke (CS)-dependent chronic obstructive pulmonary disease (COPD). RAGE ligands contained in CS bind to alveolar RAGE leading to oxidative stress, macrophage activation and lung parenchyma pathogenesis, ultimately resulting in emphysema, which is prevented by deletion or pharmacologic inhibition of RAGE [128]. Interestingly, smoking is most likely associated with the negative progression and adverse outcomes of COVID-19 patients, in which RAGE-dependent alveolar damage might be amplified [129].
4.5. RAGE and virus infection
Van Zoelen et al. demonstrated that RAGE deficiency resulted in a better outcome from pulmonary seasonal influenza A virus infection, as indicated by a relative protection from lethality in mice [130]. This was accompanied by improved viral clearance, enhanced T cell response and activation of neutrophils, suggesting that endogenous RAGE impairs the cellular immunity against respiratory tract infection with Influenza A virus.
In preclinical and clinical studies, it has been demonstrated that severe respiratory infections, including influenza and infections caused by human respiratory syncytial virus (RSV), generate substantial release of HMGB1 driving pulmonary inflammation, and that HMGB1-specific antagonists ameliorate these conditions [131,132]. HMGB1 also increased the replication of RSV, which is a negative-sense RNA virus [131]. Human adenovirus type 7 (HAdV-7) causes severe paediatric pneumonia characterized by cell necrosis, release of a large number of inflammatory mediators, high incidence of sequelae and mortality. HMGB1 levels gradually increase in the media supernatants or BALF of HAdV-7-infected A549 cells and mice, respectively, and HMGB1 promotes HAdV-7 replication and signals through TLR-4, TLR-9 and RAGE. Blocking HMGB1 leads to a reduction of the adenoviral pathology in lung tissues, suggesting that HMGB1 could be a therapeutic target in HAdV-7 infection [133].
Interestingly, HMGB1 has been implicated in the mechanism of AIM2 (absent in melanoma 2)-mediated viral evasion [6,134]. AIM2, a member of the PYRIN protein family, has affinity for viral DNA derived from murine cytomegalovirus (MCMV) and Vaccinia virus; activation of AIM2 leads to secretion of IL-1β and IL-18, polarization in M1 macrophages mediating the inflammation in response to infection [135]. HMGB1 induces excess activation of AIM2 inflammasome in alveolar macrophages response to virus infection via RAGE, causing cytokine storm and compromising the innate immune response, which is a critical factor for the outcome of virus infection [6,134]. Furthermore, the use of the HMGB1 inhibitor, glycyrrhizin, was able to rescue IL-35 secretion by Treg lymphocytes reducing cytokine storm and restoring immune homeostasis in a murine model of ALI [136]. One might speculate that HMGB1 utilizes similar strategies to modulate the host innate immune response in the presence of SARS-CoV-2, which has been able to evade the immune system in severe COVID-19 patients [[35], [36], [37], [38]].
The involvement of RAGE/HMGB1 axis has been recently speculated in the development of the Kawasaki disease (KD), which is an acute and usually self-limiting vasculitis of the medium calibre vessels affecting almost exclusively children [[137], [138], [139]]. An aberrant response of the immune system to an infectious agent in genetically predisposed children seems to trigger the cascade that causes the illness, which has been recently associated with the common respiratory viruses, such as enteroviruses, adenoviruses, rhinoviruses, and coronaviruses [138,139]. A strong association between an outbreak of Kawasaki-like disease and the SARS-CoV-2 epidemic in the Bergamo, Italy has been recently reported, suggesting that SARS-CoV-2 might represent one of the triggers of the disease [138]. Particularly, as suggested by data obtained in animal model of KD, HMGB1 might accumulate in coronary endothelial cells causing coronary vasculitis and promoting coronary artery dilatation via RAGE/NF-κB signalling. HMGB1 levels were found higher in children with KD than in healthy controls suggesting serum HMGB1 as an indicator of inflammation and coronary artery injury in this pathology [137].
4.6. RAGE and COVID-19: state of the art
A potential correlation between RAGE activity and the pathogenesis of SARS infection as well as the clinical severity of COVID-19 has been recently proposed by others [28,29].
Starting from the knowledges about HMGB1, several authors propose HMGB1 as therapeutic target for COVID-19. In SARS patients HMGB1 can be actively released from innate immune cells stimulated by elevated proinflammatory cytokines (e.g., IL-1, IL-6, TNF-α and IFN-γ), and passively released by infected alveolar cells, endothelial cells or macrophages undergoing to virus-mediated cytolysis. Once released, extracellular HMGB1 may mediate an injurious pulmonary inflammatory response including neutrophil infiltration, derangement of epithelial barrier, lung oedema and injury contributing to respiratory failure and patient death [29]. As an RNA-binding molecule, HMGB1 might bind RNA of SARS-CoV-2 and bring it to the cytosol via the RAGE-lysosomal pathway, offering an additional pathway apart from the ACE2 enabling intracellular virus replication [29]. The administration of an α7 nicotinic acetylcholine receptor agonist was able to reduce hyperoxia-induced acute inflammatory lung injury by inhibiting RAGE expression and the release and accumulation of HMGB1 in the airways and the circulation [140]. This suggests that reducing HMGB1 might alleviate inflammation in severe COVID-19 patients with concurrent oxygen-induced lung injury [140].
Among several pro-inflammatory cytokines investigated, only the expression of the S100A12 gene, encoding EN-RAGE and considered as a biomarker of pulmonary injury, was found significantly increased in the peripheral blood mononuclear cells from COVID-19 patients [141]. The expression of EN-RAGE in myeloid cells strongly correlated with its plasma levels, and was inversely related to lack of type I IFNs response and the expression of genes encoding the antigen presentation machinery, suggesting a strong role of EN-RAGE/RAGE axis in the mechanism of virus escape [141]. Significantly higher levels of EN-RAGE were observed in extracellular vesicles derived from plasma of severe COVID-19 patients compared to patients with moderate COVID-19. Interestingly, plasma exosomes contain ACE2, which makes recipient cells susceptible to virus entry thus contributing to spread of infection [142]. Moreover, serum S100B has been suggested as a marker of clinical severity in SARS-CoV-2-infected patients since S100B concentration was associated with inflammation markers [143].
Experimental studies have shown that sRAGE enhances ACE2 expression and high sRAGE levels have been found in individuals with asymptomatic course of COVID-19, suggesting a strong potential role of sRAGE in reducing the RAGE-dependent hyperactivation of Ang II/AT1R [144]. The prognostic and therapeutic potential role of circulating RAGE isoforms to help treatment of COVID-19 has been hypothesized after a pilot study in diabetic and non-diabetic COVID-19 patients [145,146] .
In a study based on interactome analysis, an activated AGE-RAGE signalling emerged as a critical pathway in older people and patients with diabetes, cancer or cardiovascular disorders, which are all fatal comorbidities of COVID-19 [147]. The AGE-RAGE signalling system was also identified among the common molecular pathways associated with SARS-CoV-2 infection and rheumatoid arthritis, malaria, hepatitis B, and influenza A [148]. Interestingly, the natural compounds Dayuanyin and N. sativa showed strong ability to reduce lung inflammation and oxidative stress in severe COVID-19 by inhibiting the activation of AGE-RAGE pathway in pre-clinical and clinical studies [149,150].
A curious link has been proposed between RAGE signalling and the gender dependent differences in SARS-CoV-2 infection and disease progression, with increased susceptibility in male patients. In particular, estrogen receptors and RAGE seem to synergize to maintain homeostasis of metabolic pathways, the dysregulation of which is at the basis of the comorbidities worsening the clinical conditions of COVID-19 patients [151]. Increasing evidence show also that estrogens, similarly to RAGE, are involved in the regulation of RAS, including ACE2, also in differentiated airway epithelial cells [152]. A recent study demonstrated an unexpected gender-specific stress response and HMGB1 release in isolated murine pulmonary endothelial cells [153]. Indeed, upon exposure to hypoxia, cells from males responded with necrosis thus releasing significant amount of HMGB1, whereas female cells preferentially responded with apoptosis, avoiding the HMGB1-mediated inflammatory response. The increased release of HMGB1 might contribute to the pro-inflammatory phenotype associated with the male gender in COVID-19 [153].
Lastly, the DAMPs-RAGE enrichment in blood vessels or adipose tissue in diabetic and obesity conditions, respectively, and in the lungs and heart during active infection, is proposed to contribute to the wide spread tissue damage induced by SARS-CoV-2 [154].
4.7. The RAGE/RAS axis
Recent studies have suggested a functional deleterious link between RAGE signalling and some members of RAS cascade in various cell types. In cultured podocytes, which express RAGE, AT1R and AT2R, Ang II induces RAGE mRNA and protein expression through AT2R and NF-κB [155]. On the converse, treatment with AGEs increases Ang II expression by phosphoinositide 3-kinase and supports Ang II/AT1R action, which have a crucial role in the pathogenesis of podocyte injury leading to proteinuria [25]. Similarly, the activation of the AGE/RAGE pathway augments renal proximal tubular angiotensinogen production via ERK1/2, which can contribute to the development of diabetic nephropathy [156]. On the other hand, AGE-RAGE axis induces damage of mesangial cells by activating RAS and leading to VCAM-1 gene expression, and by decreasing ACE2 gene expression and Ang 1–7 production, which plays a protective role against renal injury (Fig. 2). RAGE-dependent dangerous effects were completely abolished by olmesartan, an AT1R blockers (ARBs) [156]. The decrease in ACE2 mRNA level in AGE-exposed mesangial cells depends on RAGE-mediated superoxide generation [157].
In cultured vascular smooth muscle cells, Ang II increases RAGE protein levels and directly upregulates the ligand-RAGE pathway via AT1R stimulation. This step seems essential to the mechanism leading to diabetic atherosclerosis by promoting diffuse inflammatory response [26].
A link between RAS and RAGE pathways has been demonstrated in the Ang II-induced endothelial hyperpermeability, which is one of the earliest signs of endothelial dysfunction and is associated with the development of various diseases, such as, inflammation, hypertension, atherosclerosis, diabetes, thrombosis, and cancer. In the mechanism proposed by Jeong et al., Ang II activates AT1R, which subsequently activates NF-κB, leading to the expression and release of HMGB1 along with the expression of AT1R, RAGE and the adaptor protein mammalian diaphanous-1 (mDia1). Extracellular HMGB1 activates RAGE, which in turn activates again NF-κB to further increase the expression level and release of HMGB1. Blockade of RAGE signalling using HMGB1 blocking antibodies or sRAGE attenuates Ang II-induced endothelial barrier permeability in vitro and in vivo, indicating the therapeutic potential of modulating RAGE activity in controlling vascular permeability under pathological conditions. Interestingly, Ang II increased the expression of the AT1R and RAGE, and increased the production of HMGB1 via NF-κB to induce disruption of vascular endothelial cadherin (VE-cadherin) adhesion in HUVECs [27] (Fig. 2). Moreover, the cross-talk between HMGB1 and Ang II has been reported also in in vitro model of sepsis-induced ALI leading to loss of barrier integrity [125].
The association of RAGE with Ang II-induced signalling pathways has been demonstrated also in cardiomyocyte hypertrophy, in which RAGE regulates an NF-κB/NLRP3 (NOD-like receptor protein 3)/IL-1β-mediated signalling pathway as well as the traditional PKC/ERK hypertrophic signalling pathway. Accordingly, sRAGE attenuated Ang II-induced cardiomyocyte hypertrophy by downregulating RAGE and AT1R expression and the secretion of HMGB1 and IL-1β. Thus, RAGE-NLRP3 may be important mediators of Ang II-induced cardiomyocyte hypertrophy [158] (Fig. 2).
A recent study suggests that Ang II leads to myocardial inflammation in diabetic mice by increasing RAGE expression in monocyte/macrophage and furthers AGE-RAGE-mediated leucocyte myocardial infiltration [159].
Consistent with the interaction between HMGB1 and the RAS, numerous evidences have been presented that the levels of ACE2 can reduce the secretion/activity of HMGB1 [[160], [161], [162]]. Indeed, circulating HMGB1 levels increased in infarcted normal or ACE2 transgenic mice, and tissue expression of HMGB1 increased in the hearts of normal but not ACE2 transgenic infarcted mice leading to reduction of infiltration of inflammatory cells. ACE2 activation ameliorated hypoxia-induced cell death, and downregulated HMGB1 in cardiomyocytes, linking the protective effect of ACE2 to downregulation of HMGB1 and reduced downstream proinflammatory cascades. How ACE2 overexpression affects HMGB1 levels is unclear [[160], [161], [162]].
In line with these data: 1) the ACE inhibitor, ramipril, attenuated AGE accumulation in diabetic animals [163]; 2) ARBs and ACE inhibitors significantly attenuated AGE production in vitro [164]; 3) ARBs, such as olmesartan and valsartan, ameliorated diabetic nephropathy by repressing renal RAGE expression [157,165]; 4) treatment of diabetic mice with ARBs suppressed atherosclerosis, ligand-RAGE expression and inflammatory changes, implying that ARB might decrease diabetic atherogenesis by inhibiting ligand-RAGE signals [26]; and 5) treatment of diabetic rats with losartan or enalapril, reduced the proinflammatory action of RAGE in myocardium [159].
These results strongly correlate the reduction of RAS deleterious hyperactivity with the reduction of RAGE signalling.
Underlining the important role of RAGE in RAS activity, the formation of heteromeric complexes between AT1R and RAGE was demonstrated at the plasma membrane [166]. The heterodimerization of RAGE with other receptors might be functional to the trigger of RAGE's signalling pathways [167]. In particular, independently of the liberation of RAGE ligands and the ligand-binding ectodomain of RAGE, the activation of the AT1R by Ang II triggers transactivation of the cytosolic tail of RAGE and NF-κB–driven proinflammatory gene expression (Fig. 2). Consequently, Ang II–dependent inflammation and atherogenesis induced by RAS were attenuated when RAGE was deleted or when RAGE transactivation was inhibited by the RAGE specific intracellular inhibitor, S391A-RAGE362–404 [166].
4.8. RAGE inhibitors
The involvement of RAGE and its ligands in several diseases have prompted studies to identify endogenous inhibitors and synthesize chemical ones, so that a variety of RAGE antagonists is now available for preclinical and clinical studies (Table 1, Table 2 ) [168,169].
Table 1.
RAGE-based therapeutic approaches in preclinical studies.
| Inhibitors | Mechanisms of action | Diseases |
|---|---|---|
| Anti-RAGE antibodies | Blocking polyclonal or monoclonal antibodies against RAGE extracellular domains | Crash injury [198] Duchenne muscular dystrophy [99] Intra-abdominal sepsis [178] Systemic inflammation [199] Type 1 diabetes [179] Type 2 diabetes [180] |
| FPS-ZM1 | High-affinity chemical inhibitor of RAGE V domain | Alzheimer's disease [187,200] Asthma [188] Amyotrophic lateral sclerosis [201] Breast cancer [190] Cerebral ischemic stroke [202] Disuse-induced muscle atrophy [203] Emphysema [191] Hypertension and heart failure [204] Parkinson's disease [205] Pneumococcal meningitis [206] |
| Inhibitors of RAGE–mDia1 interaction | Small-molecules binding RAGE intracellular domain responsible for mDia1 interaction | Ischemia/reperfusion in diabetic heart [197] |
| RAGE-aptamers | Short, single-stranded DNA oligonucleotides able to inhibit RAGE-ligands binding | Melanoma [186] Pulmonary arterial hypertension [185] Renal diseases [183] Type 1 diabetes [184] |
| RAGE mutant peptide S391A-RAGE362–404 | Mutant cytosolic RAGE oligomeric peptide inhibiting the formation of RAGE/AT1R heteromeric complex | Type 1 diabetes [166] |
| RAGE siRNA | Non-coding small interfering RNAs targeting RAGE mRNA | Hepatic fibrosis [182] Ischemia/reperfusion injury [181] |
| RAP (RAGE antagonistic peptide) | S100P-derived peptide competing with RAGE for ligand binding | ARDS [175] Pancreatic tumor and glioma [207] |
| sRAGE | Soluble form of RAGE lacking the transmembrane and cytoplasmic domains, acting as a decoy receptor | ARDS [175] Chronic colitis [208] Heart failure [209] Myocardial ischemic injury [210] Neutrophilic asthma [211] Type 1 diabetes [212] Type 2 diabetes [213,214] |
| Synthetic fragments of RAGE | RAGE peptide sequences binding RAGE ligands | Alzheimer's disease [177] |
Table 2.
Clinical studies involving PF-04494700.
| Inhibitor | Mechanism of action | Disease | Phase clinical Trial | Administration | Effects | Adverse effects |
|---|---|---|---|---|---|---|
| PF-04494700 (TTP-488, Azeliragon) by Pfizer, TransTech Pharma, Inc., vTv Therapeutics LL) |
Oral, small-molecule inhibitor of RAGE | Type 2 diabetes (Diabetic Nephropathy) | Phase 2 NCT00287183 Safety and efficacy study (Completed, 2006–2009) |
Orally 60 mg/day for 6 days followed by 20 mg/day for 6 months |
Not reported | Not reported |
| Alzheimer's disease [194] |
Phase 2 NCT00141661 Safety and efficacy studies (Completed, 2005–2009) |
Orally 30 mg/day for 6 days, followed by 10 mg/day; 60 mg/day for 6 days, followed by 20 mg/day for 10 weeks |
No significant changes in terms of vital signs, electrocardiogram, plasma level of Aβ, inflammatory biomarkers and cognitive outcome | No severe adverse effects; Both doses resulted safe and well-tolerated |
||
| Alzheimer's disease [195,196] |
Phase 2 NCT00566397 Safety and efficacy studies (Completed, 2007–2018; high doses discontinued prematurely) |
Orally 15 mg/day for 6 days followed by daily dosing of 5 mg/day; 60 mg/day for 6 days followed by daily dosing of 20 mg/day for 18 months |
Low-dose regimen slowed down cognitive decline | No adverse effects were associated with the low dose. The high dose showed adverse events and cognitive decline |
||
| Alzheimer's disease | Phase 3 NCT02080364 (2014–2019; discontinued) | Orally 5 mg once daily for 18 months |
Lack of efficacy | Not reported | ||
| Alzheimer's disease and impaired glucose tolerance |
Phase 2 NCT03980730 Active |
Orally 5 mg once daily for 6 months (Part 1) or 18 months (Part 2) |
As a decoy receptor for RAGE ligands, sRAGE is considered a strong potential blocker of RAGE/ligand interaction, and was shown to play a protective role against accumulation of RAGE ligands [170,171]. Extensive studies in mice demonstrated the role of sRAGE, systemically or directly administrated to target organs, in counteracting RAGE-mediated pathogenesis [168]. In humans, plasma levels of sRAGE is reduced in disease conditions such as atherosclerosis, coronary artery disease, hypertension, hypercholesterolemia, chronic obstructive lung disease, heart failure, and AD [170,172,173]. In a clinical perspective, since patients infected with SARS-CoV-2 show low sRAGE plasma levels [145] they would benefit from treatment with sRAGE as a drug, as reported for emphysema and chronic lung damage [174]. In line with this possibility, preclinical studies have shown that sRAGE treatment attenuated lung injury and restored alveolar fluid clearance (AFC) in a mouse model of ARDS [175] (Table 1). Interestingly, ACE inhibition reduces accumulation of AGEs partly by increasing sRAGE plasma levels in hypertension [176], confirming the cross-talk between RAS and RAGE signalling. However, the potential use of sRAGE in humans is limited by some caveats. Firstly, the bacterial expression system for human sRAGE should lead to the non-glycosylated form of sRAGE, which is important for binding ligands in the V-type domain and preventing them from reaching cell-surface RAGE [81]. Secondly, sRAGE administration would prevent physiological binding of ligands to receptors that share ligands with RAGE. Thus, novel strategies mimicking the physiological effects of sRAGE have been developed. Small synthetic fragments of RAGE able to bind RAGE ligands showed efficacy in pre-clinical models of AD [177]; blocking antibodies against RAGE extracellular domains reduced systemic inflammation in LPS-injected rats [178] and kidney damage in animal models of diabetes [179,180]; the intravenous administration of a small peptide antagonist, RAGE antagonistic peptide (RAP), which prevents RAGE from binding with several ligands, (including HMGB1, S100P, and S100A4), improved AFC and oxygenation, and decreased alveolar inflammation and lung injury in Landrace piglets with ARDS, at similar extent of sRAGE [175].
Besides conventional therapeutics, non-coding RNA targeting RAGE have been synthesized, showing ability in reducing the progression of hepatic fibrosis, and inflammation and apoptosis in ischemic mice [181,182]. Moreover, infusion of short, single-stranded DNA oligonucleotides, called RAGE aptamers, which are able to inhibit RAGE-ligands binding, showed efficacy in several diseases including melanoma, pulmonary arterial hypertension, renal diseases and diabetes [[183], [184], [185], [186]].
Several small-molecule inhibitors have been developed able to bind RAGE's extracellular ligand-site or the intracellular signalling domain. The compound FPS-ZM1 (N-Benzyl-N-cyclohexyl-4-chlorobenzamid 12) was selected through a screening of 5000 compounds in the search for inhibitors of RAGE/Aβ interaction. FPS-ZM1 is able to cross the blood-brain barrier (BBB), block the inflammatory signalling in the mouse brain, reduce Aβ accumulation and improve cognitive performance without causing toxic side effects in mice, even at high doses [187]. The efficacy of FPS-ZM1 has been explored in other animal models of neuropathology (such as amyotrophic lateral sclerosis, Parkinson's disease, cerebral ischemic stroke, and pneumococcal meningitis), heart failure, hypertension, asthma, emphysema, COPD and cancer progression/metastasis of breast cancer [[187], [188], [189], [190], [191]]. The results demonstrated that the administration of FPS-ZM1 reduces or abrogates the pathogenic effects caused by RAGE activity by interfering with RAGE-ligand interactions. Interestingly, the treatment with FPS-ZM1 plus the ARB, valsartan, alleviates renal tubular epithelial cell injury in diabetic rats more efficiently than single treatment partly by suppressing renal inflammation and oxidative stress [192], suggesting the use of RAGE inhibitor as a potential adjunctive for the management of diabetic nephropathy.
Azeliragon is an orally bioavailable small molecule able to cross the BBB discovered by TransTech Pharma (now vTv Therapeutics) as TTP488 ([3-(4-{2-butyl-1-[4-(4-chlorophenoxy)-phenyl]-1H-imidazole-4-yl}-phenoxy)-propyl]-diethylamine1) and then licensed to Pfizer as PF-04494700 in 2006 (Table 2). TTP488 is able to inhibit the interaction from sRAGE or RAGE and Aβ, S100B, HMGB1 and the AGE compound, carboxymethyl-lysine. Preclinical studies have demonstrated that TTP488 efficiently contrasts complications due to RAGE activity in diabetes and AD [193,194]. Based on the results obtained in a mouse model of AD, in which TTP488 administration inhibited inflammation, neuronal Aβ accumulation and neurocognitive decline, a phase II clinical trial demonstrated no adverse effects associated with the dose of 15 mg/day for 6 days followed by daily dosing of 5 mg/day [195,196]. A phase II trial (NCT00287183) in type 2 diabetes subjects has been completed in 2009, and a recruitment for a phase II/III clinical trial (NCT03980730) is currently in progress.
Lastly, RAGE modulator compounds able to block the binding of RAGE cytoplasmic domain with signalling partners, which are critical for downstream effects, have been identified. Starting from 58,000 molecules, 13 small intracellular inhibitors that prevent the engagement of mDia1 inhibit AGE–RAGE proinflammatory signalling and decrease the cardiac injury in ischemic mouse models [197]. Interestingly, the mutant RAGE peptide S391A-RAGE362–404, which inhibits the formation of RAGE/AT1R heteromeric complex, attenuates Ang II–dependent atherosclerosis in Ace2/ApoE-dKO mice and reduces plaque accumulation in diabetic mice [166]. These compounds represent attractive molecular scaffolds for the development of therapeutics against RAGE-mediated diseases but show several caveats in terms of solubility, permeability, stability and half-life.
Despite the promising results, further studies are needed to establish chemical refinement, tolerance, selectivity and pharmacokinetics of the above-mentioned compounds, and to test whether intracellular and extracellular RAGE inhibitors are effective therapeutics in the different diseases.
5. Our hypothesis
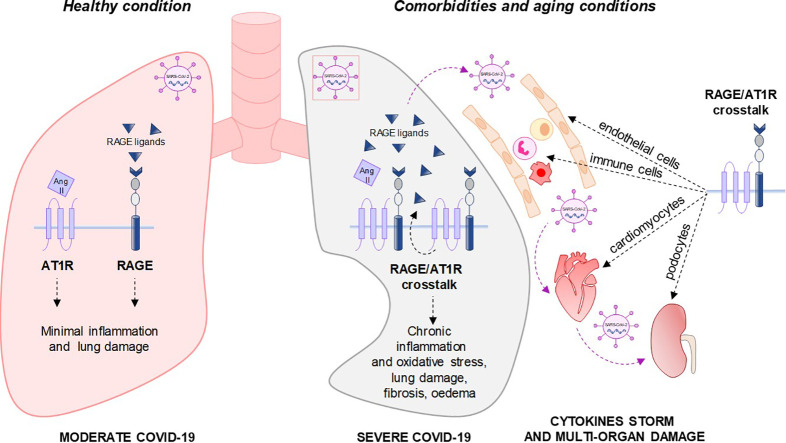
We assume that upon binding of SARS-CoV-2 to lung ACE2 and virus replication and release, the AT-2 infected cells undergo RAGE mediated pyroptosis and release DAMPs, including HMGB1. In healthy subjects, activation of RAGE expressed by neighbouring epithelial lung cells, endothelial cells and alveolar macrophages mediates an efficacious immune response leading to clearance of the virus. In this condition, SARS-CoV-2-dependent ACE2 downregulation leads to a compensable hyperactivation of the ACE/Ang II/AT1R pathway, which is not able to induce lung parenchymal damage (Fig. 1A). Instead, in the presence of comorbidities, such as chronic kidney, cardiovascular or lung disease, hypertension, obesity and diabetes, or in aged or smoking subjects, high levels of RAGE ligands and RAGE expression are elevated in lungs and in the immune system, where RAGE sustains a chronic grade inflammation. Upon SARS-CoV-2 infection, alveolar necrosis and local lung inflammation might lead to additional release of RAGE ligands and excess RAGE activation. In these conditions, RAGE reinforces and sustains the deleterious crosstalk between AT1R and RAGE signalling cascades leading to the establishment of a pro-inflammatory loop in the lung leading to systemic cytokine storm, a defective immune response leading to virus escape and diffusion, and lung irreversible damage, i.e. ARDS (Fig. 1B). RAGE-AT1R transactivation might occur in many other tissues, including heart, vessels and kidney leading to widespread inflammation and multi-tissue damage (Fig. 2). This is also a possible explanation for the effects of the virus in several organs distant from the attack site of the virus in severe COVID-19 patients (Fig. 1B).
This hypothesis, if experimentally demonstrated might lead to the development of novel therapies able to specifically disrupt the crosstalk between AT1R and RAGE signalling, reducing a broad range of pathological states in which RAGE might be reasonably responsible for the exacerbation of the Ang II action during the course of SARS-CoV-2 infection. The reduction of RAGE activity with specific inhibitors, in particular TTP488, which has been already tested in human patients, might be important to improve the immune response, reduce the cytokine storm, and increase the humoral response.
Preclinical and clinical studies evaluating the efficacy of RAGE inhibition in the course of coronavirus infections are urgent also in view of possible development of new SARS-CoVs in the future.
In the presence of several diseases and conditions, high levels of RAGE ligands are present in the serum, and RAGE expression is increased in tissues and the immune system. Upon recognition and binding of the viral spike protein by angiotensin-converting enzyme (ACE)2 receptor, SARS-CoV-2 enters the cell and leads to down-regulation of ACE2, resulting in unopposed accumulation of Ang II. Infected cells undergo pyroptosis and release DAMPs (damage-associated molecular patterns), including HMGB1 (high mobility group 1). DAMPs, cytokines and RAGE ligands lead to excess RAGE expression sustained by NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells). In these conditions, RAGE induces a further down-regulation of ACE2, increases AT1R (type 1 angiotensin receptor) expression, and transactivates with AT1R reinforcing the deleterious effects of ACE/angiotensin (Ang) II/AT1R pathway. At the same time, Ang II/AT1R induces NF-κB activation and the release of RAGE ligands with the establishment of a detrimental loop between AT1R and RAGE signalling. RAGE-AT1R crosstalk might occur in lung, vessels, immune cells, heart and kidney causing irreversible multi-organ damage.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors were supported by Associazione Italiana per la Ricerca sul Cancro (Project 17581) and Fondazione Cassa di Risparmio di Perugia (Project 2019.0321.026).
References
- 1.Snijder E.J., Horzinek M.C., Spaan W.J. The coronavirus like superfamily. Adv. Exp. Med. Biol. 1993;342:235–244. doi: 10.1007/978-1-4615-2996-5_37. [DOI] [PubMed] [Google Scholar]
- 2.Fehr A.R., Channappanavar R., Perlman S. Middle East Respiratory Syndrome: emergence of a pathogenic human coronavirus. Annu. Rev. Med. 2017;68:387–399. doi: 10.1146/annurev-med-051215-031152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.J., Luo C.M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.Y., Wang L.F., Daszak P., Shi Z.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Webster R.G. Wet markets—a continuing source of severe acute respiratory syndrome and influenza? Lancet. 2004;363:234–236. doi: 10.1016/S0140-6736(03)15329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern [published correction appears in Lancet. 2020 Jan 29] Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., Tai Y., Bai C., Gao T., Song J., Xia P., Dong J., Zhao J., Wang F.S. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published correction appears in Lancet Respir Med 2020 Feb 25] Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Food and Drug Administration https://www.fda.gov/drugs/coronavirus-covid-19-drugs/coronavirus-treatment-acceleration-program-ctap
- 9.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., Shawa I., Adams A.C., Van Naarden J., Custer K.L., Shen L., Durante M., Oakley G., Schade A.E., Sabo J., Patel D.R., Klekotka P., Skovronsky D.M., BLAZE-1 Investigators SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 2021;384:229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration Casirivimab and Imdevimab EUA letter of authorization. https://www.fda.gov/media/143891/download Available at:
- 11.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., ACTT-1 Study Group Members Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Food and Drug Administration https://www.covid19treatmentguidelines.nih.gov/therapeutic-management
- 13.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., Angus B., Baillie V.L., Barnabas S.L., Bhorat Q.E., Bibi S., Briner C., Cicconi P., Collins A.M., Colin-Jones R., Cutland C.L., Darton T.C., Dheda K., Duncan C.J.A., Emary K.R.W., Ewer K.J., Fairlie L., Faust S.N., Feng S., Ferreira D.M., Finn A., Goodman A.L., Green C.M., Green C.A., Heath P.T., Hill C., Hill H., Hirsch I., Hodgson S.H.C., Izu A., Jackson S., Jenkin D., Joe C.C.D., Kerridge S., Koen A., Kwatra G., Lazarus R., Lawrie A.M., Lelliott A., Libri V., Lillie P.J., Mallory R., Mendes A.V.A., Milan E.P., Minassian A.M., McGregor A., Morrison H., Mujadidi Y.F., Nana A., O'Reilly P.J., Padayachee S.D., Pittella A., Plested E., Pollock K.M., Ramasamy M.N., Rhead S., Schwarzbold A.V., Singh N., Smith A., Song R., Snape M.D., Sprinz E., Sutherland R.K., Tarrant R., Thomson E.C., Török M.E., Toshner M., Turner D.P.J., Vekemans J., Villafana T.L., Watson M.E.E., Williams C.J., Douglas A.D., Hill A.V.S., Lambe T., Gilbert S.C., Pollard A.J., Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. Erratum in: Lancet. 397 (2021) 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., Bao L., Zhang B., Liu G., Wang Z., Chappell M., Liu Y., Zheng D., Leibbrandt A., Wada T., Slutsky A.S., Liu D., Qin C., Jiang C., Penninger J.M. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kai H., Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors-lessons from available evidence and insights into COVID-19. Hypertens. Res. 2020;43(7) doi: 10.1038/s41440-020-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt A.M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J. Biol. Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- 20.van Zoelen M.A., Achouiti A., van der Poll T. The role of receptor for advanced glycation end products (RAGE) in infection. Crit. Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierhaus A., Humpert P.M., Morcos M., Wendt T., Chavakis T., Arnold B., Stern D.M., Nawroth P.P. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med (Berl) 2005;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- 22.Lohwasser C., Neureiter D., Weigle B., Kirchner T., Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J Invest Dermatol. 2006;126:291–299. doi: 10.1038/sj.jid.5700070. [DOI] [PubMed] [Google Scholar]
- 23.Buckley S.T., Ehrhardt C. The receptor for advanced glycation end products (RAGE) and the lung. J. Biomed. Biotechnol. 2010;917108 doi: 10.1155/2010/917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oczypok E.A., Perkins T.N., Oury T.D. All the “RAGE” in lung disease: the receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses. Paediatr. Respir. Rev. 2017;23:40–49. doi: 10.1016/j.prrv.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng C.L., Tang Y., Zheng Z., Liu X., Ye Z.C., Wang C., Lou T.Q. Advanced glycation end-products activate the renin-angiotensin system through the RAGE/PI3-K signaling pathway in podocytes. Clin Invest Med. 2012;35 doi: 10.25011/cim.v35i5.18701. [DOI] [PubMed] [Google Scholar]
- 26.Ihara Y., Egashira K., Nakano K., Ohtani K., Kubo M., Koga J., Iwai M., Horiuchi M., Gang Z., Yamagishi S., Sunagawa K. Upregulation of the ligand-RAGE pathway via the angiotensin II type I receptor is essential in the pathogenesis of diabetic atherosclerosis. J. Mol. Cell. Cardiol. 2007;43:455–464. doi: 10.1016/j.yjmcc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 27.Jeong J., Lee J., Lim J., Cho S., An S., Lee M., Yoon N., Seo M., Lim S., Park S. Soluble RAGE attenuates AngII-induced endothelial hyperpermeability by disrupting HMGB1-mediated crosstalk between AT1R and RAGE. Exp. Mol. Med. 2019;51:1–15. doi: 10.1038/s12276-019-0312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojas A., Gonzalez I., Morales M.A. SARS-CoV-2-mediated inflammatory response in lungs: should we look at RAGE? Inflamm. Res. 2020;69:641–643. doi: 10.1007/s00011-020-01353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andersson U., Ottestad W., Tracey K.J. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol. Med. 2020;26 doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholls J.M., Poon L.L., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A., Schipper D., van Run P., Leijten L., Sikkema R., Verschoor E., Verstrepen B., Bogers W., Langermans J., Drosten C., Fentener van Vlissingen M., Fouchier R., de Swart R., Koopmans M., Haagmans B.L. Comparative pathogenesis of COVID-19, MERS, and SARS in a non human primate model. Science. 2020;368:1012–1015. doi: 10.1126/science.abb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., Worlock K.B., Yoshida M., Barnes J.L., HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N., Yuen K.Y., Sung J.J. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology. 2003;125:1011–1017. doi: 10.1016/s0016-5085(03)01215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mandavilli A. Immune response to SARS sets up puzzling paradox. Nat. Med. 2004;10:1268. doi: 10.1038/nm1204-1268a. [DOI] [PubMed] [Google Scholar]
- 40.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 41.Oscar T.P. A multiple therapy hypothesis for treatment of COVID-19 patients. Med. Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.F. Jiang, J. Yang, Y. Zhang, M. Dong, S. Wang, Q. Zhang, F.F. Liu, K. Zhang, C. Zhang, (2014) Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets, Nat. Rev. Cardiol. 11 (2014) 413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed]
- 45.Mossel E.C., Wang J., Jeffers S., Edeen K.E., Wang S., Cosgrove G.P., Funk C.J., Manzer R., Miura T.A., Pearson L.D., Holmes K.V., Mason R.J. SARS-CoV replicates in primary human alveolar type II cell cultures but not in type I-like cells. Virology. 2008;372:127–135. doi: 10.1016/j.virol.2007.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M., Sigl V., Hanada T., Hanada R., Lipinski S., Wild B., Camargo S.M., Singer D., Richter A., Kuba K., Fukamizu A., Schreiber S., Clevers H., Verrey F., Rosenstiel P., Penninger J.M. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Douglas G.C., O'Bryan M.K., Hedger M.P., Lee D.K., Yarski M.A., Smith A.I., Lew R.A. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 48.López-Romero R., Nambo-Lucio M.J., Salcedo-Carrillo E., Hernández-Cueto M.L.Á., Salcedo-Vargas M. The big challenge of SARS-CoV-2 latency: testes as reservoir. Gac. Med. Mex. 2020;156:328–333. doi: 10.24875/GMM.20000295. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang X.H., Deng W., Tong Z., Liu Y.X., Zhang L.F., Zhu H., Gao H., Huang L., Liu Y.L., Ma C.M., Xu Y.F., Ding M.X., Deng H.K., Qin C. Mice transgenic for human angiotensin-converting enzyme 2 provide a model for SARS coronavirus infection. Comp Med. 2007;57:450–459. (PMID: 17974127) [PubMed] [Google Scholar]
- 51.Sparks M.A., Crowley S.D., Gurley S.B., Mirotsou M., Coffman T.M. Classical renin-angiotensin system in kidney physiology. Compr Physiol. 2014;4:1201–1228. doi: 10.1002/cphy.c130040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel V.B., Zhong J.C., Grant M.B., Oudit G.Y. Role of the ACE2/angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ. Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paz Ocaranza M., Riquelme J.A., García L., Jalil J.E., Chiong M., Santos R.A.S., Lavandero S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020;17:116–129. doi: 10.1038/s41569-019-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52:731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuba K., Imai Y., Penninger J.M. Angiotensin-converting enzyme 2 in lung diseases. Curr. Opin. Pharmacol. 2006;6:271–276. doi: 10.1016/j.coph.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papp M., Li X., Zhuang J., Wang R., Uhal B.D. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am. J. Phys. Lung Cell. Mol. Phys. 2002;282:L713–L718. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 58.Lin C.W., Huang Y.Y. Does the direct renin inhibitor have a role to play in attenuating severity of the outbreak coronavirus disease 2019 (COVID-19)? Ther Adv Endocrinol Metab. 2020;11 doi: 10.1177/2042018820916430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., Zhang Z., Wang L., Peng L., Chen L., Qin Y., Zhao D., Tan S., Yin L., Xu J., Zhou C., Jiang C., Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu H., Xie Z., Li T., Zhang S., Lai C., Zhu P., Wang K., Han L., Duan Y., Zhao Z., Yang X., Xing L., Zhang P., Wang Z., Li R., Yu J.J., Wang X., Yang P. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016;6 doi: 10.1038/srep19840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.G.J. Rey -Parra, A. Vadivel, L. Coltan, A. Hall, F. Eaton, M. Schuster, H. Loibner, J.M. Penninger, Z. Kassiri, G.Y. Oudit, B. Thébaud, Angiotensin converting enzyme 2 abrogates bleomycin-induced lung injury, J Mol Med (Berl). 90 (2012) 637–647. doi: 10.1007/s00109-012-0859-2. [DOI] [PMC free article] [PubMed]
- 64.Shenoy V., Kwon K.C., Rathinasabapathy A., Lin S., Jin G., Song C., Shil P., Nair A., Qi Y., Li Q., Francis J., Katovich M.J., Daniell H., Raizada M.K. Oral delivery of angiotensin-converting enzyme 2 and angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension [published correction appears in hypertension. 2015 Mar;65 (3):e8] Hypertension. 2015;64:1248–1259. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim S., Rigatto K., Gazzana M.B., Knorst M.M., Richards E.M., Pepine C.J., Raizada M.K. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension. 2020;75:1063–1071. doi: 10.1161/HYPERTENSIONAHA.119.14294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang P., Gu H., Zhao Z., Wang W., Cao B., Lai C., Yang X., Zhang L., Duan Y., Zhang S., Chen W., Zhen W., Cai M., Penninger J.M., Jiang C., Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci. Rep. 2014;4:7027. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou Z., Yan Y., Shu Y., Gao R., Sun Y., Li X., Ju X., Liang Z., Liu Q., Zhao Y., Guo F., Bai T., Han Z., Zhu J., Zhou H., Huang F., Li C., Lu H., Li N., Li D., Jin N., Penninger J.M., Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza a H5N1 infections. Nat. Commun. 2014;5 doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D., Hall R., Poirier G., Ronco J.J., Tidswell M., Hardes K., Powley W.M., Wright T.J., Siederer S.K., Fairman D.A., Lipson D.A., Bayliffe A.I., Lazaar A.L. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care. 2017;21 doi: 10.1186/s13054-017-1823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zoufaly A., Poglitsch M., Aberle J.H., Hoepler W., Seitz T., Traugott M., Grieb A., Pawelka E., Laferl H., Wenisch C., Neuhold S., Haider D., Stiasny K., Bergthaler A., Puchhammer-Stoeckl E., Mirazimi A., Montserrat N., Zhang H., Slutsky A.S., Penninger J.M. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020;8:1154–1158. doi: 10.1016/S2213-2600(20)30418-5. Erratum in: Lancet Respir Med. 8 (2020) e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imai Y., Kuba K., Penninger J.M. The discovery of angiotensin-converting enzyme 2 and its role in acute lung injury in mice. Exp. Physiol. 2008;93:543–548. doi: 10.1113/expphysiol.2007.040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bali A., Singh N., Jaggi A.S. Renin-angiotensin system in pain: existing in a double life? J. Renin-Angiotensin-Aldosterone Syst. 2014;15:329–340. doi: 10.1177/1470320313503694. [DOI] [PubMed] [Google Scholar]
- 72.Neeper M., Schmidt A.M., Brett J., Yan S.D., Wang F., Pan Y.C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992;267:14998–15004. (PMID: 1378843) [PubMed] [Google Scholar]
- 73.Sessa L., Gatti E., Zeni F., Antonelli A., Catucci A., Koch M., Pompilio G., Fritz G., Raucci A., Bianchi M.E. The receptor for advanced glycation end-products (RAGE) is only present in mammals, and belongs to a family of cell adhesion molecules (CAMs) PLoS One. 2014;9 doi: 10.1371/journal.pone.0086903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudson B.I., Carter A.M., Harja E., Kalea A.Z., Arriero M., Yang H., Grant P.J., Schmidt A.M. Identification, classification, and expression of RAGE gene splice variants. FASEB J. 2008;22:1572–1580. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- 75.Hudson B.I., Kalea A.Z., Del Mar Arriero M., Harja E., Boulanger E., D’Agati V., Schmidt A.M. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J. Biol. Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim W., Hudson B.I., Moser B., Guo J., Rong L.L., Lu Y., Qu W., Lalla E., Lerner S., Chen Y., Yan S.S., D’Agati V., Naka Y., Ramasamy R., Herold K., Yan S.F., Schmidt A.M. Receptor for advanced glycation end products and its ligands: a journey from the complications of diabetes to its pathogenesis. Ann. N. Y. Acad. Sci. 2005;1043:553–561. doi: 10.1196/annals.1338.063. [DOI] [PubMed] [Google Scholar]
- 77.Chavakis T., Bierhaus A., Al-Fakhri N., Schneider D., Witte S., Linn T., Nagashima M., Morser J., Arnold B., Preissner K.T., Nawroth P.P. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J. Exp. Med. 2003;198:1507–1515. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hudson B.I., Lippman M.E. Targeting RAGE signaling in inflammatory disease. Annu. Rev. Med. 2018;69:349–364. doi: 10.1146/annurev-med-041316-085215. [DOI] [PubMed] [Google Scholar]
- 79.Gefter J.V., Shaufl A.L., Fink M.P., Delude R.L. Comparison of distinct protein isoforms of the receptor for advanced glycation end-products expressed in murine tissues and cell lines. Cell Tissue Res. 2009;337:79–89. doi: 10.1007/s00441-009-0791-0. [DOI] [PubMed] [Google Scholar]
- 80.Kalea A.Z., Reiniger N., Yang H., Arriero M., Schmidt A.M., Hudson B.I. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. FASEB J. 2009;23:1766–1774. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hanford L.E., Enghild J.J., Valnickova Z., Petersen S.V., Schaefer L.M., Schaefer T.M., Reinhart T.A., Oury T.D. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J. Biol. Chem. 2004;279:50019–50024. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dahlin K., Mager E.M., Allen L., Tigue Z., Goodglick L., Wadehra M., Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am. J. Respir. Cell Mol. Biol. 2004;31:309–316. doi: 10.1165/rcmb.2003-0423OC. [DOI] [PubMed] [Google Scholar]
- 83.Demling N., Ehrhardt C., Kasper M., Laue M., Knels L., Rieber E.P. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323:475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 84.Katsuoka F., Kawakami Y., Arai T., Imuta H., Fujiwara M., Kanma H., Yamashita K. Type II alveolar epithelial cells in lung express receptor for advanced glycation end products (RAGE) gene. Biochem. Biophys. Res. Commun. 1997;238:512–516. doi: 10.1006/bbrc.1997.7263. [DOI] [PubMed] [Google Scholar]
- 85.Constien R., Forde A., Liliensiek B., Gröne H.J., Nawroth P., Hämmerling G., Arnold B. Characterization of a novel EGFP reporter mouse to monitor Cre recombination as demonstrated by a Tie2 Cre mouse line. Genesis. 2001;30:36–44. doi: 10.1002/gene.1030. [DOI] [PubMed] [Google Scholar]
- 86.Englert J.M., Hanford L.E., Kaminski N., Tobolewski J.M., Tan R.J., Fattman C.L., Ramsgaard L., Richards T.J., Loutaev I., Nawroth P.P., Kasper M., Bierhaus A., Oury T.D. A role for the receptor for advanced glycation end products in idiopathic pulmonary fibrosis. Am. J. Pathol. 2008;172:583–591. doi: 10.2353/ajpath.2008.070569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bartling B., Hofmann H.S., Weigle B., Silber R.E., Simm A. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis. 2005;26:293–301. doi: 10.1093/carcin/bgh333. [DOI] [PubMed] [Google Scholar]
- 88.Al-Robaiy S., Weber B., Simm A., Diez C., Rolewska P., Silber R.E., Bartling B. The receptor for advanced glycation end-products supports lung tissue biomechanics. Am. J. Phys. Lung Cell. Mol. Phys. 2013;305:L491–L500. doi: 10.1152/ajplung.00090.2013. [DOI] [PubMed] [Google Scholar]
- 89.Brett J., Schmidt A.M., Yan S.D., Zou Y.S., Weidman E., Pinsky D., Nowygrod R., Neeper M., Przysiecki C., Shaw A. Survey of the distribution of a newly characterized receptor for advanced glycation end products in tissues. Am. J. Pathol. 1993;143:1699–1712. (PMID: 8256857) [PMC free article] [PubMed] [Google Scholar]
- 90.Yonekura H., Yamamoto Y., Sakurai S., Petrova R.G., Abedin M.J., Li H., Yasui K., Takeuchi M., Makita Z., Takasawa S., Okamoto H., Watanabe T., Yamamoto H. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003;370:1097–1109. doi: 10.1042/BJ20021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chuah Y.K., Basir R., Talib H., Tie T.H., Nord N. Receptor for advanced glycation end products and its involvement in inflammatory diseases. Int. J. Inf. Secur. 2013;2013 doi: 10.1155/2013/403460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lin L., Park S., Lakatta E.G. RAGE signaling in inflammation and arterial aging. Front Biosci (Landmark Ed) 2009;14:1403–1413. doi: 10.2741/3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kierdorf K., Fritz G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 94.Byun K., Yoo Y., Son M., Lee J., Jeong G.B., Park Y.M., Salekdeh G.H., Lee B. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol. Ther. 2017;177:44–55. doi: 10.1016/j.pharmthera.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 95.Roh J.S., Sohn D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18 doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bierhaus A., Stern D.M., Nawroth P.P. RAGE in inflammation: a new therapeutic target? Curr. Opin. Investig. Drugs. 2006;7:985–991. (PMID: 17117586) [PubMed] [Google Scholar]
- 97.Stern D., Yan S.D., Yan S.F., Schmidt A.M. Receptor for advanced glycation endproducts: a multiligand receptor magnifying cell stress in diverse pathologic settings. Adv. Drug Deliv. Rev. 2002;54:1615–1625. doi: 10.1016/s0169-409x(02)00160-6. [DOI] [PubMed] [Google Scholar]
- 98.Chiappalupi S., Sorci G., Vukasinovic A., Salvadori L., Sagheddu R., Coletti D., Renga G., Romani L., Donato R., Riuzzi F. Targeting RAGE prevents muscle wasting and prolongs survival in cancer cachexia. J. Cachexia. Sarcopenia Muscle. 2020;11:929–946. doi: 10.1002/jcsm.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sagheddu R., Chiappalupi S., Salvadori L., Riuzzi F., Donato R., Sorci G. Targeting RAGE as a potential therapeutic approach to Duchenne muscular dystrophy. Hum. Mol. Genet. 2018;27:3734–3746. doi: 10.1093/hmg/ddy288. [DOI] [PubMed] [Google Scholar]
- 100.Riuzzi F., Sorci G., Sagheddu R., Chiappalupi S., Salvadori L., Donato R. RAGE in the pathophysiology of skeletal muscle. J. Cachexia. Sarcopenia Muscle. 2018;9:1213–1234. doi: 10.1002/jcsm.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Donato R., Sorci G., Riuzzi F., Arcuri C., Bianchi R., Brozzi F., Tubaro C., Giambanco I. S100B’s double life: intracellular regulator and extracellular signal. Biochim. Biophys. Acta. 2009;1793:1008–1022. doi: 10.1016/j.bbamcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 102.Riuzzi F., Chiappalupi S., Arcuri C., Giambanco I., Sorci G., Donato R. S100 proteins in obesity: liaisons dangereuses. Cell. Mol. Life Sci. 2020;77:129–147. doi: 10.1007/s00018-019-03257-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riuzzi F., Beccafico S., Sagheddu R., Chiappalupi S., Giambanco I., Bereshchenko O., Riccardi C., Sorci G., Donato R. Levels of S100B protein drive the reparative process in acute muscle injury and muscular dystrophy. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-12880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teissier T., Boulanger É. The receptor for advanced glycation end-products (RAGE) is an important pattern recognition receptor (PRR) for inflammaging. Biogerontology. 2019;20:279–301. doi: 10.1007/s10522-019-09808-3. [DOI] [PubMed] [Google Scholar]
- 105.Grossin N., Auger F., Niquet-Leridon C., Durieux N., Montaigne D., Schmidt A.M., Susen S., Jacolot P., Beuscart J.B., Tessier F.J., Boulanger E. Dietary CML-enriched protein induces functional arterial aging in a RAGE-dependent manner in mice. Mol. Nutr. Food Res. 2015;59:927–938. doi: 10.1002/mnfr.201400643. [DOI] [PubMed] [Google Scholar]
- 106.Bonafè M., Prattichizzo F., Giuliani A., Storci G., Sabbatinelli J., Olivieri F. Inflamm-aging: why older men are the most susceptible to SARS-CoV-2 complicated outcomes. Cytokine Growth Factor Rev. 2020;53 doi: 10.1016/j.cytogfr.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ramsgaard L., Englert J.M., Tobolewski J., Tomai L., Fattman C.L., Leme A.S., Kaynar A.M., Shapiro S.D., Enghild J.J., Oury T.D. The role of the receptor for advanced glycation end-products in a murine model of silicosis. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Queisser M.A., Kouri F.M., Königshoff M., Wygrecka M., Schubert U., Eickelberg O., Preissner K.T. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am. J. Respir. Cell Mol. Biol. 2008;39:337–345. doi: 10.1165/rcmb.2007-0244OC. [DOI] [PubMed] [Google Scholar]
- 109.He M., Kubo H., Ishizawa K., Hegab A.E., Yamamoto Y., Yamamoto H., Yamaya M. The role of the receptor for advanced glycation end-products in lung fibrosis. Am. J. Phys. Lung Cell. Mol. Phys. 2007;293:L1427–L1436. doi: 10.1152/ajplung.00075.2007. [DOI] [PubMed] [Google Scholar]
- 110.Chen L., Wang T., Wang X., Sun B.B., Li J.Q., Liu D.S., Zhang S.F., Liu L., Xu D., Chen Y.J., Wen F.Q. Blockade of advanced glycation end product formation attenuates bleomycin-induced pulmonary fibrosis in rats. Respir. Res. 2009;10 doi: 10.1186/1465-9921-10-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morbini P., Villa C., Campo I., Zorzetto M., Inghilleri S., Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod. Pathol. 2006;19:1437–1445. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 112.Zhao J., Randive R., Stewart J.A. Molecular mechanisms of AGE/RAGE-mediated fibrosis in the diabetic heart. World J. Diabetes. 2014;5:860–867. doi: 10.4239/wjd.v5.i6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Foell D., Seeliger S., Vogl T., Koch H.G., Maschek H., Harms E., Sorg C., Roth J. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax. 2003;58:613–617. doi: 10.1136/thorax.58.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makam M., Diaz D., Laval J., Gernez Y., Conrad C.K., Dunn C.E., Davies Z.A., Moss R.B., Herzenberg L.A., Herzenberg L.A., Tirouvanziam R. Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5779–5783. doi: 10.1073/pnas.0813410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beucher J., Boëlle P.Y., Busson P.F., Muselet-Charlier C., Clement A., Corvol H., French C.F., Modifier Gene Study Investigators AGER -429T/C is associated with an increased lung disease severity in cystic fibrosis. PLoS One. 2012;7 doi: 10.1371/journal.pone.0041913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iannitti R.G., Casagrande A., De Luca A., Cunha C., Sorci G., Riuzzi F., Borghi M., Galosi C., Massi-Benedetti C., Oury T.D., Cariani L., Russo M., Porcaro L., Colombo C., Majo F., Lucidi V., Fiscarelli E., Ricciotti G., Lass-Flörl C., Ratclif L., Esposito A., De Benedictis F.M., Donato R., Carvalho A., Romani L. Hypoxia promotes danger-mediated inflammation via receptor for advanced glycation end products in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2013;188:1338–1350. doi: 10.1164/rccm.201305-0986OC. [DOI] [PubMed] [Google Scholar]
- 117.Uchida T., Shirasawa M., Ware L.B., Kojima Y. Hata, Makita K., Mednick G., Matthay Z.A., Matthay M.A. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am. J. Respir. Crit. Care Med. 2006;173:1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Reynolds R.S., Schmitt R.E., Kasteler S.D., Sturrock A., Sanders K., Bierhaus A., Nawroth P.P., Paine R., 3rd, Hoidal J.R. Receptors for advanced glycation end-products targeting protect against hyperoxia-induced lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2010;42:545–551. doi: 10.1165/rcmb.2008-0265OC. [DOI] [PubMed] [Google Scholar]
- 119.Jabaudon M., Blondonnet R., Roszyk L., Pereira B., Guérin R., Perbet S., Cayot S., Bouvier D., Blanchon L., Sapin V., Constantin J.M. Soluble forms and ligands of the receptor for advanced glycation end-products in patients with acute respiratory distress syndrome: an observational prospective study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nakamura T., Sato E., Fujiwara N., Kawagoe Y., Maeda S., Yamagishi S. Increased levels of soluble receptor for advanced glycation end products (sRAGE) and high mobility group box1 (HMGB1) are associated with death in patients with acute respiratory distress syndrome. Clin. Biochem. 2011;44:601–604. doi: 10.1016/j.clinbiochem.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 121.Jabaudon M., Blondonnet R., Roszyk L., Bouvier D., Audard J., Clairefond G., Fournier M., Marceau G., Déchelotte P., Pereira B., Sapin V., Constantin J.M. Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2015;192:191–199. doi: 10.1164/rccm.201501-0020OC. [DOI] [PubMed] [Google Scholar]
- 122.L. Qu, C. Chen, Y. Chen, Y. Li, F. Tang, H. Huang, W. He, R. Zhang, L. Shen, High-mobility group box 1 (HMGB1) and autophagy in acute lung injury (ALI): a review, Med. Sci. Monit.. 25 (2019) 1828–1837. doi:10.12659/MSM.912867. [DOI] [PMC free article] [PubMed]
- 123.Suda K., Takeuchi H., Ishizaka A., Kitagawa Y. High-mobility-group box chromosomal protein 1 as a new target for modulating stress response. Surg. Today. 2010;40:592–601. doi: 10.1007/s00595-009-4232-1. [DOI] [PubMed] [Google Scholar]
- 124.M. Deng, Y. Tang, W. Li, X. Wang, R. Zhang, X. Zhang, X Zhao, J. Liu, C. Tang, Z. Liu, Y. Huang, H. Peng, L. Xiao, D. Tang, M.J. Scott, Q. Wang, J. Liu, X. Xiao, S. Watkins, J. Li, H. Yang, H. Wang, F. Chen, K.J. Tracey, T.R. Billiar, B. Lu, The endotoxin delivery protein HMGB1 mediates caspase-11-dependent lethality in sepsis, Immunity. 49 (2018) 740–753. e7. doi: 10.1016/j.immuni.2018.08.016. [DOI] [PMC free article] [PubMed]
- 125.Wolfson R.K., Chiang E.T., Garcia J.G. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvasc. Res. 2011;81:189–197. doi: 10.1016/j.mvr.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.van Zoelen M.A., Schouten M., de Vos A.F., Florquin S., Meijers J.C., Nawroth P.P., Bierhaus A., van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J. Immunol. 2009;182:4349–4356. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 127.Achouiti A., de Vos A.F., van ’t Veer C., Florquin S., Tanck M.W., Nawroth P.P., Bierhaus A., van der Poll T., van Zoelen M.A. Receptor for advanced glycation end products (RAGE) serves a protective role during Klebsiella pneumoniae - induced pneumonia. PLoS One. 2016;11 doi: 10.1371/journal.pone.0141000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sanders K.A., Delker D.A., Huecksteadt T., Beck E., Wuren T., Chen Y., Zhang Y., Hazel M.W., Hoidal J.R. RAGE is a critical mediator of pulmonary oxidative stress, alveolar macrophage activation and emphysema in response to cigarette smoke. Sci. Rep. 2019;9 doi: 10.1038/s41598-018-36163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vardavas C.I., Nikitara K. COVID-19 and smoking: a systematic review of the evidence. TobInduc Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Zoelen M.A., van der Sluijs K.F., Achouiti A., Florquin S., Braun-Pater J.M., Yang H., Nawroth P.P., Tracey K.J., Bierhaus A., van der Poll T. Receptor for advanced glycation end products is detrimental during influenza A virus pneumonia. Virology. 2009;391:265–273. doi: 10.1016/j.virol.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Manti S., Harford T.J., Salpietro C., Rezaee F., Piedimonte G. Induction of high-mobility group Box-1 in vitro and in vivo by respiratory syncytial virus. Pediatr. Res. 2018;83:1049–1056. doi: 10.1038/pr.2018.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hatayama K., Nosaka N., Yamada M., Yashiro M., Fujii Y., Tsukahara H., Liu K., Nishibori M., Matsukawa A., Morishima T. Combined effect of anti-high-mobility group box-1 monoclonal antibody and peramivir against influenza A virus-induced pneumonia in mice. J. Med. Virol. 2019;91:361–369. doi: 10.1002/jmv.25330. [DOI] [PubMed] [Google Scholar]
- 133.Fu Y., Tang Z., Ye Z., Mo S., Tian X., Ni K., Ren L., Liu E., Zang N. Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect. Dis. 2019;19 doi: 10.1186/s12879-018-3651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu L., Yang M., Kang R., Dai Y., Yu Y., Gao F., Wang H., Sun X., Li X., Li J., Wang H., Cao L., Tang D. HMGB1-DNA complex-induced autophagy limits AIM2 inflammasome activation through RAGE. Biochem. Biophys. Res. Commun. 2014;450:851–856. doi: 10.1016/j.bbrc.2014.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang H., Luo J., Alcorn J.F., Chen K., Fan S., Pilewski J., Liu A., Chen W., Kolls J.K., Wang J. AIM2 inflammasome is critical for influenza-induced lung injury and mortality. J. Immunol. 2017;198:4383–4393. doi: 10.4049/jimmunol.1600714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xie K., Chen Y.Q., Chai Y.S., Lin S.H., Wang C.J., Xu F. HMGB1 suppress the expression of IL-35 by regulating Naïve CD4+ T cell differentiation and aggravating Caspase-11-dependent pyroptosis in acute lung injury. Int. Immunopharmacol. 2021;91 doi: 10.1016/j.intimp.2020.107295. [DOI] [PubMed] [Google Scholar]
- 137.Qian B., Huang H., Cheng M., Qin T., Chen T., Zhao J. Mechanism of HMGB1-RAGE in Kawasaki disease with coronary artery injury. Eur. J. Med. Res. 2020;25 doi: 10.1186/s40001-020-00406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chang L.Y., Lu C.Y., Shao P.L., Lee P.I., Lin M.T., Fan T.Y., Cheng A.L., Lee W.L., Hu J.J., Yeh S.J., Chang C.C., Chiang B.L., Wu M.H., Huang L.M. Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 2014;113:148–154. doi: 10.1016/j.jfma.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Andersson U. The cholinergic anti-inflammatory pathway alleviates acute lung injury. Mol. Med. 2020;26 doi: 10.1186/s10020-020-00184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R.A.P.M., Scott M., Hagan T., Sigal N., Feng Y., Bristow L., Tak-Yin Tsang O., Wagh D., Coller J., Pellegrini K.L., Kazmin D., Alaaeddine G., Leung W.S., Chan J.M.C., Chik T.S.H., Choi C.Y.C., Huerta C., Paine McCullough M., Lv H., Anderson E., Edupuganti S., Upadhyay A.A., Bosinger S.E., Maecker H.T., Khatri P., Rouphael N., Peiris M., Pulendran B. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Krishnamachary B., Cook C., Spikes L., Chalise P., Dhillon N.K. 2020. The potential role of extracellular vesicles in COVID-19 associated endothelial injury and Pro-inflammation, medRxiv. [DOI] [Google Scholar]
- 143.Aceti A., Margarucci L.M., Scaramucci E., Orsini M., Salerno G., Di Sante G., Gianfranceschi G., Di Liddo R., Valeriani F., Ria F., Simmaco M., Parnigotto P.P., Vitali M., Romano Spica V., Michetti F. Serum S100B proteinas a marker of severity in Covid-19 patients. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-75618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yalcin Kehribar D., Cihangiroglu M., Sehmen E., Avci B., Capraz A., Yildirim Bilgin A., Gunaydin C., Ozgen M. The receptor for advanced glycation end product (RAGE) pathway in COVID-19. Biomarkers. 2020;13:1–5. doi: 10.1080/1354750X.2020.1861099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dozio E., Sitzia C., Pistelli L., Cardani R., Rigolini R., Ranucci M., Corsi Romanelli M.M. Soluble receptor for advanced glycation end products and its forms in COVID-19 patients with and without diabetes mellitus: a pilot study on their role as disease biomarkers. J. Clin. Med. 2020;9:3785. doi: 10.3390/jcm9113785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.De Francesco E.M., Vella V., Belfiore A. COVID-19 and diabetes: the importance of controlling RAGE. Front Endocrinol (Lausanne) 2020;11:526. doi: 10.3389/fendo.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chakrabarty B., Dibyajyoti D., Gopalakrishnan B., Arijit R. Network-based analysis of fatal comorbidities of COVID-19 and potential therapeutics. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12136470.v1. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Alsamman A.M., Zayed H. The transcriptomic profiling of SARS-CoV-2 compared to SARS, MERS, EBOV, and H1N1. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ruan X., Du P., Zhao K., Huang J., Xia H., Dai D., Huang S., Cui X., Liu L., Zhang J. Mechanism of Dayuanyin in the treatment of coronavirus disease 2019 based on network pharmacology and molecular docking. Chin. Med. 2020;15 doi: 10.1186/s13020-020-00346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jakhmola Mani R., Sehgal N., Dogra N., Saxena S., Pande Katare D. Deciphering underlying mechanism of Sars-CoV-2 infection in humans and revealing the therapeutic potential of bioactive constituents from Nigella sativa to combat COVID19: in-silico study. J. Biomol. Struct. Dyn. 2020;28:1–13. doi: 10.1080/07391102.2020.1839560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stilhano R.S., Costa A.J., Nishino M.S., Shams S., Bartolomeo C.S., Breithaupt-Faloppa A.C., Silva E.A., Ramirez A.L., Prado C.M., Ureshino R.P. SARS-CoV-2 and the possible connection to ERs, ACE2, and RAGE: focus on susceptibility factors. FASEB J. 2020;34:14103–14119. doi: 10.1096/fj.202001394RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Tsang H.F., Chan L.W.C., Cho W.C.S., Yu A.C.S., Yim A.K.Y., Chan A.K.C., Ng L.P.W., Wong Y.K.E., Pei X.M., Li M.J.W., Wong S.C. An update on COVID-19 pandemic: the epidemiology, pathogenesis, prevention and treatment strategies. Expert Rev. Anti-Infect. Ther. 2020;29:1–12. doi: 10.1080/14787210.2021.1863146. [DOI] [PubMed] [Google Scholar]
- 153.Zemskov M., Kurdyukov S., James J., McClain N., Rafikov R., Rafikova O. Sex-specific stress response and HMGB1 release in pulmonary endothelial cells. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Roy D., Ramasamy R., Schmidt A.M. Journey to a receptor for advanced glycation end products connection in severe acute respiratory syndrome coronavirus 2 infection: with stops along the way in the lung, heart, blood vessels, and adipose tissue. ArteriosclerThrombVasc Biol. 2020;41 doi: 10.1161/ATVBAHA.120.315527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rüster C., Bondeva T., Franke S., Tanaka N., Yamamoto H., Wolf G. Angiotensin II upregulates RAGE expression on podocytes: role of AT2 receptors. Am. J. Nephrol. 2009;29:538–550. doi: 10.1159/000191467. [DOI] [PubMed] [Google Scholar]
- 156.Garagliano J.M., Katsurada A., Miyata K., Derbenev A.V., Zsombok A., Navar L.G., Satou R. Advanced glycation end products stimulate angiotensinogen production in renal proximal tubular cells. Am J Med Sci. 2019;357:57–66. doi: 10.1016/j.amjms.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ishibashi Y., Matsui T., Yamagishi S. Olmesartan blocks advanced glycation end products-induced vcam-1 gene expression in mesangial cells by restoring angiotensin-converting enzyme 2 level. Horm. Metab. Res. 2014;46:379–383. doi: 10.1055/s-0033-1361114. [DOI] [PubMed] [Google Scholar]
- 158.Lim S., Lee M.E., Jeong J., Lee J., Cho S., Seo M., Park S. sRAGE attenuates angiotensin II-induced cardiomyocyte hypertrophy by inhibiting RAGE-NFκB-NLRP3 activation. Inflamm. Res. 2018;67:691–701. doi: 10.1007/s00011-018-1160-9. [DOI] [PubMed] [Google Scholar]
- 159.Muñoz N., Pedreañez A., Mosquera J. Angiotensin II induces increased myocardial expression of receptor for advanced glycation end products (RAGE), monocyte/macrophage infiltration and circulating endothelin-1 in rats with experimental diabetes. Can. J. Diabetes. 2020;44(2020):651–656. doi: 10.1016/j.jcjd.2020.03.010. [DOI] [PubMed] [Google Scholar]
- 160.Kikuchi K., Tancharoen S., Ito T., Morimoto-Yamashita Y., Miura N., Kawahara K., Maruyama I., Murai Y., Tanaka E. Potential of the angiotensin receptor blockers (ARBs) telmisartan, irbesartan, and candesartan for inhibiting the HMGB1/RAGE axis in prevention and acute treatment of stroke, [published correction appears in Int J Mol Sci] J Mol Sci. 2013;14:18899–18924. doi: 10.3390/ijms140918899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakamura T., Sato E., Fujiwara N., Kawagoe Y., Yamada S., Ueda Y., Koide H. Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. Am J Med Sci. 2012;343:46–51. doi: 10.1097/MAJ.0b013e31821f0552. [DOI] [PubMed] [Google Scholar]
- 162.Luft F.C. High-mobility group box 1 protein, angiotensins, ACE2, and target organ damage. J Mol Med (Berl) 2016;94:1–3. doi: 10.1007/s00109-015-1372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Forbes J.M., Thorpe S.R., Thallas-Bonke V., Pete J., Thomas M.C., Deemer E.R., Bassal S., El-Osta A., Long D.M., Panagiotopoulos S., Jerums G., Osicka T.M., Cooper M.E. Modulation of soluble receptor for advanced glycation end products by angiotensin-converting enzyme-1 inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 2005;16:2363–2372. doi: 10.1681/ASN.2005010062. [DOI] [PubMed] [Google Scholar]
- 164.Miyata T., van Ypersele de Strihou C., Ueda Y., Ichimori K., Inagi R., Onogi H., Ishikawa N., Nangaku M., Kurokawa K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J. Am. Soc. Nephrol. 2002;13:2478–2487. doi: 10.1097/01.asn.0000032418.67267.f2. [DOI] [PubMed] [Google Scholar]
- 165.Lozano-Maneiro L., Puente-García A. Renin-angiotensin-aldosterone system blockade in diabetic nephropathy. Present evidences. J. Clin. Med. 2015;4:1908–1937. doi: 10.3390/jcm4111908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pickering R.J., Tikellis C., Rosado C.J., Tsorotes D., Dimitropoulos A., Smith M., Huet O., Seeber R.M., Abhayawardana R., Johnstone E.K., Golledge J., Wang Y., Jandeleit-Dahm K.A., Cooper M.E., Pfleger K.D., Thomas M.C. Transactivation of RAGE mediates angiotensin-induced inflammation and atherogenesis. J. Clin. Invest. 2019;129:406–421. doi: 10.1172/JCI99987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zong H., Madden A., Ward M., Mooney M.H., Elliott C.T., Stitt A.W. Homodimerization is essential for the receptor for advanced glycation end products (RAGE)-mediated signal transduction. J. Biol. Chem. 2010;285:23137–23146. doi: 10.1074/jbc.M110.133827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bongarzone S., Savickas V., Luzi F., Gee A.D. Targeting the receptor for advanced Glycation Endproducts (RAGE): a medicinal chemistry perspective. J. Med. Chem. 2017;60:7213–7232. doi: 10.1021/acs.jmedchem.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rojas A., Morales M., Gonzalez I., Araya P. Inhibition of RAGE axis signaling: a pharmacological challenge. Curr. Drug Targets. 2019;20:340–346. doi: 10.2174/1389450119666180820105956. [DOI] [PubMed] [Google Scholar]
- 170.Asadipooya K., Uy E.M. Advanced glycation end products (AGEs), receptor for AGEs, diabetes, and bone: review of the literature. J Endocr Soc. 2019;3:1799–1818. doi: 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Schmidt A.M. Soluble RAGEs - prospects for treating & tracking metabolic and inflammatory disease. Vasc. Pharmacol. 2015;72:1–8. doi: 10.1016/j.vph.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wautier M.P., Guillausseau P.J., Wautier J.L. Activation of the receptor for advanced glycation end products and consequences on health. Diabetes Metab Syndr. 2017;11:305–309. doi: 10.1016/j.dsx.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 173.Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int. J. Angiol. 2014;23:11–16. doi: 10.1055/s-0033-1363423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Miniati M., Monti S., Basta G., Cocci F., Fornai E., Bottai M. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respir. Res. 2011;12 doi: 10.1186/1465-9921-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Audard J., Godet T., Blondonnet R., Joffredo J.B., Paquette B., Belville C., Lavergne M., Gross C., Pasteur J., Bouvier D., Blanchon L., Sapin V., Pereira B., Constantin J.M., Jabaudon M. Inhibition of the receptor for advanced glycation end-products in acute respiratory distress syndrome: a randomised laboratory trial in piglets. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-45798-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Geroldi D., Falcone C., Emanuele E., D’Angelo A., Calcagnino M., Buzzi M.P., Scioli G.A., Fogari R. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J. Hypertens. 2005;23:1725–1729. doi: 10.1097/01.hjh.0000177535.45785.64. [DOI] [PubMed] [Google Scholar]
- 177.Volpina O.M., Koroev D.O., Volkova T.D., Kamynina A.V., Filatova M.P., Zaporozhskaya Y.V., Samokhin A.N., Aleksandrova I.Y., Bobkova N.V. A fragment of the receptor for advanced glycation end products restores the spatial memory of animals in a model of Alzheimer’s disease. Russian J Bioorganic Chem. 2015;41:638–644. doi: 10.1134/S1068162015060187. [DOI] [PubMed] [Google Scholar]
- 178.Susa Y., Masuda Y., Imaizumi H., Namiki A. Neutralization of receptor for advanced glycation end-products and high mobility group box- 1 attenuates septic diaphragm dysfunction in rats with peritonitis. Crit. Care Med. 2009;37:2619–2624. doi: 10.1097/CCM.0b013e3181a930f7. [DOI] [PubMed] [Google Scholar]
- 179.Jensen L.J., Denner L., Schrijvers B.F., Tilton R.G., Rasch R., Flyvbjerg A. Renal effects of a neutralising RAGE-antibody in long-term streptozotocin-diabetic mice. J. Endocrinol. 2006;188:493–501. doi: 10.1677/joe.1.06524. [DOI] [PubMed] [Google Scholar]
- 180.Flyvbjerg A., Denner L., Schrijvers B.F., Tilton R.G., Mogensen T.H., Paludan S.R., Rasch R. Long-term renal effects of a neutralizing RAGE antibody in obese type 2 diabetic mice. Diabetes. 2004;53:166–172. doi: 10.2337/diabetes.53.1.166. [DOI] [PubMed] [Google Scholar]
- 181.Park H., Ku S.H., Park H., Hong J., Kim D., Choi B.R., Pak H.N., Lee M.H., Mok H., Jeong J.H., Choi D., Kim S.H., Joung B. RAGE siRNA-mediated gene silencing provides cardioprotection against ventricular arrhythmias in acute ischemia and reperfusion. J. Control. Release. 2015;217:315–326. doi: 10.1016/j.jconrel.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 182.Cai X.G., Xia J.R., Li W.D., Lu F.L., Liu J., Lu Q., Zhi H. Anti-fibrotic effects of specific-siRNA targeting of the receptor for advanced glycation end products in a rat model of experimental hepatic fibrosis. Mol. Med. Rep. 2014;10:306–314. doi: 10.3892/mmr.2014.2207. [DOI] [PubMed] [Google Scholar]
- 183.Taguchi K., Yamagishi S.I., Yokoro M., Ito S., Kodama G., Kaida Y., Nakayama Y., Ando R., Yamada-Obara N., Asanuma K., Matsui T., Higashimoto Y., Brooks C.R., Ueda S., Okuda S., Fukami K. RAGE-aptamer attenuates deoxycorticosterone acetate/salt-induced renal injury in mice. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-21176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Matsui T., Higashimoto Y., Nishino Y., Nakamura N., Fukami K., Yamagishi S.I. RAGE-Aptamer blocks the development and progression of experimental diabetic nephropathy. Diabetes. 2017;66:1683–1695. doi: 10.2337/db16-1281. [DOI] [PubMed] [Google Scholar]
- 185.Nakamura K., Akagi S., Ejiri K., Yoshida M., Miyoshi T., Sakaguchi M., Amioka N., Suastika L.O.S., Kondo M., Nakayama R., Takaya Y., Higashimoto Y., Fukami K., Matsubara H., Ito H. Inhibitory effects of RAGE-aptamer on development of monocrotaline-induced pulmonary arterial hypertension in rats. J. Cardiol. 2020;29 doi: 10.1016/j.jjcc.2020.12.009. S0914-5087(20)30410-X. [DOI] [PubMed] [Google Scholar]
- 186.Nakamura N., Matsui T., Nishino Y., Sotokawauchi A., Higashimoto Y., Yamagishi S.I. Long-term local injection of RAGE-aptamer suppresses the growth of malignant melanoma in nude mice. J Oncol. 2019;4 doi: 10.1155/2019/7387601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Deane R., Singh I., Sagare A.P., Bell R.D., Ross N.T., La Rue B., Love R., Perry S., Paquette N., Deane R.J., Thiyagarajan M., Zarcone T., Fritz G., Friedman A.E., Miller B.L., Zlokovic B.V. A multimodal RAGE-specific inhibitor reduces amyloid β-mediated brain disorder in a mouse model of Alzheimer disease. J. Clin. Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhao W., Lin Y., Xiong J., Wang Y., Huang G., Deng Q., Yao L., Yu C., Dong H., Cai S., Zhao H. RAGE mediates β-catenin stabilization via activation of the Src/p-Cav-1 axis in a chemical-induced asthma model. Toxicol. Lett. 2018;299:149–158. doi: 10.1016/j.toxlet.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 189.H. Lee, J. Lee, S.H. Hong, I. Rahman, S.R. Yang, Inhibition of RAGE attenuates cigarette smoke-induced lung epithelial cell damage via RAGE-mediated Nrf2/DAMP signaling, Front. Pharmacol.. 9 (2018) 684. doi: 10.3389/fphar.2018.00684. [DOI] [PMC free article] [PubMed]
- 190.Kwak T., Drews-Elger K., Ergonul A., Miller P.C., Braley A., Hwang G.H., Zhao D., Besser A., Yamamoto Y., Yamamoto H., El-Ashry D., Slingerland J.M., Lippman M.E., Hudson B.I. Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene. 2017;36:1559–1572. doi: 10.1038/onc.2016.324. [DOI] [PubMed] [Google Scholar]
- 191.Lee H., Park J.R., Kim W.J., Sundar I.K., Rahman I., Park S.M., Yang S.R. Blockade of RAGE ameliorates elastase-induced emphysema development and progression via RAGE-DAMP signalling. FASEB J. 2017;31:2076–2089. doi: 10.1096/fj.201601155R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sanajou D., Ghorbani Haghjo A., Argani H., Roshangar L., Rashtchizadeh N., Ahmad S.N.S., Ashrafi-Jigheh Z., Bahrambeigi S., Asiaee F., Rashedi J., Aslani S. Reduction of renal tubular injury with a RAGE inhibitor FPS-ZM1, valsartan and their combination in streptozotocin-induced diabetes in the rat. Eur. J. Pharmacol. 2019;842:40–48. doi: 10.1016/j.ejphar.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 193.Burstein A.H., Grimes I., Galasko D.R., Aisen P.S., Sabbagh M., Mjalli A.M. Effect of TTP488 in patients with mild to moderate Alzheimer’s disease. BMC Neurol. 2014;14 doi: 10.1186/1471-2377-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sabbagh M.N., Agro A., Bell J., Aisen P.S., Schweizer E., Galasko D. PF-04494700, an oral inhibitor of receptor for advanced glycation end products (RAGE), in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2011;25:206–212. doi: 10.1097/WAD.0b013e318204b550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Burstein A.H., Sabbagh M., Andrews R., Valcarce C., Dunn I., Altstiel L. Development of azeliragon, an oral small molecule antagonist of the receptor for advanced glycation endproducts, for the potential slowing of loss of cognition in mild Alzheimer’s disease. J Prev Alzheimers Dis. 2018;5:149–154. doi: 10.14283/jpad.2018.18. [DOI] [PubMed] [Google Scholar]
- 196.Galasko D., Bell J., Mancuso J.Y., Kupiec J.W., Sabbagh M.N., van Dyck C., Thomas R.G., Aisen P.S., Alzheimer's Disease Cooperative Study Clinical trial of an inhibitor of RAGE-Aβ interactions in Alzheimer disease. Neurology. 2014;82:1536–1542. doi: 10.1212/WNL.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Manigrasso M.B., Pan J., Rai V., Zhang J., Reverdatto S., Quadri N., DeVita R.J., Ramasamy R., Shekhtman A., Schmidt A.M. Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction. Sci. Rep. 2016;6 doi: 10.1038/srep22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matsumoto H., Matsumoto N., Shimazaki J., Nakagawa J., Imamura Y., Yamakawa K., Yamada T., Ikeda M., Hiraike H., Ogura H., Shimazu T. Therapeutic effectiveness of anti-RAGE antibody administration in a rat model of crush injury. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-12065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gasparotto J., Ribeiro C.T., Bortolin R.C., Somensi N., Fernandes H.S., Teixeira A.A., Guasselli M.O.R., Agani C.A.J.O., Souza N.C., Grings M., Leipnitz G., Gomes H.M., de Bittencourt Pasquali M.A., Dunkley P.R., Dickson P.W., Moreira J.C.F., Gelain D.P. Anti-RAGE antibody selectively blocks acute systemic inflammatory responses to LPS in serum, liver, CSF and striatum. Brain Behav. Immun. 2017;62:124–136. doi: 10.1016/j.bbi.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 200.Hong Y., Shen C., Yin Q., Sun M., Ma Y., Liu X. Effects of RAGE-specific inhibitor FPS-ZM1 on amyloid-β metabolism and AGEs-induced inflammation and oxidative stress in rat hippocampus. Neurochem. Res. 2016;41:1192–1199. doi: 10.1007/s11064-015-1814-8. [DOI] [PubMed] [Google Scholar]
- 201.Liu L., Killoy K.M., Vargas M.R., Yamamoto Y., Pehar M. Effects of RAGE inhibition on the progression of the disease in hSOD1G93A ALS mice. Pharmacol. Res. Perspect. 2020;8 doi: 10.1002/prp2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Shen L., Zhang T., Yang Y., Lu D., Xu A., Li K. FPS-ZM1 alleviates neuroinflammation in focal cerebral ischemia rats via blocking ligand/RAGE/DIAPH1 pathway. ACS Chem. Neurosci. 2021;12:63–78. doi: 10.1021/acschemneuro.0c00530. [DOI] [PubMed] [Google Scholar]
- 203.Egawa T., Kido K., Yokokawa T., Fujibayashi M., Goto K., Hayashi T. Involvement of receptor for advanced glycation end products in microgravity-induced skeletal muscle atrophy in mice. Acta Astronautica. 2020;176:332–340. doi: 10.1016/j.actaastro.2020.07.002. [DOI] [Google Scholar]
- 204.Liu Y., Shen W., Chen Q., Cao Q., Di W., Lan R., Chen Z., Bai J., Han Z., Xu W. Inhibition of RAGE by FPS-ZM1 alleviates renal injury in spontaneously hypertensive rats. Eur. J. Pharmacol. 2020;882 doi: 10.1016/j.ejphar.2020.173228. [DOI] [PubMed] [Google Scholar]
- 205.Gasparotto J., Ribeiro C.T., Bortolin R.C., Somensi N., Rabelo T.K., Kunzler A., Souza N.C., Pasquali M.A.B., Moreira J.C.F., Gelain D.P. Targeted inhibition of RAGE in substantia nigra of rats blocks 6-OHDA–induced dopaminergic denervation. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-09257-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Giridharan V.V., Generoso J.S., Collodel A., Dominguini D., Faller C.J., Tardin F., Bhatti G.S., Petronilho F., Dal-Pizzol F., Barichello T. Receptor for advanced glycation end products (RAGE) mediates cognitive impairment triggered by Pneumococcal meningitis. Neurotherapeutics. 2020 doi: 10.1007/s13311-020-00917-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Arumugam T., Ramachandran V., Gomez S.B., Schmidt A.M., Logsdon C.D. S100P-derived RAGE antagonistic peptide reduces tumor growth and metastasis. Clin. Cancer Res. 2012;18:4356–4364. doi: 10.1158/1078-0432.CCR-12-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hofmann M.A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M.F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A.M. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 209.Liu Y., Yu M., Zhang Z., Yu Y., Chen Q., Zhang W., Zhao X. Blockade of receptor for advanced glycation end products protects against systolic overload-induced heart failure after transverse aortic constriction in mice. Eur. J. Pharmacol. 2016;791:535–543. doi: 10.1016/j.ejphar.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 210.Dang M., Zeng X., Chen B., Wang H., Li H., Liu Y., Zhang X., Cao X., Du F., Guo C. Soluble receptor for advance glycation end-products inhibits ischemia/reperfusion-induced myocardial autophagy via the STAT3 pathway. Free Radic. Biol. Med. 2019;130:107–119. doi: 10.1016/j.freeradbiomed.2018.10.437. [DOI] [PubMed] [Google Scholar]
- 211.Zhang F., Su X., Huang G., Xin X.F., Cao E.H., Shi Y., Song Y. sRAGE alleviates neutrophilic asthma by blocking HMGB1/RAGE signalling in airway dendritic cells. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Park L., Raman K.G., Lee K.J., Lu Y., Ferran L.J., Jr., Chow W.S., Stern D., Schmidt A.M. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 213.Goova M.T., Li J., Kislinger T., Qu W., Lu Y., Bucciarelli L.G., Nowygrod S., Wolf B.M., Caliste X., Yan S.F., Stern D.M., Schmidt A.M. Blockade of receptor for advanced glycation end-products restores effective wound healing in diabetic mice. Am. J. Pathol. 2001;159:513–525. doi: 10.1016/S0002-9440(10)61723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wendt T., Harja E., Bucciarelli L., Qu W., Lu Y., Rong L.L., Jenkins D.G., Stein G., Schmidt A.M., Yan S.F. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–77. doi: 10.1016/j.atherosclerosis.2005.06.013. [DOI] [PubMed] [Google Scholar]